Abstract
Infertility can result from a wide range of defects, from behavioral, through germ cell development and maturation, to fertilization or embryo development. Many of the hormones regulating these processes signal via G protein-coupled receptors, which in turn activate a range of plasma membrane enzymes including phospholipase C (PLC)-β isoforms. Transgenic mice lacking functional Plc-β1 (Plc-β1 KO mice) have been noted to have severely impaired fertility, but there has been little study of the reproductive processes affected by lack of this enzyme. This study examined reproductive behavior, gonadal development, fertilization, and implantation in Plc-β1 KO mice. Male and female Plc-β1 KO mice exhibited impaired reproductive behavior. No other defect in reproduction was noted in males, raising the possibility that the reduced fertility of Plc-β1 KO males could be due solely to impaired behavior. In contrast, female Plc-β1 KO mice exhibited both behavioral and nonbehavioral defects. Plc-β1 KO females ovulated only in response to exogenous hormones, with a large proportion of in vivo embryos recovered on embryonic d 4.5 exhibiting abnormal morphology. In addition, uteri of pregnant Plc-β1 KO females exhibited an implantation defect, with poor embryo attachment and a failure to up-regulate cyclooxygenase-2 mRNA.
Signaling via the plasma membrane enzyme PLC-β1 is involved in multiple aspects of reproduction, including behavior, ovulation, pre-implantation embryo development, and implantation.
Many of the hormones regulating a wide range of reproductive processes signal through G protein-linked cell surface receptors, notably the gonadotropins FSH and LH; GnRH; oxytocin; and, in some cases, prostaglandins. Activation of G protein-coupled receptors leads to dissociation of bound heterotrimeric G proteins, which then activate one of several plasma enzymes, such as adenylyl cyclase or phospholipase C (PLC). PLC enzymes hydrolyze phosphatidylinositol 4,5-bisphosphate to form inositol-1,3,4-trisphosphate and diacylglycerol, which in turn stimulate Ca2+ release and protein kinase C activity, respectively. There are five subfamilies of mammalian PLC; β, γ, δ, ε, and ζ. Within the PLC-β subfamily, there are four subtypes, PLC-β1 to PLC-β4, activated by G protein-linked receptors. For most reproductive hormones, it is still not known which PLC-β isoform is activated in response to which receptor stimulation. Furthermore, for some hormones there has been a focus on other plasma enzymes, with the role of PLC-β1 activation unclear. For example, FSH is thought to act primarily via adenylyl cyclase and hence cAMP action (1). There is therefore limited information available about the role of PLC-β1 in reproductive tissues. It has been implicated in ovarian function, specifically in the regulation of meiotic timing in oocytes: location of PLC-β1 in oocytes changes from nuclear to cytoplasmic during follicle maturation, followed by movement back into the nucleus immediately before germinal vesicle breakdown (2,3). Furthermore, at ovulation, it may be involved in both oocyte and somatic cell signaling, with meiosis delayed in oocytes injected with an antibody to PLC-β1, and PLC-β1 activated in somatic granulosa cells in response to progesterone (2,4).
Several different transgenic mice have been generated lacking a functional copy of Plc-β1 (Plc-β1−/− mice) (5,6,7). All exhibit impaired growth and reduced viability, and two lines (6,7) exhibit epileptic-like seizures. Ballester et al. (5) reported that the Plc-β1−/− mice were severely subfertile, which we also found to be the case in mice obtained from Kim et al. (7), whereas Bohm et al. (6) reported late-onset fertility defects. There has been little examination of the cause of the subfertility in any of these lines beyond a report that sperm from the Kim et al. (7) Plc-β1−/− mice exhibited an impaired acrosome reaction, with fertilization rates reduced in vitro (8).
We report here that female Plc-β1−/− mice (7) have a range of reproductive defects, affecting ovulation, support of preimplantation embryo development, implantation and behavior but that the reduced fertility of Plc-β1−/− males could be due solely to altered reproductive behavior.
Materials and Methods
Animals
Mice were housed in an environmentally controlled room on 12-h light, 12-h dark photoperiod. Animals were provided food and water ad libitum, and kept in accordance with U.K. legal requirements.
Transgenic mice had insertion of a neocassette in the plecstrin homology domain of the 5′ region of Plc-β1, with the colony on a mixed 129Sv × C57BL6J background (7). Mice were fed high-protein Teklad 2019 diet (Harlan UK Ltd., Bicester, UK) to provide additional nutrition for Plc-β1−/− offspring (because these mice can have stunted general development when provided with standard rodent food). Heterozygous Plc-β1+/− pairs were bred to provide Plc-β1+/+ [wild type (WT)], Plc-β1+/− (heterozygous), and Plc-β1−/− [knockout (KO)] offspring. Offspring were earmarked for identification upon weaning and DNA prepared from ear punch material for subsequent genotyping.
For all experiments, female mice were 6 wk to 6 months of age and males 2–9 months of age. Control mice were usually age-matched (sibling if possible) WTs, although occasionally heterozygous mice were used for nonbehavioral experiments because they are fully fertile. Stud males were proven WT males from the same colony. For in vitro fertilization (IVF), 6- to 8-wk-old females and 12- to 16-wk-old males from C57BL/6J × CBA/Ca crosses (referred to as F1 mice) were used as a control for the IVF process.
Females were identified as in estrus in which vaginal smears showed primarily cornified cells. Smears to establish cyclicity were carried out at the same time each day over a 5-d period.
Behavioral experiments
Behavioral observations were carried out at the same time of day and in a separate, environmentally controlled room that contained no other mice. Experiments were performed in which a KO or control male was placed in the cage of an estrous WT female, and in which a stud male was placed in the cage of an estrous KO or control female. In each case, the cage was videotaped for 30 min immediately after introduction of the male to allow analysis of his behavior.
Genotyping transgenic mice
Genotyping was carried out from PCRs on DNA extracted from ear punches and confirmed with DNA extracted from tail tips at culling. Sense primer 5′-GTTAAGTCCTCAGGCAAACACC and antisense primer 5′-ACCTTGGGAGCTTTGGCGTG were used to amplify a 180-bp Plc-β1+/+ band, and sense primer 5′-GTTAAGTCCTCAGGCAAACACC and antisense primer 5′-CTGACTAGGGGAGGAGTAGAAG were used to amplify a 290 bp Plc-β1−/− band (modified from Ref. 7).
Histology
Gonads were fixed in 4% (wt/vol) paraformaldehyde overnight and embedded in wax. Sections (5 μm) were counterstained with hematoxylin and eosin for routine histological examination by light microscopy.
IVF and embryo culture
Spermatozoa suspensions were prepared from males as described previously (9) and allowed to capacitate for 2 h in T6 medium (10). Oocyte-cumulus complexes were recovered from oviducts of superovulated females and transferred into droplets of T6 medium, with standard concentrations of capacitated spermatozoa then added. After 4–5 h, oocytes were transferred into glutamine-free KSOM medium (11) supplemented with 1 mg ml−1 fatty acid-free BSA. Media were made in house (chemicals obtained from Sigma-Aldrich, St. Louis, MO). As a control for the IVF system, oocytes were obtained from superovulated F1 females. All oocytes were examined the following day and two-cell embryos transferred to fresh drops of KSOM medium at a ratio of one embryo to 2 μl medium and cultured for 4 d. Number of oocytes reaching blastocyst stage of development was noted. Droplets of medium were covered with silicon fluid (VWR, Lutterworth, UK), and incubation environment was 5% CO2 in air at 37 C.
For culture of in vivo fertilized embryos, zygotes were dissected from oviducts of superovulated, mated females and transferred into droplets of first T6 and subsequently KSOM medium, as with embryos fertilized in vitro.
Superovulations and matings
Animals were injected ip with 5 IU pregnant mare serum gonadotropin, followed by 5 IU human chorionic gonadotropin (hCG) 48 h later (both from Intervet, Milton Keynes, UK), both administered after 1600 h. Oocytes were collected from oviducts 18 h later, or, in experiments in which mating was investigated or embryos obtained, animals were housed with a stud male for a single night immediately after hCG administration. The following day at 1200 h was regarded as d 0.5 of pregnancy with embryos at embryonic d (E) 0.5.
Dye injection, oviduct and uterine flushings and dissection, of implantation sites
At 1200 h on E4.5, females received an ip injection of 0.1ml of 54.7 mg/ml−1 sodium pentobarbitone (Ceva Sante Animale, Libourne, France). Under anesthesia, 1 ml of 0.5% Chicago Sky Blue 6B (Sigma-Aldrich) was perfused through the heart. Chicago Sky Blue stains areas of increased vascular permeability, thus visualizing implantation sites from E4.5 onward (12). Syringes fitted with 34-G square-end luer lock needles (Cooper Needle Works, Birmingham, UK) were filled with Liebovitz L-15 medium (Invitrogen, Renfrew, UK) containing 3 mg ml−1 BSA (Sigma-Aldrich). Needles were inserted into infundibulum of oviducts or upper opening of uterine tracts and medium flushed through to collect any embryos. Implantation sites (blue stained uterine pieces) and interimplantation sites (undyed uterine pieces between two blue stained bands) were dissected out, frozen on dry ice, and stored at −70 C.
RNA extraction and cDNA synthesis
RNA was extracted from implantation sites and interimplantation sites of E4.5 uteri using RNeasy mini kit (QIAGEN, Crawley, UK), with RNA extracts treated with deoxyribonuclease to eliminate traces of genomic DNA (ribonuclease free deoxyribonuclease set; QIAGEN); cDNA was synthesized using QIAGEN′s quantitect reverse transcription kit (kits used according to manufacturer’s directions).
Real-time RT-PCR
Levels of cyclooxygenase-2 (Cox-2) cDNA were quantified using real-time RT-PCR and normalized against the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Forward and reverse primers for Cox-2 were 5′-AAGCGAGGACCTGGGTTCA and 5′-AAGGCGCAGTTTATGTTGTCTGT, respectively, whereas forward and reverse primers for GAPDH were 5′-GGGTGTGAACCACGAGAAAT and 5′-CCTTCCACAATGCCAAAGTT, respectively. QIAGEN′s Quantitect SYBR Green PCR kit was used according to manufacturer’s directions.
Statistics
Statistical significance of differences between mean ± sem of groups was compared by Student’s t tests (paired for male behavioral experiments) or, where more than two groups, by ANOVA followed by Student’s t tests of planned comparisons. mRNA expression differences relative to control tissue were tested with a one-sample t test. Differences between proportions were assessed using χ2.
Results
Behavior is impaired in male and female mice lacking PLC-β1
KO males placed in the cage of an estrous female exhibited reduced appetitive aspects of reproductive behavior (Fig. 1A), with significantly less time spent investigating the female (sniffing female’s head, P < 0.05; sniffing female’s genitals, P < 0.005) and attempted mounting or mounting (P < 0.05). Time spent mating (intromission and ejaculation) was not significantly different, probably because only three of five controls mated during the 30-min observation period, but this was in stark contrast to the KOs, where none of five mated. A similar nonsignificant trend was seen in self-grooming of genitals, a behavior usually observed immediately after mating. Latency of behaviors showed the same general pattern (see supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). There was no significant difference in the time spent eating or drinking, but controls spent more time performing other activities (e.g. investigating environment, self-grooming; P < 0.05), whereas KO males were more inactive (P < 0.05).
Figure 1.
Reproductive behavior is affected in KO mice. Graph shows time spent by male carrying out various behaviors when placed in the cage of an estrous female and videotaped for 30 min (mating covers both intromission and ejaculation, behaviors that are difficult to differentiate in mice). A, Mean ± sem time spent by control (open boxes) or KO (shaded boxes) males carrying out different behaviors when caged with estrous female. B, Mean ± sem time spent by stud males carrying out different reproductive behaviors when caged with control (open boxes) or KO (shaded boxes) estrous females. *, Significant difference between grouped values (P < 0.001).
To determine whether mating would take place over a longer time period, control or KO males were caged with superovulated females overnight and females examined the following morning for copulatory plugs (indicating that mating had taken place). Plugs were found in eight of nine females caged overnight with control males, whereas in contrast, a copulatory plug was found in only one of 11 females caged overnight with KO males (significant difference: P < 0.001).
Where a stud male was introduced into the cage of an estrous KO or control female, analysis of the male’s behavior showed that both estrous KO and estrous control females were equally capable of inducing appetitive aspects of the male’s reproductive behavior (Fig. 1B), with no difference in time spent investigating the female or attempted mounting/mounting. In contrast, stud males virtually never proceeded to the consummatory aspects (P < 0.05), with mating observed in only one of five KO females, and then only briefly, in contrast to more prolonged periods of mating with three of five control females. Likewise, significantly less time was spent in self-grooming of genitals (P < 0.05). Latency of behaviors showed the same general pattern (supplemental Fig. 1).
When superovulated KO or control females were examined for copulatory plugs after overnight caging with a stud male, vaginal plugs were found in almost all females, regardless of phenotype. Thus, despite the reduction in mating of stud males with KO females during the video-observation period, stud males did mate when left overnight, with no significant difference between the rate of mating of stud males and control females and that of stud males and KO females. KO females therefore delay but do not abolish mating behavior in stud males.
Pheromonal cues from female mice lacking PLC-β1
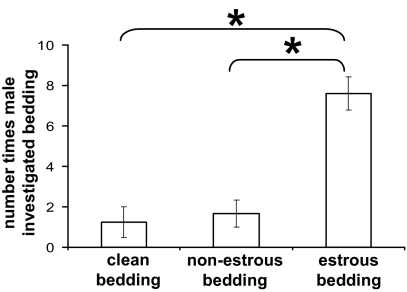
The delay in mating of stud males with estrous KO females could be explained by a lack of production of estrous-indicating pheromonal cues by the females. To test this hypothesis, daily vaginal smears were obtained from unstimulated KO females and bedding obtained from animals that were in estrus (smears showing primarily cornified cells) or metestrus/diestrus (here termed nonestrus: smears showing only leukocyte cells). Bedding, clean or from nonestrous KO females or estrous KO females, was placed at one end of a clean cage. A novel object was placed at the other end. A stud male was introduced to the cage and after a 5-min settling-in period, his behavior was observed every 10 sec for a further 5-min period. Stud males were more likely to investigate bedding from KO estrous females than bedding that was either clean or from KO nonestrous females (P < 0.001 for both; Fig. 2), indicating that the bedding of KO females in estrus does contain pheromonal signals attractive to the males.
Figure 2.
Stud males respond to bedding from KO estrous females. Individual stud males were introduced into a cage containing a novel object and bedding that was unused or from a nonestrous KO female or an estrous KO female (n = 5 for each). Males were observed for a 5-min period, with behavior noted every 10 sec. Graph shows mean ± sem of number of observations during which the animal was investigating bedding. *, Significant difference between grouped values (P < 0.001).
Spermatozoa from males lacking PLC-β1 are able to fertilize oocytes both in vitro and in vivo
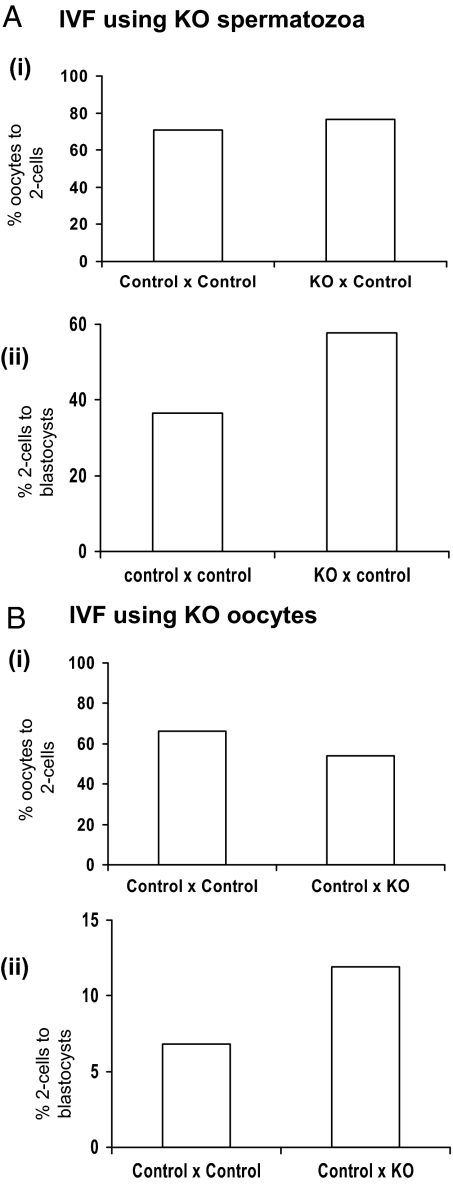
IVF was carried out using spermatozoa preparations from experimental KO or control males and oocytes obtained from superovulated females. Source of spermatozoa preparations had no effect on the rate of fertilization or the development of two-cell embryos to the blastocyst stage in vitro (Fig. 3A).
Figure 3.
IVF: spermatozoa and oocytes from KO mice are able to fertilize in vitro as successfully as spermatozoa and oocytes from control mice, with embryos subsequently able to develop to the blastocyst stage. All treatments are shown as spermatozoa source × oocyte source. A, IVF using experimental KO or control spermatozoa added to superovulated oocytes. i, Rate of fertilization of oocytes, as indicated by percentage of two-cell embryos obtained (two cell embryo number obtained: KO = 34; control = 62); ii, rate of blastocyst development from two-cell embryos. B, IVF using stud male spermatozoa added to experimental KO or control oocytes. i, Rate of fertilization of oocytes, as indicated by percentage of two-cell embryos obtained (two cell embryo number obtained: KO = 187; control = 89); ii, rate of blastocyst development from two-cell embryos.
To examine fertilization in vivo, superovulated females were caged overnight with experimental KO or control males and subsequently examined for implanted embryos at E6.5 (animals were from the overnight behavior experiment detailed above). Embryos were found in all eight plugged females that had been mated with control males and in the single plugged female that had been mated with a KO male. This KO male was subsequently placed in a permanent pair with a WT female, during which time he sired four litters. This particular animal is the only KO male to have sired pups during the course of this study, with no pups obtained from 10 other KO × WT permanent breeding pairs. From the embryos and offspring sired by this particular KO male, we can determine that in vivo, as in vitro, KO spermatozoa that have developed within the testes of KO mice are able to fertilize oocytes.
Female mice lacking PLC-β1 do not usually ovulate, although ovarian follicles develop to the preovulatory stage and mice exhibit estrous cycles
All ovaries, from both KO and control adult mice, contained follicles at all stages of development from primordial to preovulatory, along with corpora lutea (data not shown). Daily vaginal smears of seven KO and 12 control mice showed that all mice exhibited a pattern of smears indicative of estrous cycles. Despite this, healthy ovulated oocytes have not yet been obtained from the fallopian tubes of KO females mated on the night of an estrous smear and culled the following day (although in one instance, the fallopian tube did contain a single degenerate oocyte), whereas histological examination of adult KO ovaries have shown instances of oocytes retained within corpora lutea (data not shown). This situation is in marked contrast to the healthy oocytes routinely recovered from the fallopian tubes of control mice on the day after an estrous smear.
Female mice lacking PLC-β1 respond to superovulation treatment, with oocytes obtained able to undergo fertilization in vitro
Twelve KO and 12 control female mice were superovulated. The day after hCG injection, mice were culled and superovulated oocytes collected from fallopian tubes. In contrast to the lack of oocytes found in the fallopian tubes of unstimulated KO females the day after production of an estrous smear, KO females did ovulate after superovulation. It was therefore possible to obtain KO oocytes for IVF. In each run of IVF, oocytes from two to four KO or two to four control females were pooled; thus, numbers of oocytes from individual animals were not recorded. Counts of pooled oocytes, however, showed no evidence of any marked decrease in the number of oocytes obtained per superovulated KO mouse, compared with that obtained per superovulated control mouse (average number of oocytes obtained per female from 14 KO females = 30; average number of oocytes obtained per female from nine control females = 40). IVF was carried out on KO and control oocytes using spermatozoa preparations from both KO and control males (because we had already determined that sperm from KO mice are able to fertilize oocytes in vitro; see above), with the same spermatozoa preparation always used for both KO and control oocytes within each IVF run. Source of oocytes had no significant effect on fertilization rate (Fig. 3B).
The experiment established that KO oocytes that have developed within the ovaries of KO females are able to fully support embryonic development to the blastocyst stage, even when fertilized by KO spermatozoa that have developed within the testes of KO males.
Ovulated eggs in females lacking PLC-β1 can be fertilized in vivo
Five KO and five control females were superovulated and then caged with a stud male overnight. Copulatory plugs were found in all females the following morning. Females were culled and oocytes/zygotes collected from the fallopian tubes and placed in culture medium as for IVF. Examination of embryo development showed that in four of five KO females and five of five control females, fertilization had taken place in vivo after mating with stud males, with zygotes from these females subsequently developing into healthy-looking blastocysts in vitro.
Mice lacking PLC-β1 have an implantation defect
Expression of PLC-β1 is up-regulated through early pregnancy and marked in cells adjacent to the implanting embryo, consistent with a role in implantation (supplemental Fig. 2). KO and control females were superovulated and placed in a cage with a stud male overnight, as above. When females were culled at presumptive E6.5, no implanted embryos were found in any of the four KO females, in contrast to the implanted embryos found in four of five control females.
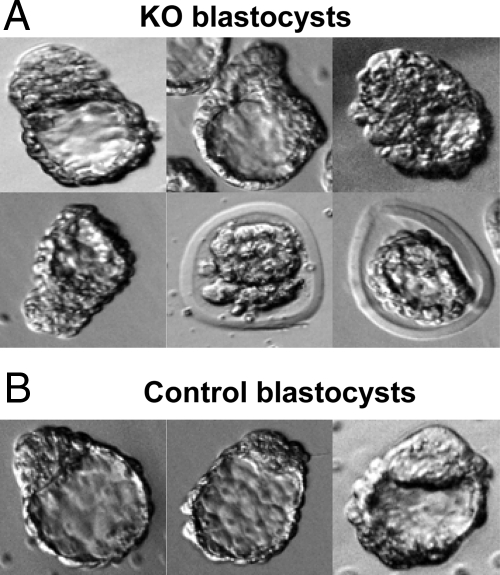
To examine the initial stages of implantation, KO (n = 7) and control (n = 9) females were superovulated and caged overnight with a stud male. Females were then culled at between 1100 and 1300 h on presumptive E4.5. Females were injected with Chicago Sky Blue to visualize implantation bands, the implantation and nonimplantation bands were collected, and fallopian tubes and uteri flushed, all as detailed in Materials and Methods. No embryos were collected from fallopian tube flushings for either KO or control females. In some instances, embryos were collected from uterine flushings of both KO and control females. Embryos were almost all at the blastocyst stage, although many of those from KO females were severely suboptimal, with several retaining zona pellucida (Fig. 4).
Figure 4.
Many E4.5 blastocysts from KO mice have abnormal morphology. Photomicrographs showing typical examples of blastocysts obtained after uterine flushing of KO (A) or control (B) females at E4.5.
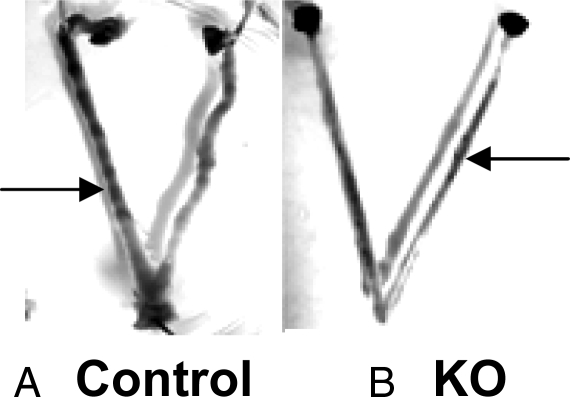
All nine control females had clear implantation bands (Fig. 5A): uterine flushings from these females produced only a small number of embryos, if any. This is most likely to be due to the degree to which implantation had already proceeded. In no instance did the number of embryos flushed from the uterus of a control female exceed the number of implantation sites found, indicating that all embryos in control females had already initiated implantation. This was in marked contrast to the situation in KO females, in which uterine flushings often produced a large number of embryos but few corresponding implantation bands. Two of seven KO females had no implantation bands despite the presence of blastocysts in the uteri. Five of seven KO females had uteri with some implantation bands, but the bands were less strongly stained than in controls (Fig. 5B), and even the female with the most implantation bands (six) had markedly fewer bands than any of the control females. In addition, in two of the five KO females that exhibited bands, there were fewer bands than blastocysts obtained from uterine flushings, indicating that not all embryos had yet initiated implantation.
Figure 5.
KO mice have poorly defined implantation bands at E4.5 compared with those of controls. Photomicrographs showing uteri dissected from E4.5 pregnant mice injected with Chicago Sky Blue, which allows visualization of implantation bands. A, Control uterus. B, KO uterus. Arrows indicate examples of implantation bands.
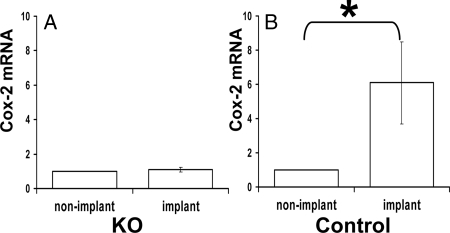
Uteri were dissected from all females that exhibited implantation sites; in each case, strips of implantation bands and strips from areas exhibiting no implantation bands were collected. Real-time RT-PCR was carried out to determine transcription levels of Cox-2, which should be up-regulated in response to implantation (13). Implantation bands obtained from KO females did not exhibit up-regulated Cox-2 expression relative to the level in nonimplantation sites, in contrast to those from control females, in which there was marked up-regulation of Cox-2 mRNA in the implantation sites (Fig. 6; P < 0.05).
Figure 6.
Implantation sites in KO mice fail to up-regulate Cox-2 mRNA. mRNA was extracted from KO and control implantation and nonimplantation sites and the amount of Cox-2 quantified using real-time PCR normalized against GAPDH. A total of four runs were performed for KO (A) and control (B) animals, with three nonimplantation sites (nonimplant) and three implantation sites (implant) assayed from each uterus. In each case, the amount of Cox-2 mRNA in the implantation sites were calculated relative to levels in the nonimplantation sites. Graphs show means ± sem of the Cox-2 mRNA fold change across the four runs. *, Significant difference between grouped values (P < 0.05).
Discussion
All four members of the Plc-β subfamily have been knocked out in mice (5,6,7,14,15), but notably only Plc-β1 KO animals have been reported to be infertile or severely subfertile (5,6). This indicates that PLC-β1 is associated with processes essential for reproductive competence that cannot be compensated by other PLC-βs. Despite this fact, there has been limited investigation on the infertility of Plc-β1 KO mice. Here we report that Plc-β1 KOs exhibit a multitude of reproductive defects that include altered reproductive behavior in both male and female KO mice as well as defective ovulation, early embryonic development and implantation in KO females, whereas in contrast, spematogenesis and fertilization appear to be unaffected.
Behavior
Reproductive behavior of KO animals was significantly different from controls. KO males exhibited greatly reduced appetitive aspects of reproductive behavior, whereas KO estrous females produced pheromonal cues and were as attractive to stud males as control estrous females but delayed the consummatory aspects of the stud males’ reproductive behavior.
The cause of the lack of KO males’ appetitive behavior is unknown. It could result from the animals’ general lack of activity. Increased fear is unlikely because the animals exhibited none of the classical fear responses. It appears not to be due to lack of testosterone because there is no significant difference between serum testosterone levels of WT and KO males (Millar, R., personal communication).
Sexual behavior in mice is mediated by many areas of the limbic and hypothalamic regions of the brain, such as the bed nucleus of the stria terminalis, preoptic area, and the paraventricular and supraoptic nuclei. Neurons from many of these regions express PLC-β1 (16) and are coactivated with nuclear Fos protein during sexual behavior in male mice (17). It will be important to determine whether loss of PLC-β1 expression from these hypothalamic neurons is mediating the behavior deficits described here. Nonreproductive behavioral deficits have been noted in male mice with a forebrain-specific knockout of GluR-B-containing α-amino-3-hydroxy-5-methyl-isoxazoleproprionic acid receptors (18). PLC-β1 is heavily expressed in the forebrain of developing adult rodents (19). Furthermore, both PLC-β1 and GluR-B regulate plasticity at forebrain synapses. Therefore it is possible that any nonreproductive behavioral deficits in Plc-β1 KO mice (such as general lack of activity) may also result in part from nonhypothalamic brain defects.
Gametogenesis and fertilization
Ovaries from KO females contained all stages of follicles as well as corpora lutea. KOs also exhibited estrous cycles and produced pheromonal cues during estrous. Ovulation of oocytes into fallopian tubes, however, almost never occurred naturally, with oocytes instead retained within the forming corpora lutea. This defective ovulation phenotype was rescued by superovulation. It is at present unclear whether lack of natural ovulation in KOs is due to reduced gonadotropin release from the pituitary gland and/or suboptimal gonadotropin action within the ovary. At present, only one paper (20) establishes a link between LH secretion in response to GnRH and PLC-β1 in cell culture of LβT2 pituitary cells. Romoser et al., (20) also report that proteins of PLC-β1 and PLC-β3, but not PLC-β2 and PLC-β4, are expressed in murine pituitaries. It is possible therefore that because Plc-β3 KOs are fertile, PLC-β1 may have essential role(s) in the release of gonadotropins or may be able to compensate for lack of PLC-β3. It is more likely that LH release and/or action is affected, rather than that of FSH, given that mature, preovulatory follicles are routinely observed in KO ovaries.
PLC-β1 has been implicated in murine oocyte maturation (2) and resumption of meiosis after fertilization (2) as well as Ca2+ mobilization of porcine granulosa cells in response to progesterone (4). Taken together along with the lack of ovulation in unprimed females, it might have been expected that oocytes produced by KO females would be likely to be incompetent. Surprisingly, however, oocytes from superovulated KOs fertilized in vitro or in vivo developed to the blastocyst stage in vitro with rates comparable with control oocytes. Oocyte PLC-β1 therefore does not have an obligatory role in oocyte maturation, fertilization competency, or early embryonic development.
Despite the altered reproductive behavior of KO males, their sperm were able to fertilize oocytes from control females in vitro, with resulting embryos developing to blastocysts with rates comparable with WT embryos. This contradicts the findings of Choi et al. (8), who report that Plc-β1 KO sperm show impaired acrosome reaction and reduced rates of fertilization as well as a reduced rate of embryo development to the morula or blastocyst stages. The reason for this discrepancy is unclear; Choi et al. (8) examined the same Plc-β1 mutation on a similar genetic background as used in the experiments detailed here (although the relative levels of C57BL6J to 129Sv were not controlled in either study). The discrepancy might perhaps be the result of different media used for the IVF or due to other as yet unknown differences that led to the overall higher rates of IVF reported here. Most KO mice did not mate, so routine examination of the ability of spermatozoa from KO mice to fertilize oocytes in vivo was not possible. The colony did, however, produce one male KO mouse that mated and produced a series of normal-sized litters during the course of this study. The production of spermatozoa from Plc-β1 KO mice routinely able to fertilize oocytes in vitro, and capable of fertilizing oocytes in vivo in the sole male that mated with females, shows that the process of spermatogenesis itself is unaffected by lack of PLC-β1. There have also been occasional documented occurrences of offspring sired by KO males in the colony before the start of this study, as has occurred in other colonies of these mice (Upton, L,, personal communication,). It is thus possible that the lack of mating behavior usually found in male KOs is the only affect of loss of PLC-β1 on male reproduction.
Early embryo development and implantation
Despite the efficiency of fertilization of KO oocytes in vivo and in vitro, no embryos were found implanted in the uteri of KO animals at E6.5. In mice, embryos reach the uterus at E3.5 in which they hatch from zona pellucida and start implanting at E4.5. It was observed here that at E4.5 all embryos in KO animals had reached the uterus, with none retained in the fallopian tube. However, when uteri of both KO and control animals were flushed, many more blastocysts were retrieved from KO than control animals. This indicates that implantation is impaired in KO females because blastocysts have usually initiated attachment to the uterine epithelium by E4.5, a process that should result in retrieval of only occasional embryos by that stage.
Many of the blastocysts collected from KO uteri at E4.5 were abnormal looking, in contrast to those collected from control uteri. They exhibited gross morphological abnormalities despite the fact that in vivo- and in vitro-fertilized oocytes successfully developed into morphologically normal-looking blastocysts in vitro. Because all embryos in KO females were heterozygous, generated from matings with stud WT males (and because KO embryos can, in any case, implant successfully in heterozygous females), it appears that the reproductive tract of KOs provides inadequate and/or detrimental cues to early embryonic development. There are few documented maternal effects on preimplantation embryo development.
One system of potential relevance here that, when disrupted, impairs both preimplantation embryo development and implantation is endocannabinoid signaling (21,22). Activation of G(q/11)-coupled receptors induces endocannabinoid release from hippocampal neurons in a PLC-β1-dependent process (23). The endocannabinoid anandamide is found in the fallopian tube (24), whereas both anandamide and 2-arachidonoylglycerol are present in vast amounts in the uterus (25). Proper preimplantation embryo development is dependent on the concentration of endocannabinoids; small amounts have beneficial effects on embryo development, whereas larger amounts are detrimental (26,27). Implantation is also regulated by endocannabinoids, with implantation sites having lower concentrations of anandamide and 2-arachidonoylglycerol than nonimplantation sites (25). It is possible therefore that the implantation defect found here may be due to disrupted PLC-β1-dependent endocannabinoid signaling in the reproductive tract.
PLC-β1 was expressed in uteri with up-regulation before implantation, and KO females could not successfully implant embryos. KO females were, however, able to at least initiate some implantation events, despite the abnormal appearance of some of the blastocysts. This was evident from the presence of a small number of Chicago Sky-dyed implantation sites (although these occasional sites were less strongly dyed than sites from control uteri). It was further found that the expected physiological up-regulation of Cox-2 does not happen in these E4.5 KO implantation sites, although uteri up-regulate Cox-2, even in response to oil droplets (28). It is therefore possible that KO uteri are unable to respond to signals from embryos, that embryos developing in the KO reproductive tract fail to produce these signals, or that both scenarios may happen at the same time. The links between Cox-2 and fertility are well documented, with Cox-2 KO animals showing defective ovulation, fertilization, implantation, and decidualization (for review see Ref. 28). It is unclear whether the Cox-2 deficiency is a cause or a result of the impaired initiation of implantation documented here.
Conclusion
This paper reports a multitude of reproductive problems in Plc-β1 KO mice, ranging from abnormal sexual behavior to defects in ovulation, early embryonic development, and implantation. The data signify that G protein-linked cell surface receptor activation of PLC-β1 plays important roles in controlling reproductive success.
Supplementary Material
Acknowledgments
We thank He Sup Shin for Plc-β1 transgenic mice; Rowena Smith for technical assistance; Vanessa Vanneck for help with initial behavioral and estrous studies; Celine Caquineau and Emma Wood for advice on the design of behavioral studies; and Richard Anderson and Evelyn Telfer for comments on an earlier version of the manuscript.
Footnotes
This work was supported by the Moray Endowment Fund (University of Edinburgh) and The Wellcome Trust (077636).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 2, 2009
Abbreviations: Cox-2, Cyclooxygenase-2; E, embryonic day; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; hCG, human chorionic gonadotropin; IVF, in vitro fertilization; KO, knockout; PLC, phospholipase C; WT, wild type.
References
- Conti M 2002 Specificity of the cyclic adenosine 3′,5′-monophosphate signal in granulosa cell function. Biol Reprod 67:1653–1661 [DOI] [PubMed] [Google Scholar]
- Avazeri N, Courtot AM, Pesty A, Duquenne C, Lefèvre B 2000 Cytoplasmic and nuclear phospholipase C-β1 relocation: role in resumption of meiosis in the mouse oocyte. Mol Biol Cell 11:4369–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avazeri N, Courtot AM, Pesty A, Lefevre B 2003 Meiosis resumption, calcium-sensitive period, and PLC-β1 relocation into the nucleus in the mouse oocyte. Cell Signal 15:1003–1010 [DOI] [PubMed] [Google Scholar]
- Lieberherr M, Grosse B, Machelon V 1999 Phospholipase C-β and ovarian sex steroids in pig granulosa cells. J Cell Biochem 74:50–60 [PubMed] [Google Scholar]
- Ballester M, Molist J, Lopez-Bejar M, Sánchez A, Santaló J, Folch JM, Ibáñez E 2004 Disruption of the mouse phospholipase C-β1 gene in a β-lactoglobulin transgenic line affects viability, growth, and fertility in mice. Gene 341:279–289 [DOI] [PubMed] [Google Scholar]
- Böhm D, Schwegler H, Kotthaus L, Nayernia K, Rickmann M, Köhler M, Rosenbusch J, Engel W, Flügge G, Burfeind P 2002 Disruption of PLC-β 1-mediated signal transduction in mutant mice causes age-dependent hippocampal mossy fiber sprouting and neurodegeneration. Mol Cell Neurosci 21:584–601 [DOI] [PubMed] [Google Scholar]
- Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS 1997 Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature 389:290–293 [DOI] [PubMed] [Google Scholar]
- Choi D, Lee E, Hwang S, Jun K, Kim D, Yoon BK, Shin HS, Lee JH 2001 The biological significance of phospholipase Cβ1 gene mutation in mouse sperm in the acrosome reaction, fertilization, and embryo development. J Assist Reprod Genet 18:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears N, Boland NI, Murray AA, Gosden RG 1994 Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod 9:527–532 [DOI] [PubMed] [Google Scholar]
- Quinn P, Warnes GM, Kerin JF, Kirby C 1984 Culture factors in relation to the success of human in vitro fertilization and embryo transfer. Fertil Steril 41:202–209 [DOI] [PubMed] [Google Scholar]
- Devreker F, Hardy K 1997 Effects of glutamine and taurine on preimplantation development and cleavage of mouse embryos in vitro. Biol Reprod 57:921–928 [DOI] [PubMed] [Google Scholar]
- Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK 2002 Cytosolic phospholipase A2α is crucial [correction of A2α deficiency is crucial] for ‘on-time’ embryo implantation that directs subsequent development. Development 129:2879–2889 [DOI] [PubMed] [Google Scholar]
- Scherle PA, Ma W, Lim H, Dey SK, Trzaskos JM 2000 Regulation of cyclooxygenase-2 induction in the mouse uterus during decidualization. An event of early pregnancy. J Biol Chem 275:37086–37092 [DOI] [PubMed] [Google Scholar]
- Jiang H, Kuang Y, Wu Y, Xie W, Simon MI, Wu D 1997 Roles of phospholipase Cβ2 in chemoattractant-elicited responses. Proc Natl Acad Sci USA 94:7971–7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Samoriski GM, McLaughlin JP, Romoser VA, Smrcka A, Hinkle PM, Bidlack JM, Gross RA, Jiang H, Wu D 1999 Genetic alteration of phospholipase Cβ3 expression modulates behavioral and cellular responses to mu opioids. Proc Natl Acad Sci USA 96:10385–10390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Nakamura M, Sato K, Kano M, Simon MI, Inoue Y 1998 Patterns of expression for the mRNA corresponding to the four isoforms of phospholipase Cβ in mouse brain. Eur J Neurosci 10:2016–2025 [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Lunn K, Kind P, Spears N 2006 Phospholipase Cβ1-positive neurons in the brain are activated during sexual behavior in male mice. Front Neuroendocrinol 27:146 (Abstract) [Google Scholar]
- Shimshek DR, Bus T, Grinevich V, Single FN, Mack V, Sprengel R, Spergel DJ, Seeburg PH 2006 Impaired reproductive behavior by lack of GluR-B containing AMPA receptors but not of NMDA receptors in hypothalamic and septal neurons. Mol Endocrinol 20:219–231 [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Kind PC, Blakemore C 1998 Phospholipase C-β1 expression correlates with neuronal differentiation and synaptic plasticity in rat somatosensory cortex. Neuropharmacology 37:593–605 [DOI] [PubMed] [Google Scholar]
- Romoser VA, Graves TK, Wu D, Jiang H, Hinkle PM 2001 Calcium responses to thyrotropin-releasing hormone, gonadotropin-releasing hormone and somatostatin in phospholipase css3 knockout mice. Mol Endocrinol 15:125–135 [DOI] [PubMed] [Google Scholar]
- Paria BC, Song H, Wang X, Schmid PC, Krebsbach RJ, Schmid HH, Bonner TI, Zimmer A, Dey SK 2001 Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem 276:20523–20528 [DOI] [PubMed] [Google Scholar]
- Wang H, Xie H, Guo Y, Zhang H, Takahashi T, Kingsley PJ, Marnett LJ, Das SK, Cravatt BF, Dey SK 2006 Fatty acid amide hydrolase deficiency limits early pregnancy events. J Clin Invest 116:2122–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M 2005 Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 45:257–268 [DOI] [PubMed] [Google Scholar]
- Schuel H 2006 Tuning the oviduct to the anandamide tone. J Clin Invest 116:2087–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK 1997 Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc Natl Acad Sci USA 94:4188–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Ma W, Andrenyak DM, Schmid PC, Schmid HH, Moody DE, Deng H, Makriyannis A, Dey SK 1998 Effects of cannabinoids on preimplantation mouse embryo development and implantation are mediated by brain-type cannabinoid receptors. Biol Reprod 58:1490–1495 [DOI] [PubMed] [Google Scholar]
- Wang J, Paria BC, Dey SK, Armant DR 1999 Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol Reprod 60:839–844 [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK 2005 Lipid signaling in embryo implantation. Prostaglandins Other Lipid Mediat 77:84–102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.