Abstract
Human skin is constantly exposed to UV light, the most ubiquitous environmental stressor. Here, we investigated the expression and regulation of Nrf1-3, transcription factors crucially involved in protection against oxidative stress in human skin cells in vitro, ex vivo, and in situ. In particular, we examined whether α-MSH, a UV-induced peptide, is capable of modulating Nrf2 and Nrf-dependent gene expression. Nrf1, -2, and -3 were found to be expressed in various cutaneous cell types in vitro. Surprisingly, UVB irradiation at physiological doses (10 mJ/cm2) reduced Nrf2 and Nrf-dependent gene expression in normal keratinocytes and melanocytes in vitro as well as ex vivo in skin organ cultures. α-MSH alone significantly increased Nrf2 as well as Nrf-dependent heme oxygenase-1, γ-glutamylcysteine-synthetase, and glutathione-S-transferase Pi gene expression in both keratinocytes and melanocytes. This effect of α-MSH occurred at physiological doses and was due to transcriptional induction, mimicked by the artificial cAMP inducer forskolin, and blocked by protein kinase A pathway inhibition. In silico promoter analysis of Nrf2 further identified several putative binding sites for activator protein 1 and cAMP response element-binding protein, transcription factors typically activated by α-MSH. Importantly, α-MSH prevented or even overcompensated the UVB-induced suppression of Nrf2 and Nrf-dependent genes not only in normal keratinocytes and melanocytes in vitro but also in skin organ cultures. These findings, for the first time, show regulation of Nrf2 and Nrf-dependent genes by α-MSH. Our data also highlight a novel facet in the cytoprotective and antioxidative effector mechanisms of α-MSH and perhaps of related melanocortin peptides.
α-MSH counteracts UV-induced Nrf suppression.
UV irradiation represents the most ubiquitous environmental stressor to human skin and is crucially involved in various forms of skin damage including the development of melanoma, nonmelanoma skin cancer, and photoaging (1). The UV spectrum reaching the earth’s surface is divided into the more energetic wavelength UVB (280–320 nm) and the less energetic but more skin-penetrating UVA (320–400 nm). Albeit both UVA and UVB have distinct biological effects, one overlapping capacity is the generation of reactive oxygen species (ROS) (2,3,4). If not properly detoxified, excess amounts of UV-induced ROS oxidize biomolecules leading to photoaging and promoting photo-carcinogenesis.
In light of the prooxidant effects of UV irradiation, human skin must be equipped with a panel of defense mechanisms. One key regulatory module orchestrating the expression of antioxidative enzymes is the family of transcription factors Nrfs. Nrf1-3 belong to the cap’n’ collar family of basic leucine zipper proteins. Whereas Nrf1 and -2 are broadly expressed, Nrf3 is present only in few tissues such as placenta, B cell, and monocyte lineages (5). The basic region is responsible for DNA binding and is located upstream of the leucine zipper region (6). Under normal conditions, Nrf2 is bound to the Kelch-like ECH-associated protein and retained in the cytoplasm. Upon exposure to oxidative stress, Nrf2 undergoes translocation to the nucleus where it activates its target genes in combination with other transcription factors (7). Nrf2 recognizes and binds the antioxidant response element in the promoter regions of its target genes and induces phase II detoxifying enzymes such as heme oxygenase-1 (HO-1) (8), γ-glutamylcysteine synthetase (γ-GCS) (9), or glutathione-S-transferase Pi (GSTPi) (10). The antioxidant response element is a cis-acting enhancer that mediates not only the transcriptional activation of genes in response to various chemical and electrophilic agents but also the basal expression of many of these genes under physiological conditions (11,12). Recent studies have shown that the presence of Nrf1 and Nrf2 is critical for cells to cope with oxidative stress. Nrf1-deficient fibroblasts have lower levels of glutathione and are more sensitive to oxidative stress-producing compounds (13,14). Nrf2 was also shown to exert a protective effect against apoptosis induced by Fas (15). The role of Nrf2 in the regulation of protective genes and within the cellular stress response is further reflected by the phenotype of Nrf2 knockout mice, which under normal laboratory conditions show no obvious phenotype. Induction of phase II detoxifying enzymes, however, is strongly reduced in the liver and intestine of the knockout mice by a phenolic antioxidant (16).
α-MSH is a melanocortin peptide derived from proopiomelanocortin (17). It binds to melanocortin receptors (MC-R), which are G protein-coupled receptors with seven transmembrane domains and which increase intracellular cAMP (18). Research during the last decade has shown that α-MSH has many additional effects beyond pigmentation such as regulation of exocrine gland function (19), immunomodulation (20), or collagen metabolism (21). Recently, it was shown that α-MSH possesses another important effect related to maintenance of epidermal homeostasis and genetic stability upon UVB irradiation. We and others reported that α-MSH protects human melanocytes from UVB-induced apoptosis by decreasing UVB-induced DNA damage (22,23). The precise mechanism by which α-MSH and related peptides (24) exert this effect is still incompletely understood. During our ongoing research aiming at identifying effector mechanisms by which α-MSH protects from UV-induced skin damage, we addressed the role of Nrf1-3. Our data provide an expression analysis of Nrf1-3 in human skin cells and disclose a surprising effect of UVB on Nrf2 and Nrf-dependent genes. Most interestingly, we found that α-MSH modulates the expression of Nrf2 and Nrf-dependent genes as a novel cytoprotective effector mechanism of this hormone.
Materials and Methods
Cell culture
Normal human keratinocytes (NHK) and human dermal fibroblasts (HDF) were all derived from newborn foreskin and purchased from PromoCell (Heidelberg, Germany). Normal human melanocytes (NHM; Tebu-bio, Portland, OR) and HDF were from individual donors, whereas NHK were pooled from several donors. NHM were routinely grown in MGM-M2 plus supplements (Cascade Biologics, Portland, OR). NHK were maintained in KBM-2 with all supplements (PromoCell). HDF and HaCaT cells (a gift from Dr. Fusenig, Heidelberg, Germany) were cultured in RPMI 1640 (PAA, Cölbe, Germany) with 1% l-Glu, 1% penicillin/streptomycin (both from PAA), and 10% fetal calf serum (Biochrom, Berlin, Germany). The human microvascular endothelial cell line HMEC-1 was a gift from the Department of Dermatology, Emory University School of Medicine (Atlanta, GA) and cultured in endothelial cell medium with all supplements (PAA). All cells were maintained in a humidified atmosphere of 5% CO2 at 37 C.
Cell treatment and UV irradiation
For all experiments involving α-MSH (Calbiochem, Schwalbach, Germany), cells were cultured in routine medium for 2 d with all supplements except bovine pituitary extract. UVB irradiation was performed as described before (22). In some experiments, α-MSH was preincubated for 24 h. In other experiments, forskolin (FSK) (Calbiochem) was used at 1 μm, or actinomycin D (AD) (5 μg/ml; Sigma, Steinheim, Germany) or H89 (Calbiochem) were preincubated for 30 min before stimulation with α-MSH.
Determination of melanin
Measurement of melanin content was performed on 2 × 105 cells seeded in tissue culture plates. After incubation with α-MSH (at 10−6 m) for 2 and 24 h, cells were trypsinized and centrifuged, and pellets were dissolved in 1 N NaOH. Melanin concentration was determined by measuring the OD at 405 nm in relation to a standard curve generated by synthetic melanin (Sigma).
RNA extraction and conventional and real-time RT-PCR
Total RNA was isolated from cells using the RNeasy mini kit (QIAGEN, Hilden, Germany). DNA digestion was performed on RNeasy columns with the RNase-Free deoxyribonuclease set (QIAGEN). One microgram of total RNA was reverse transcribed with Revert Aid first-strand cDNA synthesis kit (Fermentas Life Sciences, St. Leon-Rot, Germany) using oligo(deoxythymidine) primers. Conventional PCR amplification was performed with GoTaq polymerase (Promega, Mannheim, Germany) and commercially synthesized Nrf1-3 primers (25). PCR products were separated on 1.5% tris-acetate-EDTA-agarose gels, stained with ethidium bromide, and photographed under UV light. Negative controls consisting of RNA instead of the cDNA template were consistently negative.
Quantification of mRNA steady-state levels of the tested genes was carried out by real-time fluorescence detection using Absolute SYBR Green ROX mix (Applied Biosystems, Foster City, CA). Real-time RT-PCR was performed in a total volume of 20 μl with SYBR Green PCR Master Mix and a 200 nm concentration of each primer. Primers were either retrieved from the literature or designed with the computer program Primer Express (Applied Biosystems), using parameters recommended by the manufacturer. Reactions were carried out in duplicate in an ABI Prism 7300 sequence detector supplied with SDS 2.1 software (Applied Biosystems), using the following conditions: an initial activation step (2 min at 50 C) and a single denaturation step (15 min at 95 C) followed by 40 cycles of 15 sec at 95 C and 60 sec at 60 C and a final cycle of 15 sec at 95 C, 15 sec at 60 C, and 15 sec at 95 C. Levels of gene expression in each sample were quantified using the cycle threshold method (after validation assays for each gene primer set) as described (26), using GAPDH as an internal standard. For each condition, the ground condition was set as 1. The primer sequences were as follows: Nrf2 sense, 5′-TTCTGTTG CTCAGGTAGCCCC-3′, and antisense, 5′-TCA GTTTGGCTTCTGGACTTGG-3′; HO-1 (27); γ-GCS (28); GSTPi (29); and GAPDH sense, 5′-TGCACCACCAACTGCTTAGC-3′, and antisense, 5′-GGCATGGACTGTGGTCA TGAG-3′. Expression of each gene was assessed by at least three independent PCR analyses. Significance of the data was determined by the Student’s t test.
Western immunoblotting
Cells were seeded into 35-mm dishes, and protein lysates were collected by hot lysis using 2× Laemmli sample buffer. SDS-PAGE was performed under reducing conditions using precast 4–12% Bis-Tris or 7% Tris-acetate gels (NuPAGE; Invitrogen, Karlsruhe, Germany). After transfer, the membrane was blocked in 10% BSA (PAA) in Tris-buffered saline overnight at 4 C and immunoprobed with a rabbit anti-Nrf2 antibody (R&D Systems, Minneapolis, MN). HO-1 was detected using a mouse monoclonal anti-HO-1 antibody (StressGen, Ann Arbor, MI). After washing, membranes were probed with a horseradish peroxidase-conjugated antirabbit or antimouse IgG antibody (Amersham Biosciences, Freiburg, Germany) and developed using the enhanced chemiluminescence kit (Amersham). A mouse anti-α-tubulin antibody (Oncogene, San Diego, CA) was used as housekeeping gene to verify equal loading of cell lysates after stripping the blots. All Western blots were repeated at least three times.
Skin organ cultures
Cultured skin explants (different donors, n = 4) were prepared from skin biopsies immediately after routine surgery as described before (30). These experiments were approved from and performed under the guidelines of the local ethical committee of University and Hospital Center of Rennes, France (Comité Consultativ pour la Protection des Personnes daus la Recherche Biomedicale 04/36-517). Cultured skin explants were irradiated with 10 mJ/cm2 UVB (peak emission, 312 nm; UV Stratalinker 2400; Stratagene, La Jolla, CA). After 4 and 12 h of incubation in presence or absence of α-MSH (10−6 m), samples were homogenized and processed for RNA preparation using the NucleoSpin RNA II purification kit (Macherey Nagel, Hoerd, France) with slight modifications. Reverse transcription reactions were carried out using the high-capacity cDNA archive kit (Applied Biosystems), with 1 μg total RNA.
Immunohistochemistry
Samples of normal skin from healthy individuals undergoing routine plastic surgery (n > 3) or from human skin organ cultures were fixed in paraformaldehyde, dehydrated, embedded in paraffin, and mounted on Tissue-Tek (Mikrom, Walldorf, Germany). All experiments involving skin biopsies were approved by the local ethical committee of the University of Münster (Münster, Germany; registration no. 5 VI Böhm). Sections were deparaffinized, epitope-demasked by microwave treatment, and quenched for endogenous peroxidase activity. Sections were blocked with 2% BSA for 25 min at room temperature followed by incubation with an anti-Nrf2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA; 1:200). Bound antibodies were reacted with a biotinylated antirabbit antibody (Dako, Hamburg, Germany) followed by incubation with a streptavidin-peroxidase conjugate and 3-amino-9-ethylcarbazole (Sigma). Negative controls consisted of incubation of sections with rabbit IgG at the same concentration as the Nrf2 antbody. Double staining of Nrf2 and melanocytes was performed by combining Nrf2 immunostaining with immunogold labeling/silver enhancement as previously described for fibroblasts (31). For the detection of melanocytes, the pan-Mel antibody consisting of monoclonal anti-HMB45, anti-Mart1 and anti-tyrosinase antibodies (Biocare, Concord, CA) was used.
In silico promoter analysis
The Java program TOUCAN was the primary software used for comparative promoter analysis (32). Proximal promoter sequences (3 kb upstream) were extracted from the ENSEMBL genomic database. The TOUCAN tool MotifLocator, which searches the TRANSFAC 7.0 database, was used to detect transcription factor binding sites in the sets of sequences. The stringency level was set to a value of 0.9, and the human promoter set of the Eukaryotic Promoter Database was chosen as third-order background model. The statistics tool of TOUCAN was applied to the data produced by MotifLocator in combination with the appropriate expected frequencies file, thereby detecting overrepresented transcription factor binding sites.
Oxidation of α-MSH
α-MSH was oxidized as reported by others (33) with slight modifications. In brief, α-MSH was exposed to hydrogen peroxide (1 mm) for 45 min followed by neutralization of excess hydrogen peroxide by addition of 40 mm bovine liver catalase (Sigma). NHM were then pretreated with normal α-MSH or oxidized α-MSH for 24 h followed by irradiation with UVB (10 mJ/cm2) and, after incubation for another 2 h, RNA processing and RT-PCR as described above.
Results
Expression pattern of Nrf1-3 in human skin cells in vitro and in situ
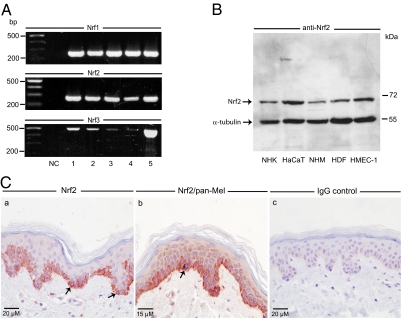
To get a first insight into the expression of Nrf1-3 in human skin, we performed in vitro studies on various cutaneous cell types using conventional RT-PCR. The examined cell types were NHK, the immortalized human keratinocyte-like cell line HaCaT, NHM, HDF, and a human microvascular endothelial cell line, HMEC-1. All cell types were found to express significant levels of Nrf1 and -2 at the mRNA level (Fig. 1A). The amplification products were of the expected size (Nrf1, 255 bp; Nrf2, 275 bp). Interestingly, low levels of Nrf3 mRNA (size of amplicon, 506 bp) were also detected in all tested cell types with the highest abundance in HMEC-1.
Figure 1.
Expression analysis of Nrf1-3 in human skin cells in vitro and in situ. A, Detection of Nrf1-3 in various human skin cell types in vitro as shown by conventional RT-PCR analysis. NC, Negative control, H2O as template; 1, NHK; 2, HaCaT; 3, NHM; 4, HDF; 5, HMEC-1. B, Protein expression of Nrf2 in human skin cell types. Total cell lysates (50 μg/lane) were subjected to Western immunoblot analysis using an anti-Nrf2 antibody. The same blot was reprobed with an anti-α-tubulin antibody. C, Expression of Nrf2 in healthy human skin. Nrf2 was detected by immunohistochemistry using the immunoperoxidase technique (a). Note specific cytoplasmic Nrf2 immunoreactivity within the basal layer of epidermal keratinocytes (red cells, arrows) but not in the IgG control (c). Double immunostaining of Nrf2 and melanocytes by the immunoperoxidase (Nrf2) and immunogold-silver enhancement technique (melanocytes) using a pan-Mel antibody (b). Note gray-blackish precipitate within a melanocyte but lack of Nrf2 staining within (arrow).
Nrf2 Western immunoblot analysis of total cell extracts derived from the above cutaneous cell types disclosed the presence of one specific immunoreactive band at approximately 70 kDa (Fig. 1B). The size of this detected band is in close agreement with the predicted molecular mass of Nrf2 (66.1 kDa, 589 amino acids) and also in accordance with previously published data (6,35). HaCaT cells and HMEC-1 cells expressed more Nrf2 protein than NHM and HNK (Fig. 1B). In contrast to the detected Nrf2-related band, immunoblotting with all commercially available anti-Nrf1 and -3 antibodies failed to generate reliable and/or specific signals upon extensive testing. These antibodies in our hands either produced no detectable signals or generated multiple bands of unknown specificity (data not shown).
To finally assess the expression of Nrf2 in situ, immunohistochemistry on normal skin was performed. Nrf2 immunoreactivity appeared as a very specific cytoplasmic staining confined to the basal layer of the epidermis (Fig. 1C). Double staining of Nrf2 and melanocytes employing the immunoperoxidase plus the immunogold-silver enhancement technique revealed that Nrf2 immunoreactivity is primarily detectable in basal keratinocytes, whereas it is largely absent within melanocytes (Fig. 1C). Negative controls consisting of IgG did not produce any specific staining (Fig. 1C).
In summary, this expression analysis revealed that Nrf1-3 are constitutively expressed at the RNA level at various degree in human skin cell types in vitro. Nrf2 protein expression could also be demonstrated in various skin cell types in vitro, whereas Nrf2 immunoreactivity in human skin in situ was mainly confined to basal epidermal keratinocytes. In light of the detected Nrf2 at RNA and protein level in skin cells, we focused on Nrf2 only as well as on Nrf-dependent enzymes in all subsequent experiments.
UVB irradiation reduces Nrf2 as well as Nrf-dependent gene expression in human skin cells in vitro and ex vivo
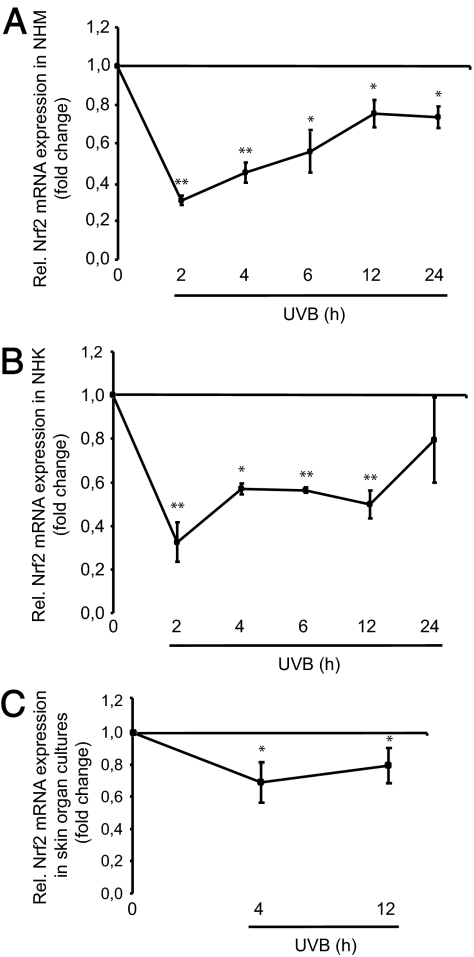
Next we examined the impact of the most ubiquitous environmental stressor to human skin, UV irradiation, on Nrf2 expression. To this end we concentrated on UVB because this UV spectrum is mostly concerned with photocarcinogenesis. NHK and NHM were exposed to a physiological dose of UVB irradiation, i.e. 10 mJ/cm2. Expression levels of Nrf2 were determined by real-time RT-PCR analysis. Time kinetic studies revealed a dramatic decline in Nrf2 mRNA levels as early as 2 h after UVB exposure in NHM (nadir, −69 ± 2%) and NHK (nadir, −68 ± 9%) (Fig. 2, A and B). This was followed by a gradual increase in mRNA amounts of Nrf2 up to basal levels in NHK by 24 h, whereas Nrf2 mRNA levels in NHM remained significantly reduced as long as 24 h after UVB exposure. To substantiate these in vitro findings, human skin organ cultures (30) were irradiated with UVB (10 mJ/cm2), and Nrf2 mRNA levels were determined at two representative time points. UVB exposure again reduced the steady-state levels of Nrf2 significantly after 4 h (nadir, −38 ± 7%) and 12 h (nadir, −21 ± 11%) (Fig. 2C). However, the overall decline in Nrf2 mRNA expression after UVB was not as strong as in NHM and NHK in vitro, possibly due to interference of other cell types in the skin organ cultures whose Nrf2 expression is not affected by UVB exposure (e.g. adnexal epithelia).
Figure 2.
UVB-induced suppression of Nrf2 expression in human skin cells in vitro and ex vivo. A, Real-time RT-PCR analysis of Nrf2 in NHM. Cells were irradiated with UVB (10 mJ/cm2) and harvested after 2–24 h. *, P < 0.01; **, P < 0.001 vs. control. B, mRNA expression analysis of Nrf2 in NHK by real-time RT-PCR. Cells were treated as described above. *, P < 0.01; **, P < 0.001 vs. control. C, Real-time RT-PCR analysis of Nrf2 in skin organ explants exposed to UVB (10 mJ/cm2). After UVB exposure, skin organ explants were cultured for 4 or 12 h. *, P < 0.01 vs. control.
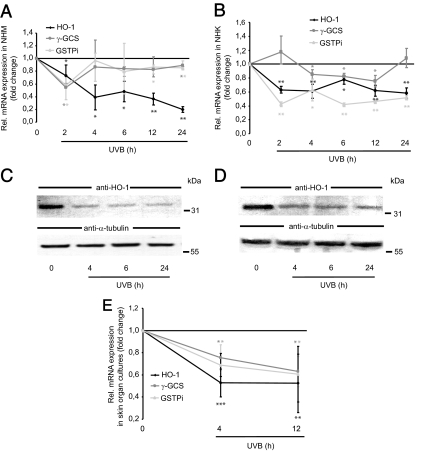
To clarify whether UVB reduces the levels of Nrf-dependent phase II detoxifying enzymes, expression analysis of HO-1, γ-GCS, and GSTPi was performed using real-time RT-PCR. In NHM, mRNA expression of HO-1 was most prominently affected (Fig. 3A). As early as 2 h after UVB exposure (10 mJ/cm2) of NHM, HO-1 mRNA levels were reduced and exhibited an expression nadir 24 h after UVB irradiation (−80 ± 5%). In contrast, γ-GCS and GSTPi mRNA levels dropped mainly 2 h after UVB exposure and began to rise again at subsequent time points with only marginal changes compared with nonirradiated cells. In NHK, both HO-1 and GSTPi mRNA levels were found to be consistently reduced at all tested time points, whereas mRNA expression levels of γ-GCS oscillated with little evidence for regulation (Fig. 3B). In accordance with the UVB-mediated suppression of the mRNA levels of HO-1 in both NHM and NHK, protein expression of this enzyme was strongly diminished after UVB irradiation at all tested time points as shown by Western immunoblotting (Fig. 3, C and D). Importantly, the UVB-mediated decline in mRNA expression of HO-1 and related enzymes in vitro could be confirmed in human skin organ cultures. UVB (10 mJ/cm2) reduced the expression of HO-1, γ-GCS and GSTPi 12 h after UV irradiation compared with nonexposed skin (Fig. 3E).
Figure 3.
UVB-induced suppression of Nrf2-dependent gene expression in human skin cells in vitro and ex vivo. A, Real-time RT-PCR analysis of HO-1, γ-GCS, and GSTPi in NHM. Time kinetic studies were performed with NHM exposed to UVB (10 mJ/cm2). *, P < 0.01; **, P < 0.001 vs. control. B, Determination of HO-1, γ-GCS and GSTPi mRNA levels in NHK using real-time RT-PCR. Time kinetics with UVB was performed as described above. *, P < 0.01; **, P < 0.001 vs. control. C and D, Western immunoblot analysis of HO-1 protein expression after UVB in NHM and NHK. Cells were exposed to UVB as indicated above. Cell lysates were harvested after different time points. Reprobing with an anti-α-tubulin antibody ensured equal loading of cell lysates. E, Real-time RT-PCR analysis of HO-1, γ-GCS, and GSTPi mRNA expression after UVB exposure in skin organ cultures. Human skin explants were exposed to UVB (10 mJ/cm2) and cultured for 4 and 12 h followed by RNA extraction. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. control.
Taken together, these findings indicate that UVB irradiation at a physiological dose is capable of lowering the basal expression levels of Nrf2 in human epidermal cell types, which is associated with cellular depletion of phase II detoxifying enzymes, especially HO-1.
α-MSH in vitro up-regulates Nrf2 as well as Nrf-dependent gene expression in human melanocytes and keratinocytes
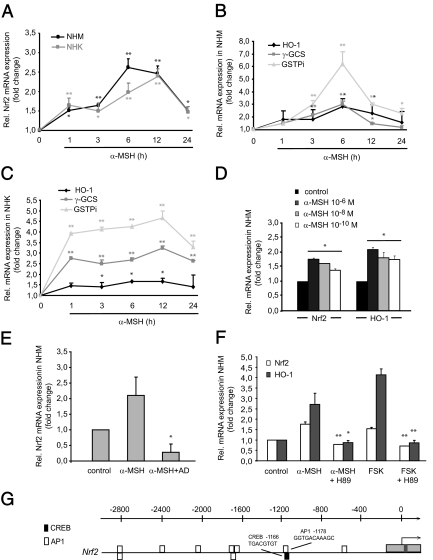
In light of the emerging role of α-MSH not only as a pigment inducer but also as a guardian of epidermal homeostasis and genomic stability (22,23), we hypothesized that α-MSH may induce Nrf2 and Nrf-dependent gene expression. Indeed, NHM but also NHK have been reported to express the MC-1R, which binds α-MSH (18,36,37,38). As demonstrated by real-time RT-PCR analysis, α-MSH (10−6 m) significantly up-regulated the mRNA levels of Nrf2 in a time-dependent manner in both NHM and NHK (Fig. 4A). Peak induction was observed 6–12 h after α-MSH, but elevated levels were detected up to 24 h after stimulation. Moreover, α-MSH proved to strongly up-regulate mRNA levels of Nrf-dependent genes, namely HO-1, γ-GCS, and especially GSTPi in NHM and NHK (Fig. 4, B and C). Indeed, physiological doses of α-MSH (i.e. at 10−8 and 10−10 m as representatively shown for NHM for 6 h) likewise induced Nrf2 and HO-1 gene expression (Fig. 4D). These data show for the first time that α-MSH at physiological levels is capable of inducing Nrf2 as well as expression of Nrf-dependent phase II detoxifying enzymes in NHM and NHK.
Figure 4.
α-MSH induces Nrf2 and Nrf2-dependent gene expression in vitro and activates the cAMP/PKA pathway. A, Real-time RT-PCR analysis of α-MSH-induced Nrf2 expression in NHM and NHK. Cells were stimulated with α-MSH (10−6 m) for various time points. *, P < 0.01; **, P < 0.001 vs. control. B and C, α-MSH induces mRNA expression of HO-1, γ-GCS, and GSTPi in NHM and HNK as shown by real-time RT-PCR. Cells were stimulated with α-MSH as outlined above. *, P < 0.05; **, P < 0.001 vs. control. D, Dose kinetic analysis of the up-regulating effect of α-MSH on Nrf2 and HO-1 expression in NHM. Cells were stimulated with α-MSH for 6 h as indicated. Nrf2 and HO-1 RNA expression levels were determined by real-time RT-PCR. *, P < 0.001 vs. control. E, Blockade of transcription abrogates α-MSH-induced Nrf2 mRNA expression. NHM were stimulated with α-MSH (10−6 m) for 6 h in the absence or presence of AD (5 μg/ml) followed by real-time RT-PCR analysis of Nrf2. *, P < 0.01 vs. α-MSH. F, Identification of the cAMP/PKA pathway in α-MSH-mediated Nrf2 and HO-1 induction. NHM were treated with α-MSH (10−6 m) or FSK (1 μm) in the presence or absence of the PKA inhibitor H89 (10 μm) for 6 h followed by real-time RT-PCR analysis of Nrf2 and HO-1. *, P < 0.01; **, P < 0.001 vs. α-MSH/FSK. G, Result of the in silico promoter analysis of the promoter regions of Nrf2 for putative CREB and AP1 binding sites. Each block identified by the TOUCAN MotifLocator represents a possible binding site. Black bars, CREB sites; white bars, AP1 sites. The numbers on the axis refer to bases upstream from the transcription start site.
Mechanism of α-MSH-mediated induction of Nrf2 and Nrf-dependent genes
To shed light into the mechanism of α-MSH-mediated Nrf2 induction, NHM were preincubated with AD, a RNA polymerase inhibitor. AD totally blocked α-MSH-mediated Nrf2 mRNA induction in NHM after 6 h, indicating that α-MSH exerts its effect by transcriptional induction but not by increased mRNA stability of Nrf2 (Fig. 4E). Moreover, the protein kinase A (PKA) inhibitor H89 attenuated the α-MSH-mediated effect on Nrf2 and HO-1 mRNA expression (Fig. 4F). On the other hand, the artificial cAMP inducer FSK mimicked the effect of α-MSH on induction of Nrf2 and HO-1. Again, preincubation with H89 blocked FSK-induced Nrf2 and HO-1 mRNA expression, as representatively shown for NHM after 6 h (Fig. 4F). These results provide a first insight into the molecular mechanism of α-MSH-mediated Nrf2 and HO-1 induction and suggest that the cAMP pathway mediates the observed effect of α-MSH.
Promoter analysis of Nrf2
To further elucidate the mechanism by which α-MSH exerts its inductive effect on Nrf2 gene expression, we performed in silico promoter analysis of the human Nrf2 gene. Here we focused on binding sites for activator protein 1 (AP1) and cAMP response element-binding protein (CREB), transcription factors activated in B16 melanoma cells in response to α-MSH or FSK (39) and in α-MSH-treated HEK293 cells stably expressing MC-1R (40). Using the TOUCAN software for comparative promoter analyses, a CREB and several putative AP1 binding sites were detected in the promoter regions of Nrf2 (Fig. 4G).
α-MSH counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin
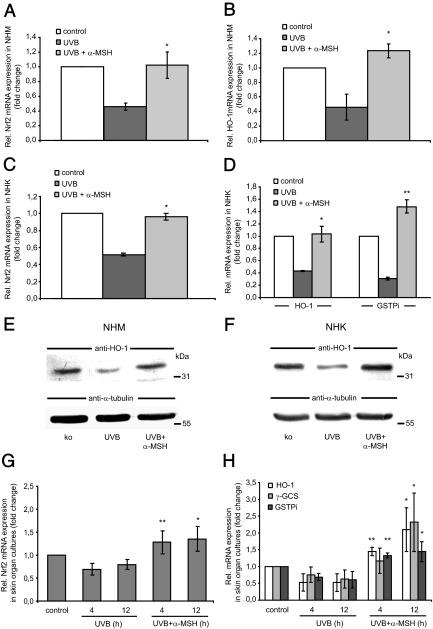
Because α-MSH was found to induce Nrf2 in a time- and dose-dependent manner, we tested whether preincubation of the peptide can counteract the suppressive effect of UVB on the levels of Nrf2 and Nrf-dependent phase II detoxifying enzymes. NHM and NHK were thus preincubated with α-MSH (10−6 m) for 24 h and then irradiated with UVB (10 mJ/cm2). After 2 and 24 h of incubation, steady-state levels of Nrf2, HO-1, GSTPi, and γ-GCS were determined by real-time RT-PCR analysis. In NHM, α-MSH significantly attenuated and even partially overcompensated the suppressive effect of UVB on Nrf2 as well as on HO-1 mRNA expression (Fig. 5A and B). RNA expression analysis of the enzymes γ-GCS and GSTPi exhibited a similar but lower compensatory effect of α-MSH on UVB-induced suppression of these genes in NHM (data not shown).
Figure 5.
α-MSH attenuates the suppressive effect of UVB on Nrf2 and Nrf2-dependent gene expression in NHM and NHK in vitro. A and B, Expression of Nrf2 and HO-1 in NHM after UVB or treatment together with α-MSH. NHM were preincubated with α-MSH (10−6 m) for 24 h, exposed to UVB (10 mJ/cm2), and harvested after 2 h for Nrf2 or 24 h for HO-1 expression analysis by real-time RT-PCR analysis. *, P < 0.01 vs. UVB. C and D. Analysis of Nrf2, HO-1, and GSTPi gene expression in NHK. Cells were treated with α-MSH and UVB as outlined for NHM above followed by real-time RT-PCR. *, P < 0.001 vs. UVB. E and F, Protein expression of HO-1 in NHM and NHK. Cells were pretreated with α-MSH for 24 h, irradiated with UVB, harvested after 24 h, and subjected to Western immunoblot analysis. The same blot was stripped and reprobed with an anti-α-tubulin antibody to ensure equal loading of cell lysates. G and H, mRNA expression of Nrf2 and Nrf-dependent genes in human skin organ cultures exposed to UVB and/or UVB plus α-MSH. Explants (n = 4) were treated with UVB (10 mJ/cm2) alone or in combination with α-MSH (10−6 m) and cultured for 4 or 12 h followed by real-time PCR analysis. *, P < 0.05; **, P < 0.01 vs. UVB.
Similar results were obtained with α-MSH on UVB-treated NHK (Fig. 5, C and D). Here, not only UVB-induced suppression of HO-1 was abrogated but also that of GSTPi overcompensated by α-MSH (Fig. 5D). Western immunoblot analysis of HO-1 confirmed the counteracting effects of α-MSH on UVB-induced suppression of HO-1 protein expression in both NHM and NHK (Fig. 5, E and F).
In accordance with these in vitro findings, UVB-mediated suppression of both Nrf2 and Nrf-dependent mRNA expression was largely counteracted by α-MSH (10−6 m) in human skin organ cultures (Fig. 5, G and H). At 4 and 12 h after UVB irradiation, α-MSH had abrogated or even overcompensated the decrease in Nrf2, HO-1, and GSTPi gene expression (Fig. 5, G and H).
To rule out that the counteracting effect of α-MSH on UVB-mediated reduction of Nrf2 and Nrf-dependent gene expression is mediated by increased melanin content, we performed melanin assays. Indeed, no significant changes in the melanin content of NHM treated with α-MSH for up to 24 h were detectable.
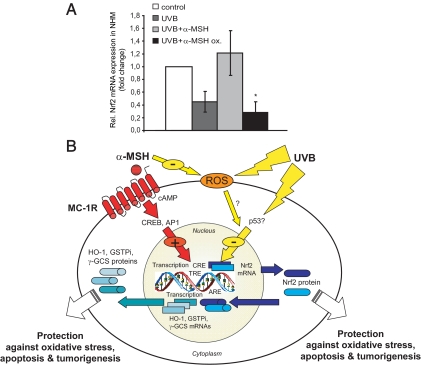
In a last set of experiments, we explored the possibility that α-MSH per se may act as a ROS scavenger due to oxidation of susceptible amino acids in its sequence (33). To this end, we used oxidized α-MSH vs. normal α-MSH and compared its impact on UVB-induced reduction in mRNA expression of Nrf2 in NHM (Fig. 6A). Interestingly, oxidized α-MSH appeared to lose its property to counteract UVB-induced suppression of Nrf2 mRNA expression, suggesting a direct effect of the peptide during UVB exposure of these cells.
Figure 6.
Impact of α-MSH oxidation on its modulatory effect on UVB-mediated reduction of Nrf2 expression in NHM and proposed molecular mechanism of the modulatory effect of α-MSH. A, RNA expression analysis of Nrf2 in NHM exposed to UVB, α-MSH plus UVB, or oxidized (ox.) α-MSH plus UVB. Cells were preincubated with α-MSH (10−6 m) or oxidized α-MSH at the same dose for 24 h, exposed to UVB (10 mJ/cm2), and harvested after 2 h. Nrf2 mRNA expression was monitored by real-time RT-PCR. *, P < 0.05; **, P < 0.01 vs. UVB plus α-MSH. B, Schematic illustration depicting the proposed role of α-MSH as an endogenous protector against UVB-induced oxidative stress via induction of Nrf2 and Nrf-dependent genes or directly by scavenging ROS in keratinocytes and melanocytes. ARE, Antioxidant response element; CRE, cAMP response element; TRE, 12-O-tetradecanoylphorbol-13-acetate response element.
Taken together, these finding highlight a novel non-pigment-cell-specific biological activity of α-MSH, namely a counteracting capacity on UVB-induced suppression of Nrf2 gene expression and on UVB-mediated depletion of phase II detoxifying enzymes.
Discussion
In this study, we have investigated the expression pattern of Nrf1-3 in human skin cells, the effect of UVB on these transcription factors including its dependent phase II detoxifying enzymes, and the role of α-MSH.
Until now, few data exist on the expression and regulation of Nrfs in skin. Expression of Nrf2 in vitro was found in HaCaT cells (35,41) as well as in human dermal fibroblasts (42). Keratinocyte growth factor, epidermal growth factor, and basic fibroblast growth factor enhanced Nrf2 expression in HaCaT cells (35). The latter authors also detected Nrf3 mRNA in HaCaT cells and in wounded murine skin of Nrf2 knockout animals (35). Marrot et al. (43) further reported mRNA expression of Nrf2 in primary NHM and NHK in vitro. In the murine system, Nrf2 protein expression was found in primary keratinocytes and dermal fibroblasts (42,44,45).
Our expression analysis of Nrfs in human skin cells in vitro as well in normal human skin revealed that Nrf2 is constitutively expressed by epidermal keratinocytes. This finding is in accordance with data from others in which Nrf2 mRNA was found in the basal keratinocytes especially after murine skin wounding (35). Interestingly, although Nrf2 protein was detectable in NHM in vitro, Nrf2 immunoreactivity was absent within melanocytes of normal skin. It is possible that growth factors routinely used for the propagation of NHM in vitro, e.g. insulin or basic fibroblast growth factor, increase Nrf2 protein expression in these cells.
Surprisingly, UVB exposure of both NHM and NHK in vitro as well as of human skin organ cultures markedly suppressed Nrf2 and Nrf-dependent gene expression in a time-dependent manner. These findings extend a previous report in which UVB irradiation (10 and 20 J/m2) of mouse hepatoma (Hepa-1) cells led to nuclear exclusion of Nrf2. Similar observations were obtained with UVB irradiation of mouse keratinocytes and human dermal fibroblasts at 20 J/m2 UVB (42). In human dermal and mouse keratinocytes exposed to the above UVB doses, a decline in Nrf2-regulated reduced nicotinamide adenine dinucleotide phosphate quinone oxidoreductase-1 gene expression and antioxidant response element-mediated luciferase gene expression was further detected. However, in the latter study, no kinetics of Nrf2 expression over time after UVB irradiation was made. In contrast to the herein detected suppressive effect of UVB on Nrf2 expression, UVA (10 J/cm2) increased whole-cell and nuclear Nrf2 amounts in murine dermal fibroblasts (44). It is likely that the different effects of UVA vs. UVB on Nrf expression are due to the relative amounts of ROS being generated after UV exposure. UVA is more potent in inducing ROS compared with UVB (2). The mechanism by which UVB leads to suppression of Nrf2 and Nrf-dependent phase II detoxifying enzyme as shown by our study remains unknown. Recent data point toward a direct interaction between Nrf2-dependent phase II detoxifying enzymes and the tumor suppressor gene product p53. Increased mRNA expression of these antioxidative genes induced by electrophilic stimulation or Nrf2 overexpression was blocked by endogenous p53 activation or by p53 overexpression. Chromatin immunoprecipitation analysis supported the concept that p53 trans-represses Nrf2-dependent gene activating by directly interacting with antioxidant response element-containing promoters (46). Because p53 is induced by UVB, it will be fascinating to check whether p53 may likewise reduce Nrf2 expression in NHM or NHK by trans-repressing the activation of distinct promoter regions within the genes of these transcription factors.
The novelty of our data further consists in the regulatory effect of α-MSH on Nrf2 and Nrf-dependent enzymes. These findings add another facet to the so-far identified protective properties of α-MSH and related peptides. ACTH was shown to up-regulate HO-1 gene expression in murine adrenal cells (47) and in the murine macrophage cell line RAW264.7 (48). Both α-MSH and ACTH also induce another ROS-detoxifying enzyme, superoxide dismutase-2 (34,49). However, superoxide dismutase-2 is not an Nrf-dependent enzyme.
Further studies have to address the mechanism by which α-MSH increases Nrf2 gene transcription and Nrf-dependent expression. Similar to ACTH-mediated HO-1 induction in RAW264.7 cells (48), α-MSH-mediated Nrf2 and HO-1 expression was blocked by inhibition of PKA. As suggested by our in silico promoter analysis, several putative CREB and AP1 binding sites were detected in the promoter regions of Nrf2. These findings and the up-regulatory effect of FSK on Nrf2 mRNA expression suggest that activation and binding of CREB to its respective consensus sites within the promoter regions of Nrf2 alone, or in synergy with AP1, could mediate the effect of α-MSH (Fig. 6B).
As can be expected from the inductive effect of α-MSH on Nrf2, we found that the peptide attenuates the suppressive effect of UVB irradiation on Nrf2 and HO-1 expression in NHM and NHK in vitro. In NHK, not only HO-1 but also UVB-induced suppression of GSTPi was antagonized or even overcompensated by α-MSH. These findings were supported by the impact of α-MSH in UVB-treated skin organ cultures. α-MSH-mediated attenuation of this UVB-induced Nrf2 and Nrf-dependent gene expression may thus represent a novel natural defense mechanism by which UVB-induced melanocortins protect against accumulation of ROS within the epidermis after sun exposure.
The molecular mechanism by which α-MSH attenuates UVB-mediated suppression of Nrf2 and Nrf-dependent genes appears to be complex (Fig. 6B). Whereas the mechanism of UVB-mediated reduction in Nrf2 and Nrf-dependent gene expression remains elusive, our pharmacological studies with pathway inhibitors and stimulators (H89 and FSK) support a MC-1R-cAMP-PKA-mediated effect of α-MSH. This is corroborated by our in silico promoter analysis, which identified a cAMP response element within the promoter regions of Nrf2. Because α-MSH activates not only CREB but also AP1 in melanocytes (39), Nrf2 mRNA expression may be further enhanced by this transcription factor family. On the other hand, we could show that oxidized α-MSH loses its capacity to counteract the effect of UVB on Nrf2 expression. This finding would suggest a MC-1R-independent pathway in which the tridecapeptide per se acts as a ROS scavenger. However, we cannot totally exclude that the lack of the observed effect of oxidized α-MSH on Nrf2 expression is due to loss of ligand binding and MC-1R signaling by the altered peptide. Experiments with MC-1R-signaling-deficient NHM would clarify this issue.
Regardless of these mechanistic possibilities, the net effect of α-MSH is compensation of the UVB-mediated depletion of Nrf2 and Nrf-dependent genes. Because α-MSH suppresses UVB-induced apoptosis of epidermal cells (22,23,24), it is tempting to speculate that α-MSH-mediated induction of Nrf2 (or its dependent genes) participates in the antiapoptotic and DNA damage-preventing effect of this peptide. This is currently under investigation in our laboratory.
Acknowledgments
We thank Britta Ringelkamp, Andrea Wissel, and Nicole Gross for their expert technical assistance.
Footnotes
M.B. was supported by a grant from the Deutsche Forschungsgemeinschaft (BO 1075/5-3) and by a grant from the Rolf-Dierichs- Stiftung.
This work is part of the Ph.D. thesis of A.K.
Disclosure Summary: A.K., D.M., N.M., M.-D.G., M.S., and T.A.L. have nothing to declare.
First Published Online March 12, 2009
Abbreviations: AD, Actinomycin D; AP1, activator protein 1; CREB, cAMP response element-binding protein; FSK, forskolin; γ-GCS, γ-glutamylcysteine synthetase; GSTPi, glutathione-S-transferase Pi; HDF, human dermal fibroblasts; HO-1, heme oxygenase-1; MC-R, melanocortin receptor; NHK, normal human keratinocytes; NHM, normal human melanocytes; PKA, protein kinase A; ROS, reactive oxygen species.
References
- MacKie RM 2006 Long-term health risk to the skin of ultraviolet radiation. Prog Biophys Mol Biol 92:92–96 [DOI] [PubMed] [Google Scholar]
- Morliere P, Moysan A, Tirache I 1995 Action spectrum for UV-induced lipid peroxidation in cultured human skin fibroblasts. Free Radic Biol Med 19:365–371 [DOI] [PubMed] [Google Scholar]
- Heck DE, Vetrano AM, Mariano TM, Laskin JD 2003 UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem 278:22432–22436 [DOI] [PubMed] [Google Scholar]
- Yasui H, Hakozaki T, Date A, Yoshii T, Sakurai H 2006 Real-time chemiluminescent imaging and detection of reactive oxygen species generated in the UVB-exposed human skin equivalent model. Biochem Biophys Res Commun 347:83–88 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Ito E, Toki T, Kogame K, Takahashi S, Igarashi K, Hayashi N, Yamamoto M 1999 Molecular cloning and functional characterization of a new cap’n’ collar family transcription factor Nrf3. J Biol Chem 274:6443–6452 [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW 1994 Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the β-globin locus control region. Proc Natl Acad Sci USA 91:9926–9930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Ishii T, Wakabayashi N, Yamamoto M 1999 Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res 31:319–324 [DOI] [PubMed] [Google Scholar]
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL 1999 Nrf2, a cap’n’collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274:26071–26078 [DOI] [PubMed] [Google Scholar]
- Wild AC, Moinova HR, Mulcahy RT 1999 Regulation of γ-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem 274:33627–33636 [DOI] [PubMed] [Google Scholar]
- Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M 2000 The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem Soc Trans 28:33–41 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB 2003 Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:223–260 [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB 1991 The antioxidant responsive element: activation by oxidative stress and indentification of the DNA consensus sequence required for functional activity. J Biol Chem 266:11632–11639 [PubMed] [Google Scholar]
- Kwong M, Kan YW, Chan JY 1999 The CNC basic leucine zipper factor, Nrf1, is essential for cell survival in response to oxidative stress-inducing agents. J Biol Chem 274:37491–37498 [DOI] [PubMed] [Google Scholar]
- Leung L, Kwong M, Hou S, Lee C, Chan JY 2003 Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem 278:48021–48029 [DOI] [PubMed] [Google Scholar]
- Morito N, Yoh K, Itoh K, Hirayama A, Koyama A, Yamamoto M, Takahashi S 2003 Nrf2 regulates the sensitivity of death receptor signals by affecting intracellular glutathione levels. Oncogene 22:9275–9281 [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y 1997 An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313–322 [DOI] [PubMed] [Google Scholar]
- Eipper BA, Mains RE 1980 Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev 1:1–27 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD 1992 The cloning of a family of genes that encode the melanocortin receptors. Science 257:1248–1251 [DOI] [PubMed] [Google Scholar]
- Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD 1997 Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 91:789–798 [DOI] [PubMed] [Google Scholar]
- Brzoska T, Luger TA, Maaser C, Abels C, Böhm M 2008 α-Melanocyte-stimulating hormone and related tripeptides. Biochemistry, anti-inflammatory effects in vitro and in vivo, and future implications for the treatment of immune-mediated inflammatory diseases. Endocr Rev 29:581–602 [DOI] [PubMed] [Google Scholar]
- Böhm M, Raghunath M, Sunderkötter C, Schiller M, Ständer S, Brzoska T, Cauvet T, Schiöth HB, Schwarz T, Luger TA 2004 Collagen metabolism is a novel target of the neuropeptide α-melanocyte-stimulating hormone. J Biol Chem 278:6959–6966 [DOI] [PubMed] [Google Scholar]
- Böhm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A 2005 α-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem 280:5795–5802 [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, Scott G, Abdel-Malek ZA 2005 α-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res 65:4292–4299 [DOI] [PubMed] [Google Scholar]
- Abdel-Malek ZA, Kadekaro AL, Kavanagh RJ, Todorovic A, Koikov LN, McNulty JC, Jackson PJ, Millhauser GL, Schwemberger S, Babcock G, Haskell-Luevano C, Knittel JJ 2006 Melanoma prevention strategy based on using tetrapeptide α-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxcity. FASEB J 20:1561–1563 [DOI] [PubMed] [Google Scholar]
- Terui K, Takahashi Y, Kitazawa J, Toki T, Yokoyama M, Ito E 2000 Expression of transcription factors during megakaryocytic differentiation of CD34+ cells from human cord blood induced by thrombopoietin. Tohoku J Exp Med 192:259–273 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Colombrita C, Lombardo G, Scapagnini G, Abraham NG 2003 Heme oxygenase-1 expression levels are cell cycle dependent. Biochem Biophys Res Commun 308:1001–1008 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB 2003 Increased protein stability as a mechanism that enhanced Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biochem 278:4536–4541 [DOI] [PubMed] [Google Scholar]
- Brasch-Andersen C, Christiansen L, Tan Q, Haagerup A, Vestbo J, Kruse TA 2004 Possible gene dosage effect of glutathione-S-transferase on atopic asthma: using real-time PCR for quantification of GSTM1 and GSTT1 gene copy numbers. Hum Mutat 24:208–214 [DOI] [PubMed] [Google Scholar]
- Corre S, Mekideche K, Adamski H, Mosser J, Watier E, Galibert MD 2006 In vivo and ex vivo UV-induced analysis of pigmentation gene expression. J Invest Dermatol 126:916–918 [DOI] [PubMed] [Google Scholar]
- Schiller M, Raghunath M, Kubitscheck U, Scholzen TE, Fisbeck T, Metze D, Luger TA, Böhm M 2001 Human dermal fibroblasts express prohormone convertases 1 and 2 and produce proopiomelanocortin-derived peptides. J Invest Dermatol 117:227–235 [DOI] [PubMed] [Google Scholar]
- Aerts S, Van Loo P, Thijs G, Mayer H, de Martin R, Moreau Y, De Moor B 2005 TOUCAN 2: the all-inclusive open source workbench for regulatory sequence analysis. Nucleic Acids Res 33:W393–W396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JD, Gibbons NC, Rokos H, Peters EM, Wood JM, Schallreuter KU 2007 Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J Invest Dermatol 127:411–420 [DOI] [PubMed] [Google Scholar]
- Chinn AM, Ciais D, Bailly S, Chambaz E, LaMarre J, Feige JJ 2002 Identification of two novel ACTH-responsive genes encoding manganese-dependent superoxide dismutase (SOD2) and the zinc finger protein TIS11b [tetradecanoyl phorbol acetate (TPA)-inducible sequence 11b]. Mol Endocrinol 16:1417–1427 [DOI] [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S 2002 Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol 22:5492–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA 1996 Binding of melanocortin hormones to the melanocortin receptor MC1R in human melanocytes stimulates proliferation and melanogenesis. Endocrinology 137:1627–1633 [DOI] [PubMed] [Google Scholar]
- Curry JL, Pinto W, Nickoloff BJ, Slominski AT 2001 Human keratinocytes express functional α-MSH (MC1-R) receptors. In Vitro Cell Dev Biol Anim 37:234–236 [DOI] [PubMed] [Google Scholar]
- Elliott RJ, Szabo M, Wagner MJ, Kemp EH, MacNeil S, Haycock JW 2004 α-Melanocyte-stimulating hormone, MSH 11–13 KPV and adrenocorticotropic hormone signalling in human keratinocyte cells. J Invest Dermatol 122:1010–1019. [DOI] [PubMed] [Google Scholar]
- Englaro W, Rezzonico R, Durand-Clément M, Lallemand D, Ortonne JP, Ballotti R 1995 Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells. J Biol Chem 270:24315–24320 [DOI] [PubMed] [Google Scholar]
- Newton RA, Smit SE, Barnes CC, Pedley J, Parsons PG, Sturm RA 2005 Activation of the cAMP pathway by variant human MC1R alleles expressed in HEK and in melanoma cells. Peptides 26:1818–1824 [DOI] [PubMed] [Google Scholar]
- Devling TW, Lindsay CD, McLellan LI, McMahon M, Hayes JD 2005 Utility of siRNA against Keap1 as a strategy to stimulate a cancer chemopreventive phenotype. Proc Natl Acad Sci USA 102:7280A–7285A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Jaiswal AK 2006 Low and high dose UVB regulation of transcription factor NF-E2-related factor 2. Cancer Res 66:8421–8429 [DOI] [PubMed] [Google Scholar]
- Marrot L, Jones C, Perez P, Meunier JR 2008 The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res 21:79–88 [DOI] [PubMed] [Google Scholar]
- Hirota A, Kawachi Y, Itoh K, Nakamura Y, Xu X, Banno T, Takahashi T, Yamamoto M, Otsuka F 2005 Ultraviolet A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: protective role in UVA-induced apoptosis. J Invest Dermatol 124:825–832 [DOI] [PubMed] [Google Scholar]
- Durchdewald M, Beyer TA, Johnson DA, Johnson JA, Werner S, auf dem Keller U 2007 Electrophilic chemicals but not UV irradiation or reactive oxygen species activate Nrf2 in keratinocytes in vitro and in vivo. J Invest Dermatol 127:646–653 [DOI] [PubMed] [Google Scholar]
- Faraonio R, Vergara P, Di Marzo D, Pierantoni MG, Napolitano M, Russo T, Cimino F 2006 p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem 281:39776–39784 [DOI] [PubMed] [Google Scholar]
- Pomeraniec Y, Grion N, Gadda L, Pannunzio V, Podesta EJ, Cymeryng CB 2004 Adrenocorticotropin induces heme oxygenase-1 expression in adrenal cells. J Endocrinol 180:113–124 [DOI] [PubMed] [Google Scholar]
- Lam CW, Getting SJ, Perretti M 2005 In vitro and in vivo induction of heme oxygenase 1 in mouse macrophages following melanocortin receptor activation. J Immunol 174:2297–2304 [DOI] [PubMed] [Google Scholar]
- Kokot A, Sindrilaru A, Schiller M, Sunderkötter C, Kerkhoff C, Scharffetter-Kochanek K, Luger TA, Böhm M 2009 α-Melanocyte-stimulating hormone suppresses bleomycin-induced collagen synthesis and reduces tissue fibrosis in a mouse model of scleroderma. Arthritis Rheum 60:592–603 [DOI] [PubMed] [Google Scholar]