Abstract
Pulsatile release of GnRH-1 is critical to stimulate gonadotropes of the anterior pituitary. This secretory pattern seems to be inherent to GnRH-1 neurons, however, the mechanisms underlying such episodical release remain unknown. In monkey nasal explants, the GnRH-1 population exhibits synchronized calcium events with the same periodicity as GnRH-1 release, suggesting a link, though the sequence of events was unclear. GnRH-1 neurons in mouse nasal explants also exhibit synchronized calcium events. In the present work, GnRH-1 release was assayed in mouse nasal explants using radioimmunology and its relationship with calcium signaling analyzed. GnRH-1 neurons generated episodical release as early as 3 d in vitro (div) and maintained such release throughout the period studied (3–21 div). The pulse frequency remained constant, suggesting that the pulse generator is operative at an early developmental stage. In contrast, pulse amplitude increased 2-fold between 3 and 7 div, and again between 7 and 14 div, suggesting maturation in synthesizing and/or secretory mechanisms. To evaluate these possibilities, total GnRH-1 content was measured. Only a small increase in GnRH-1 content was detected between 7 and 14 div, whereas a large increase occurred between 14 and 21 div. These data indicate that GnRH-1 content was not a limiting factor for the amplitude of the pulses at 7 div but that the secretory mechanisms mature between 3 and 14 div. The application of kisspeptin-10 revealed the ability of GnRH-1 neurons to integrate signals from natural ligands into a secretory response. Finally, simultaneous sampling of medium and calcium imaging recordings indicated that the synchronized calcium events and secretory events are congruent.
Developing GnRH-1 neurons exhibit pulsatile secretion that correlates with synchronized somal calcium events and show maturation in secretory processes prior to entering the brain.
GnRH-1 controls reproductive physiology by triggering secretion of pituitary hormones, LH, and FSH, which subsequently act on the gonads (1). Because the secretion of pituitary hormones is abrogated under continuous GnRH-1 stimulation (2), GnRH-1 release has to be pulsatile for secretion of gonadotropin hormones to occur. Measurements of GnRH-1 levels have been hampered in small animal models due to accessibility of the portal capillary system in free moving animals and quantity of GnRH-1 released, however, peripheral detection of LH reveals pulsatile release reflecting the activity of the GnRH-1 pulse generator (3,4).
During embryogenesis in vertebrates, GnRH-1 neurons originate outside the brain from the nasal placodes (5). They migrate along the olfactory, vomeronasal, and terminal nerves before entering the forebrain just under the medial aspect of the olfactory bulbs. Once in the forebrain, GnRH-1 neurons continue to migrate, turning ventrally to their final location, which is dependent on the species. In rodents, GnRH-1 cells are bilaterally distributed in a continuum that spans from the olfactory bulbs to the caudal hypothalamus (5). During the nasal and forebrain migration, these neurons express GnRH-1, as shown by immunocytochemical and in situ hybridization studies performed in various species [mouse (6,7), sheep (8,9), and monkey (10)].
A number of groups have used this developmental feature to access GnRH-1 cellular physiology by generating in vitro nasal explants (11,12,13,14). Several studies performed on prenatal GnRH-1 neurons, devoid of central nervous system (CNS) inputs, have shown that GnRH-1 is secreted in an episodical manner, with mean durations of interpulse intervals (IPIs) consistent with those observed in vivo in castrated animals, i.e. in the absence of steroid negative feedback [monkey ∼50 min (15), sheep ∼50 min (16), and rat ∼30 min (17)]. These data indicate that periodic release is inherent to GnRH-1 neurons, and is consistent with data obtained from cultures of native dissociated cells and immortalized GnRH-1 secreting cells, GT1 (18,19), exhibiting pulsatile GnRH-1 release while devoid of any CNS neuronal input.
Periodic neuronal secretion (20) is a cellular process that requires a transient increase in intracellular calcium ([Ca2+]i), and data suggest that an increase in [Ca2+]i involves l-type voltage-gated calcium channels (15,18). In addition, maturation of a network seems required for pulsatile release to establish: 1) secretion at the level of single neurons (21), and 2) coordination across a cohort of neurons (22). In mouse nasal explants, GnRH-1 cells cease migration after 5–6 d in vitro (div) (13) and exhibit synchronized [Ca2+]i oscillations approximately every 20 min by 7 div, revealing coordination within the GnRH-1 neuronal population at this early stage of development (23). However, the temporal relationship between synchronized [Ca2+]i oscillations and GnRH-1 secretion are unclear. Data obtained in GT1 cells showed a relationship, at the cellular level, between secretion and calcium events (24), but no direct information on the dynamics of these processes at the level of the GnRH-1 neuronal population exists. The aims of this study were to investigate the development of GnRH-1 secretion in nasal explants, using a highly sensitive RIA (25), and to investigate the relationship between secretion and calcium signaling, using calcium imaging on the GnRH-1 neuronal population.
Materials and Methods
All animal procedures done at Institut National de la Recherche Agronomique were performed in agreement with the European legislation for animal experimentation (Authorization A37801 from the French Ministry of Agriculture). All animal procedures done at the National Institute of Neurological Disorders and Stroke were in accordance with National Institutes of Health, National Institute of Neurological Stroke and Disorders guidelines.
Nasal explants
Nasal explants were cultured as previously described (Fig. 1A) (13). Briefly, embryos were obtained from timed pregnant animals. Nasal pits of embryonic d 11.5 staged Swiss mice were isolated under aseptic conditions and refrigerated for 1 h in Gey’s balanced salt solution (Eurobio, Les Ulis, France) enriched with glucose (Sigma-Aldrich Corp., St. Louis, MO). Nasal explants were adhered onto coverslips by a chicken plasma (local source)/thrombin (Sigma-Aldrich) clot. The explants were maintained in a defined serum-free media (SFM). On culture d 3, fresh media containing uridine (5 mg/ml; Sigma-Aldrich) and 5′-fluoro-2-deoxyuridine (2 mg/ml; Sigma-Aldrich) were given to inhibit proliferation of dividing olfactory neurons and non-neuronal explant tissue. On culture d 6 and every 2 d afterward, the media were changed to fresh SFM. Explants were used for experiments from 3–21 div, encompassing developmental stages known to exhibit synchronization of calcium oscillations in GnRH-1 neurons (23).
Figure 1.
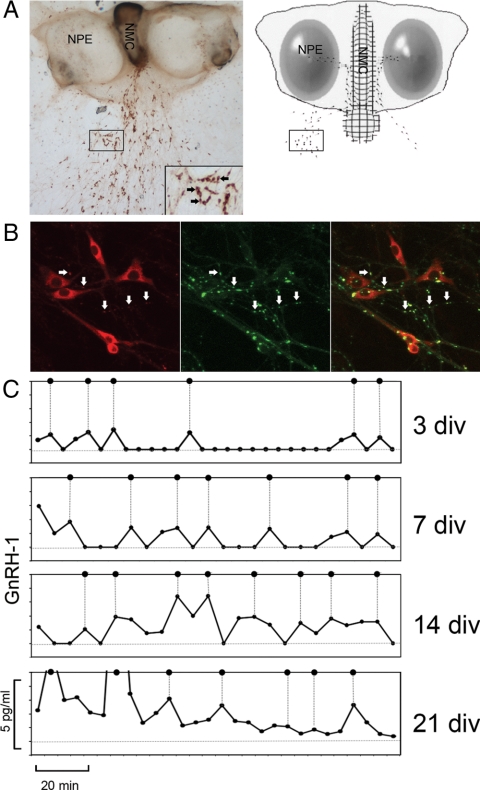
Kinetics of GnRH-1 secretion. A, GnRH-1 immunostaining of a nasal explant obtained from embryonic d 11.5 mouse, maintained for 7 div, and the corresponding schematic showing nasal pit epithelium (NPE) (ovals) and the nasal midline cartilage (NMC) (meshed area), surrounded by mesenchyme. GnRH-1 neurons (dots) migrate from NPE and follow olfactory axons to the nasal midline cartilage and off the explant into the periphery. The boxes indicate the similar area on a low-magnification picture and schematic (the inset shows the corresponding area at a higher magnification). The inset box width is equivalent to 150 μm. B, Immunocytochemical labeling of a 10-div explant, showing GnRH-1 (red) and synapsin-I (green), a neuron-specific phosphoprotein associated with the membranes of synaptic vesicles, reveals putative active sites of GnRH-1 release (arrows). Box width represents 50 μm. C, Representative GnRH-1 secretion profiles obtained from mouse nasal explants at 3, 7, 14, and 21 div. Samples were collected every 5 min. All samples were assayed in duplicates. Data were obtained from two assays using the same GnRH-1 references. Pulses are indicated by dotted lines. PULSAR parameters are summarized in Table 1.
Immunocytochemistry
Nasal explants were fixed in 4% formaldehyde [45–60 min, rinsed in PBS (3 × 10 min)], blocked (1 h, 10% normal goat serum, 0.3% Triton X-100, and 0.1% sodium azide), rinsed in PBS (3 × 8 min), and incubated overnight in anti-GnRH-1 [1:3000, SW-1, polyclonal (26)] or anti-GnRH-1 plus anti-synapsin-I (1:1000, monoclonal; Calbiochem, San Diego, CA). Antibodies were diluted in PBS containing 10% normal goat serum, 0.3% Triton X-100, and 0.1% sodium azide. The next day, the explants were rinsed in PBS (3 × 8 min), incubated in secondary antibody (0.3% Triton X-100 and biotinylated antirabbit; Vector Laboratories, Burlingame, CA) or antirabbit Alexa 546 plus antimouse Alexa 488 (1:500, 1 h; Molecular Probes, Inc., Eugene OR) and rinsed in PBS (4 × 8 min). Explants incubated with fluorescent antibodies were mounted with VectorShield (Vector Laboratories), and explants incubated with biotinylated antibodies were processed for avidin-biotin horseradish peroxidase/3′3-diamino-benzidine cytochemistry (13).
Medium sampling for determining profiles of GnRH-1 secretion
Explants were covered with 300 μl SFM. Supernatant was collected from each explant (250 μl removed; 50 μl left to maintain the explants in SFM) and replaced with the same volume of fresh SFM every 5 or 6 min for a 3-h period. Samples were kept at −20 C until RIA. Medium collection was performed on 3-div (n = 5), 7-div (n = 8), 14-div (n = 9), and 21-div (n = 11) explants. For the agonist trial, an 80-min control period was followed by an 80-min period of kisspeptin-10 (kp-10) application at 100 nm (17 div, n = 5), and secretion profiles were compared with a 160-min control period subdivided into two periods (17 div, n = 5).
Total GnRH-1 content
GnRH-1 was extracted from nasal explants using a procedure described in Ref. 27. Briefly, explants were washed twice in ice-cold PBS, then 100 μl ice-cold 0.1 n HCl plus 5 mg/ml protease inhibitor CPI (Roche Diagnostics Corp., Indianapolis, IN) was added. Coverslips with explants were placed on dry ice until freezing. Frozen drops were collected from the coverslips and put in 1.7 ml silicone microtubes, homogenized for 10 sec, centrifuged 10 min at 300 × g at 4 C. Supernatants were collected, dried using a Speedvac (Thermo Fisher Scientific, Inc., Waltham, MA), and frozen at −80 C (28). GnRH-1 was extracted from 1-(n = 10), 2-(n = 14), and 3-(n = 5) week-old explants.
Calcium imaging
Calcium imaging recordings were performed between 6 and 10 div. The Calcium Green-1 AM (Molecular Probes) was diluted to a 2.7-mm concentration in 80% dimethylsulfoxide and 20% pluronic F-127 solution (Molecular Probes). This solution was diluted 1:200 with SFM to a final Calcium Green-1 concentration of 13.5 μm. For long recordings (3 h), Calcium Green-1 was diluted at a 10-μm final concentration in SFM supplemented with 10 mm HEPES and 2.5 mm probenecid (Molecular Probes), an inhibitor of organic anion transporters (29).
Nasal explants, maintained at 37 C in a 5% CO2 humidified incubator, were incubated with this loading solution for 20–30 min, then washed twice with fresh SFM (10 min each). Explants were mounted into a perfusion chamber and were continuously superfused with medium, at a rate of approximately 60 μl/min, using a peristaltic pump. For a posteriori GnRH-1 measurement, medium was collected every 5 min and kept at −20 C until RIA. The perfusion chamber was maintained at 36 C using a temperature controller (Warner Instruments, Hamden, CT).
Calcium Green-1 was visualized using an inverted microscope (DM-IRB; Leica Microsystems GmbH, Wetzlar, Germany), through a ×20 fluorescence objective, and acquired using a cooled intensified charge-coupled device camera (CoolSNAP fx; Ropper Instruments, Photometrics, Tucson, AZ). Experiments were piloted by Metafluor (Molecular Devices, Downingtown, PA), controlling the shutter and the acquisition (every 20 sec for 3 h). Excitation wavelengths were provided through a medium-width excitation bandpass filter at 465–495 nm, and emission was monitored through a 40-nm bandpass centered on 535 nm. Fluctuations in [Ca2+]i were analyzed a posteriori with Metafluor software. Each cell, individually identified, was circled. Calcium Green-1 fluorescence intensity was plotted and analyzed with Excel (Microsoft Corp., Redmond, WA). All recordings were terminated by a 40-mm KCl stimulation to ensure the viability of the recorded cells, increasing basal secretion by 2.5-fold (28).
Acquired every 20 sec, a calcium peak was defined when a value was greater than 1.5 × sd on the average of the five previous and five subsequent points. Synchronicity was defined as the percentage of cells exhibiting a peak simultaneously (23).
RIA
GnRH-1 concentration was measured by RIA (30) in 100 μl duplicate aliquots after methanol extraction. The GnRH-1 assay detection level was 0.2 pg/ml; the mean interassay and intraassay coefficients of variation were 13 and 9%, respectively (n = 4). Anti-GnRH-1 BDS-037 antibody is a specific N-terminal-directed antibody that binds the first seven amino acids of the GnRH-1 peptide. Thus, this antibody recognizes both native GnRH-1 and variant forms such as pro-GnRH and Hyp9-GnRH-1 (30).
Pulsatility was determined using the PULSAR algorithm (31). The G parameters (number of sd values by which a peak must exceed the baseline to be accepted) were set at 3, 2.6, 2.2, 1.8, and 1.4 for G1-5, these being the requirements for pulses composed of one to five samples that exceed the baseline, respectively. The Baxter parameters describing the parabolic relationship between concentration of a hormone in a sample and the sd (assay variation) about the concentration were 2.86 (c, the intercept), 0.57 (b, the first order coefficient), and 0.004 (a, the second order coefficient), estimated from the intraassay coefficient of variation (n = 4 assays).
Statistical analysis
ANOVA using R software (GNU General Public License) was performed to compare the different parameters of pulsatility between the different ages of culture, followed by a post hoc Bonferroni test. For the agonist trial, a paired t test was used between the control period and the agonist-treated period. A P value of 0.05 was chosen for significance.
For testing the correlation between RIA and calcium imaging, random data were generated using MATLAB (The MathWorks, Inc., Natick, MA).
Results
Potential active sites for GnRH-1 release
Synapsin-I is a nerve terminal-specific phosphoprotein associated with synaptic vesicles, involved in their recruitment and/or maturation (32). Thus, explants were stained for GnRH-1 (Fig. 1B, red) and synapsin-I (Fig. 1B, green). GnRH-1 positive varicosities colabeled for synapsin-I (Fig. 1B, white arrows) identify putative sites for GnRH-1 release. Although non-GnRH-1 varicosities (Fig. 1B, green only) are detected, many, if not all, of the GnRH-1 varicosities exhibited immunoreactivity for synapsin-I.
Profiles of GnRH-1 secretion
GnRH-1 was released in an episodical manner at all ages tested (Fig. 1C), with a mean IPI of approximately 20 min [27.18 ± 2.41 min at 3 div (n = 5), 25.46 ± 3.10 min at 7 div (n = 8), 21.2 ± 1.21 min at 14 div (n = 9), and 19.78 ± 1.16 min at 21 div (n = 11)]. The IPIs were similar between ages (ANOVA, P > 0.05; Fig. 2A).
Figure 2.
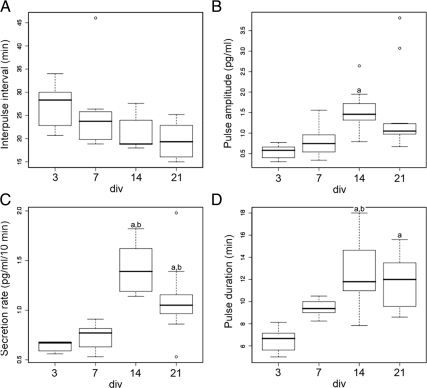
Pulsatility parameters with the age in culture: A, IPI; B, pulse amplitude; C, secretion rate; D, pulse duration. Data are represented using Tukey’s box plot [box lower limit, lower quartile Q1; box upper limit, upper quartile Q3; black line, median Q2; whisker, 1.5 × interquartile range (IQR); circles, extreme observation]. Age of culture had a statistically significant effect on secretion rate (C), pulse amplitude (B), and pulse duration (D) (one-way ANOVA, P < 0.05). Post hoc pair-wise comparison using the Bonferroni method. a, significantly different from 3 div; b, significantly different from 7 div (P < 0.05).
The mean pulse amplitude estimated by PULSAR showed an increase with time in vitro (ANOVA, P < 0.05). By post hoc Bonferroni evaluation, any consecutive days in vitro were significantly different. However, a 1.5-fold increase was observed between 3 (0.54 ± 0.08 pg/ml) and 7 div (0.80 ± 0.13 pg/ml; Bonferroni test, P < 0.05), and an additional 2-fold increase was found between 7 and 14 div (1.59 ± 0.17 pg/ml; Bonferroni test, P < 0.05). Between 14 and 21 div, no changes were detected (21 div; 1.44 ± 0.31 pg/ml; Bonferroni test, P > 0.05; Fig. 2B).
The mean level determined by PULSAR gives an estimation of the mean secretion rate/10 min and was found to increase significantly with days in vitro (ANOVA, P < 0.05). There was a significant 2-fold increase in GnRH-1 mean level between 7 (0.70 ± 0.05 pg/ml) and 14 div (1.44 ± 0.09 pg/ml; Bonferroni test, P < 0.05). The mean level at 3 div (0.63 ± 0.02 pg/ml) was comparable to 7 div (Bonferroni test, P > 0.05), and the level at 21 div (1.10 ± 0.11 pg/ml) was not significantly different from 14 div (Bonferroni test, P > 0.05; Fig. 2C).
The mean pulse duration also showed a significant increase with days in vitro (ANOVA, P < 0.05), reaching a plateau at 14 div (3 div, 6.51 ± 0.55 min; 7 div, 9.44 ± 0.26 min; 14 div, 12.52 ± 0.97 min; and 21 div, 11.78 ± 0.71 min; Fig. 2D). The results are summarized in Table 1.
Table 1.
Comparison of PULSAR parameters estimated from GnRH-1 secretion kinetics at different ages of culture
| 3 div (n = 5) | 7 div (n = 8) | 14 div (n = 9) | 21 div (n = 11) | |
|---|---|---|---|---|
| Secretion rate (pg/ml/10 min) | 0.63 ± 0.02 | 0.70 ± 0.01 | 1.44 ± 0.09a,b | 1.10 ± 0.11a,b |
| No. of pulses | 7.0 ± 0.4 | 5.6 ± 0.5 | 7.0 ± 0.5 | 7.9 ± 0.7 |
| Pulse amplitude (pg/ml) | 0.54 ± 0.08 | 0.80 ± 0.13 | 1.59 ± 0.17a | 1.44 ± 0.31 |
| Pulse duration (min) | 6.51 ± 0.55 | 9.44 ± 0.26 | 12.52 ± 0.97a,b | 11.78 ± 0.71a |
| Interpulse duration (min) | 27.18 ± 2.41 | 25.46 ± 3.10 | 21.22 ± 1.21 | 19.78 ± 1.16 |
One-way ANOVA P < 0.05, post hoc pair-wise comparison using the Bonferroni method.
Different from 3 div.
Different from 7 div.
These data indicate that synchronicity of the cells, leading to the episodical release of GnRH-1, is a precocious characteristic of GnRH-1 neurons, but that maturational events occur endogenously that refine the GnRH-1 secretion pattern.
Total GnRH-1 content
An increase in the amount of secreted GnRH-1 could be the consequence of: 1) an increase in the synthesis of GnRH-1 by the cells, 2) greater cellular efficiency to secrete, or 3) a combination of both. To evaluate the underlying change, the content of GnRH-1 was measured over in vitro development.
The amount of GnRH-1 in nasal explants did not reveal a developmental pattern similar to any of the changes observed previously. At 7 and 14 div, 19 ± 3 and 26 ± 5 pg were extracted from each explant, respectively, whereas a 5-fold increase was observed at 21 div (108 ± 14 pg; ANOVA and post hoc Bonferroni test, P < 0.05; Fig. 3). These data indicate that the secreted amount of GnRH-1 was not correlated to the total content (correlation coefficient ∼0.4). The fact that secretion doubled between 7 and 14 div, without any significant increase in the total GnRH-1 content, suggests a maturation of secretory mechanisms rather than overall synthesis.
Figure 3.
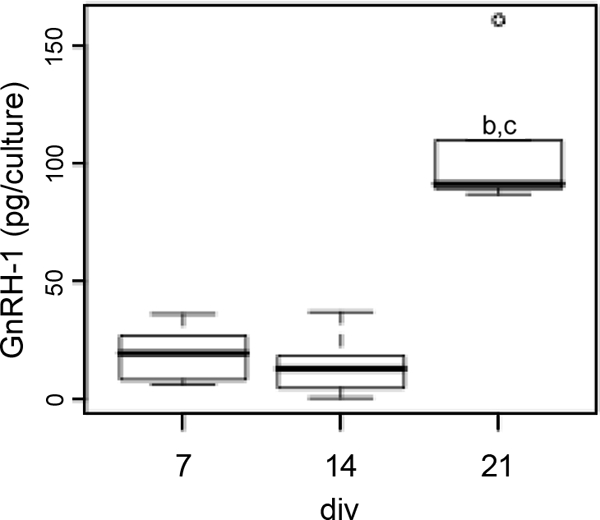
GnRH-1 content as a function of the age in culture. Tukey’s box plot [box lower limit, lower quartile Q1; box upper limit, upper quartile Q; black line, median Q2; whisker, 1.5 × interquartile range (IQR); circles, extreme observation] of GnRH-1 contents. GnRH-1 content was significantly higher at 21 div (one-way ANOVA, P < 0.050). Post hoc pair-wise comparison using the Bonferroni method (P < 0.05). b, significantly different from 7 div; c, significantly different from 14 div.
Agonist-induced secretion
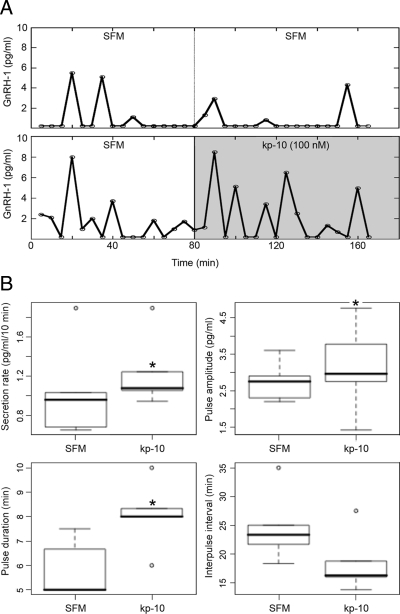
kp-10 appears to be one of the most potent excitatory signals for GnRH-1 neurons postnatally (33). Recently, Constantin et al. (34) showed that kp-10 also modulates GnRH-1 neuronal activity in nasal explants. Thus, to determine whether GnRH-1 could be triggered by a natural ligand, the secretory responsiveness of GnRH-1 neurons was investigated using kp-10 (100 nm; Fig. 4A). Bath application of kp-10 (n = 5) increased the secretion rate (1.34 ± 0.10 pg/ml/10 min in kp-10 vs. 0.89 ± 1.21 pg/ml/10 min in SFM) by reducing the IPI (18.45 ± 2.39 min in kp-10 vs. 24.66 ± 2.81 min in SFM), increased the pulse length (8.06 ± 0.63 min in kp-10 vs. 5.83 ± 0.52 min in SFM; paired t test, P < 0.05), without modifying the pulse amplitude (3.13 ± 0.55 pg/ml in kp-10 vs. 2.75 ± 0.25 pg/ml in SFM; paired t test, P > 0.05; Fig. 4B). These data show the ability of GnRH-1 neurons, without CNS cues, to integrate signals from a natural ligand into a secretory response.
Figure 4.
Stimulation of GnRH-1 release by a natural ligand. Representative secretion profile at 17 div in SFM (A, upper) and SFM and kp-10 (A, lower). B, Corresponding pulsatility parameters in SFM and kp-10 (stars indicate P < 0.05, paired t test). Tukey’s box plot [box lower limit, lower quartile Q1; box upper limit, upper quartile Q; black line, median Q2; whisker, 1.5 × interquartile range (IQR); circles, extreme observation].
In control experiments (n = 5), none of the parameters showed a significant change (rate: 0.62 ± 0.13 pg/ml/10 min in period 2 vs. 0.89 ± 1.21 pg/ml/10 min in period 1; IPI: 22.60 ± 2.80 min in period 2 vs. 22.50 ± 4.10 min in period 1; pulse length: 7.67 ± 1.55 min in period 2 vs. 5.93 ± 0.41 min in period 1; pulse amplitude: 1.71 ± 0.27 pg/ml in period 2 vs. 2.29 ± 0.60 pg/ml in period 1; paired t test, P > 0.05).
GnRH-1 neuronal synchronization and secretion
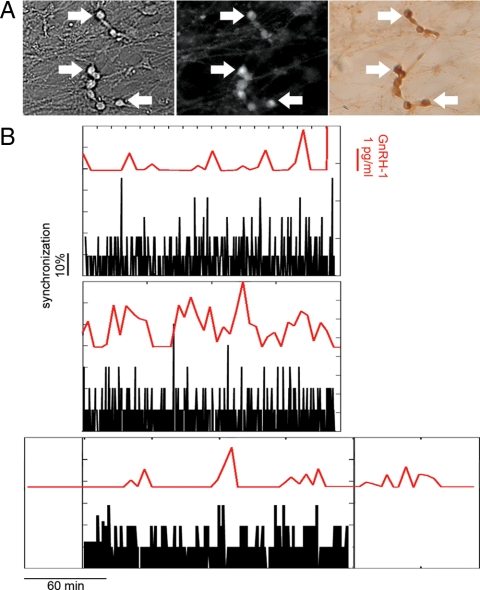
Because GnRH-1 neurons maintained in nasal explants exhibit synchronized [Ca2+]i oscillations (23,35), which occur with the same periodicity as GnRH-1 (36) or LH release in vivo (4), it has been assumed that these events reflect the synchronicity required for the genesis of a secretory pulse. However, no data exist showing these two events on the same time line. Simultaneous recordings of [Ca2+]i fluctuations (Fig. 5A) and sampling of medium were performed to investigate the relationship between synchronized [Ca2+]i events and secretion in GnRH-1 neurons. The plot of the percentage of GnRH-1 cells exhibiting calcium peaks during a time window and secretory pulse suggest that a minimum of 30% of the GnRH-1 neuronal population is required for a secretory episode (Fig. 5B).
Figure 5.
Pulsatile GnRH-1 secretion correlates with synchronized calcium events across the GnRH-1 neuronal population. A, High magnification (box width represents 150 μm) of GnRH-1 neurons before (left, bright field), during (middle, calcium green-1 fluorescence), and after (right, immunocytochemical GnRH-1 staining) calcium imaging recordings (arrows indicate the same cells). B, Three examples of simultaneous recordings of GnRH-1 secretion and [Ca2+]i events. GnRH-1 secretion profile and the proportion of GnRH-1 neurons having a simultaneous [Ca2+]i event are plotted with the red line and black histogram, respectively.
To investigate the strength of the relationship between synchronized [Ca2+]i events and secretion, a cross-correlation was required. Getting a stringent cross-correlation with events whose frequency was as low as approximately 1/20 min required long calcium recordings. However, with respect to the acquisition frequency required for the dynamics of the calcium signaling and the limitation imposed by the bleaching of the calcium dyes, calcium recordings were not long enough to perform this statistic. Therefore, to ensure that the correlation obtained between synchronized [Ca2+]i events (instantaneous values every 20 sec) and secretion (cumulated values obtained over 5 min periods) was not due to sampling and thereby occurring by chance when the two data sets (whose acquisition frequency were different) were superposed, a comparison between experimental data and random data was performed. The delay between every measured synchronized event (>30%) and the next secretory episode was determined in four explants (over ∼3 h each). The same analysis was then performed on random recordings, generated for calcium oscillations and secretion, both exhibiting the same probability as the experimental recordings for, respectively, synchronized events and secretory events. A delay of 4.1 ± 1.0 min (15 pairs of events) was observed between measured synchronized events and secretory events in experimental recordings, whereas 21.9 ± 3.3 min was observed in random recordings (ANOVA, P < 0.05), strengthening the fact that this delay reflects a relationship between cellular events, with calcium synchronization preceding GnRH-1 pulsatile secretion.
Discussion
Pulsatile release of GnRH-1 is essential for reproduction. This study demonstrates that mouse prenatal GnRH-1 neurons maintained in nasal explants exhibit pulsatile GnRH-1 secretion, and show maturation in secretory processes during the first 2 wk in vitro. In addition, this work provides experimental data for a relationship between synchronized [Ca2+]i oscillations and secretory events in the GnRH-1 neuronal population, supporting a hypothesis emitted 10 yr ago based on work in nasal explants from rhesus monkey (35).
In vivo, it is clear that GnRH-1 secretion can occur physiologically during late prenatal [sheep (37)] or early postnatal life [monkey (38)], or pathologically in precocious puberty (39). The transient activation of the hypothalamic-pituitary-gonadal axis, which results in an increase in circulating gonadal steroids that participate in sexual differentiation (40), also suggests pulsatile release of GnRH-1 at early stages followed by quiescence of the system until the time of puberty (41). In accordance with the ability of prenatal GnRH-1 cells to secrete, our data indicate the presence of synapsin-I in many GnRH-1 varicosities, suggesting a large number of potential active sites for GnRH-1 release and the ability of GnRH-1 neurons to secrete in a pulsatile pattern at very early developmental stages. Pulsatile GnRH-1 secretion occurred as early as 3 div in mouse nasal explants and was maintained throughout the time of culturing (21 div).
Pulsatile secretion requires synchronization among GnRH-1 neurons. Two groups have described synchronized [Ca2+] oscillations in GnRH-1 neurons, consistent with the periodicity of LH release in vivo (15,23). Therefore, it was supposed that it reflected the synchronization necessary for generating a pulse. In this study, pulses in GnRH-1 released were detected with approximately 25-min intervals at 7 div, which is slightly higher than the approximate 20-min interval described for synchronized [Ca2+]i oscillations. Because the amplitude of the secretory pulses was relatively small at 7 div, the difference might be due to pulses less than 0.2 pg/ml. This would artificially increase the mean secretory IPI. In agreement with this hypothesis, approximately 20-min IPIs were found at 14 and 21 div, when the amplitude of pulses had increased.
Developmentally, the amount of GnRH-1 released per pulse increased between 3 and 7 div and 7 and 14 div. These increases might reflect enhanced efficiency of the secretory and/or synthesizing processes. In support of maturation of the synthesizing mechanisms, an increase in total GnRH-1 content was observed, consistent with in vivo and in vitro data (28). However, in our experiments the increase in total content was between 14 and 21 div, after the change in GnRH-1 release occurred. Therefore, it appears that the GnRH-1 content is not a limiting factor at 7 div for secretion but that the secretory mechanisms become optimized between 7 and 14 div. These data are consistent with previous data showing that, at 7 div, a potassium-induced depolarization increased basal secretion by 2.5-fold (28), indicating that the secreted amount does not reflect a limitation in the available releasable pool but a regulated release. In accordance with this hypothesis, kp-10 application, known to stimulate GnRH-1 neurons in vitro (34), showed that even at early stages, GnRH-1 neurons are able to adapt their secretion rate to their surrounding input.
Neurosecretion can be subdivided into two active processes, after peptide maturation: translocation of the vesicles (in close vicinity of the membrane and docking to the membrane) and excitation-release coupling (20) and both processes of neurosecretion are known to evolve with development (21). Evidence exists indicating that the GnRH-1 peptide processing occurs in developing GnRH-1 neurons (5), indicating the peptide efficiency. Few data are available in general on the development of protein scaffolding involved in vesicular trafficking indicating dynamic mechanisms. It is known that the transition of the growth cone into a fully functional presynaptic terminal requires a switch in the expression of soluble N-ethylmaleimide-sensitive factor attached protein receptor (SNARE) proteins, that SNARE proteins exhibit specific and independent expression patterns and that even the recruitment of partners by the SNARE complex is regulated by several elements within the terminals. Only a few factors modulating SNARE levels have been identified, but hormones, intracellular messengers, and electrical activity play a fundamental role at the time when secretory processes are activated (21).
Excitation-release coupling requires an increase in [Ca2+]i close to the vesicles, via influx through plasma membrane channels. GnRH-1 release requires calcium influx through l-type voltage-gated calcium channels (15). Early neuronal development is usually divided into three major periods: proliferation, migration, and differentiation. It is well known that mechanisms that do not depend on classical voltage- or neurotransmitter-gated channels regulate the early excitability of cells, and those early forms of electrical activity shape the intrinsic excitability, defining a time window during which the developmental program occurs (42). GnRH-1 neurons in nasal explants are postmitotic neurons (6), however, they do undergo a migratory period (13), and our data suggest in vitro differentiation.
This study demonstrates a correlation between synchronization of [Ca2+]i oscillations and secretory events in prenatal GnRH-1 neurons in the mouse, and indicates that both [Ca2+]i oscillations in GnRH-1 cells and GnRH-1 secretion are species dependent and occur before GnRH-1 cells reaching the brain. This is yet another interesting facet of the GnRH-1 system that suggests intrinsic regulation that is species modified.
Acknowledgments
We appreciate the help of Monique Ottogalli with cell culture and Didier Lomet with RIA.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 12, 2009
Abbreviations: [Ca2+]i, Intracellular calcium; CNS, central nervous system; div, days in vitro; IPI, interpulse interval; kp-10, kisspeptin-10; SFM, serum-free media; SNARE, soluble N-ethylmaleimide-sensitive factor attached protein receptor.
References
- Herbison AE 2006 Physiology of the gonadotropin-releasing hormone neuronal betwork. In: Knobil E, Neill J, eds. The physiology of reproduction. 3rd ed. San Diego: Academic Press; 1415–1482 [Google Scholar]
- Stojilkovic SS 2006 Pituitary cell type-specific electrical activity, calcium signaling and secretion. Biol Res 39:403–423 [DOI] [PubMed] [Google Scholar]
- Kawakami M, Uemura T, Hayashi R 1982 Electrophysiological correlates of pulsatile gonadotropin release in rats. Neuroendocrinology 35:63–67 [DOI] [PubMed] [Google Scholar]
- Kokoris GJ, Lam NY, Ferin M, Silverman AJ, Gibson MJ 1988 Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hpg) male mice. Neuroendocrinology 48:45–52 [DOI] [PubMed] [Google Scholar]
- Wray S 2002 Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol 23:292–316 [DOI] [PubMed] [Google Scholar]
- Wray S, Grant P, Gainer H 1989 Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW 1989 Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161–164 [DOI] [PubMed] [Google Scholar]
- Caldani M, Antoine M, Batailler M, Duittoz A 1995 Ontogeny of GnRH systems. J Reprod Fertil Suppl 49:147–162 [PubMed] [Google Scholar]
- Bruneau G, Izvolskaia M, Ugrumov M, Tillet Y, Duittoz AH 2003 Prolonged neurogenesis during early development of gonadotropin-releasing hormone neurones in sheep (Ovis aries): in vivo and in vitro studies. Neuroendocrinology 77:177–186 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Resko JA 1990 Ontogeny of gonadotropin-releasing hormone-containing neurons in early fetal development of rhesus macaques. Endocrinology 126:498–511 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P 1993 A primary cell culture system of luteinizing hormone-releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology 133:2379–2390 [DOI] [PubMed] [Google Scholar]
- Daikoku S, Koide I, Chikamori-Aoyama M, Shimomura Y 1993 Migration of LHRH neurons derived from the olfactory placode in rats. Arch Histol Cytol 56:353–370 [DOI] [PubMed] [Google Scholar]
- Fueshko S, Wray S 1994 LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol 166:331–348 [DOI] [PubMed] [Google Scholar]
- Duittoz AH, Batailler M, Caldani M 1997 Primary cell culture of LHRH neurones from embryonic olfactory placode in the sheep (Ovis aries). J Neuroendocrinol 9:669–675 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P 1999 Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- Duittoz AH, Batailler M 2000 Pulsatile GnRH secretion from primary cultures of sheep olfactory placode explants. J Reprod Fertil 120:391–396 [PubMed] [Google Scholar]
- Funabashi T, Daikoku S, Shinohara K, Kimura F 2000 Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology 71:138–144 [DOI] [PubMed] [Google Scholar]
- Krsmanoviæ LZ, Stojilkoviæ SS, Merelli F, Dufour SM, Virmani MA, Catt KJ 1992 Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci USA 89:8462–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de la Escalera G, Choi AL, Weiner RI 1992 Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1–1 GnRH neuronal cell line. Proc Natl Acad Sci USA 89:1852–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kits KS, Mansvelder HD 2000 Regulation of exocytosis in neuroendocrine cells: spatial organization of channels and vesicles, stimulus-secretion coupling, calcium buffers and modulation. Brain Res Brain Res Rev 33:78–94 [DOI] [PubMed] [Google Scholar]
- Hepp R, Langley K 2001 SNAREs during development. Cell Tissue Res 305:247–253 [DOI] [PubMed] [Google Scholar]
- Belin V, Moos F 1986 Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol 377:369–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Jr JP, Shang E, Wray S 2002 In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci 22:8932–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez L, Villalobos C, Boockfor FR, Frawley LS 2000 The relationship between pulsatile secretion and calcium dynamics in single, living gonadotropin-releasing hormone neurons. Endocrinology 141:2012–2017 [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Martin GB 1989 Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol 123:375–382 [DOI] [PubMed] [Google Scholar]
- Wray S, Gähwiler BH, Gainer H 1988 Slice cultures of LHRH neurons in the presence and absence of brainstem and pituitary. Peptides 9:1151–1175 [DOI] [PubMed] [Google Scholar]
- Maurer JA, Wray S 1999 Luteinizing hormone-releasing hormone quantified in tissues and slice explant cultures of postnatal rat hypothalami. Endocrinology 140:791–799 [DOI] [PubMed] [Google Scholar]
- Moore Jr JP, Wray S 2000 Luteinizing hormone-releasing hormone (LHRH) biosynthesis and secretion in embryonic LHRH. Endocrinology 141:4486–4495 [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Steinberg TH, Silverstein SC 1990 Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium 11:57–62 [DOI] [PubMed] [Google Scholar]
- Caraty A, Antoine C, Delaleu B, Locatelli A, Bouchard P, Gautron JP, Evans NP, Karsch FJ, Padmanabhan V 1995 Nature and bioactivity of gonadotropin-releasing hormone (GnRH) secreted during the GnRH surge. Endocrinology 136:3452–3460 [DOI] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW 1982 Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Südhof TC 1995 Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 375:488–493 [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Caligioni CS, Stojilkovic S, Wray S 2009 Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in GnRH-1 neurons. Endocrinology 150:1400–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Schanhofer WK, Keen KL, Luchansky L 1999 Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel PW, Araki S, Ferin M 1976 Pituitary stalk portal blood collection in rhesus monkeys: evidence for pulsatile release of gonadotropin-releasing hormone (GnRH). Endocrinology 99:243–248 [DOI] [PubMed] [Google Scholar]
- Foster DL, Mickelson IH, Ryan KD, Coon GA, Drongowski RA, Holt JA 1978 Ontogeny of pulsatile luteinizing hormone and testosterone secretion in male lambs. Endocrinology 102:1137–1146 [DOI] [PubMed] [Google Scholar]
- Plant TM 1982 Pulsatile luteinizing hormone secretion in the neonatal male rhesus monkey (Macaca mulatta). J Endocrinol 93:71–74 [DOI] [PubMed] [Google Scholar]
- Rage F, Hill DF, Sena-Esteves M, Breakefield XO, Coffey RJ, Costa ME, McCann SM, Ojeda SR 1997 Targeting transforming growth factor α expression to discrete loci of the neuroendocrine brain induces female sexual precocity. Proc Natl Acad Sci USA 94:2735–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL 2004 The neural basis of puberty and adolescence. Nat Neurosci 7:1040–1047 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL 2001 Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- Spitzer NC 2006 Electrical activity in early neuronal development. Nature 444:707–712 [DOI] [PubMed] [Google Scholar]