Abstract
Prolactin (PRL) affects the development and function of the reproductive system by binding to two types of receptors, which differ by the size of their intracellular domain in rodents. Whereas the signaling pathway through the long form of the receptor (PRL-RL) is well characterized, signaling through the short form (PRL-RS) remains obscure. In this investigation, we examined transcription factors regulated by PRL in the ovary and decidua of mice expressing only PRL-RS in a PRL receptor null background. These mice provide a powerful in vivo model to study the selective signaling mechanism of PRL through PRL-RS independent of PRL-RL. We also examined the regulation of transcription factors in ovarian and uterine cell lines stably transfected with PRL-RS or PRL-RL. We focused our investigation on transcription factors similarly regulated in both these tissues and clearly established that signaling through PRL-RS does not activate the JaK/Stat in vivo but leads to severe down-regulation of Sp1 expression, DNA binding activity, and nuclear localization, events that appear to involve the calmodulin-dependent protein kinase pathway. Our in vivo and in culture data demonstrate that the PRL-RS activates a signaling pathway distinct from that of the PRL-RL.
Prolactin signaling through PRL-RS in ovarian and decidual cells does not activate the Jak2/Stat pathway in vivo, but leads to severe repression of Sp1 transcription factor.
Prolactin (PRL), a hormone mainly secreted by the pituitary, regulates many functions in diverse target tissues through multiple prolactin receptor (PRL-R) isoforms. A large body of literature has established the important role of PRL in the ovary and its critical contribution to the development and survival of the corpus luteum (CL) and progesterone synthesis (reviewed in Refs. 1,2,3,4). In addition to the pituitary, the decidua of humans (5), primates (6), and rodents (7,8) not only express the genes for PRL and its cognate receptor (6,9) but also is the site of PRL production and action (7,10,11,12,13). The generation of PRL and PRL-R null mice (14,15,16) have confirmed the role of PRL in the ovary (4,14) and have also revealed a key role for decidual PRL in the maintenance of pregnancy and fetal survival (8). Decidual PRL is shown to silence, locally, the expression of decidual genes detrimental to pregnancy (8,17,18).
PRL is known to activate multiple isoforms of membrane-bound receptors. These isoforms are alternative splice variants of the primary transcript. PRL-R is a member of the class I cytokine receptor superfamily that includes receptors for GH, leptin, erythropoietin, and several ILs (reviewed in Refs. 19, 20). The two major PRL-R isoforms described in rodent ovaries and decidua are the short (PRL-RS) and long (PRL-RL) forms (9,21,22). These isoforms differ in the length and composition of their cytoplasmic tail. PRL signaling through the PRL-RL has been extensively studied and the well-established downstream signaling pathway of PRL is that of Janus kinase (Jak)/signal transducer and activator of transcription (Stat) (reviewed in Refs. 15, 23), an archetype signaling pathway used by all cytokine receptors. Hormonal stimulation of PRL-RL is shown to induce Jak2 activation, PRL-R phosphorylation, and the association and phosphorylation of Stat transcription factors. This triggers Stat dimerization and nuclear translocation, events necessary for PRL-dependent functions.
The sequence required for Jak2 recruitment is present in both PRL-RL and PRL-RS and Jak2 associates with both receptors (24,25,26). Whereas tyrosine phosphorylation of Jak2 occurs with PRL-RL, activation of Jak2 through PRL-RS is controversial. Kelly and associates (24) demonstrated that cotransfection of PRL-RS with Jak2 kinase in 293 fibroblast cells results in association and activation of Jak2. Similarly, sheep PRL-RS is able to phosphorylate Jak2 on PRL stimulation (26). More recently Dufau and associates (27) have shown that human PRL-RS could also activate ligand-dependent Jak2 phosphorylation. In contrast, Clevenger and associates (28,29) reported that PRL-RS homodimers are unable to activate Jak2. Their work emphasizes the importance of tyrosine phosphorylation at Y309 and Y382 residues (within the X box and the C terminus of the receptor, respectively) for the activation of Jak2, regions that are absent in the PRL-RS. Another group has shown that the box2 region, present in PRL-RL, but not in PRL-RS, is required for Jak2 activation (30). There are also controversies about the activation of Stat5 through PRL-RS (26,31). However, all of these studies were performed using cell culture transfection systems, and there are no in vivo data available about Jak2/Stat phosphorylation through PRL-RS in either the ovary or decidua.
The conflicting data reported for the PRL-RS center around the question of whether the PRL-RS signals through a pathway distinct from that of the PRL-RL or acts instead as a dominant negative, serving only to decrease PRL-RL signaling (32,33,34). Recent findings from our laboratory suggest that the PRL-RS has a distinct signaling pathway. In transgenic mice expressing only PRL-RS (PRLR−/−RS), PRL causes early follicular recruitment followed by severe follicular death and premature ovarian failure (35). Additionally, overexpression of PRL-RS induces mammary gland differentiation and rescues the defects in mammopoiesis observed in PRL receptor knockout mice (36). These studies suggest a unique role for the PRL-RS in the mammary gland and ovary (35,36). However, no studies have examined the role of the PRL-RS in the decidua. The in vivo data presented here demonstrate a specific signaling role for PRL-RS and argue against a simple dominant-negative effect for this receptor. In contrast with in vitro studies, our work establishes that PRL-RS signaling does not activate the Jak2/Stat pathway in vivo. We demonstrate that down-regulation of the transcription factor Sp1 in the ovary and decidua is specifically mediated by the PRL-RS and that this pathway involves calmodulin-dependent protein kinase (CamK). This work provides the first in vivo evidence for a unique signaling pathway mediated by the PRL-RS.
Materials and Methods
Animal model and tissue preparation
PRL-R−/−RS transgenic mice were originally generated by microinjecting the eF1-PRL-R-PR-1 transgenic construct encoding the mouse cDNA for receptor short into fertilized PRL-R+/− oocytes derived from 129SV pure background mice (36). Animals were genotyped by PCR using genomic DNA isolated from tail as described previously (35). Mice were kept at 25 C with a 14-h light, 10-h dark cycle and were fed a commercial pellet diet ad libitum.
PRLR−/−RS transgenic mice were mated with vasectomized males to induce pseudopregnancy. Progesterone pellet (25 mg; Innovative Research of America, Sarasota, FL) was implanted sc, and decidualization was induced with intrauterine administration of sesame oil on d 4 of pseudopregnancy. Ergocryptine (200 μg, sc; Sigma, St. Louis, MO) was injected to block PRL secretion and recombinant ovine PRL [oPRL; 60 μg, purchased from Dr. Arieh Gertler (Protein Laboratories Rehovot Ltd., Rehovot, Israel)] was injected ip. Control mice received the vehicle (0.1% BSA). Ovaries and decidua obtained on d 9 of pseudopregnancy were frozen in liquid nitrogen and stored at −80 C until processing for RNA or protein extraction.
All experimental procedures were performed in accordance with the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Protein/DNA binding assay
Nuclear extracts from ovarian and luteal tissue and cultured cells were prepared as previously described (37). Activation of transcription factors was screened using the TranSignal protein/DNA approach according to the manufacturer’s instructions (Panomics, Inc., Redwood City, CA).
EMSA
Five picomoles of Sp1 annealed oligonucleotide probes were labeled using 10 U of T4 polynucleotide kinase (Invitrogen, Carlsbad, CA) and 25 μCi of γ-32P ATP (Amersham, Piscataway, NJ) to a specific activity of more than 8000 cpm/fmol. Five micrograms of nuclear extract were incubated with 1 μg of polydeoxyinosinic-deoxycytidylic acid (Amersham) and 50 fmol of probe in 1× binding buffer on ice for 30 min. Cold competitor probes were added to a final concentration of 2.5 pmol, and Sp1 antibody in supershift studies were used according to the manufacturer’s protocol. Antibody to Sp1 (PEP2, sc-59) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Samples were run on a 4% nondenaturing polyacrylamide gel in 0.5× Tris borate EDTA buffer at 200 V for 2–3 h. The gels were then dried and analyzed by autoradiography.
Cell culture
GG-CL cells, a rat luteal cell line generated in our laboratory (38), were incubated in a humidified atmosphere of 5% CO2 at 33 C. The stable transfection of GG-CL cells with the rat PRL-R was previously described (39).
The rat uterine stromal cells, UIII, and ovarian cell line, RCLP, were grown as described previously (37,40). For transient transfection cells were grown at 50–60% confluency in 2% Charcoal Dextran-Treated fetal bovine serum (FBS) (Hyclone, Logan, UT) in six-well plates. Cells are transfected using Lipofectamine 2000 (Invitrogen) or Effectine (QIAGEN, Valencia, CA) according to the manufacturer’s protocol. The cells were transfected with or without wild-type Jak2 expression vector in the presence or absence of rat PRL-RL or PRL-RS expression vectors, each at 0.8 μg/well. Cells were treated with oPRL (1 μg/ml) in medium supplemented with 1% CDT-FBS. At different time points, cells were rinsed twice with ice cold PBS and were frozen at −80 C until RNA and protein extraction.
RNA extraction and RT-PCR
Total RNA was extracted from ovary and decidua using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. One microgram of total RNA was reverse transcribed using Superscript Polymerase II kit according to the manufacturer’s instructions (Invitrogen). Mouse Sp1, rat Sp1, mouse L19, and rat L19 mRNA expression were detected using mouse Sp1, 5′-CGT GCA AAA GGA GAT CAA GC-3′ (forward) and 5′-AAT CAA GGC CAG GAA GTC G-3′ (reverse); rat Sp1 5′-TGA ATG CTG CTC AAC TGT CC-3′ (forward) and 5′-CTC CAC CTG CTG TCT CAT CA-3′ (reverse); mouse L19 5′-AGC GCC TCC AGG CCA AGA AGG-3′ (forward), 5′-CCA GGC CGC TAT GTA CAG ACA CGA-3′ (reverse) and rat L19 5′-GGA CAG AGT CTT GAT GAT CTC-3′ (forward), 5′-CTG AAG GTC AAA GGG AAT GTG C-3′ (reverse) primers, respectively. The PCR products were then separated by gel electrophoresis on a 0.7% agarose gel, and the intensity was measured using UV transilluminator and a digital camera (Eastman Kodak Co., New Haven, CT).
Western blot analysis
Antibodies to phospho-Jak2 (Cell Signaling, Danvers, MA or Abcam Inc., Cambridge, MA), Jak2, phopho-Stat5a/b, phospho-Stat3, Stat3 (Upstate Biotechnology, Lake Placid, NY), Stat5a/b (BioSource International, Inc., Camarillo, CA), β-actin (Abcam), and Sp1 (Santa Cruz Biotechnology) were used. Western blots were performed as described previously (37).
Immunocytochemistry
GG-CL and UIII cells were grown for 24 h in M199 medium supplemented with 2% CDT-FBS on Lab-Tek chamber slides (Nalge Nunc International, Rochester, NY). Cells were then cultured with either recombinant oPRL (1 μg/ml) or vehicle for 2 h and processed for immunocytochemistry as described previously (40). A polyclonal antibody to Sp1 (1:200; Santa Cruz Biotechnology) and Cy3-conjugated donkey antirabbit IgG (1:800; Jackson ImmunoResearch Laboratories, West Grove, PA) were used as primary and secondary antibodies, respectively.
Immunohistochemistry
Paraffin-embedded sections were subjected to the avidin-biotin-peroxidase complex method using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA) as described previously (35). Slides were incubated overnight at 4 C with a polyclonal antibody to Sp1 (1:100; Santa Cruz Biotechnology) and processed as described (35).
Statistical analysis
Data were examined by t test and one-way ANOVA followed by the Tukey test using Prism software (GraphPad Software Inc., San Diego, CA). Values are considered statistically significant at *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Results
Analysis of transcription factors regulated similarly by PRL in the ovary and decidua of transgenic mice expressing PRL-RS
To have a better understanding of how PRL signals through PRL-RS, we examined the activation/deactivation of transcription factors regulated in the ovary and decidua of PRL-RS expressing mice using the TranSignal protein/DNA binding assay. For this purpose, we induced pseudopregnancy by mating female mice with vasectomized males, treated them with progesterone the day of mating, and induced decidualization with an intrauterine oil injection on d 4 of pseudopregnancy. On d 9, mice were treated with ergocryptine to block endogenous PRL secretion, followed by PRL injections. Ovaries and deciduas were harvested on d 9 at different time periods after PRL administration and nuclear extracts were isolated. The size and weight of the decidua of PRL-RS expressing mice was not different from that of wild-type mice. Only a few transcription factors were similarly regulated by PRL in the ovary and decidua of PRL-RS-expressing mice. Among these are the Sp1 and Stat transcription factors (data not shown).
Effect of PRL activation of PRL-RS on the Stats and Jak2 phosphorylation in vivo and in vitro
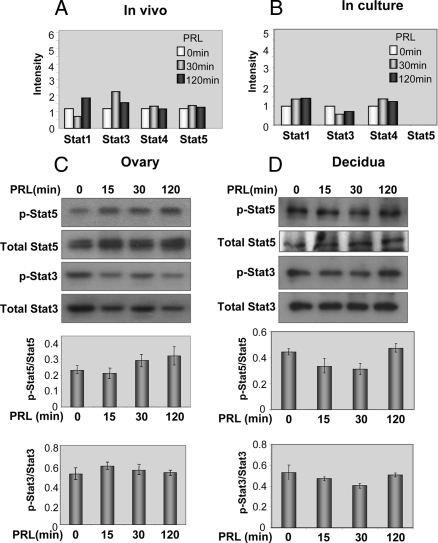
Stat transcription factors, Stat5 and Stat3, have been shown to be activated by PRL (15,41). The results of the protein-DNA binding assay in the PRL-RS expressing ovary showed no in vivo regulation of the Stat family of transcription factors by PRL at any of the time points examined. Only differences greater than 2-fold in the intensity of the spots were considered significant (Fig. 1A). We also examined the lack of Stat activation by PRL in vitro using GG-CL cells (38). A similar absence of Stat activation (Fig. 1B, right panel) was found in GG-CL cells, stably transfected with PRL-RS. Specific expression of PRL-RS mRNA in GG-CL cells is shown in supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Western analysis further established the inability of PRL to stimulate phosphorylation of Stat5 and Stat3 in both the ovary (Fig. 1C) and decidua (Fig. 1D) of mice expressing only PRL-RS.
Figure 1.
Effect of PRL on DNA binding activity and phosphorylation status of Stat transcription factors. Decidualization was induced in pseudopregnant PRLR−/−RS mice as previously described (8). Day 9 pseudopregnant PRLR−/−RS mice were injected with ergocryptine (200 μg, sc) for 6 h followed by a single ip injection of oPRL (60 μg). Ovaries and decidua were obtained after 0, 15, 30, and 120 min of PRL treatment. Protein-DNA binding assay was performed using nuclear extracts from these tissues. A, DNA binding activity of Stat transcription factors in the ovary of these mice was analyzed by protein-DNA binding assay. The intensity of the spots less than 2-fold compared with control (0 min) was not considered significant. B, DNA binding activity of Stat transcription factors activated by PRL in GG-CL cells stably transfected with PRL-RS. Using specific phospho-antibodies against Stat5 (p-Stat5) and Stat3 (p-Stat3), protein expression of activated Stat5 and Stat3 was analyzed by Western blot, in the ovary (C) and decidua (D) of pseudopregnant PRLR−/−RS mice as described in Materials and Methods. Total Stat5 and Stat3 antibodies were used as loading control. Comparative intensities are shown at the bottom.
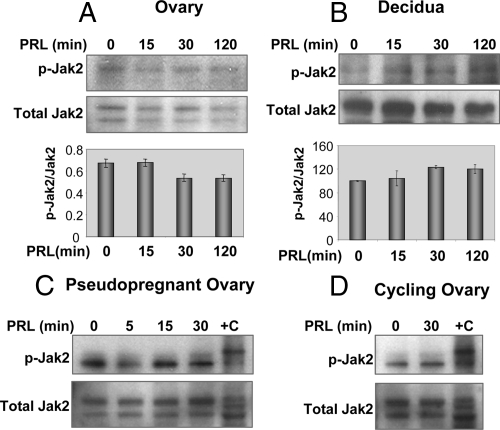
As shown in Fig. 2, A and B, we also found an absence of Jak2 phosphorylation on Tyr 1007/1008 by PRL in both target tissues expressing PRL-RS. No Jak2 phosphorylation is observed despite the presence of Jak2 in both PRL-RS expressing ovaries and decidua. Three possibilities may explain the lack of Jak2 activation in the ovary: 1) Jak2 activation occurs earlier than 15 min, 2) pseudopregnant mice do not have functional CL, and Jak2 activation through PRL-RS may occur only in functional CL; and/or 3) there is an intrinsic property/signaling of PRL-RS that prevents phosphorylation of Jak2 in vivo.
Figure 2.
Lack of Jak2 activation by PRL through PRL-RS in the ovary and decidua. A and B, Phosphorylation of Jak2 (p-Jak2) in response to PRL treatment (0, 15, 30, and 120 min) was measured by Western analysis using specific phospho-Jak2 antibody in the ovary and decidua. Total Jak2 was used as loading control. Comparative intensities are shown at the bottom. C, Phosphorylation of Jak2 in response to earlier time points of PRL treatment (0, 5, 15, and 30 min) was measured by Western analysis using specific phospho-Jak2 antibody in the ovary. HepG2 cells treated with insulin (Cell Signaling Technology) was used as the positive control (+C). D, Cycling PRLR−/−RS female mice (diestrous 1) were injected with ergocryptine (200 μg, sc) for 6 h followed by a single ip injection of PRL (60 μg). Ovaries were obtained after 0 and 30 min of PRL treatment. Phosphorylation of Jak2 was measured by Western analysis using specific phospho Jak2 antibody.
To address the first point, PRL-RS-expressing mice were treated with PRL for shorter time periods. As shown in Fig. 2C, Jak2 phosphorylation did not change at the earlier time point (5 min), suggesting that Jak2 cannot be activated through PRL-RS in vivo. To address the second possibility, we used mice in diestrus 1 (in which CL are functional), and treated them with the ergocryptine/PRL regimen. As shown in Fig. 2D, the results indicate that, even in the presence of normal CL, Jak2 is not activated by PRL signaling through PRL-RS. These results led us to examine the third possibility, namely that lack of Jak2/Stat activation through PRL-RS signaling is specific to the in vivo system.
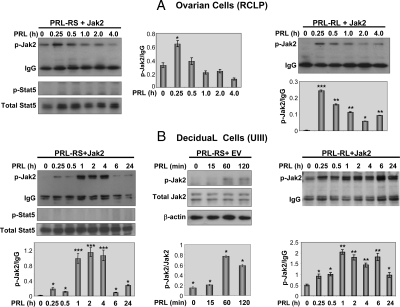
To address this, we used the RCLP ovarian-derived cell line (37). We first established that Stat5a as well as Stat5b mRNA is expressed in RCLP cells (data not shown). Because RCLP cells do not express the PRLR, we transiently transfected these cells with either PRL-RS or PRL-RL together with the Jak2 expression vector. In contrast to the in vivo results, PRL treatment activated Jak2 phosphorylation in RCLP cells expressing either PRL-RS (Fig. 3A, left panel) or PRL-RL (Fig. 3A, right panel). Jak2 activation reached its maximum at 15 min and then gradually declined in cells expressing either receptor. Despite Jak2 activation, no Stat5a/b phosphorylation was observed in PRL-RS expressing cells (Fig. 3A, left panel). We further investigated the phosphorylation of Jak2/Stat5 in a decidual cell line (UIII) previously characterized in our laboratory (42). We found a robust activation of Jak2 by PRL in PRL-RS expressing cells transfected with either Jak2 expression vector (Fig. 3B, left panel) or empty vector (Fig. 3B, middle panel). However, no Stat5a/b phosphorylation was observed (Fig. 3B, left panel). Interestingly, the pattern of Jak2 activation by PRL in UIII cells expressing PRL-RS differed from that in PRL-RL-expressing cells. Whereas levels of Jak2 phosphorylation declined after 4 h of PRL treatment in PRL-RS-expressing cells, (Fig. 3B, left panel), they remained elevated in cells expressing PRL-RL (Fig. 3B, right panel).
Figure 3.
Activation of Jak2 by PRL signaling through PRL-RS in the ovarian (RCLP) and decidual (UIII) cell lines. A, RCLP cells were cotransfected with either PRL-RS (left panel) or PRL-RL (right panel) and with Jak2 expression vector. Cells were then treated with PRL (1 μg/ml) for various time points as indicated. Phosphorylation of Jak2 (p-Jak2) and Stat5 (p-Stat5) was examined by Western analysis using specific phosphoantibodies. IgG and total Stat5 were used as loading controls. B, UIII cells were transfected with PRL-RS and either Jak2 expression vector (left panel) or empty vector (middle panel). In the right panel, cells were transfected with PRL-RL and Jak2. Cells were treated with PRL (1 μg/ml) for various time points as indicated. Phosphorylation of Jak2 and Stat5 was examined by Western analysis using specific phosphoantibodies. Comparative intensities are shown within the corresponding panels. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. control.
Inhibition of Sp1 DNA binding activity, nuclear localization, and expression by PRL signaling through PRL-RS
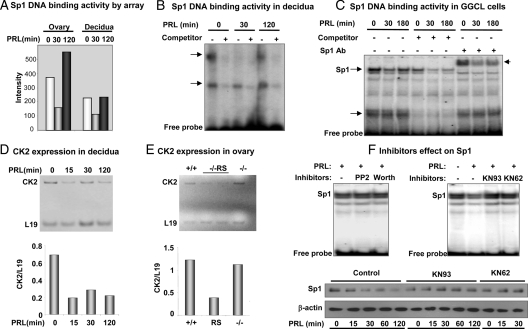
The protein-DNA binding assay indicates that DNA binding activity of Sp1 is inhibited within 30 min of PRL treatment in both the ovary and decidua of PRL-RS expressing mice (Fig. 4A). We also confirmed these results by EMSA. As shown in Fig. 4B, two protein-DNA complexes are formed with decidual nuclear extracts and oligos containing the Sp1 site. Also, whereas both complexes are inhibited with excess cold oligo, only the upper one is down-regulated by PRL. We further examined the PRL/PRL-RS mediated down-regulation of Sp1 DNA binding activity using the Sp1 expressing GG-CL cells, stably transfected with PRL-RS. Cells were treated with PRL for different time periods and Sp1 DNA binding activity was examined by EMSA (Fig. 4C). Here also we found two major protein/DNA complexes inhibited by excess cold oligo. However, only the upper complex was inhibited by PRL activation of PRL-RS. This complex was entirely supershifted with Sp1 antibody, indicating that it is Sp1 specific. The inhibition of Sp1 binding activity by PRL is clearly apparent in this complex (Fig. 4C).
Figure 4.
Inhibition of DNA binding activity of Sp1 transcription factor by PRL signaling through PRL-RS and role of kinases. Day 9 pseudopregnant PRLR−/−RS mice were injected with ergocryptine (200 μg, sc) for 6 h followed by a single ip injection of PRL (60 μg). Decidua and ovaries were obtained after 0, 30, and 120 min of PRL treatment. A, DNA binding activity of Sp1 transcription factor was measured by protein-DNA binding assay using nuclear extracts from decidua and ovary. B, Nuclear extract from decidua was subjected to EMSA using oligonucleotide-specific probe to Sp1. C, GG-CL cells stably transfected with PRL-RS, were treated with PRL (1 μg/ml) for 0, 30, and 180 min. Nuclear extracts from these cells were subjected to EMSA using radiolabeled oligonucleotides specific to Sp1 in the presence or absence of either 50-fold molar excess unlabeled competitor or Sp1 antibody (Ab). D, CK2 mRNA expression was examined by RT-PCR in the decidua of pseudopregnant PRLR−/−RS mice treated with PRL. E, CK2 mRNA expression was examined in the ovaries of wild-type (+/+) and PRLR−/−RS and in PRL−/− females by RT-PCR. The relative intensities of CK2 in both decidua and ovary was quantified by densitometry and normalized to corresponding L19 intensities. F, Upper panel, Ovarian cells (GG-CL) stably transfected with PRL-RS were cultured in the presence of serine phosphatase inhibitor [4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP2)], kinase inhibitor (wortmanin), or CamK inhibitors (KN-93 or KN-62) for 1 h. They were then treated with PRL (1 μg/ml) for 30 min. Nuclear extracts were subjected to EMSA using oligonucleotide specific to Sp1. Lower panel, Decidual cells (UIII) were transfected with PRL-RS using Effectene reagent. Twelve to 15 h later, cells were washed and cultured in serum-free media for 2 h with or without KN-93 or KN62, followed by treatment with PRL (1 μg/ml) for different time points as indicated. Total protein was isolated and subjected to Western analysis for Sp1. β-Actin was used as loading control.
The primary sequence of Sp1 protein contains consensus sites for numerous kinases (reviewed in Ref. 43); however, casein kinase II (CK2) is the only known kinase that phosphorylates Sp1, leading to the inhibition of its activity (44). Because CK2 activity depends on the level of its expression, we examined the effect of PRL on mRNA expression of this kinase in the decidua (Fig. 4D) and ovary (Fig. 4E) of PRL-RS expressing mice. Instead of stimulation, we found PRL inhibition of CK2 expression. These results suggest that PRL-mediated inhibition of Sp1 DNA binding activity is not due to phosphorylation of Sp1 by CK2. To determine the kinase involved in PRL mediated Sp1 inhibition, we performed gel shift experiments using GG-CL cells stably transfected with PRL-RS and treated with either kinase or phosphatase inhibitors. We found no change in Sp1 binding activity with 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP2) and wortmannin (Fig. 4F, upper panel). However, we were able to prevent PRL-mediated inhibition of Sp1 binding activity with CamK inhibitors, KN93 and KN62 (Fig. 4F, upper panel). As shown in Fig. 4F, lower panel, these inhibitors appear to affect the level of Sp1 protein expression. PRL induced a sharp decrease in Sp1 protein levels in cells expressing PRL-RS. This decrease was prevented by both CamK inhibitors.
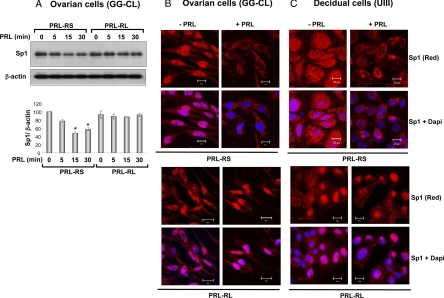
We further established that PRL-mediated inhibition of Sp1 protein expression is specific to PRL-RS and does not occur in GG-CL cells expressing PRL-RL (Fig. 5A). We found similar results in UIII cells (data not shown). Immunocytochemical analysis and confocal microscopy revealed that Sp1 is principally localized in the nucleus of untreated GG-CL cells expressing PRL-RS (Fig. 5B, upper panel), although Sp1 is also detectable in the cytoplasm. PRL treatment induced a remarkable decline in Sp1 nuclear localization. In contrast, Sp1 localization in the nucleus was not affected by PRL in GG-CL cells transfected with PRL-RL (Fig. 5B, lower panel). Similar results were obtained with the UIII cells (Fig. 5C, lower panel). Sp1 is uniformly present in the nucleus and cytoplasm of the untreated PRL-RS expressing UIII cells. In these cells, PRL treatment also caused a reduction in nuclear as well as an overall Sp1 protein expression. In UIII cells transfected with PRL-RL, Sp1 was abundantly expressed in the nucleus and this expression remained unchanged upon PRL treatment. Interestingly after PRL treatment and concomitant with the loss of Sp1, cell death was invariably noticed in both ovarian and decidual cells expressing PRL-RS. No such cell death was observed in PRL-RL expressing cells.
Figure 5.
Inhibition of Sp1 by PRL in the ovarian and decidual cell lines. A, GG-CL cells were transfected with PRL-RS or PRL-RL using Effectene reagent. Twelve to 15 h later, cells were washed and cultured in serum-free media for 2 h followed by treatment with PRL (1 μg/ml) for different time points as indicated. Total protein was isolated and subjected to Western blot analysis for Sp1. The relative intensity of Sp1 was quantified by densitometry and normalized to corresponding β-actin intensities. *, P < 0.05 vs. control. B and C, GG-CL and UIII cells, cultured on chamber slides, were transfected with PRL-RS (upper panel) or PRL-RL (lower panel) and Sp1 expression vector. The cells were serum starved for 2 h and then treated with PRL (1 μg/ml) or vehicle for 120 min. Cells were prepared for immunocytochemistry as described previously (40). Cells treated with vehicle (left panel) or PRL (right panel) were stained with polyclonal antibody to Sp1 (1:200, final dilution). Nuclei were stained with 4′,6-diamino-2-phenylindole (DAPI). Sp1, Red; nucleus, blue. Scale bars, 20 μm.
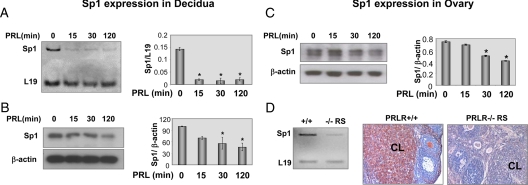
Next, we examined whether the PRL-mediated inhibition of Sp1 protein in the ovarian and decidual cell lines also occurs in vivo in PRL-RS expressing mice. In vivo injection of ergocryptine/PRL regimen to PRL-RS-expressing mice induced a rapid reduction of Sp1 mRNA followed by a decrease in Sp1 protein in the decidua (Fig. 6, A and B). A decrease in Sp1 expression was also seen in the ovaries of PRL-RS mice challenged with the same ergocryptine/PRL regimen (Fig. 6C). Furthermore, we observe diminished expression of Sp1 mRNA (Fig. 6D, left panel) in ovaries of d 2.5 pregnant PRL-RS mice, which are normally subjected to high levels of PRL, and negligible levels of Sp1 protein compared with wild-type control (Fig. 6D, right panel), thus further establishing the inhibitory role of PRL on Sp1.
Figure 6.
Inhibition of Sp1 expression in the decidua and ovary of PRLR−/−RS mice. Day 9 pseudopregnant PRLR−/−RS mice were injected with ergocryptine (200 μg, sc) for 6 h followed by a single ip injection of PRL (60 μg). Total mRNA and protein from decidua and ovary were obtained after 0, 15, 30, and 120 min of PRL treatment. Then Sp1 expression was examined by RT-PCR (A) and Western blot (B) in the decidua. Ovarian expression of Sp1 was quantified by RT-PCR (E) and Western blot (C) and normalized to L19 or β-actin, respectively. D, Sp1 expression was also examined in the ovaries obtained from 2.5 d pregnant PRLR−/−RS females (left panel). *, P < 0.05 vs. control. Ovaries were processed for immunohistochemistry and Sp1 expression was detected using antibody specific to Sp1 (right panel). Sp1, as shown in red, is expressed in the CL and theca interstitial cells.
Discussion
Ovary and decidua express PRL-RS as well as PRL-RL (9) and are two well-known target tissues of PRL action in reproduction (reviewed in Refs. 1,2,3,4, 45). It has been argued that PRL signals only through PRL-RL and that PRL-RS plays only a dominant-negative role preventing PRL signaling (31). This possibility was challenged by several laboratories including ours (35,36,46,47).
In this investigation we used mice expressing the PRL-RS in a PRL-R knockout background as well as ovarian and decidual cell lines expressing the PRL-RS to study its signaling pathways in the absence of PRL-RL. Our results clearly established that PRL signaling through PRL-RS does not affect the Jak2/Stat pathway in vivo; however, it leads to severe down-regulation of Sp1 expression, DNA binding activity, and nuclear localization, events that appear to involve the CamK pathway. Our in vivo and in culture data demonstrate that the PRL-RS activates a signaling pathway distinct from that of the PRL-RL.
Because there are controversial reports about activation of Stat5 and Jak2 through PRL-RS (24,25,26,27,28,29,30,31), we examined PRL-RS mediated Stat activation both in vivo and culture by DNA binding assay as well as Western blot. The lack of Stat activation through PRL-RS was shown in both PRL-RS-expressing mice and ovarian and decidual cell lines transfected with PRL-RS. These results do not support the finding that PRL can activate Stat5 through PRL-RS (26) and confirm earlier results indicating that PRL activation of Stat transcription factors does not involve this receptor type (48).
Association of Jak2 with PRL-RS and PRL-RL is a well-established event; however, activation of Jak2 through PRL-RS is controversial. Whereas some investigators (24,26,27) demonstrated that cotransfection of PRL-RS with Jak2 results in activation of Jak2 on PRL stimulation, others (28,29) were unable to activate Jak2 through PRL-RS. However, these results were obtained using cell culture systems and transfection studies. There are no in vivo data available on Jak2 phosphorylation through PRL-RS alone. Our in vivo results indicate that PRL treatment to mice expressing only the PRL-RS fails to increase Jak2 phosphorylation in either ovaries or decidua. The fact that Jak2 is not activated by PRL in mice expressing only PRL-RS provides physiological evidence that this activation does not take place in the whole animal.
To examine whether the inability of PRL-RS to activate Jak2 is exclusive to our in vivo system, the Jak2 phosphorylation status was examined in ovarian and uterine-derived cells transfected with either PRL-RS or PRL-RL and treated with PRL. As expected, Jak2 was activated by PRL signaling through PRL-RL; however, in contrast to results obtained in vivo, activation of PRL-RS in these cells caused a clear phosphorylation of transfected Jak2. This led us to address the issue of whether activation of Jak2 seen in vitro was due to overexpression of Jak2. In normal cells, truncation of the C terminus of the PRL-R renders the receptor unable to phosphorylate Jak2 (49). Expression of this truncated receptor was shown to induce ligand-dependent Jak2 activation in cells constitutively overexpressing Jak2. It appears that overexpression of Jak2 overrides the requirement of certain regions of PRL-R to activate Jak2 kinase activity (49). Moreover, we and others have traditionally studied activation of Jak2 using transient transfection studies with Jak2 and the PRL-R (8,18,24,27). However, our results show that PRL activation of PRL-RS can lead to the phosphorylation of the endogenously expressed Jak2 and that the phosphorylation is not merely due to overexpression of Jak2. One possible reason that PRL can activate Jak2 in cultured cells, but not in ovaries and decidua expressing PRL-RS, may be due to the absence of protein(s) that associate with the PRL-RS and prevent Jak2 phosphorylation in vivo. We have previously shown that both the ovaries (50) and decidua (51,52) express a protein (PRAP/HSD17B7) that associates specifically with PRL-RS but not PRL-RL (50). This protein is not expressed in either of the cell lines used. Whether this association with PRL-RS and not PRL-RL prevents Jak2 phosphorylation in vivo is an interesting possibility. Alternatively, PRL/PRL-RS may very rapidly activate protein tyrosine phosphatase(s), yet to be identified, causing dephosphorylation of Jak2.
Another salient result of this investigation is that whereas PRL activation of PRL-RS does not affect Stat transcription factors it causes a severe inhibition in Sp1 activity. The down-regulation of Sp1 peaked our interest because of the recent discovery that Sp1 stimulates the expression and promoter activity of FOXO3 (53), a transcription factor whose expression is repressed by PRL in ovaries of mice expressing only PRL-RS (35).
The activity of Sp1 is known to be largely regulated by posttranslational modifications (54,55,56,57). Sp1 is phosphorylated and activated by a number of cellular kinases (reviewed in Refs. 43, 54, and 57). The only kinase known to inhibit the activity of Sp1 is CK2, which phosphorylates Sp1 on the C terminus and prevents its DNA binding property (44,58). Whereas protein kinases are tightly controlled signaling molecules that are activated in response to specific stimuli, CK2 is an exceptional protein kinase that, owing to unique structural features, is constitutively active and its activity depends on its concentration in the cell (reviewed in Refs. 59 and 60). The report that Sp1 activity is repressed by CK2 suggested that PRL signaling through PRL-RS could enhance the expression and thus the activity of CK2, leading to phosphorylation of Sp1 and a decrease in Sp1 binding activity. It was therefore surprising to find that CK2 expression was inhibited, rather than stimulated, by PRL in the ovary and decidua of PRL-RS-expressing mice. In fact, from all the kinase inhibitors examined, only CamK inhibitors, KN93 or KN62, were effective in preventing PRL-mediated inhibition of Sp1 DNA binding activity. Sp1 contains putative CamK phosphorylation sites (reviewed in Ref. 61); however, the significance of such phosphorylation remains unknown. In any case, our finding that CamKII inhibitors prevent PRL mediated inhibition of Sp1 DNA binding and expression clearly suggests that CamKII might be a key player in the regulation of Sp1 activity.
We further examined the specificity of PRL-RS signaling using ovarian and decidual cell lines. We found that PRL induces a rapid decline in Sp1 protein level in the cells transfected with PRL-RS but not PRL-RL. These results confirmed that regulation of Sp1 expression by PRL is specific to PRL-RS signaling and that inhibition of Sp1 protein expression is a mechanism by which Sp1 activity is regulated by PRL. Additionally, the immunocytochemical study revealed that this PRL mediated inhibition of Sp1 is most affected in the nucleus, suggesting that PRL/PRL-RS signaling may also affect posttranslational modifications of Sp1 that may allow exclusion of Sp1 from the nucleus (62,63,64).
We also found dramatic inhibition of Sp1 mRNA by PRL treatment followed by a decline in protein levels in both target tissues. These results suggest that the repressed DNA-binding activity of Sp1 in response to PRL treatment is due in large part to decreased levels of Sp1 protein. Our result showing that Sp1 is profoundly inhibited in the ovary of PRL-RS mice, compared with wild-type in which both PRL-RS and PRL-RL are present, indicates that the presence of PRL-RL could prevent repression of Sp1 through PRL-RS. These data further emphasize our previous report (35) that coexpression of PRL-RL with PRL-RS prevents PRL inhibition of genes such as GALT and FOXO3. We also show that Sp1 is abundantly expressed in CL and theca interstitial cells in the normal ovary and that its expression is remarkably inhibited in the PRL-RS ovary. This suggests that theca cell function is affected by PRL/PRL-RS signaling. We have already shown that steroidogenic capacity of theca cells in the ovary of PRL-RS is severely compromised (35).
Sp1 is traditionally considered to be a constitutive activator of housekeeping genes and other TATA-less genes. Indeed, countless Sp1 target genes encode proteins for intermediary metabolism (65). In recent years it has become clear that Sp1 is also intimately involved in multiple cell responses (reviewed in Ref. 43). Sp1 is shown to stimulate survival in cells under oxidative stress (62,66). Our observation that cells expressing PRL-RS die after PRL treatment, concomitant with the loss of Sp1, suggests that this transcription factor is critical for cell survival in the ovary and decidua.
Taken together, our results have established a role for PRL-RS in PRL activation/deactivation of specific transcription factors. Sp1 is one of the few transcription factors whose DNA binding activity, nuclear localization, and expression is repressed by PRL in vivo and in vitro. We also found that PRL is unable to stimulate the phosphorylation of Jak2 and Stat transcription factors in both ovaries and decidua of mice expressing PRL-RS, even though Jak2 is clearly activated in cultured cells expressing only this receptor. Further studies should reveal the specific signaling pathways involved in the PRL/PRL-RS regulation of Sp1.
Supplementary Material
Acknowledgments
We are grateful to O. Silvennoinen and R. Tjian for the Jak2 and Sp1 constructs, respectively, and Laura T. Goldsmith for the RCLP cell line. We acknowledge Kristin Luther and Konstantina Heretis for their technical help, Witchuda Saengsawang for the characterization of the RCLP cell line, and Patricia Mavrogianis for tissue embedding and processing. We are most thankful to Evelyn Maizels for helpful suggestions.
Footnotes
This work was supported by National Institutes of Health Grants HD11119, U54 HD 40093, and HD 12356 (to G.G.), and T32 HL007692 (to J.L. and A.M.S.), and Institut National de la Santé et de la Recherche Médicale (to N.B.).
Present address for C.S.: Department of Obstetrics, Gynecology and Reproductive Sciences, Yale University School of Medicine, New Haven, Connecticut 06520.
M.L. is on sabbatical from Technion-Israel Institute of Technology, Haifa, Israel.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 2, 2009
Abbreviations: CamK, Calmodulin-dependent protein kinase; CK2, casein kinase II; CL, corpus luteum; FBS, fetal bovine serum; Jak, Janus kinase; oPRL, ovine PRL; PRL, prolactin; PRL-R, PRL receptor; PRL-RL, long form of PRL-R; PRL-RS, short form of PRL-R.
References
- Risk M, Gibori G 2001 Mechanisms of luteal cell regulation by prolactin. In: Horseman N, ed. Prolactin. Dordrecht: Kluwer Academic Publisher; 265–295 [Google Scholar]
- Bowen-Shauver J, Gibori G 2004 The corpus luteum of pregnancy. In: Adashi EY, Leung CK, eds. The ovary. New York: Raven Press; 201–232 [Google Scholar]
- Stocco C, Telleria C, Gibori G 2007 The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 [DOI] [PubMed] [Google Scholar]
- Bachelot A, Binart N 2007 Reproductive role of prolactin. Reproduction 133:361–369 [DOI] [PubMed] [Google Scholar]
- Handwerger S, Richards R, Markoff E 1991 Autocrine/paracrine regulation of prolactin release from human decidual cells. Ann NY Acad Sci 622:111–119 [DOI] [PubMed] [Google Scholar]
- Frasor J, Gaspar CA, Donnelly KM, Gibori G, Fazleabas AT 1999 Expression of prolactin and its receptor in the baboon uterus during the menstrual cycle and pregnancy. J Clin Endocrinol Metab 84:3344–3350 [DOI] [PubMed] [Google Scholar]
- Prigent-Tessier A, Tessier C, Hirosawa-Takamori M, Boyer C, Ferguson-Gottschall S, Gibori G 1999 Rat decidual prolactin. Identification, molecular cloning, and characterization. J Biol Chem 274:37982–37989 [DOI] [PubMed] [Google Scholar]
- Bao L, Tessier C, Prigent-Tessier A, Li F, Buzzio OL, Callegari EA, Horseman ND, Gibori G 2007 Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology 148:2326–2334 [DOI] [PubMed] [Google Scholar]
- Gu Y, Srivastava RK, Clarke DL, Linzer DI, Gibori G 1996 The decidual prolactin receptor and its regulation by decidua-derived factors. Endocrinology 137:4878–4885 [DOI] [PubMed] [Google Scholar]
- Gu Y, Jayatilak PG, Parmer TG, Gauldie J, Fey GH, Gibori G 1992 α2-Macroglobulin expression in the mesometrial decidua and its regulation by decidual luteotropin and prolactin. Endocrinology 131:1321–1328 [DOI] [PubMed] [Google Scholar]
- Barkai U, Prigent-Tessier A, Tessier C, Gibori GB, Gibori G 2000 Involvement of SOCS-1, the suppressor of cytokine signaling, in the prevention of prolactin-responsive gene expression in decidual cells. Mol Endocrinol 14:554–563 [DOI] [PubMed] [Google Scholar]
- Tessier C, Deb S, Prigent-Tessier A, Ferguson-Gottschall S, Gibori GB, Shiu RP, Gibori G 2000 Estrogen receptors α and β in rat decidua cells: cell-specific expression and differential regulation by steroid hormones and prolactin. Endocrinology 141:3842–3851 [DOI] [PubMed] [Google Scholar]
- Tessier C, Prigent-Tessier A, Ferguson-Gottschall S, Gu Y, Gibori G 2001 PRL antiapoptotic effect in the rat decidua involves the PI3K/protein kinase B-mediated inhibition of caspase-3 activity. Endocrinology 142:4086–4094 [DOI] [PubMed] [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K 1997 Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16:6926–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA 1998 Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocrine Rev 19:225–268 [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA 1997 Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 11:167–178 [DOI] [PubMed] [Google Scholar]
- Deb S, Tessier C, Prigent-Tessier A, Barkai U, Ferguson-Gottschall S, Srivastava RK, Faliszek J, Gibori G 1999 The expression of interleukin-6 (IL-6), IL-6 receptor, and gp130-kilodalton glycoprotein in the rat decidua and a decidual cell line: regulation by 17β-estradiol and prolactin. Endocrinology 140:4442–4450 [DOI] [PubMed] [Google Scholar]
- Tessier C, Prigent-Tessier A, Bao L, Telleria CM, Ferguson-Gottschall S, Gibori GB, Gu Y, Bowen-Shauver JM, Horseman ND, Gibori G 2003 Decidual activin: its role in the apoptotic process and its regulation by prolactin. Biol Reprod 68:1687–1694 [DOI] [PubMed] [Google Scholar]
- Bazan JF 1990 Haemopoietic receptors and helical cytokines. Immunol Today 11:350–354 [DOI] [PubMed] [Google Scholar]
- Kelly PA, Djiane J, Postel-Vinay MC, Edery M 1991 The prolactin/growth hormone receptor family. Endocr Rev 12:235–251 [DOI] [PubMed] [Google Scholar]
- Davis JA, Linzer DI 1989 Expression of multiple forms of the prolactin receptor in mouse liver. Mol Endocrinol 3:674–680 [DOI] [PubMed] [Google Scholar]
- Telleria CM, Parmer TG, Zhong L, Clarke DL, Albarracin CT, Duan WR, Linzer DI, Gibori G 1997 The different forms of the prolactin receptor in the rat corpus luteum: developmental expression and hormonal regulation in pregnancy. Endocrinology 138:4812–4820 [DOI] [PubMed] [Google Scholar]
- Frasor J, Gibori G 2003 Prolactin regulation of estrogen receptor expression. Trends Endocrinol Metab 14:118–123 [DOI] [PubMed] [Google Scholar]
- Lebrun JJ, Ali S, Ullrich A, Kelly PA 1995 Proline-rich sequence-mediated Jak2 association to the prolactin receptor is required but not sufficient for signal transduction. J Biol Chem 270:10664–10670 [DOI] [PubMed] [Google Scholar]
- Goupille O, Daniel N, Bignon C, Jolivet G, Djiane J 1997 Prolactin signal transduction to milk protein genes: carboxy-terminal part of the prolactin receptor and its tyrosine phosphorylation are not obligatory for JAK2 and STAT5 activation. Mol Cell Endocrinol 127:155–169 [DOI] [PubMed] [Google Scholar]
- Bignon C, Daniel N, Belair L, Djiane J 1999 In vitro expression of long and short ovine prolactin receptors: activation of Jak2/STAT5 pathway is not sufficient to account for prolactin signal transduction to the ovine β-lactoglobulin gene promoter. J Mol Endocrinol 23:125–136 [DOI] [PubMed] [Google Scholar]
- Qazi AM, Tsai-Morris CH, Dufau ML 2006 Ligand-independent homo- and heterodimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol Endocrinol 20:1912–1923 [DOI] [PubMed] [Google Scholar]
- Chang WP, Clevenger CV 1996 Modulation of growth factor receptor function by isoform heterodimerization. Proc Natl Acad Sci USA 93:5947–5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Ye Y, Clevenger CV 1998 Stoichiometric structure-function analysis of the prolactin receptor signaling domain by receptor chimeras. Mol Cell Biol 18:896–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva L, Rui H, Erwin RA, Howard OM, Kirken RA, Malabarba MG, Hackett RH, Larner AC, Farrar WL 1996 Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol Cell Endocrinol 117:131–140 [DOI] [PubMed] [Google Scholar]
- Lesueur L, Edery M, Ali S, Paly J, Kelly PA, Djiane J 1991 Comparison of long and short forms of the prolactin receptor on prolactin-induced milk protein gene transcription. Proc Natl Acad Sci USA 88:824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga JJ, Garcia-Ruiz JP, Perrot-Applanat M, Kelly PA, Edery M 1997 The short form of the prolactin (PRL) receptor silences PRL induction of the β-casein gene promoter. Mol Endocrinol 11:1449–1457 [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M, Gualillo O, Pezet A, Vincent V, Edery M, Kelly PA 1997 Dominant negative and cooperative effects of mutant forms of prolactin receptor. Mol Endocrinol 11:1020–1032 [DOI] [PubMed] [Google Scholar]
- Saunier E, Dif F, Kelly PA, Edery M 2003 Targeted expression of the dominant-negative prolactin receptor in the mammary gland of transgenic mice results in impaired lactation. Endocrinology 144:2669–2675 [DOI] [PubMed] [Google Scholar]
- Halperin J, Devi SY, Elizur S, Stocco C, Shehu A, Rebourcet D, Unterman TG, Leslie ND, Le J, Binart N, Gibori G 2008 Prolactin signaling through the short form of its receptor represses forkhead transcription factor FOXO3 and its target gene galt causing a severe ovarian defect. Mol Endocrinol 22:513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binart N, Imbert-Bollore P, Baran N, Viglietta C, Kelly PA 2003 A short form of the prolactin (PRL) receptor is able to rescue mammopoiesis in heterozygous PRL receptor mice. Mol Endocrinol 17:1066–1074 [DOI] [PubMed] [Google Scholar]
- Risk M, Shehu A, Mao J, Stocco CO, Goldsmith LT, Bowen-Shauver JM, Gibori G 2005 Cloning and characterization of a 5′ regulatory region of the prolactin receptor-associated protein/17β hydroxysteroid dehydrogenase 7 gene. Endocrinology 146:2807–2816 [DOI] [PubMed] [Google Scholar]
- Sugino N, Zilberstein M, Srivastava RK, Telleria CM, Nelson SE, Risk M, Chou JY, Gibori G 1998 Establishment and characterization of a simian virus 40-transformed temperature-sensitive rat luteal cell line. Endocrinology 139:1936–1942 [DOI] [PubMed] [Google Scholar]
- Telleria CM, Zhong L, Deb S, Srivastava RK, Park KS, Sugino N, Park-Sarge OK, Gibori G 1998 Differential expression of the estrogen receptors α and β in the rat corpus luteum of pregnancy: regulation by prolactin and placental lactogens. Endocrinology 139:2432–2442 [DOI] [PubMed] [Google Scholar]
- Bao L, Devi S, Bowen-Shauver J, Ferguson-Gottschall S, Robb L, Gibori G 2006 The role of interleukin-11 in pregnancy involves up-regulation of α2-macroglobulin gene through janus kinase 2-signal transducer and activator of transcription 3 pathway in the decidua. Mol Endocrinol 20:3240–3250 [DOI] [PubMed] [Google Scholar]
- Russell DL, Richards JS 1999 Differentiation-dependent prolactin responsiveness and stat (signal transducers and activators of transcription) signaling in rat ovarian cells. Mol Endocrinol 13:2049–2064 [DOI] [PubMed] [Google Scholar]
- Prigent-Tessier A, Barkai U, Tessier C, Cohen H, Gibori G 2001 Characterization of a rat uterine cell line, U(III) cells: prolactin (PRL) expression and endogenous regulation of PRL-dependent genes; estrogen receptor β, α(2)-macroglobulin, and decidual PRL involving the Jak2 and Stat5 pathway. Endocrinology 142:1242–1250 [DOI] [PubMed] [Google Scholar]
- Safe S, Abdelrahim M 2005 Sp1 transcription factor family and its role in cancer. Eur J Cancer 41:2438–2448 [DOI] [PubMed] [Google Scholar]
- Armstrong SA, Barry DA, Leggett RW, Mueller CR 1997 Casein kinase II-mediated phosphorylation of the C terminus of Sp1 decreases its DNA binding activity. J Biol Chem 272:13489–13495 [DOI] [PubMed] [Google Scholar]
- Gibori G 1995 The decidual hormones and their role in pregnancy recognition. In: Glasser SM, Mulholland J, Psychoyos A, eds. Endocrinology of embryo-endometrium interactions. Plenum Press; 217–222 [Google Scholar]
- Huang K, Ueda E, Chen Y, Walker AM 2008 Paradigm-shifters: phosphorylated prolactin and short prolactin receptors. J Mamm Gland Biol Neoplasia 13:69–79 [DOI] [PubMed] [Google Scholar]
- Das R, Vonderhaar BK 1995 Transduction of prolactin’s (PRL) growth signal through both long and short forms of the PRL receptor. Mol Endocrinol 9:1750–1759 [DOI] [PubMed] [Google Scholar]
- Gouilleux F, Wakao H, Mundt M, Groner B 1994 Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J 13:4361–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui H, Kirken RA, Farrar WL 1994 Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem 269:5364–5368 [PubMed] [Google Scholar]
- Duan WR, Parmer TG, Albarracin CT, Zhong L, Gibori G 1997 PRAP, a prolactin receptor associated protein: its gene expression and regulation in the corpus luteum. Endocrinology 138:3216–3221 [DOI] [PubMed] [Google Scholar]
- Nokelainen P, Peltoketo H, Mustonen M, Vihko P 2000 Expression of mouse 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase type 7 in the ovary, uterus, and placenta: localization from implantation to late pregnancy. Endocrinology 141:772–778 [DOI] [PubMed] [Google Scholar]
- Shehu A, Mao J, Gibori GB, Halperin J, Le J, Devi YS, Merrill B, Kiyokawa H, Gibori G 2008 Prolactin receptor-associated protein/17β-hydroxysteroid dehydrogenase type 7 gene (Hsd17b7) plays a crucial role in embryonic development and fetal survival. Mol Endocrinol (Baltimore, Md) 22:2268–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehu A, Devi YS, Stocco S, Mao J, Halperin J, Le J, Binart N, Gibori G Repression of Sp1 mediates inhibition of Foxo3 transcriptional activity by PRL signaling through PRL-RS. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA 2008, p 632, (Abstract P127) [Google Scholar]
- Rohlff C, Ahmad S, Borellini F, Lei J, Glazer RI 1997 Modulation of transcription factor Sp1 by cAMP-dependent protein kinase. J Biol Chem 272:21137–21141 [DOI] [PubMed] [Google Scholar]
- Alroy I, Soussan L, Seger R, Yarden Y 1999 Neu differentiation factor stimulates phosphorylation and activation of the Sp1 transcription factor. Mol Cell Biol 19:1961–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojas de Borja P, Collins NK, Du P, Azizkhan-Clifford J, Mudryj M 2001 Cyclin A-CDK phosphorylates Sp1 and enhances Sp1-mediated transcription. EMBO J 20:5737–5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger K, Kaufmann R, Gille J 2003 Increased Sp1 phosphorylation as a mechanism of hepatocyte growth factor (HGF/SF)-induced vascular endothelial growth factor (VEGF/VPF) transcription. J Cell Sci 116:225–238 [DOI] [PubMed] [Google Scholar]
- Jackson SP, MacDonald JJ, Lees-Miller S, Tjian R 1990 GC box binding induces phosphorylation of Sp1 by a DNA-dependent protein kinase. Cell 63:155–165 [DOI] [PubMed] [Google Scholar]
- Buchou T, Cochet C 2003 [Protein kinase CK2: an enzyme that likes to be different]. Med Sci (Paris) 19:709–716 [DOI] [PubMed] [Google Scholar]
- Pinna LA 2003 The raison d’etre of constitutively active protein kinases: the lesson of CK2. Acc Chem Res 36:378–384 [DOI] [PubMed] [Google Scholar]
- Samson SL, Wong NC 2002 Role of Sp1 in insulin regulation of gene expression. J Mol Endocrinol 29:265–279 [DOI] [PubMed] [Google Scholar]
- Ryu H, Lee J, Zaman K, Kubilis J, Ferrante RJ, Ross BD, Neve R, Ratan RR 2003 Sp1 and Sp3 are oxidative stress-inducible, antideath transcription factors in cortical neurons. J Neurosci 23:3597–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar G, Harmon A, Candelaria R, Martinez-Hernandez A, Raghow R, Solomon SS 2003 O-glycosylation of Sp1 and transcriptional regulation of the calmodulin gene by insulin and glucagon. Am J Physiol Endocrinol Metab 285:E584–E591 [DOI] [PubMed] [Google Scholar]
- Banchio C, Schang LM, Vance DE 2004 Phosphorylation of Sp1 by cyclin-dependent kinase 2 modulates the role of Sp1 in CTP:phosphocholine cytidylyltransferase α regulation during the S phase of the cell cycle. J Biol Chem 279:40220–40226 [DOI] [PubMed] [Google Scholar]
- Wierstra I 2008 Sp1: emerging roles—beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun 372:1–13 [DOI] [PubMed] [Google Scholar]
- Lee J, Kosaras B, Aleyasin H, Han JA, Park DS, Ratan RR, Kowall NW, Ferrante RJ, Lee SW, Ryu H 2006 Role of cyclooxygenase-2 induction by transcription factor Sp1 and Sp3 in neuronal oxidative and DNA damage response. FASEB J 20:2375–2377 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.