Abstract
Normal postnatal growth is dependent in part on overlapping actions of GH and IGF-I. These actions reflect GH stimulation of IGF-I production in liver and extrahepatic tissues, representing respectively the endocrine and autocrine/paracrine arms of the IGF system. Recent experiments in genetically modified mice show that each source of IGF-I can compensate for absence of the other but do not resolve their relative role in postnatal growth. In an effort to address this issue, we studied the GH responsiveness of mice harboring a null mutation of the acid-labile subunit (ALS). Null ALS mice have a substantial reduction in endocrine IGF-I but, unlike other models of plasma IGF-I deficiency, have no obvious additional endocrine defects. Wild type and null ALS mice of both sexes received daily sc injections of saline or recombinant bovine GH between d 35 and 63 of postnatal age. The GH-stimulated body weight gain of null ALS mice was reduced by more than 30% relative to wild type mice, irrespective of sex. Reductions in GH responsiveness were also seen for kidney and linear growth. Absence of ALS eliminated the ability of GH to increase plasma IGF-I despite intact GH-dependent stimulation of IGF-I expression in liver, adipose tissue, and skeletal muscle. GH treatment was also less efficient in antagonizing insulin action in null ALS mice. Overall, these results suggest that the GH effects mediated by endocrine IGF-I depends on ALS, and accordingly null ALS mice are less responsive to exogenous GH therapy.
Growth hormone effects are attenuated in mice deficient for the acid-labile subunit.
Normal postnatal growth requires the combined actions of GH and IGF-I (1,2). Thus, postnatal growth is severely impaired in viable mice with gene deletions preventing either GH or IGF-I actions (3,4). Lupu et al. (2) estimated the contribution of each signal by comparing the postnatal growth of mice harboring null GH receptor or IGF-I genes. They found that GH and IGF-I account, respectively, for 14 and 35% of postnatal growth whereas the contribution of GH mediated by IGF-I is 34%.
During postnatal life, most of plasma IGF-I is synthesized by the liver and circulates in ternary complexes of 150 kDa composed of one molecule each of IGF-I, IGF binding protein (IGFBP)-3 or IGFBP-5 and the acid labile subunit (ALS) (5,6,7). IGF-I is also synthesized by most tissues (8,9) in which it is usually bound to one member of the IGFBP family to yield binary complexes of 40–50 kDa (10). Various knockout models have been created to address the relative contribution of plasma and locally derived IGF-I for postnatal growth (11,12,13). Models devoid of either source of IGF-I provide the most extreme comparison. On one hand, liver IGF-I-deficient mice (LID) grow normally after birth suggesting little functional significance for plasma IGF-I (11). On the other hand, postnatal growth of IGF-I knockout mice is restored by exclusive reexpression of IGF-I in the liver (14). These experiments show that each source can compensate for absence of the other but do not resolve their relative role in postnatal growth.
The liver is ideally positioned to sense anabolic inputs represented by the combination of nutrients and anabolic hormones such as GH and to increase synthesis of IGF-I accordingly (15,16,17). In this manner, liver-derived IGF-I could fine-tune the growth response in accordance with the prevailing metabolic state by supplementing the locally produced pool of IGF-I. To test this hypothesis, we studied the ability of exogenous GH to increase the postnatal growth of null ALS mice. Null ALS mice have a substantially reduced plasma IGF-I reservoir because they cannot incorporate liver-derived IGF-I into long-lived ternary complexes (12,18). We hypothesized that in the absence of ALS, the ability of GH to increase plasma IGF-I and to stimulate growth would be impaired. This experiment also provided the opportunity to determine whether null ALS mice are equally susceptible to the antagonistic effects of GH on insulin action.
Materials and Methods
Animals
Experimental procedures involving live animals were conducted with the approval of the Cornell University Institutional Animal Care and Use Committee. All animals were fed a standard rodent chow (Harlan Teklad 8640 containing 22% protein, 5% fat; Harlan, Madison, WI) and were offered water ad libitum. The facility was kept at constant temperature and humidity (∼22 C, ∼65%) with controlled lighting (14 h light, 10 h dark cycle).
Null ALS mice were of mixed genetic background (BALB/c × 129sv) and were described previously (12). Male and female mice heterozygous for the null ALS allele were intercrossed to generate wild-type (WT) and null ALS littermates. Mice were weaned at 3 wk of age and genotyped by PCR using forward primers forward Neo1, GAC ATA GCG TTG GCT ACC CGT GA, and forward ALS, TGC AGC TGG GCC ACA ATC GAA TC with the single reverse primer R-mPrB, GCA AGG AGT TAT TCC TGA GGT TG. This combination of primers yielded bands of 602 bp for the WT ALS allele and 395 bp for the null ALS allele.
Chronic GH treatment
A total of 61 mice were used (n = 14–16 mice for each genotype × sex combination). On d 35, they were randomly allocated to receive twice-daily sc injections of saline or recombinant bovine GH (3 μg/g body weight per injection; Protiva, St. Louis, MO) for 4 wk. On a daily basis, animals were weighed and injected at 0900 h, followed by a second injection at 2100 h. On the last day of treatment, mice were killed within 2–4 h of the last injection by carbon dioxide asphyxiation. Blood was collected by cardiac puncture and plasma was stored at −20 C until further analysis. Nose to anus length was measured. Liver, kidney, heart, spleen, and brain were dissected, blotted dry, and weighed. A subsample of gonadal adipose tissue was also obtained. All tissues were frozen immediately in liquid nitrogen.
Insulin tolerance test during short-term GH treatment
Eight-week-old male mice (n = 9–10 for each genotype) were used in a cross-over design with 3.5-d periods separated by a 2-wk interval. Treatments were twice-daily sc injections of saline or recombinant bovine GH (GH, 3 μg/g body weight per injection) for 3.5 d. After the final injection at 0900 h, feed was removed and an insulin tolerance test was performed 6 h later. Briefly, a blood sample was obtained from the dorsal tail artery and assayed for glucose with an Ascensia Contour glucometer (Bayer Health Care, Tarrytown, NY). This was followed by an ip injection of human insulin (0.6 μU/g body weight, Humulin R; Eli Lilly and Co., Indianapolis, IN). Blood glucose determinations were repeated 30 and 60 min after insulin injection.
Analysis of plasma variables
Plasma IGF-I and insulin were measured using commercially available rat RIA assays (Diagnostic Systems Laboratories, Webster, TX; Linco Research, Inc., St. Charles, MO). Plasma glucose concentrations were measured by the glucose oxidase method (Sigma, St. Louis MO).
Plasma IGFBP-3 abundance was determined by ligand blotting (12). Plasma samples (0.7 μl) were resolved on a 10% sodium dodecyl sulfate-polyacrylamide gel under nonreducing conditions. After electrophoresis, proteins were transferred onto a 0.45 μm nitrocellulose membrane (Life Technologies, Gaithersburg, MD), and IGFBP-3 was detected by incubation with 100,000 cpm/ml of recombinant [125I]IGF-I. Membranes were washed and exposed to x-ray film at −80 C. Signals were quantified using a FUJIX bioimaging analyzer BAS1000 (Fuji Medical Systems USA, Inc., Stanford, CT).
For ALS determination, plasma samples (0.5 μl) were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels under reducing conditions and transferred to nitrocellulose as above. Membranes were blocked in Tris-buffered saline with Tween 20 [50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.1% Tween 20] containing 5% (wt/vol) nonfat dried skim milk. They were incubated sequentially with the primary antibody (1:1000 dilution, goat antimouse ALS AF1436; R&D Systems, Inc., Minneapolis, MN) and the secondary antibody (1:5000 dilution, rabbit antigoat; KPL, Gaithersburg, MD). The ALS signal was developed with LumiGLO chemiluminescent reagent (KPL). Signals were recorded onto x-ray film and quantified by densitometry using the NIH Image 1.6.3 software (National Institutes of Health, Bethesda, MD).
Total RNA isolation and Northern analysis
Total RNA was extracted from liver using Qiazol and RNeasy columns (QIAGEN, Valencia, CA). The concentration of total RNA was measured by absorbance at 260 nm and its integrity confirmed by staining ribosomal RNA with Sybr Green II (Molecular Probes, Eugene, OR). Total RNA was analyzed by Northern blotting using a 406 bp rat IGF-I cDNA and a 423 rat IGFBP-3 cDNA (12). Equality of loading was verified by hybridization signals obtained with an oligonucleotide corresponding to the 18S ribosomal RNA. Signals were quantified using a FUJIX bioimaging analyzer BAS1000.
Real-time PCR assays were used to measure IGF-I and IGFBP-3 expression in skeletal muscle, adipose tissue, and kidney. Total RNA was extracted as above with the exception that on-column sodium dodecyl sulfate-free deoxyribonuclease treatment was also performed (QIAGEN). Quantity and integrity of RNA was determined using the RNA nano lab chip kit and bioanalyzer (Agilent, Palo Alto, CA). Reverse transcriptase reactions were performed with 2 μg of total RNA using a cDNA synthesis kit (high-capacity cDNA synthesis kit; Applied Biosystems, Foster City, CA).
Real-time SYBR green PCR assays were performed using primers described elsewhere (19) for IGFBP-3 forward primer, CAG TTC GTG TGT GGA CCG AG and reverse primer, GCT CCG GAA GCA ACA CTC AT for IGF-I, and forward primer CAT GGC TCG CTC GGT GAC C and reverse primer AAT GTG AGG CGG GTG GAA CTG for β2-microglobulin. Reactions were performed in duplicate in a 25-μl volume using ABI power SYBR mix with ROX dye (Applied Biosystems). Reactions contained 25 ng of cDNA and 400 nm of gene specific primers. A relative standard curve was constructed for each tissue and consisted of seven serial 2-fold dilutions of pooled cDNAs prepared from random samples. Unknown sample expression was determined from the appropriate standard curve and expressed as a fold difference as indicated in the figure legends. β2-Microglobulin expression was unaffected by genotype and treatments across all tissues and was used as the invariant control.
Statistical analyses
GH-dependent gain was analyzed by a model accounting for genotype (G; WT or null ALS), sex (S; female or male), and their interaction (G × S). All other data were analyzed by sex using a model accounting for G (WT or null ALS), hormone (H; saline or GH), and their interaction (G × H). Covariates were included in the model when appropriate (final body weight when analyzing organ weights and β2-microglobulin when analyzing IGF-I and IGFBP-3 expression). Statistical significance was set at P < 0.05 for main effects and P < 0.10 for interactions.
Results
Absence of ALS attenuates GH-dependent body weight gain
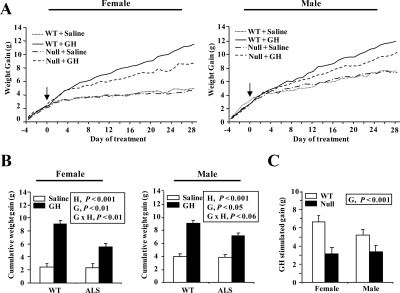
Body weights were recorded daily, and the cumulative weight gain was calculated by difference from initial body weight at d 30. Male and female null ALS mice grew at the exact same rate as their WT counterpart over the 4 d preceding treatment (Fig. 1A, d −4 to 0 relative to the start of treatment). At the start of treatment on d 35, female and male null ALS mice had growth deficits of 15 and 21% (null vs. WT females, 14.8 vs. 17.5 g, P < 0.05; null vs. WT males, 16.3 vs. 20.5 g, P < 0.05).
Figure 1.
Effect of chronic GH treatment on body weight gain. WT and null ALS mice (null) were treated twice daily with GH (3 μg/g body weight per injection) or an equivalent volume of saline from 35 to 63 d of age. A, Body weights were recorded daily and the cumulative gain calculated by difference from body weight on d 30. The arrow indicates the start of treatment. Data are shown for female and male mice. B, The cumulative weight gain during the treatment period was calculated as the difference between weight on d 63 and 35. Data are shown for female and male mice. C, GH-stimulated gain in female mice. This variable corresponds to the gain, depending exclusively on exogenous GH, and was calculated for the entire treatment period by subtracting the average gain of saline-treated mice from the gain of each GH-treated mice. The significant effects of G (WT or null), H (saline or GH), and their interaction (G × H) are reported. Each line and bar represents means of seven to nine mice.
The role of ALS in GH-dependent growth was assessed by administering exogenous GH between d 35 and 63 of age. During the treatment period, GH stimulated body weight gain in both genotype but less so in null than WT mice (Fig. 1A). The reduced effect of GH in null mice was seen in both sexes (Fig. 1B, G × H, P < 0.06 or less).
This analysis also showed that the gain of saline-treated WT and null ALS mice was identical throughout the treatment period (Fig. 1, A and B). Therefore, the gain depending exclusively on exogenous GH, referred to in the following text as GH-stimulated gain, was calculated by subtracting the average gain of saline-treated mice from the gain of each GH-treated mice. In the case of females, the GH-stimulated gain of null mice was reduced by 50% relative to their WT counterparts after the first week of treatment (1.2 vs. 2.4 g, P < 0.001). This reduction remained constant in null ALS female mice during each subsequent week of treatment (results not shown) such that the overall reduction was 52% over the 4-wk treatment period (Fig. 1C; G, P < 0.001). The reduction in GH responsiveness persisted, even after using initial body weight as a covariate in the statistical analysis. These data indicate that ALS is necessary for complete effectiveness of exogenous GH on body weight gain.
The GH-stimulated body weight gain appeared to be less in male than female mice (Fig. 1C), and accordingly absence of ALS also appeared to reduce GH responsiveness to a lesser in male mice (34 vs. 52%); these effects, however, were never statistically different between male and female mice, irrespective of the treatment period considered (S and G × S effects, P > 0.15 or more). A similar lack of a sex effect was seen for all other variables, and accordingly the results reported in the next sections are for female mice unless specifically noted.
Absence of ALS attenuates GH-dependent linear and organ growth
We next asked whether ALS was also necessary for the complete stimulatory effects of GH on body length and organ growth. At the end of treatment, GH increased linear growth as well as the absolute weight of liver, kidney, heart, and spleen (Table 1; H, P < 0.001). With the exception of the heart, these positive GH effects were attenuated in null ALS mice (G × H, P < 0.08 or less).
Table 1.
Effect of chronic GH treatment on body length and organ weights in WT and null ALS female mice
| WTa
|
Null ALSa
|
Significance levelb
|
||||||
|---|---|---|---|---|---|---|---|---|
| Saline | GH | Saline | GH | sd | G | H | G × H | |
| Body length (cm) | 8.5 | 9.4 | 8.2 | 8.7 | 0.32 | 0.001 | 0.001 | 0.05 |
| Absolute weight (g) | ||||||||
| Liver | 0.75 | 1.31 | 0.77 | 1.07 | 0.15 | NS | 0.001 | 0.03 |
| Kidney | 0.26 | 0.38 | 0.27 | 0.33 | 0.02 | 0.03 | 0.001 | 0.002 |
| Heart | 0.10 | 0.13 | 0.09 | 0.11 | 0.01 | 0.05 | 0.001 | NS |
| Brain | 0.42 | 0.46 | 0.40 | 0.40 | 0.03 | 0.001 | NS | 0.08 |
| Spleen | 0.06 | 0.10 | 0.07 | 0.08 | 0.02 | NS | 0.001 | 0.02 |
| Weight adjusted by covariate analysis for final body weight (g) | ||||||||
| Liver | 0.84 | 1.17 | 1.00 | 1.12 | 0.10 | 0.02 | 0.02 | NS |
| Kidney | 0.28 | 0.33 | 0.31 | 0.34 | 0.01 | 0.02 | 0.001 | 0.02 |
| Heart | 0.10 | 0.11 | 0.10 | 0.12 | 0.01 | NS | 0.07 | NS |
| Brain | 0.42 | 0.46 | 0.40 | 0.40 | 0.03 | 0.02 | NS | NS |
| Spleen | 0.07 | 0.08 | 0.08 | 0.08 | 0.02 | NS | NS | NS |
WT and null ALS mice were injected twice daily with GH (3 μg/g body weight) or an equivalent volume of saline from 35 to 63 d of age (n = 7–9 mice for each treatment).
Type I error probability for G, H, and their interaction (G × H). NS = nonsignificant at P > 0.05 for main effects and P > 0.1 for the interaction.
Next we determined whether the attenuation of GH-stimulated organ growth by ALS was accounted entirely by its effect on overall growth. This was done by using final body weight as a covariate in the analysis of organ weights (Table 1). The attenuation of GH-stimulated organ growth by ALS persisted for kidney (G × H, P < 0.02), approached significance for spleen (G × H, P < 0.13), but was eliminated in the case of liver. This analysis also revealed that null ALS mice have disproportionately heavier liver and kidney than their WT counterparts but smaller brain (G, P < 0.02). Overall, these data suggest that ALS is required for the full organomegalic effect of exogenous GH on kidney and for its stimulation of linear growth.
Absence of ALS prevents the GH stimulation of plasma IGF-I
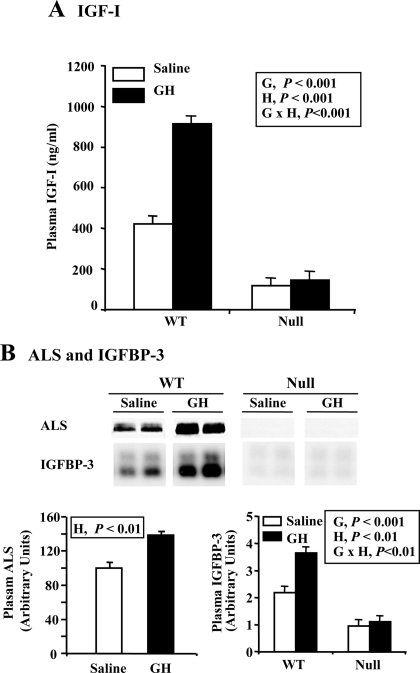
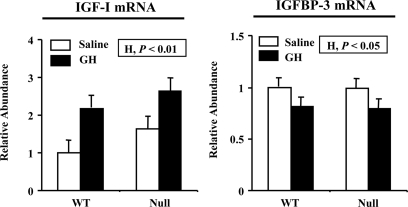
Impact of GH and null ALS genotype on the plasma IGF system was assessed at the end of the treatment period. In WT mice, GH treatment doubled plasma IGF-I concentration and increased the abundance of plasma ALS and IGFBP-3 by 39 and 64%, respectively (Fig. 2, A and B; H, P < 0.01 or less). Absence of ALS reduced plasma IGF-I and IGFBP-3 by 73% and 57%, respectively, and completely eliminated the ability of GH to increase these plasma proteins (Fig. 2, A and B; G × H, P < 0.01 or less). In the case of IGF-I, this occurred despite similar GH-dependent stimulation of hepatic expression in both WT and null liver (Fig. 3; H, P < 0.01). GH reduced hepatic IGFBP-3 expression, but again this effect was similar across genotype (Fig. 3; H, P < 0.05) and therefore cannot explain the lack of GH effect on plasma IGFBP-3 in null ALS mice. Therefore, in the absence of ALS, the liver loses the ability of using plasma IGF-I to mediate GH effects on extrahepatic tissues.
Figure 2.
Effect of chronic GH treatment on plasma IGF-I, IGFBP-3, and ALS. WT and null ALS mice (null) were treated twice daily with GH (3 μg/g body weight per injection) or an equivalent volume of saline from 35 to 63 d of age. Blood was collected on d 63 of treatment. A, Plasma was analyzed for the concentration of IGF-I by RIA. B, Plasma was analyzed for the abundance of IGFBP-3 by ligand blotting and for the abundance of ALS by Western immunoblotting (top panel). Signals were quantified and shown in graphical forms (bottom panel). The significant effects of G (WT or null), H (saline or GH), and their interaction (G × H) are reported. Each bar represents the mean ± se of seven to nine female mice.
Figure 3.
Effect of chronic GH treatment on the expression of IGF-I and IGFBP-3 mRNA in liver. WT and null ALS mice (null) were treated twice daily with GH (3 μg/g body weight per injection) or an equivalent volume of saline from 35 to 63 d of age. Total RNA was extracted from liver at the end of treatment and analyzed for the abundance of IGF-I or IGFBP-3 mRNA by Northern blotting. Results are expressed relative to the mean expression level of saline-treated WT mice. The significant effects of G (WT or null), H (saline or GH), and their interaction (G × H) are reported. Each bar represents the mean ± se of seven to nine female mice.
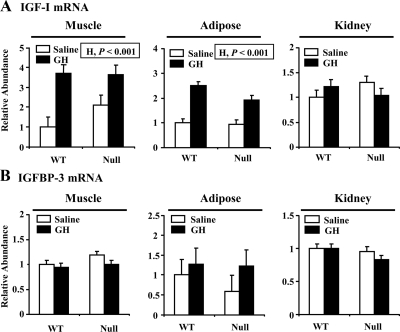
Next we asked whether the absence of ALS impacted the ability of GH to induce IGF-I expression in extrahepatic tissues. In skeletal muscle, IGF-I expression appeared higher in saline-treated null than WT mice, but this effect was not significant (Fig. 4A; G, P > 0.3). More, importantly, GH caused an increase in muscle IGF-I expression (H, P < 0.001) that was statistically similar between genotype (G × H, P > 0.2). GH had a similar positive effect on IGF-I expression in adipose tissue (Fig. 4A; H, P < 0.001). There was no effect of GH on IGFBP-3 expression in any of the extrahepatic tissues studied. These data suggest that absence of ALS does not modify the ability of GH to raise IGF-I production in extrahepatic tissues.
Figure 4.
Effect of chronic GH treatment on the expression of IGF-I and IGFBP-3 mRNA in extrahepatic tissues. WT and null ALS mice (null) were treated twice daily with GH (3 μg/g body weight per injection) or an equivalent volume of saline from 35 to 63 d of age. Total RNA was extracted from skeletal muscle, adipose tissue, or kidney at the end of treatment and analyzed by real-time quantitative RT-PCR for abundance of IGF-I mRNA (A) or IGFBP-3 mRNA (B). Results are expressed relative to the mean expression level of saline-treated WT mice. The significant effects of G (WT or null), H (saline or GH), and their interaction (G × H) are reported. Each bar represents the mean ± se of seven to nine female mice.
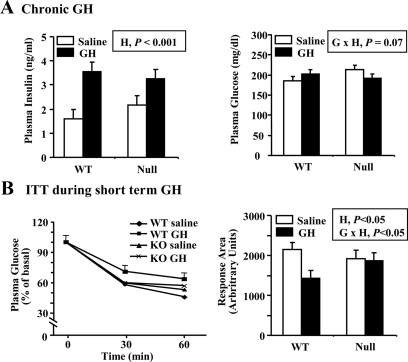
Effect of ALS attenuates GH regulation of carbohydrate metabolism
Plasma insulin and glucose were measured at the end of treatment under fed conditions in both male and female mice. Chronic GH treatment increased plasma insulin similarly in both WT and null mice (Fig. 5A; H, P < 0.001). Chronic GH treatment increased plasma glucose in WT mice but had the opposite effect in null ALS mice (G × H, P < 0.07). These data suggest that GH-treated null ALS mice might be more insulin sensitive than similarly-treated WT mice.
Figure 5.
Effect of GH treatment on indices of carbohydrate metabolism. A, WT and null ALS mice (null) were treated twice daily with GH (3 μg/g body weight per injection) or an equivalent volume of saline from 35 to 63 d of age. Plasma levels of insulin and glucose were measured at the end of the treatment period. Each bar represents the mean ± se of 14–18 male and female mice. B, Eight-week-old WT and null mice were treated twice daily with GH (3 μg/g body weight per injection) or an equivalent volume of saline for 3.5 d. Plasma glucose was measured immediately before (0 min) and after (30 and 60 min) the administration of insulin (0.6 μU/g body weight). Relative plasma glucose levels are shown on the left with pooled se shown only for WT saline group for clarity. The graph depicting areas under the glucose response curve is shown on the right. The significant effects of G (WT or null), H (saline or GH), and their interaction (G × H) are reported. Each bar represents the mean ± se of nine to 10 male mice.
To probe this interaction, WT and null ALS male mice were treated for 3.5 d with either saline or GH and subjected to an insulin tolerance test. Short-term GH treatment increased fasted plasma glucose to a greater extent in WT than null mice (GH vs. saline, 82 vs. 71 mg/dl in WT and 76 vs. 77 mg/dl in null, G × H, P < 0.01). In WT mice, GH attenuated the reduction of plasma glucose occurring 30 and 60 min after insulin administration, whereas saline- and GH-treated null ALS mice had overlapping glucose response curves (Fig. 5B). As a result, GH caused a significant reduction in the area under the glucose response curve in WT but not null ALS mice (Fig. 5B; G × H, P < 0.05). Overall, these data suggest that the ability of GH to induce peripheral insulin resistance is attenuated in null ALS mice.
Discussion
The plasma IGF-I concentration increases manyfold in early postnatal life (20,21). The events leading to this increase in plasma IGF-I are well understood in rodents. First, the liver becomes the predominant site of IGF-I synthesis (9,22,23). This predominance is facilitated by the acquisition of functional hepatic GH receptor at approximately 3 wk of age, allowing GH-dependent production of IGF-I (15,24). Second, the liver coincidentally initiates GH-dependent synthesis of ALS, a protein that recruits IGF-I and IGFBP-3 into ternary complexes (25). Ternary complexes prolong IGF-I half-life from minutes to hours, allowing the development of the plasma IGF-I reservoir (7,26,27). So far, the physiological significance of the liver-derived, plasma IGF-I reservoir for postnatal growth remains uncertain. The liver is a major peripheral sensor of anabolic inputs represented by the prevailing combination of nutrients and hormones (16,17,28). One possibility is that the increased hepatic IGF-I synthesis that occurs with high anabolic inputs supplements locally produced IGF-I to bring about the full growth response. Null ALS mice offer a unique opportunity to test this model because their ability to retain liver-derived IGF-I is impaired (12,18).
To start assessing this possibility, we used a chronic period of GH administration. This treatment provides a strong anabolic stimulus and increases whole body growth in mice and other mammals (29,30,31). We chose to administer GH between d 35 and 63 of postnatal life for two reasons. First, as shown here and in our previous work (12), null ALS mice grow at exactly the same rate as WT mice starting at approximately d 30 of postnatal age. Second, previous studies in the GH-deficient lit/lit mice identified the pubertal and immediate postpubertal periods as times of greatest GH responsiveness, both in terms of stimulation of plasma IGF-I and overall growth (29). Our results indicate that, despite an equally effective GH stimulation of hepatic IGF-I mRNA, null ALS mice did not grow as well as WT animals in response to GH. So despite mounting an appropriate IGF-I response, the liver is unable to supplement local IGF-I via the plasma IGF-I reservoir, and as a consequence GH-dependent growth is impaired. This was obvious for not only overall growth but also growth indices that have been previously shown to be responsive to plasma IGF-I, such as linear and kidney growth (18,33).
The role of the circulating IGF-I reservoir in GH-stimulated growth has also been studied in LID mice (34). LID mice resemble null ALS mice by having a small plasma IGF-I reservoir that is completely unresponsive to chronic GH therapy but differ by having a 4-fold increase in plasma GH (11,35). Despite excessive plasma GH, female LID mice responded to chronic GH therapy with a 37% increase in body weight over a 4-wk treatment period (34). It is not possible to determine from that study whether the plasma IGF-I deficiency of LID mice limited the positive effects of exogenous GH because a group of intact mice treated with GH was not included. Male LID mice, on the other hand, completely failed to respond to GH therapy (34). The authors suggested that this failure resulted from already maximal growth stimulation by endogenous GH. This appears unlikely because the final weight of GH-treated male LID mice was identical with that of untreated, age-matched WT males of the same genetic background (11). Moreover, WT mice from this genetic background responded normally to GH therapy, irrespective of sex (30). This failure, therefore, likely relates to uncharacterized defects that reprogram GH responsiveness specifically in the male. Null ALS mice have no such defects as both sexes responded to exogenous GH. Based on the reduction of GH-stimulated growth we observed in null ALS mice, we estimate that the minimum contribution of endocrine IGF-I to the effects of exogenous GH on growth is 35%.
The remaining growth effects of exogenous GH must be accounted by extrahepatic actions, which can be independent or dependent on local IGF-I production (2,6). The balance between these two classes of GH actions varies across tissues. So the first category is likely dominant in liver because it lacks a significant population of IGF-I receptor (36). Accordingly, GH increased fractional liver growth by 16% in IGF-I knockout mice despite any effects on overall growth (30). Our data are consistent with GH effects on liver being independent of IGF-I because, after adjusting for final body weight, the ability of GH to stimulate liver growth was undiminished in null ALS mice, even though plasma IGF-I failed to rise.
In most tissues, however, GH actions are dependent on local IGF-I synthesis (2). This mode of action was initially suggested by the ability of exogenous GH to stimulate IGF-I gene expression in a variety of extrahepatic tissues, including bone, skeletal muscle, and adipose tissue (9). A number of tissue-specific knockouts now provide direct evidence for this mode of action. For example, knockout of the IGF-I gene in bone cells (osteoblast and chondrocytes) reduces bone and whole-body growth (19,37), whereas muscle-specific ablation of signal transducers and activators of transcription-5, the transcription factor mediating the effect of GH on IGF-I transcription, results in lower muscle IGF-I mRNA and reduced lean mass (38). Finally, a full growth response is dependent on extrahepatic GH action as demonstrated in mice with GH-independent production of IGF-I in the liver (39). Chronic treatment of these mice with the GH antagonist pegvisomant reduced growth, even though plasma IGF-I remained nearly normal. The GH responsiveness of the local IGF-I system appears intact in null ALS mice because we found equally effective GH stimulation of IGF-I expression in muscle and adipose tissue.
LID mice suffer from insulin resistance and rely on hyperinsulinemia during both the fed and fasted state to maintain normal plasma glucose (35,40). Null ALS mice, on the other hand, have normal concentrations of plasma glucose and insulin (12,13,40). The simplest explanation for the contrasting metabolic states of LID and null ALS mice despite nearly identical reduction in plasma IGF-I resides in the molecular complexes carrying plasma IGF-I. Under normal circumstances, efficient capture of IGF-I into ternary complexes is driven by a 2- to 3-fold molar excess of ALS over IGF-I (5,41). In LID mice, however, ALS circulates at approximately 20-fold excess over IGF-I (13), increasing substantially the efficiency of sequestration of the remaining IGF-I. As a result, insulin-sensitizing effects dependent on vascular IGF-I efflux, such as direct IGF-I actions in skeletal muscle and inhibition of GH secretion (42,43), are reduced more than predicted from plasma concentration. In contrast, the fall of plasma IGF-I in null ALS mice is balanced by the existence of nearly all IGF-I in highly permeable binary complexes (12,13), allowing sufficient IGF-I efflux to sustain its metabolic effects. Our data suggest that IGF-I efflux in null ALS mice also limits the ability of exogenous GH to promote peripheral insulin resistance. So despite similar hyperinsulinemia, GH-treated null ALS mice had decreasing plasma glucose rather than the increase seen in the GH-treated WT mice. Consistent with this interpretation, we also found that short-term GH treatment failed to impair insulin-mediated glucose disposal in null ALS mice.
Our study has added relevance in view of the recent identification of 10 patients with plasma ALS deficiency (44,45,46,47,48). ALS deficiency is accounted by a variety of ALS gene mutations (missense and nonsense mutations, nucleotide duplication or deletion) leading to a premature stop codon (44,47) or alteration in protein structure (44,46). As in the case of null ALS mice, all patients have extremely low plasma concentrations of IGF-I and IGFBP-3 in the presence of normal GH concentration. They uniformly suffer from a modest growth deficit and, in some cases, delayed puberty and bone maturation. Given our results, one obvious question is whether ALS-deficient individuals would benefit from GH therapy during the postnatal growth phase. So far, three ALS-deficient patients have received GH therapy, with slight growth acceleration in one and the opposite in another (44,45,47). A second issue is the presence of fasting hyperinsulinemia in many ALS-deficient humans, suggesting some degree of insulin resistance (44,45,47). The basis for hyperinsulinemia in humans but not mice is unknown but could relate to species differences that could alter IGF-I efflux, such as the plasma concentration of the IGFBPs that do not interact with ALS. Additional studies are needed to determine whether ALS-deficient humans will resist the antagonistic effects of exogenous GH on insulin action as null ALS mice do.
In summary, our study demonstrates that null ALS mice retain the ability to respond to anabolic signals by increasing hepatic IGF-I production but loses the ability to deliver this additional IGF-I to peripheral tissues. In this context, ternary complex formation has recently been shown to limit lipid mobilization and promote survival in nutritionally deprived Drosophila (49). It will be interesting to determine whether ALS is similarly essential in mammals during catabolic states.
Acknowledgments
We thank Ramona Ehrhardt for her help analyzing plasma metabolites and hormones.
Footnotes
This work was supported by National Institutes of Health Grant DK-51624 (to Y.R.B.).
Disclosure Summary: The authors have nothing to declare.
First Published Online March 19, 2009
Abbreviations: ALS, Acid labile subunit; G, genotype; H, hormone; IGFBP, IGF binding protein; LID, liver IGF-I-deficient mice; S, sex; WT, wild type.
References
- Efstratiadis A 1998 Genetics of mouse growth. Int J Dev Biol 42:955–976 [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A 2001 Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229:141–162 [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A 1993 Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75:73–82 [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ 1997 A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA 94:13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC 1994 Insulin-like growth factor binding proteins in the human circulation: a review. Horm Res 42:140–144 [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A 2001 The somatomedin hypothesis: 2001. Endocr Rev 22:53–74 [DOI] [PubMed] [Google Scholar]
- Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT 2001 The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol 170:63–70 [DOI] [PubMed] [Google Scholar]
- D'Ercole AJ, Stiles AD, Underwood LE 1984 Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA 81:935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LJ, Bell GI, Duckworth ML, Friesen HG 1987 Identification, characterization, and regulation of a rat complementary deoxyribonucleic acid which encodes insulin-like growth factor-I. Endocrinology 121:684–691 [DOI] [PubMed] [Google Scholar]
- Rechler MM 1993 Insulin-like growth factor binding proteins. Vitam Horm 47:1–114 [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D 1999 Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96:7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki I, Ooi GT, Tremblay ML, Hurst KR, Bach LA, Boisclair YR 2000 Inactivation of the acid labile subunit gene in mice results in mild retardation of postnatal growth despite profound disruptions in the circulating insulin-like growth factor system. Proc Natl Acad Sci USA 97:6868–6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, Wu Y, Emerton K, Williams V, Jepsen K, Schaffler MB, Majeska RJ, Gavrilova O, Gutierrez M, Hwang D, Pennisi P, Frystyk J, Boisclair Y, Pintar J, Jasper H, Domene H, Cohen P, Clemmons D, LeRoith D 2008 Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J 23:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sun H, Kawashima Y, Yakar S, LeRoith D, Liver-derived, circulating (endocrine) insulin-like growth factor-1, is sufficient to support normal post-natal growth. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008, p 103 (Abstract OR15-1) [Google Scholar]
- Bichell DP, Kikuchi K, Rotwein P 1992 Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Mol Endocrinol 6:1899–1908 [DOI] [PubMed] [Google Scholar]
- Thissen J-P, Ketelslegers J-M, Underwood LE 1994 Nutritional regulation of the insulin-like growth factors. Endocr Rev 15:80–101 [DOI] [PubMed] [Google Scholar]
- Phillips LS, Pao CI, Villafuerte BC 1998 Molecular regulation of insulin-like growth factor-I and its principal binding protein, IGFBP-3. Prog Nucleic Acids Res Mol Biol 60:195–265 [DOI] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D 2002 Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S 2007 Disruption of insulin-like growth factor-I expression in type IIαI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics 30:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughaday WH, Rotwein P 1989 Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev 10:68–91 [DOI] [PubMed] [Google Scholar]
- Hwang DL, Lee PD, Cohen P 2008 Quantitative ontogeny of murine insulin-like growth factor (IGF)-I, IGF-binding protein-3 and the IGF-related acid-labile subunit. Growth Horm IGF Res 18:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews LS, Norstedt G, Palmiter RD 1986 Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci USA 83:9343–9347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Bichell DP, Rotwein P 1992 Chromatin changes accompany the developmental activation of insulin-like growth factor I gene transcription. J Biol Chem 267:21505–21511 [PubMed] [Google Scholar]
- Tiong TS, Herington AC 1992 Ontogeny of messenger RNA for the rat growth hormone receptor and serum binding protein. Mol Cell Endocrinol 83:133–141 [DOI] [PubMed] [Google Scholar]
- Dai J, Baxter RC 1994 Regulation in vivo of the acid-labile subunit of the rat serum insulin-like growth factor-binding protein complex. Endocrinology 135:2335–2341 [DOI] [PubMed] [Google Scholar]
- Guler H-P, Zapf J, Schmid C, Froesch ER 1989 Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenhagen) 121:753–758 [DOI] [PubMed] [Google Scholar]
- Zapf J, Hauri C, Futo E, Hussain M, Rutishauser J, Maack CA, Froesch ER 1995 Intravenously injected insulin-like growth factor (IGF) I/IGF binding protein-3 complex exerts insulin-like effects in hypophysectomized, but not in normal rats. J Clin Invest 95:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C, Dentin R, Girard J 2004 Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab 30:398–408 [DOI] [PubMed] [Google Scholar]
- Kasukawa Y, Baylink DJ, Guo R, Mohan S 2003 Evidence that sensitivity to growth hormone (GH) is growth period and tissue type dependent: studies in GH-deficient lit/lit mice. Endocrinology 144:3950–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, LeRoith D 1999 Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology 140:5178–5184 [DOI] [PubMed] [Google Scholar]
- Etherton TD, Bauman DE 1998 Biology of somatotropin in growth and lactation of domestic animals. Physiol Rev 78:745–761 [DOI] [PubMed] [Google Scholar]
- Arquier N, Géminard C, Bourouis M, Jarretou G, Honegger B, Paix A, Léopold P 2008 Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab 7:333–338 [DOI] [PubMed] [Google Scholar]
- Fielder P, Mortensen DL, Mallet P, Carlsson B, Baxter RC, Clark RG 1996 Differential long-term effects of insulin-like growth factor-I (IGF-I), growth hormone (GH), and IGF-I plus GH on body growth and IGF binding proteins in hypophysectomized rats. Endocrinology 137:1913–1920 [DOI] [PubMed] [Google Scholar]
- Liu JL, Yakar S, LeRoith D 2000 Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology 141:4436–4441 [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, Chernausek SD, Mejia W, Le Roith D 2001 Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes 50:1110–1118 [DOI] [PubMed] [Google Scholar]
- Clemmons DR 2006 Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol 6:620–625 [DOI] [PubMed] [Google Scholar]
- Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S 2007 Conditional deletion of insulin-like growth factor-I in collagen type 1α2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology 148:5706–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klover P, Hennighausen L 2007 Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology 148:1489–1497 [DOI] [PubMed] [Google Scholar]
- Liao L, Dearth RK, Zhou S, Britton OL, Lee AV, Xu J 2006 Liver-specific overexpression of the insulin-like growth factor-I enhances somatic growth and partially prevents the effects of growth hormone deficiency. Endocrinology 147:3877–3888 [DOI] [PubMed] [Google Scholar]
- Haluzik M, Yakar S, Gavrilova O, Setser J, Boisclair Y, LeRoith D 2003 Insulin resistance in the liver-specific IGF-1 gene-deleted mouse is abrogated by deletion of the acid-labile subunit of the IGF-binding protein-3 complex: relative roles of growth hormone and IGF-1 in insulin resistance. Diabetes 52:2483–2489 [DOI] [PubMed] [Google Scholar]
- Baxter RC, Dai J 1994 Purification and characterization of the acid-labile subunit of rat serum insulin-like growth factor binding protein complex. Endocrinology 134:848–852 [DOI] [PubMed] [Google Scholar]
- Clemmons DR 2004 The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest 113:25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D, Yakar S 2007 Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nat Clin Pract Endocrinol Metab 3:302–310 [DOI] [PubMed] [Google Scholar]
- Heath KE, Argente J, Barrios V, Pozo J, Díaz-González F, Martos-Moreno GA, Caimari M, Gracia R, Campos-Barros A 2008 Primary acid-labile subunit deficiency due to recessive IGFALS mutations results in postnatal growth deficit associated with low circulating insulin growth factor (IGF)-I, IGF binding protein-3 levels, and hyperinsulinemia. J Clin Endocrinol Metab 93:1616–1624 [DOI] [PubMed] [Google Scholar]
- Domené HM, Scaglia PA, Lteif A, Mahmud FH, Kirmani S, Frystyk J, Bedecarrás P, Gutierrez M, Jasper HG 2007 Phenotypic effects of null and haploinsufficiency of acid-labile subunit in a family with two novel IGFALS gene mutations. J Clin Endocrinol Metab 92:4444–4450 [DOI] [PubMed] [Google Scholar]
- Hwa V, Haeusler G, Pratt KL, Little BM, Frisch H, Koller D, Rosenfeld RG 2006 Total absence of functional acid labile subunit, resulting in severe insulin-like growth factor deficiency and moderate growth failure. J Clin Endocrinol Metab 91:1826–1831 [DOI] [PubMed] [Google Scholar]
- Domené HM, Bengolea SV, Martínez AS, Ropelato MG, Pennisi P, Scaglia P, Heinrich JJ, Jasper HG 2004 Deficiency of the circulating insulin-like growth factor system associated with inactivation of the acid-labile subunit gene. N Engl J Med 350:570–577 [DOI] [PubMed] [Google Scholar]
- Domené HM, Martínez AS, Frystyk J, Bengolea SV, Ropelato MG, Scaglia PA, Chen JW, Heuck C, Wolthers OD, Heinrich JJ, Jasper HG 2007 Normal growth spurt and final height despite low levels of all forms of circulating insulin-like growth factor-I in a patient with acid-labile subunit deficiency. Horm Res 67:243–249 [DOI] [PubMed] [Google Scholar]