Abstract
Nonalcoholic steatohepatitis (NASH) is commonly found in patients with obesity and is often accompanied with abnormally elevated levels of plasma leptin, i.e. hyperleptinemia. A relatively high population of NASH patients develops hepatic fibrosis, even cirrhosis. Hepatic stellate cells (HSCs) are the major effector cells during liver fibrogenesis and could be activated by leptin. The antioxidant curcumin, a phytochemical from turmeric, has been shown to suppress HSC activation in vitro and in vivo. This project is to evaluate the effect of curcumin on leptin-induced HSC activation and to elucidate the underlying mechanisms. We hypothesize that curcumin abrogates the stimulatory effect of leptin on HSC activation by interrupting leptin signaling and attenuating leptin-induced oxidative stress. Curcumin eliminates the stimulatory effects of leptin on regulating expression of genes closely relevant to HSC activation. Curcumin interrupts leptin signaling by reducing phosphorylation levels of leptin receptor (Ob-R) and its downstream intermediators. In addition, curcumin suppresses gene expression of Ob-R in HSCs, which requires the activation of endogenous peroxisome proliferator-activated receptor-γ and de novo synthesis of glutathione. In conclusion, our results demonstrate that curcumin abrogates the stimulatory effect of leptin on HSC activation in vitro by reducing the phosphorylation level of Ob-R, stimulating peroxisome proliferator-activated receptor-γ activity, and attenuating oxidative stress, leading to the suppression of Ob-R gene expression and interruption of leptin signaling. These results provide novel insights into therapeutic mechanisms of curcumin in inhibiting HSC activation and intervening liver fibrogenesis associated with hyperleptinemia in NASH patients.
Curcumin eliminates the stimulatory impact of leptin on the activation of hepatic satellite cells by interrupting leptin signaling and attenuating leptin-induced oxidative stress.
Nonalcoholic steatohepatitis (NASH) is an advanced form of nonalcoholic fatty liver disease, featured with fat accumulation and inflammation in the liver. NASH is commonly found in obese patients (1) and is often accompanied with abnormally elevated levels of plasma leptin, i.e. hyperleptinemia (2,3). Approximately one third of NASH patients develop hepatic fibrosis and even cirrhosis (4). Accumulating evidence has shown that leptin and its receptor play critical roles in the development of hepatic fibrosis in animal models with NASH (5,6,7).
The hormone leptin is an obese gene (ob) product mainly produced by adipocytes (8). It is a central mediator in a negative feedback loop regulating energy homeostasis (3). Leptin receptor (Ob-R) is a member of the family of cytokine receptors. Binding of leptin to Ob-Rb, one of the five known isoforms of Ob-Rs, induces its dimerization, leading to the phosphorylation and activation of the receptor and its downstream intermediators, including Janus kinase (JAK)-signal transducer and activator of transcription (STAT) 3, and MAPKs (9), and to the subsequent transcriptional regulation of target genes (10). Homozygous defect of leptin (ob/ob) or its receptor (db/db in mouse, or fa/fa in Zucker rat) causes obese phenotype. Leptin-deficient mice fail to develop hepatic fibrosis during steatohepatitis or in response to chronic toxic liver injury (7). Restitution of physiological levels of circulating leptin by injecting exogenous leptin restores liver fibrosis caused by dietary manipulations in leptin-deficient mice. However, human obese individuals are leptin resistant (3). Serum leptin levels are elevated in patients with alcoholic cirrhosis and are related to the degree of hepatic fibrosis (5,6). Ob-R is universally distributed in extraneural tissues, which reflects the multiplicity of biological effects of leptin (3). Increased expression of Ob-R is found in patients with NASH, especially those with hepatic fibrosis (11). Ob-R-deficient Zucker rats (fa/fa) retard the development of liver fibrosis (12). These observations collectively indicate the significance and essential of leptin and Ob-R in the development of hepatic fibrosis.
During hepatic fibrogenesis the production of extracellular matrix (ECM) components surpasses their degradation in the liver. Hepatic stellate cells (HSCs) are the primary source of the excessive production of ECM during hepatic fibrogenesis, regardless of etiology (13). During hepatic injury, quiescent HSCs undergo profound phenotypical changes, including enhanced cell proliferation, de novo expression of α-smooth muscle actin (α-SMA), and excessive production of ECM. This process is called HSC activation. Culturing quiescent HSCs on plastic plates causes spontaneous activation, mimicking the process seen in vivo, which provides a good model for elucidating underlying mechanisms of HSC activation and studying potential therapeutic intervention of the process (13). In the last decade, advances in the understanding of genes promoting HSC activation are impressive. However, there are few breakthroughs in the therapeutic intervention of hepatic fibrogenesis. Therefore, research identifying antifibrogenic agents that are innocuous is of high priority and is urgently needed. Most evolving antifibrogenic therapies will be aimed at inhibiting HSC activation.
The antioxidant curcumin is one of the most-studied natural compounds. Although the underlying mechanisms remain largely elusive, curcumin has shown its diverse and beneficial effects (14). Curcumin has recently received attention as a promising dietary supplement for liver protection (15). We recently showed that curcumin inhibited HSC activation, including inducing gene expression of endogenous peroxisome proliferator-activated receptor-γ (PPARγ) and suppressing gene expression of αI(I) collagen, α-SMA, connective tissue growth factor (CTGF), and receptors for TGF-β, platelet-derived growth factor (PDGF)-β, and epidermal growth factor, and suppressed hepatic fibrogenesis in vitro and in vivo (15,16,17,18,19).
Prior studies have shown that abnormal levels of leptin stimulate HSC activation (5,6,11,20,21,22). The purposes of this study were to evaluate the role of curcumin in inhibiting leptin-activated HSCs and elucidate the underlying mechanisms. Results in this report supported our initial hypothesis that curcumin might eliminate the stimulatory effects of leptin on HSC activation by interrupting leptin signaling and attenuating leptin-induced oxidative stress.
Materials and Methods
Chemicals
Recombinant rat leptin, curcumin (purity >94%), l-buthionine-sulfoximine (BSO), and N-acetyl-l-cysteine (NAC) were purchased from Sigma-Aldrich Corp. (St. Louis, MO). 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2), a natural PPARγ agonist, was a product from Cayman Chemical Co. (Ann Arbor, MI). PD68235, a specific PPARγ antagonist, was generously provided by Pfizer (Ann Arbor, MI).
Isolation and culture of HSCs
Primary HSCs were isolated from male Sprague Dawley rats (200–250 g), and cultured HSCs were used at passage 4–8, as we previously described (17). Animals were housed in a temperature and humidity controlled room with a 12-h light, 12-h dark cycle, and with free access to standard pellet diet and drinking water in the animal house of the School of Medicine, Saint Louis University. The animal protocol followed the regulations in the Animal Welfare Act, and the principles in the U.S. Interagency document entitled “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training,” and the standards in the Guide for the Care and Use of Laboratory Animals.
Immunoprecipitation and Western blotting analyses
Preparation of whole cell extracts, immunoprecipitation assays (IPs), and Western blotting analyses were performed as we previously described (23). Protein concentrations were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL). A total of 300 μg whole cell extracts were used for immunoprecipitation. Primary rabbit polyclonal antibodies (Abs) against PDGF-β receptor (PDGF-βR) (sc-432, 1:500 dilution), Cyclin D1 (sc-718, 1:500), Bax (sc-493, 1:500), Bcl-2 (sc-492, 1:500), TGF-β receptor (TGF-βR) I (sc-399, 1:500), TGF-βRII (sc-400, 1:500), αI(I) pro-collagen (sc-30136, 1:500), leptin (Ob) (sc-9041, 1:125), p-Ob-R (sc-16421-R, 1:125), total Ob-R (sc-8325, 1:125), p-ERK1/2 (sc-16982-R, 1:500), total ERK1 (sc-94, 1:500), total ERK2 (sc-153, 1:500), p-AKTser 473 (sc-7985-R, 1:500), total AKT(sc-8312, 1:500), p-c-Jun N-terminal kinase (JNK) (sc-12882-R, 1:500), total JNK (sc-474, 1:500), p-JAK2 (sc-16566-R, 1:250), total JAK2 (sc-294, 1:250), and total STAT3 (sc-482, 1:250), goat polyclonal Abs against PPARγ (sc-1984, 1:250), CTGF (sc-14939, 1:1000), mouse polyclonal Abs against p-STAT3 (sc-8059, 1:250), and the secondary goat antirabbit IgG-horseradish peroxidase (HRP) (sc-2004, 1:15000), goat antimouse IgG-HRP (sc-2005, 1:15000), and bovine antigoat IgG-HRP (sc-2350, 1:15000) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit polyclonal Abs against β-actin (A2066, 1:10,000) and mouse monoclonal Abs against α-SMA (A2547, 1:8,000) were purchased from Sigma-Aldrich. Densities of bands in Western blotting analyses were normalized with the internal or loading control. Levels of target protein bands were densitometrically determined using Quantity One 4.4.1 (Bio-Rad Laboratories, Inc., Hercules, CA). Variations in the density were expressed as fold changes (n = 3), compared with the control in the blots.
RNA isolation and real-time PCR
Preparation of total RNA and real-time PCR assays using SYBR green were performed, as we previously described (24). Total RNA was treated with deoxyribonuclease I before the synthesis of the first strand of cDNA. mRNA levels were expressed as fold changes after normalization with endogenous glyceraldehyde-3-phosphate dehydrogenase, as suggested by Schmittgen et al. (25). Real-time PCR primers for α1(I) collagen, α-SMA, PDGF-βR, subunits of glutamate-cysteine ligase (GCL) (GCLc and GCLm), PPARγ, TGF-βRI and II, and glyceraldehyde-3-phosphate dehydrogenase were previously described (16). The following were the additional primers for: leptin, forward 5′-GAG ACC TCC TCC ATG TGC TG-3′ and reverse 5′-CAT TCA GGG CTA AGG TCC AA-3′; Ob-Ra, forward 5′-TGA TAT CGC CAA ACA GCA AA-3′ and reverse 5′-AGT GTC CGC TCT CTT TTG GA-3′; Ob-Rb, forward 5′-TGA CCA CTC CAG ATT CCA CA-3′ and reverse 5′-CCA CTG TTT TCA CGT TGC TG-3′; and Ob-Re, forward 5′-AAG TGG CTT AAA ATC CCT TCG-3′ and reverse 5′-CAA ACA TTC TGA CCA GGC AAG-3′.
Plasmids and transient transfection assays
To generate a rat Ob-R promoter luciferase reporter plasmid, rat genomic DNA was isolated from cultured HSCs and used as a template for PCR amplification of the Ob-R promoter region (−1469/+30) (GenBank accession no. NM-010704). The primers for PCR were: forward 5′-TGG GGT ACC CCA GCA GGA TTG AGCC TCA AAG GTG CC-3′ and reverse 5′-GCC GCT AGC GGC TCT ATG CTG CTG GCT CAG AAG GTG C-3′. The primers were tailed with a KpnI site (the forward primer) or NheI site (the reverse primer). The 1.5-kb PCR product was digested with KpnI and NheI, and subcloned into the luciferase reporter vector pGL3-(basic) (Promega Corp., Madison, WI). The insert was confirmed by DNA sequencing. The cDNA expressing plasmid pPPARγcDNA contained a full fragment of cDNA encoding human PPARγ (a gift from Dr. Reed Graves, Department of Medicine, University of Chicago, Chicago, IL). Semi-confluent HSCs in six-well cell culture plates were transiently transfected using the Lipofectamine reagent (Invitrogen Corp., Carlsbad, CA), as we previously described (17). Each sample was in triplicate in every experiment. Transfection efficiency was controlled by cotransfection of the β-galactosidase reporter plasmid, pSV-β-gal (0.5 μg/well) (Promega). β-Galactosidase activities were measured using a chemiluminescence assay kit (Tropix, Bedford, MA). Luciferase activities were presented in arbitrary units, i.e. ODs per microgram of protein after normalization with β-galactosidase activities.
Determination of levels of intracellular reactive oxygen species (ROS)
Levels of ROS in HSCs were determined by analyzing dichlorofluorescein fluorescence, as described previously (26).
Analyses of lipid peroxidation (LPO)
LPO assays were performed using the Lipid Hydroperoxide Assay Kit purchased from Cayman Chemical (26).
Glutathione (GSH) assays
Levels of reduced GSH and oxidized glutathione (GSSG) were determined using the enzyme immune assay kit GSH-400 (Cayman Chemical) (26).
Analyses of GCL activity
Activities of GCL were spectrophotometrically determined using a coupled assay with pyruvate kinase and lactate dehydrogenase, as we previously described (26).
Statistical analyses
Differences between means were evaluated using an unpaired two-sided Student’s t test (P < 0.05 considered as significant). Where appropriate, comparisons of multiple treatment conditions with controls were analyzed by ANOVA with the Dunnett’s test for post hoc analysis.
Results
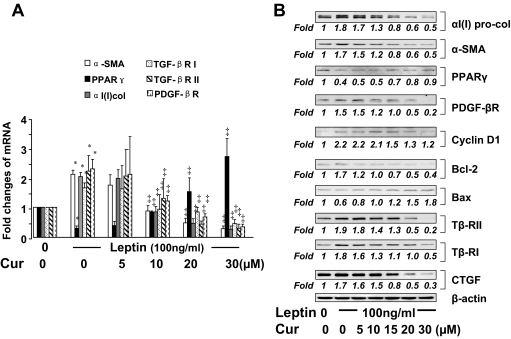
Curcumin eliminates the effects of leptin on regulating expression of genes relevant to HSC activation
Prior studies have shown that leptin stimulates the activation of HSCs in autocrine and paracrine actions (20,21). To evaluate the role of curcumin in inhibiting leptin-induced HSC activation, after serum starvation for 24 h, passaged HSCs were treated with leptin at 100 ng/ml plus or minus curcumin at various concentrations in serum-depleted media for another 24 h. Pilot experiments indicated that serum starvation rendered HSCs more sensitive to exogenous leptin (our unpublished observations). The subsequent culture in serum-depleted media excluded the interference from other factors in fetal bovine serum (FBS) (18,27). Total RNA and whole cell extracts were prepared from the cells for real-time PCR (Fig. 1A) and Western blotting analyses (Fig. 1B). As shown in Fig. 1, compared with the untreated control (first columns or wells), exogenous leptin stimulated, as expected, gene expression of αI(I) collagen and α-SMA (second columns or wells), the markers of activated HSCs, and expression of genes closely relevant to HSC activation, including pro-mitogenic PDGF-βR, Cyclin D1, pro-apoptotic Bcl-2, and pro-fibrogenic TGF-βRI and TGF-βRII, as well as CTGF. In great contrast, leptin significantly suppressed gene expression of PPARγ, an endogenous inhibitor of HSC activation, and reduced the abundance of antiapoptotic Bax. Further results indicated that curcumin dose dependently diminished the effects of leptin on regulating expression of the genes relevant to the activation of HSCs. It is noteworthy that curcumin induced gene expression of endogenous PPARγ suppressed by leptin, which has been observed to play a critical role in the curcumin-caused inhibition of HSC activation (17,18). Together, these results demonstrated that curcumin eliminated the effects of leptin on regulating expression of genes relevant to HSC activation.
Figure 1.
Curcumin (Cur) eliminates the effects of leptin on regulating expression of genes relevant to HSC activation. Serum-starved passaged HSCs were treated with or without leptin at 100 ng/ml plus curcumin at various concentrations in serum-depleted media for 24 h. Total RNA and whole cell extracts were prepared. A, Real-time PCR assays of mRNA of representative genes relevant to HSC activation. Values were presented as mean ± sd (n = 3). *, P < 0.05 vs. the untreated control (corresponding first column on the left); ‡, P < 0.05 vs. cells treated with leptin only (corresponding second column). B, Western blotting analyses of proteins of genes relevant to HSC activation. β-Actin was used as an invariant control for equal loading. Representatives were from three independent experiments. Italic numbers were fold changes in densities of the bands compared with the control with no treatment (first well) after normalization (n = 3). Because of the limited space, sd values were not presented. Tβ-RI, TGF-βRI; Tβ-RII, TGF-βRII.
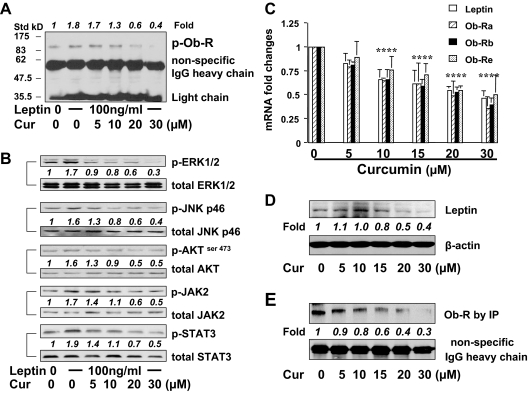
Curcumin interrupts leptin signaling in activated HSCs by reducing the phosphorylation levels of Ob-R and its downstream intermediators, and suppressing gene expression of leptin and its receptors
To explore the mechanism by which curcumin eliminated the roles of leptin in the activation of HSCs in vitro, we hypothesized that curcumin might interrupt leptin signaling. To test the hypothesis, serum-starved HSCs were pretreated with curcumin at indicated concentrations for 1 h before the stimulation with leptin at 100 ng/ml for an additional 30 min. Pilot experiments revealed that leptin rapidly activated its signaling and reached its peak within 20–30 min (data not shown here). Whole cell extracts were prepared. Total Ob-R was immunoprecipitated by Abs against Ob-R from total cell proteins (300 μg). The phosphorylation levels of Ob-R were evaluated by Western blotting analyses using Abs against phosphorylated Ob-R. It was observed that leptin significantly stimulated, as expected, tyrosine phosphorylation of Ob-R (Fig. 2A, second well), compared with the untreated control (first well), which led to increased phosphorylation of its downstream intermediators, including ERK1/2, AKTser473, JNK, JAK2, and STAT3 (Fig. 2B, second wells). The pretreatment of cells with curcumin apparently reduced the phosphorylation levels of Ob-R (Fig. 2A, third to sixth wells), as well as the downstream intermediators (Fig. 2B, third to sixth wells), in a dose-dependent manner. These results indicated that curcumin blocked leptin signaling by reducing the phosphorylation of Ob-R and its downstream intermediators.
Figure 2.
A and B, Curcumin (Cur) interrupts leptin signaling pathways in activated HSCs by reducing phosphorylation levels of Ob-R and its downstream intermediators, and suppressing gene expression of leptin and its receptors. Serum-starved HSCs were pretreated with curcumin at indicated concentrations for 1 h before the stimulation with leptin at 100 ng/ml for an additional 30 min. Whole cell extracts were prepared. Representatives were from three independent experiments. Italic numbers were fold changes in densities of the bands compared with the control with no treatment (corresponding first well) after normalization (n = 3). A, Western blotting analyses of phosphorylated (p)-Ob-R from immunoprecipitated total Ob-R. Nonspecifically recognized heavy chain of IgG was used for normalization. Molecular weights of standards (Std) were presented on the left. B, Western blotting analyses of phosphorylated (p)-ERK1/2, JNK-p46, AKTser473, JAK2, and STAT3. Corresponding total proteins were used as internal controls for normalization. C–E, Passaged HSCs were treated with curcumin at indicated concentrations for 24 h. Total RNA and whole cell extracts were prepared. Representatives from three independent Western blotting analyses were presented. Italic numbers beneath blots were fold changes in densities of the bands compared with the control without treatment in the blot (n = 3), after normalization. C, Real-time PCR assays of mRNA of leptin and three isoforms of Ob-Rs. Values were presented as mean ± sd (n = 3). *, P < 0.05 vs. the untreated control (the corresponding first column on the left). D, Western blotting analyses of leptin. β-Actin was used as an invariant control for equal loading and normalization. E, Western blotting analyses of immunoprecipitated Ob-R. Nonspecifically recognized heavy chain of IgG was used for normalization.
Additional experiments were performed to determine the role of curcumin in regulating gene expression of leptin and its receptors in HSCs. Passaged HSCs were treated with curcumin at various concentrations as indicated for 24 h. Total RNA and whole cell extracts were prepared from the cells. Real-time PCR assays demonstrated that curcumin dose dependently reduced the steady-state levels of mRNA of leptin and different isoforms of Ob-Rs, including Ob-Ra, Ob-Rb, and Ob-Re, in cultured HSCs (Fig. 2C). This observation was confirmed by Western blotting analyses of leptin (Fig. 2D) and by IPs of Ob-R (Fig. 2E). These results revealed that curcumin significantly suppressed gene expression of leptin and Ob-R in activated HSCs in vitro. Because the processes of transcription and translation were involved, it took several hours for curcumin to show its significant impact on the suppression of gene expression of leptin and Ob-R (data not shown), which lasted, however, no less than 24 h. A major difference between experiments in Fig. 2, A and E, was the duration of the curcumin treatment. Cells in Fig. 2E were treated with curcumin for 24 h. In contrast, cells in Fig. 2A were treated with curcumin only for a total of 1.5 h, which was too short to show differences in Ob-R abundance. Together, these results demonstrated that in addition to its instant action to reduce the phosphorylation levels of Ob-R and its downstream intermediators, curcumin dose dependently suppressed gene expression of leptin and Ob-R. These two-step actions respectively resulted in the instant and long-lasting interruption of leptin signaling in activated HSCs in vitro.
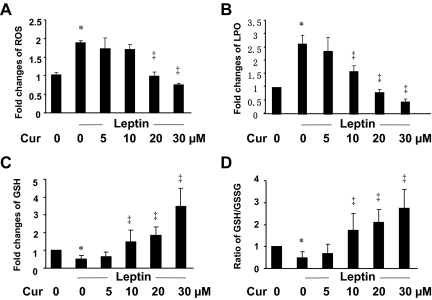
Leptin induces oxidative stress in HSCs, which is dose dependently attenuated by curcumin
The underlying mechanisms by which leptin stimulates HSC activation remain largely to be defined. Leptin induces oxidative stress in many pathological conditions and cells (28,29,30). Oxidative stress is a major factor to stimulate HSC activation and hepatic fibrogenesis (31,32). We assumed that leptin stimulated HSC activation by inducing oxidative stress, which was attenuated by curcumin by increasing the level of cellular GSH, the most abundant thiol antioxidant in mammalian cells (33). To test the assumption, serum-starved HSCs were stimulated with or without leptin at 100 ng/ml for 24 h. The levels of cellular ROS, LPO, GSH, and the ratio of reduced GSH vs. GSSG, the index of cellular oxidative stress, were determined. As shown in Fig. 3, compared with the untreated control (first columns), leptin significantly increased the levels of ROS (Fig. 3A) and LPO (Fig. 3B), and reduced the contents of cellular GSH (Fig. 3C) and the ratio of GSH to GSSG (Fig. 3D) (second columns). These results indicated that leptin induced oxidative stress in cultured HSCs.
Figure 3.
Curcumin (Cur) eliminates the effects of leptin on the induction of oxidative stress by reducing the levels of cellular ROS and LPO, increasing the contents of cellular GSH and improving the ratio of GSH to GSSG in cultured HSCs. Serum-starved HSCs were stimulated with or without leptin at 100 ng/ml plus curcumin at indicated concentrations in serum-depleted media for 24 h. Cell extracts were prepared for assays. Values were presented as fold changes (mean ± sd, n = 3), compared with the untreated control (corresponding first column on the left). *, P < 0.05 vs. the untreated control (first column); ‡, P < 0.05 vs. cells treated with leptin only (second column). A, Analyses of ROS levels. B, Analyses of LPO levels. C, Analyses of GSH contents. D, Determination of the ratio of GSH to GSSG.
Additional experiments were conducted to evaluate the role of curcumin in attenuating leptin-induced oxidative stress. Serum-starved HSCs were stimulated with leptin at 100 ng/ml in the presence of curcumin at various concentrations in serum-depleted media for 24 h. As shown in Fig. 3, curcumin dose dependently attenuated leptin-caused oxidative stress by reducing the levels of ROS (Fig. 3A) and LPO (Fig. 3B) in cultured HSCs. In addition, the phytochemical increased the contents of cellular GSH (Fig. 3C) and improved the radio of GSH to GSSG (Fig. 3D). Together, these results demonstrated that leptin induced oxidative stress in HSCs, which was, in a dose-dependent manner, attenuated by curcumin by increasing the levels of cellular GSH.
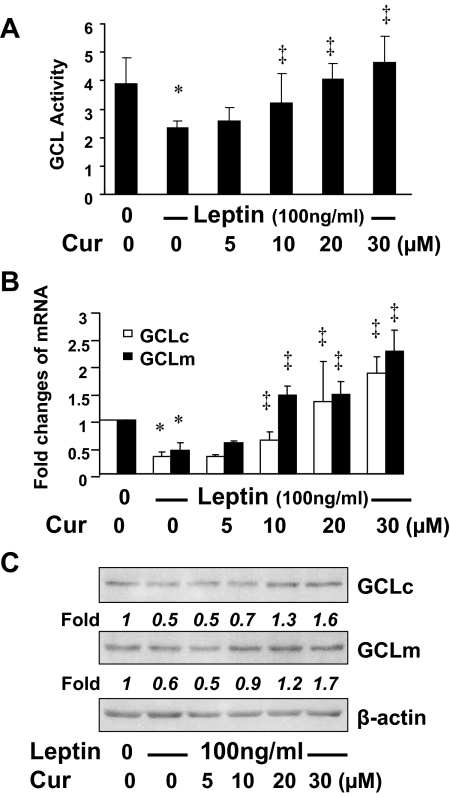
Curcumin increases GCL activities in activated HSCs in vitro by inducing gene expression of GCLc and GCLm
GCL is a rate-limiting enzyme in de novo synthesis of GSH in mammalian cells (34). To explore the mechanisms by which leptin induced oxidative stress and curcumin attenuated the oxidative stress, we postulated that leptin inhibited GCL activities in HSCs, which was eliminated by curcumin by inducing gene expression of GCL, leading to de novo synthesis of GSH and the attenuation of leptin-induced oxidative stress. To test the postulation, serum-starved HSCs were stimulated with leptin at 100 ng/ml with or without curcumin at various concentrations in serum-free media for 24 h. Cell extracts were prepared for analyses of GCL activities. As shown in Fig. 4A, compared with the untreated control (first column on the left), leptin dramatically decreased GCL activities (second column). Curcumin dose dependently eliminated the inhibitory effect and increased GCL activities in activated HSCs in vitro.
Figure 4.
Curcumin (Cur) increases GCL activities in activated HSCs in vitro by inducing gene expression of GCLc and GCLm. Serum-starved HSCs were stimulated with or without leptin at 100 ng/ml plus curcumin at indicated concentrations in serum-depleted media for 24 h. Assays were performed. *, P < 0.05 vs. the untreated control (corresponding first column); ‡, P < 0.05 vs. cells treated with leptin only (corresponding second column). A, Analyses of GCL activities. Values were presented as fold changes (mean ± sd, n = 3), compared with the untreated control (first column on the left). B, Real-time PCR analyses of GCLc and GCLm mRNA (n = 3). C, Western blotting analyses of GCLc and GCLm proteins. β-Actin was used as an invariant control for equal loading. Representatives were from three independent experiments. Italic numbers beneath blots were fold changes in densities of the bands compared with the control without treatment in the blot (n = 3), after normalization.
To understand the underlying mechanisms, further experiments were conducted using total RNA and whole cell extracts from HSCs with the aforementioned treatment. The enzyme of GCL is composed of a large catalytic subunit (GCLc, ∼73 kDa) and a small modulatory subunit (GCLm, ∼30 kDa), which are encoded by two different genes (35). Real-time PCR and Western blotting analyses demonstrated, respectively, that leptin significantly reduced the levels of mRNA (Fig. 4B) and proteins (Fig. 4C) of GCLc and GCLm (corresponding second columns or wells), compared with the control (first columns or wells), in the cells. Curcumin dose dependently eliminated this inhibitory effect and induced gene expression of GCLc and GCLm. These results collectively demonstrated that curcumin eliminated the inhibitory effect of leptin on GCL activities in activated HSCs by inducing gene expression of GCLc and GCLm, leading to the elevation of GSH contents and the attenuation of oxidative stress.
De novo synthesis of GSH is required for curcumin to suppress gene expression of Ob-R in activated HSCs in vitro
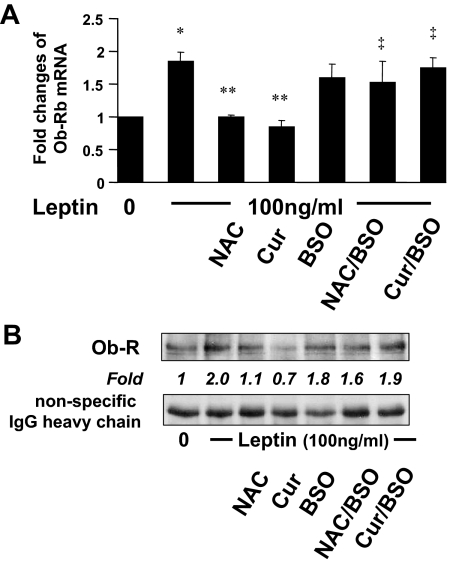
Additional experiments were performed to evaluate the role of the curcumin-caused increase in the level of cellular GSH in the suppression of Ob-R in HSCs. Cellular GSH contents were altered by two well-known manipulators of GSH synthesis. N-acetyl-cysteine (NAC) was a precursor of GSH, increasing GSH contents by supplying cysteine (36). BSO was a specific inhibitor of GCL, which could deplete cellular GSH (37). Serum-starved HSCs were divided into two groups. One was treated with leptin (100 ng/ml) plus curcumin (20 μm), or NAC (5 mm), in serum-depleted media for 24 h. The other group was pretreated with BSO (0.25 mm) for 1 h before the treatment with leptin plus curcumin (20 μm) or NAC (5 mm) in serum-depleted media for an additional 24 h. Serum-starved HSCs without treatment were used as an untreated control. Total RNA or whole cell extracts were prepared. As shown by real-time PCR (Fig. 5A) and IP plus Western blotting analyses (Fig. 5B), leptin induced, as expected, gene expression of Ob-R in cultured HSCs (second column, or well), compared with the untreated control (first column, or well). NAC (third column, or well), mimicking curcumin (fourth column, or well), significantly eliminated the inductive role of leptin and reduced the levels of the transcript (Fig. 5A) and protein of Ob-R (Fig. 5B) in HSCs. Pre-exposure of cells to BSO apparently diminished the inhibitory effect of both NAC and curcumin (last two columns or wells). Together, these results demonstrated that de novo synthesis of GSH was required for curcumin to inhibit gene expression of Ob-R in activated HSCs in vitro.
Figure 5.
The depletion of cellular GSH by BSO eliminates the suppressive effect of curcumin (Cur) on gene expression of Ob-R in activated HSCs in vitro. Serum-starved HSCs were divided into two groups. In one group, cells were stimulated with or without leptin (100 ng/ml) plus curcumin (20 μm) or NAC (5 mm) in serum-free media for 24 h. In the other group, cells were pretreated with BSO (0.25 mm) for 1 h before the addition of leptin (100 ng/ml) plus curcumin (20 μm) or NAC (5 mm) in serum-free media for an additional 24 h. Total RNA or whole cell extracts were prepared. A, Real-time PCR analyses of Ob-R mRNA (n = 3). *, P < 0.05 vs. the untreated control (first column); **, P < 0.05 vs. cells treated with leptin only (second column); ‡, P < 0.05, vs. cells treated with leptin plus NAC or curcumin (third or fourth column, respectively). B, Western blotting analyses of immunoprecipitated Ob-R. Representatives were from three independent experiments. Italic numbers were fold changes in densities of the bands compared with the untreated cells (first well) after normalization with nonspecifically recognized heavy chain of IgG (n = 3).
The activation of PPARγ by curcumin mediates the effect of the phytochemical on the suppression of gene expression of Ob-R in activated HSCs in vitro
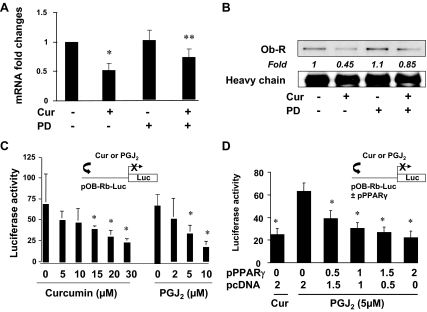
We previously demonstrated that curcumin induced gene expression of PPARγ and stimulated its activity in activated HSCs (17,18). We assumed that the curcumin-enhanced PPARγ activity mediated the effect of curcumin on the inhibition of Ob-R gene expression in activated HSCs. To test the assumption, passaged HSCs were, first of all, pretreated with or without PD68235 (20 μm), a specific PPARγ antagonist, for 30 min before the exposure to curcumin (20 μm) for an additional 24 h. Total RNA or whole cell extracts were prepared for real-time PCR and Western blotting analyses. As shown in Fig. 6A, compared with the untreated control (first column on the left), curcumin significantly decreased, as expected, the steady-state level of Ob-R mRNA in HSC (second column). The inhibition of PPARγ activity by pre-exposing cells to PD68235 apparently eliminated this inhibitory effect of curcumin (fourth column). Results of Western blotting analyses in Fig. 6B confirmed the observation. These results collectively indicated that the inhibition of PPARγ activity eliminated the inhibitory effect of curcumin on Ob-R gene expression, suggesting the requirement of PPARγ activation in this process.
Figure 6.
The activation of PPARγ by curcumin (Cur) mediates the effect of the phytochemical on the suppression of gene expression of Ob-R in activated HSCs in vitro. A and B, Semi-confluent HSCs were pretreated with or without PD68235 (PD) (20 μm) for 30 min before the exposure to curcumin (20 μm) for an additional 24 h. Total RNA or whole cell extracts were prepared. A, Real-time PCR assays of Ob-R mRNA (n = 3). *, P < 0.05 vs. the untreated control (first column); **, P < 0.05 vs. cells treated with leptin only (second column). B, Western blotting analyses of immunoprecipitated Ob-R. Italic numbers were fold changes in densities of the bands compared with the control with no treatment (first well) after normalization with nonspecifically recognized heavy chain of IgG (n = 3). C and D, HSCs were transiently transfected with indicated plasmids. After recovery, cells were treated with curcumin (20 μm) in DMEM with FBS (10%), or with PGJ2 (5 μm) in serum-depleted media, for 24 h. Luciferase activities were expressed as relative units after β-galactosidase normalization (means ± sd, n ≥ 6). The floating schema denotes plasmid(s) in use and the application of curcumin or PGJ2 to the system. C, HSCs were transfected with pOb-R-Luc. *, P < 0.05 vs. the untreated control (corresponding first column of each group). D, HSCs were cotransfected with pOb-R-Luc and pPPARγcDNA at indicated doses. *, P < 0.05 vs. the untreated control (second column).
To further evaluate the role of PPARγ activity in the inhibition of Ob-R gene expression, HSCs in six-well culture plates were transfected with the Ob-R promoter luciferase reporter plasmid pOb-R-Luc. After recovery, one group of cells was treated with curcumin in DMEM with FBS (10%) for 24 h. Prior experiments have suggested that 10% FBS in the media contains enough agonists to activate PPARγ in HSCs (17,18,38). Another group of the transfected cells was treated with PGJ2, a natural PPARγ agonist, in serum-depleted media for 24 h. Luciferase activity assays in Fig. 6C indicated that PGJ2 (right panel), like curcumin (left panel), dose dependently reduced luciferase activities, suggesting that the activation of endogenous PPARγ by PGJ2 inhibited the promoter activity of Ob-R gene in HSCs.
To verify the role of PPARγ activation in suppressing Ob-R gene expression, HSCs were cotransfected with pOb-R-Luc and the PPARγ cDNA expression plasmid pPPARγcDNA. A total of 4.5 μg of a DNA mixture was added to each well of cells in six-well culture plates, including 2 μg pOb-R-Luc, 0.5 μg pSV-β-gal, and 2.0 μg pPPARγcDNA at various doses plus the empty vector pcDNA. The latter was used to ensure an equal amount of total DNA in transfection assays. After recovery, cells were treated with curcumin (20 μm) in DMEM with FBS (10%), or with PGJ2 (5 μm) in serum-depleted media, for 24 h. As shown in Fig. 6D by luciferase assays, forced expression of exogenous PPARγ cDNA, like curcumin (first column on the left), dose dependently reduced luciferase activities, confirming the role of PPARγ activation in inhibiting the promoter activity of Ob-R gene in HSCs. Together, these results showed that the activation of PPARγ by curcumin mediated the inhibitory effect of curcumin on Ob-R gene expression in activated HSCs in vitro.
Discussion
It was reported that at concentrations seen in nonobese individuals (leptin < 10 ng/ml), leptin did not affect expression of types I and III collagen, matrix metalloproteinase-1, or tissue inhibitor of metalloproteinase-1 in cultured human HSCs (22). At concentrations seen in obesity (30–50 ng/ml), leptin caused a significant increase in collagen production in HSCs by activating the JAK and phosphatidylinositol 3-kinase pathways. In addition, leptin dose dependently (0–100 ng/ml) facilitated HSC proliferation through up-regulation of PDGF receptor (39). Prior studies have collectively and strongly suggested the roles of hyperleptinemia and leptin in stimulating HSC activation and hepatic fibrogenesis (5,6,11,20,21). We demonstrated in this paper that curcumin abrogated the stimulatory effect of leptin on HSC activation in vitro by interrupting leptin signaling. Curcumin suppressed Ob-R gene expression by inducing PPARγ activity and attenuating oxidative stress.
In this report, curcumin showed two steps to interrupt leptin signaling. Curcumin reduced the phosphorylation levels of Ob-R and its downstream intermediators. This action was instant, but short term. On the other hand, curcumin suppressed gene expression of Ob-R, leading to the reduction in the bioavailability of Ob-R to the ligand leptin. This action was relatively slow, experiencing the processes of transcript and translation, but lasted a longer time for at least 24 h. Prior studies have shown similar roles of curcumin in interrupting signal transduction pathways for PDGF, epidermal growth factor, and TGF-β (17,27,40). Curcumin inhibits the phosphorylation of receptors for PDGF-β and TGF-β, as well as suppresses gene expression of the receptors in activated HSCs in vitro. These results suggest that curcumin might be a broad spectrum inhibitor and nonspecifically interrupt signaling pathways involved in activation of HSCs. The underlying mechanisms remain elusive. Other studies have indicated the role of GSH in modulating the activity of protein tyrosine phosphatase 1B (41). We previously observed that it took several hours for curcumin to increase the level of cellular GSH in HSCs (26). Because curcumin could reduce the phosphorylation levels of Ob-R and its downstream intermediators within 20 min, it was unlikely that this inhibitory effect of curcumin resulted from the elevation of the level of cellular GSH. We recently observed that curcumin dramatically and instantly reduced the level of cellular Ca2+ in cultured HSCs (our unpublished observations). Compelling evidence has indicated the role of calcium in regulating activities of protein tyrosine kinases and phosphatases (42,43,44). Additional experiments are necessary to explore the mechanisms by which curcumin acts as a protein tyrosine kinase inhibitor and/or a phosphatase activator to reduce the phosphorylation levels of Ob-R and its downstream signaling intermediators.
The reduction in the level of PPARγ coincides with HSC activation (38,45,46). Activation of PPARγ results in the inhibition of HSC activation. Curcumin induces expression of endogenous PPARγ gene and stimulates its activity in activated HSCs in vitro (17), leading to the inhibition of HSC activation (17,18). In this report we demonstrated that the curcumin-caused inhibition of Ob-R gene expression was mediated by the activation of PPARγ. Our results were consistent with other prior studies (47). Expression of Ob-R in cultured HSCs was induced by leptin, and was suppressed by ciglitazone, a synthesized PPARγ agonist. Additional experiments are necessary to elucidate the underlying molecular mechanisms.
It is well documented that PPARγ regulates expression of genes involved in lipid synthesis (48). A major concern in this project was whether the curcumin-stimulated PPARγ activity and the interruption of leptin signaling would deteriorate the state of hepatic steatosis in vivo. Hepatocytes are the major place for fat accumulation during steatosis. HSCs account for only approximately 5% of the total mass of the liver. Few literature is available regarding the roles of curcumin in the biosynthesis of cellular fatty acids and triacylglycerol in hepatocytes or in HSCs. Our preliminary results indicated that curcumin stimulated biosynthesis of cellular fatty acids and triacylglycerol in cultured HSCs (our unpublished data) . However, curcumin significantly improved the state of hepatic steatosis with a significant reduction in the number of macrovesicular and microvesicular steatosis in hepatocytes in a rat model with hepatic fibrosis induced by CCl4 (16). PPARγ activity is negatively regulated by ERK and JNK (49,50). Curcumin inhibits the activities of ERK and JNK in HSCs (27,51). However, curcumin has shown distinct impacts on ERK and JNK activities in different types of cells (51,52,53,54). Therefore, curcumin could exert different impacts on PPARγ activity depending on cell types. Additional experiments are ongoing in our laboratory to evaluate the effects of curcumin on PPARγ activity and on leptin signaling in hepatocytes, to assess the roles of curcumin in the biosynthesis of cellular fatty acids and triacylglycerol, and to elucidate further the underlying mechanisms.
In addition to its physiological roles, abnormally enhanced levels of leptin may facilitate many pathological processes via oxidative stress-dependent pathways (28,29,55). Administration of leptin decreased the level of GSH and increased the content of GSSG in the rat kidney (56). Our experiments revealed that leptin induced oxidative stress in cultured HSCs by increasing the levels of cellular ROS and LPO. Oxidative stress is a major player in the process of HSC activation and in the induction of hepatic fibrogenesis, regardless of etiology (13). The beneficial effects of some commonly used food-derived products have recently attracted attention for preventing various pathological conditions. In this respect, flavonoids and other polyphenolic compounds have received a great deal of attention. Curcumin is a polyphenol with potent antioxidant capacity (57). Effects of curcumin on the attenuation of oxidative stress in cultured HSCs were recently addressed (26). We reported in this study that curcumin stimulated the GCL activity by inducing gene expression of GCL subunits in activated HSCs, leading to de novo synthesis of GSH and the attenuation of oxidative stress. Furthermore, we observed that the curcumin-elevated level of GSH was a prerequisite for the inhibition of Ob-R gene expression in activated HSCs. GSH is important in regulating activities of transcription factors and enzymes by altering cellular redox status (34). The unique feature of curcumin might allow it to succeed where other agents have failed to inhibit hepatic fibrogenesis.
Based on our observations, a simplified model is proposed to explain the inhibitory effects of curcumin on leptin-induced HSC activation. Abnormally enhanced level of leptin activates Ob-R and its downstream signaling pathways in HSCs, which induce oxidative stress, cell proliferation, and overproduction of ECM, leading to the activation of HSCs. Curcumin abrogates the stimulatory effects of leptin by interrupting leptin signaling via inhibiting the phosphorylation of Ob-R and suppressing Ob-R gene expression. The latter is mediated by stimulating PPARγ activity and attenuating oxidative stress. It bears emphasis that this model does not exclude any other mechanisms involved in the inhibitory role of curcumin. Our results in this report provide novel insights into mechanisms of curcumin, and a therapeutic candidate for the treatment and prevention of liver fibrogenesis induced by hyperleptinemia in NASH patients with obesity and/or type 2 diabetes mellitus.
Footnotes
The work was supported by the Grant RO1 DK 047995 from National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (to A.C.).
Disclosure Summary: The authors have nothing to declare.
First Published Online March 19, 2009
Abbreviations: Ab, Antibody; BSO, l-buthionine-sulfoximine; CTGF, connective tissue growth factor; ECM, extracellular matrix; FBS, fetal bovine serum; GCL, glutamate-cysteine ligase; GSH, glutathione; GSSG, oxidized glutathione; HRP, horseradish peroxidase; HSC, hepatic stellate cell; IP, immunoprecipitation assay; JAK, Janus kinase; JNK, c-Jun N-terminal kinase; LPO, lipid peroxidation; NAC, N-acetyl-l-cysteine; NASH, nonalcoholic steatohepatitis; Ob-R, leptin receptor; PDGF, platelet-derived growth factor; PDGF-βR, platelet-derived growth factor-β receptor; PGJ2, 15-deoxy-Δ12,14-prostaglandin J2; PPARγ, peroxisome proliferator-activated receptor-γ; ROS, reactive oxygen species; α-SMA, α-smooth muscle actin; STAT, signal transducer and activator of transcription; TGF-βR, transforming growth factor-β receptor.
References
- Sanyal AJ 2002 AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 123:1705–1725 [DOI] [PubMed] [Google Scholar]
- Yokaichiya DK, Galembeck E, Torres BB, Da Silva JA, de Araujo DR 2008 Insulin and leptin relations in obesity: a multimedia approach. Adv Physiol Educ 32:231–236 [DOI] [PubMed] [Google Scholar]
- Friedman JM 2004 Modern science versus the stigma of obesity. Nat Med 10:563–569 [DOI] [PubMed] [Google Scholar]
- Clark JM 2006 The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 40(Suppl 1):S5–S10 [DOI] [PubMed] [Google Scholar]
- García-Suárez C, Crespo J, Fernández-Gil PL, Amado JA, García-Unzueta MT, Pons Romero F 2004 [Plasma leptin levels in patients with primary biliary cirrhosis and their relationship with degree of fibrosis]. Gastroenterol Hepatol 27:47–50 [DOI] [PubMed] [Google Scholar]
- Henriksen JH, Holst JJ, Møller S, Brinch K, Bendtsen F 1999 Increased circulating leptin in alcoholic cirrhosis: relation to release and disposal. Hepatology 29:1818–1824 [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Farrell GC, Schriemer R, Robertson GR 2002 Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol 37:206–213 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM 1996 Abnormal splicing of the leptin receptor in diabetic mice. Nature 379:632–635 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell Jr JE, Stoffel M, Friedman JM 1996 Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14:95–97 [DOI] [PubMed] [Google Scholar]
- Cayón A, Crespo J, Mayorga M, Guerra A, Pons-Romero F 2006 Increased expression of Ob-Rb and its relationship with the overexpression of TGF-β1 and the stage of fibrosis in patients with nonalcoholic steatohepatitis. Liver Int 26:1065–1071 [DOI] [PubMed] [Google Scholar]
- Sakaida I, Jinhua S, Uchida K, Terai S, Okita K 2003 Leptin receptor-deficient Zucker (fa/fa) rat retards the development of pig serum-induced liver fibrosis with Kupffer cell dysfunction. Life Sci 73:2491–2501 [DOI] [PubMed] [Google Scholar]
- Friedman SL 2008 Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe B, Vijaykumar M, Lokesh BR 2004 Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr 44:97–111 [DOI] [PubMed] [Google Scholar]
- O'Connell MA, Rushworth SA 2008 Curcumin: potential for hepatic fibrosis therapy? Br J Pharmacol 153:403–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zheng S, Lin J, Ryerse J, Chen A 2008 Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol 73:399–409 [DOI] [PubMed] [Google Scholar]
- Xu J, Fu Y, Chen A 2003 Activation of peroxisome proliferator-activated receptor-γ contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol 285:G20–G30 [DOI] [PubMed] [Google Scholar]
- Zheng S, Chen A 2004 Activation of PPARγ is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J 384(Pt 1):149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Chen A 2006 Curcumin suppresses the expression of extracellular matrix genes in activated hepatic stellate cells by inhibiting gene expression of connective tissue growth factor. Am J Physiol Gastrointest Liver Physiol 290:G883–G893 [DOI] [PubMed] [Google Scholar]
- Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA 2002 Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology 35:762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, Vizzutti F, Anania FA, Milani S, Rombouts K, Laffi G, Pinzani M, Marra F 2005 Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology 42:1339–1348 [DOI] [PubMed] [Google Scholar]
- Choudhury J, Mirshahi F, Murthy KS, Yager DR, Sanyal AJ 2006 Physiologic concentrations of leptin increase collagen production by non-immortalized human hepatic stellate cells. Metabolism 55:1317–1322 [DOI] [PubMed] [Google Scholar]
- Chen A, Zhang L 2003 The antioxidant (-)-epigallocatechin-3-gallate inhibits rat hepatic stellate cell proliferation in vitro by blocking the tyrosine phosphorylation and reducing the gene expression of platelet-derived growth factor-β receptor. J Biol Chem 278:23381–23389 [DOI] [PubMed] [Google Scholar]
- Chen A, Zhang L, Xu J, Tang J 2002 The antioxidant (-)-epigallocatechin-3-gallate inhibits activated hepatic stellate cell growth and suppresses acetaldehyde-induced gene expression. Biochem J 368(Pt 3):695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW 2000 Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem 285:194–204 [DOI] [PubMed] [Google Scholar]
- Zheng S, Yumei F, Chen A 2007 De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic Biol Med 43:444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zheng S, Lin J, Zhang QJ, Chen A 2007 The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARγ in rat activated hepatic stellate cell in vitro. Lab Invest 87:488–498 [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Marumo T, Lafontan M, Busse R 1999 Leptin induces oxidative stress in human endothelial cells. FASEB J 13:1231–1238 [PubMed] [Google Scholar]
- Gülen S, Dinçer S 2007 Effects of leptin on oxidative stress in healthy and Streptozotocin-induced diabetic rats. Mol Cell Biochem 302:59–65 [DOI] [PubMed] [Google Scholar]
- Xu FP, Chen MS, Wang YZ, Yi Q, Lin SB, Chen AF, Luo JD 2004 Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation 110:1269–1275 [DOI] [PubMed] [Google Scholar]
- Greenwel P, Domínguez-Rosales JA, Mavi G, Rivas-Estilla AM, Rojkind M 2000 Hydrogen peroxide: a link between acetaldehyde-elicited α1(I) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. Hepatology 31:109–116 [DOI] [PubMed] [Google Scholar]
- Lee KS, Buck M, Houglum K, Chojkier M 1995 Activation of hepatic stellate cells by TGF α and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest 96:2461–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND 2004 Glutathione metabolism and its implications for health. J Nutr 134:489–492 [DOI] [PubMed] [Google Scholar]
- Forman HJ, Dickinson DA 2003 Oxidative signaling and glutathione synthesis. Biofactors 17:1–12 [DOI] [PubMed] [Google Scholar]
- Seelig GF, Meister A 1985 Glutathione biosynthesis; γ-glutamylcysteine synthetase from rat kidney. Methods Enzymol 113:379–390 [DOI] [PubMed] [Google Scholar]
- Cotgreave IA 1997 N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol 38:205–227 [PubMed] [Google Scholar]
- Anderson ME, Luo JL 1998 Glutathione therapy: from prodrugs to genes. Semin Liver Dis 18:415–424 [DOI] [PubMed] [Google Scholar]
- Miyahara T, Schrum L, Rippe R, Xiong S, Yee Jr HF, Motomura K, Anania FA, Willson TM, Tsukamoto H 2000 Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem 275:35715–35722 [DOI] [PubMed] [Google Scholar]
- Lang T, Ikejima K, Yoshikawa M, Enomoto N, Iijima K, Kitamura T, Takei Y, Sato N 2004 Leptin facilitates proliferation of hepatic stellate cells through up-regulation of platelet-derived growth factor receptor. Biochem Biophys Res Commun 323:1091–1095 [DOI] [PubMed] [Google Scholar]
- Zheng S, Chen A 2007 Disruption of transforming growth factor-β signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-γ in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 292:G113–G123 [DOI] [PubMed] [Google Scholar]
- Mueller AS, Bosse AC, Most E, Klomann SD, Schneider S, Pallauf J 2009 Regulation of the insulin antagonistic protein tyrosine phosphatase 1B by dietary Se studied in growing rats. J Nutr Biochem 20:235–247 [DOI] [PubMed] [Google Scholar]
- Gerthoffer WT 2005 Signal-transduction pathways that regulate visceral smooth muscle function. III. Coupling of muscarinic receptors to signaling kinases and effector proteins in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol 288:G849–G853 [DOI] [PubMed] [Google Scholar]
- Nilsson BO, Gomez MF, Swärd K, Hellstrand P 2002 Regulation of Ca2+ channel and phosphatase activities by polyamines in intestinal and vascular smooth muscle—implications for cellular growth and contractility. Acta Physiol Scand 176:33–41 [DOI] [PubMed] [Google Scholar]
- Tani E, Matsumoto T 2004 Continuous elevation of intracellular Ca2+ is essential for the development of cerebral vasospasm. Curr Vasc Pharmacol 2:13–21 [DOI] [PubMed] [Google Scholar]
- Galli A, Crabb D, Price D, Ceni E, Salzano R, Surrenti C, Casini A 2000 Peroxisome proliferator-activated receptor γ transcriptional regulation is involved in platelet-derived growth factor-induced proliferation of human hepatic stellate cells. Hepatology 31:101–108 [DOI] [PubMed] [Google Scholar]
- Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P 2000 Ligands of peroxisome proliferator-activated receptor γ modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology 119:466–478 [DOI] [PubMed] [Google Scholar]
- Lee JI, Paik YH, Lee KS, Lee JW, Kim YS, Jeong S, Kwon KS, Lee DH, Kim HG, Shin YW, Kim MA 2007 A peroxisome-proliferator activated receptor-γ ligand could regulate the expression of leptin receptor on human hepatic stellate cells. Histochem Cell Biol 127:495–502 [DOI] [PubMed] [Google Scholar]
- Sharma AM, Staels B 2007 Review: peroxisome proliferator-activated receptor γ and adipose tissue—understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab 92:386–395 [DOI] [PubMed] [Google Scholar]
- Camp HS, Tafuri SR 1997 Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. J Biol Chem 272:10811–10816 [DOI] [PubMed] [Google Scholar]
- Camp HS, Tafuri SR, Leff T 1999 c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-γ1 and negatively regulates its transcriptional activity. Endocrinology 140:392–397 [DOI] [PubMed] [Google Scholar]
- Chen A, Zheng S 2008 Curcumin inhibits connective tissue growth factor gene expression in activated hepatic stellate cells in vitro by blocking NF-κB and ERK signalling. Br J Pharmacol 153:557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YR, Tan TH 1998 Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 17:173–178 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J 2008 Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem 283:14497–14505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, Kim WK, Kim HS 2005 Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun 335:1017–1025 [DOI] [PubMed] [Google Scholar]
- Dubey L, Hesong Z 2006 Role of leptin in atherogenesis. Exp Clin Cardiol 11:269–275 [PMC free article] [PubMed] [Google Scholar]
- Beltowski J, Jamroz-Wiśniewska A, Wójcicka G, Lowicka E, Wojtak A 2008 Renal antioxidant enzymes and glutathione redox status in leptin-induced hypertension. Mol Cell Biochem 319:163–174 [DOI] [PubMed] [Google Scholar]
- Sreejayan, Rao MN 1994 Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol 46:1013–1016 [DOI] [PubMed] [Google Scholar]