Abstract
Several integral membrane proteins that constitute the blood-testis barrier (BTB) in mammalian testes, in particular rodents, are known to date. These include tight junction (TJ) proteins (e.g. occludin, junctional adhesion molecule-A, claudins), basal ectoplasmic specialization proteins (e.g. N-cadherin), and gap junction proteins (e.g. connexin43). However, the regulators (e.g. protein kinases and phosphatases) that affect these proteins, such as their interaction with the cytoskeletal actin, which in turn confer cell adhesion at the TJ, remain largely unknown. We report herein that focal adhesion kinase (FAK) is a putative interacting partner of occludin, but not claudin-11 or junctional adhesion molecule-A. Immunohistochemistry and fluorescence microscopy studies illustrated that the expression of FAK in the seminiferous epithelium of adult rat testes was stage specific. FAK colocalized with occludin at the BTB in virtually all stages of the seminiferous epithelial cycle but considerably diminished in stages VIII–IX, at the time of BTB restructuring to facilitate the transit of primary leptotene spermatocytes. Using Sertoli cells cultured in vitro with established TJ-permeability barrier and ultrastructures of TJ, basal ectoplasmic specialization and desmosome-like junction that mimicked the BTB in vivo, FAK was shown to colocalize with occludin and zonula occludens-1 (ZO-1) at the Sertoli-Sertoli cell interface. When these Sertoli cell cultures were treated with CdCl2 to perturb the TJ-barrier function, occludin underwent endocytic-mediated internalization in parallel with FAK and ZO-1. Thus, these findings demonstrate that FAK is an integrated regulatory component of the occludin-ZO-1 protein complex, suggesting that functional studies can be performed to study the role of FAK in BTB dynamics.
Focal adhesion kinase (FAK) is an integrated regulatory component of the occludin-ZO-1 protein complex, suggesting functional studies can be performed to study the role of FAK in blood-testis barrier dynamics.
In adult rat testes, during stages VIII–IX of the seminiferous epithelial cycle of spermatogenesis, preleptotene and leptotene spermatocytes are in transit at the blood-testis barrier (BTB) while differentiating into zygotene spermatocytes (1,2). Thus, it is envisioned that the BTB undergoes extensive restructuring at stages VIII (∼29.1 h) and IX (∼7.7 h) of the epithelial cycle (3) to facilitate the passage of these primary spermatocytes at the BTB. Although the structural protein complexes that constitute the BTB are known (4), such as occludin/zonula occludens (ZO-1), junctional adhesion molecule-A (JAM-A)/ZO-1, claudins/ZO-1, and N-cadherin/β-catenin, the regulators that work in concert with these structural components to determine their association and/or disassociation with the cytoskeleton are not known.
Focal adhesion kinase (FAK), a nonreceptor protein tyrosine kinase, was detected at the BTB in the seminiferous epithelium of adult rat testes by immunohistochemistry (5), whereas its activated form, p-FAK-Tyr397, was localized mostly at the apical ectoplasmic specialization (ES) at the Sertoli cell-elongating spermatid interface (from step 8 spermatids through step 19 in rats), colocalized and structurally interacted with the apical ES constituent protein β1-integrin (5). Subsequent studies have shown that the p-FAK-Tyr397 is an integrated component of the α6β1-integrin-based protein complex at the apical ES (6). Because recent studies have shown that FAK is a crucial regulator of tight junction (TJ) and adherens junction (AJ) in multiple epithelia and endothelia, as well as cell movement across the TJ barrier, such as during viral transfection (7,8,9) (for review, see Refs. 10,11,12), we thought it pertinent to perform a study to define if FAK is indeed an integrated component of the BTB in rat testes and in cultured Sertoli cells with an established TJ barrier that mimics the BTB in vivo. We also sought to identify the putative interacting protein partner(s) of FAK at the BTB.
Furthermore, there are studies in the field showing that the CdCl2-induced disruption of the Sertoli cell TJ-permeability barrier function in vitro is a novel model to study BTB dynamics (13,14). Therefore, we have used this in vitro model to assess the likely mechanism by which FAK interacts with integral membrane proteins at the BTB during cadmium-induced damage to the TJ barrier.
Materials and Methods
Animals
Male Sprague Dawley rats were purchased from Charles River Laboratories (Kingston, NY). The use of animals was approved by the Rockefeller University Laboratory Animal Research Center Animal Care and Use Committee with protocol no. 06018.
Primary Sertoli cell cultures
Sertoli cells were isolated from 20-d-old rat testes as described (15). Cells were plated on Matrigel (BD Biosciences, Franklin Lakes, NJ)-coated 12-well dishes (Matrigel diluted 1:7 in serum-free F12/DMEM; Sigma-Aldrich Corp., St. Louis, MO) or bicameral units (Millipore Millicell HA (mixed cellulose esters) filters, with an effective surface area of ∼0.6 cm2; Millipore Corp., Billerica, MA) and cultured in serum-free F12/DMEM supplemented with sodium bicarbonate, gentamicin, epidermal growth factor, insulin, transferrin, and bacitracin as described (16). Cells were cultured at 35C with 95% air-5% CO2 (vol/vol) in a humid atmosphere. About 48 h thereafter, cells were subjected to a hypotonic treatment [20 mm Tris (pH 7.4), 2.5 min, at 22 C] to lyse residual germ cells (17). As such, Sertoli cell cultures used for our studies had a cell purity of greater than 98% with negligible contamination of germ and/or Leydig cells as described (18). It is noted that these Sertoli cells were differentiated, ceased to divide, and were indistinguishable from Sertoli cells isolated from adult rat testes based on morphological and functional analysis, such as the expression of selected proteins and/or genes (e.g. myotubularins, N-Ras, Smad2, MEKK2) (19,20). In this in vitro model, Sertoli cells were shown to form a functional TJ-permeability barrier (21), as well as to possess the ultrastructures of both TJ and basal ES when examined by electron microscopy (16), mimicking the BTB in vivo.
Assessment of Sertoli cell TJ-permeability barrier by quantifying transepithelial electrical resistance (TER) across the Sertoli cell epithelium
To assess the establishment of the functional TJ-permeability barrier in Sertoli cells cultured in vitro, freshly isolated cells were plated at 1.2 × 106 cells per cm2 on Matrigel-coated bicameral units. Each unit was placed on the well of a 24-well dish containing 0.5 ml F12/DMEM in both the apical and basal chambers. The assembly of the TJ barrier was monitored daily for 7 d by quantifying the TER across the Sertoli cell epithelium as described (21). To assess the effects of cadmium on the TJ-barrier function, CdCl2 at 1 and 3 μm was included in the F12/DMEM at the apical and basal chambers of the bicameral units on d 4, when the TJ barrier had been established. TER across the epithelium was measured before and after treatment (8 h), and cells were washed twice with F12/DMEM to remove CdCl2 thereafter. Vehicle control (0.9% NaCl) and cells without any treatment served as controls. Media in each bicameral unit were replaced daily after TER measurement. Each time point had triplicate bicameral units.
Electron microscopy
Electron microscopy was performed at the Rockefeller University Bio-Imaging Resource Center, as earlier described (22), to monitor the presence of BTB ultrastructures in the Sertoli cell cultures (at 0.5 × 106 cells per cm2 on Matrigel-coated dishes and terminated on d 6) that mimicked the BTB in vivo.
Immunohistochemistry
Immunohistochemical localization of FAK in rat testes was performed using the Histostain-Plus Kit (Zymed Laboratories, Invitrogen Corp., Carlsbad, CA) as described (16). In brief, adult rat testes were fixed in Bouin’s fixative, dehydrated, embedded in paraffin, and sectioned to 5 μm. After sections were deparaffinized and treated with 3% hydrogen peroxide in methanol (vol/vol) to block endogenous peroxidase activity, sections were permeabilized with 0.1% Triton X-100 (vol/vol). Sections were subsequently incubated with rabbit anti-FAK antibody (supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) and then with biotinylated goat antirabbit IgG, and streptavidin-peroxidase, stained with the 3′3-diaminobenzidine HCl substrate-chromogen mixture, counterstained with hematoxylin, and mounted. Light micrographs were acquired using an Olympus DP71 12.5 MPa digital camera built into an Olympus BX61 microscope (Olympus America, Inc., Melville, NY). Images were acquired using the Olympus MicroSuite FIVE (Version 1224) software package (Olympus Soft Imaging Solutions Corp., Lakewood, CO), converted to TIFF format, and analyzed by Adobe PhotoShop in Adobe Creative Suite (Version 3.0; Adobe Systems, Inc., Mountain View, CA). Negative controls in which the primary antibody was substituted by rabbit IgG were also performed.
Immunofluorescence microscopy
Immunofluorescence microscopy was performed essentially as described (23). In short, frozen sections (∼7 μm) from adult rat testes were mounted onto poly-l-lysine-coated slides, fixed with paraformaldehyde, permeabilized with Triton X-100, blocked with BSA, and incubated with primary antibodies (supplemental Table 1), to be followed by an incubation with secondary antibodies conjugated with AlexaFluor488 (green) or AlexaFluor555 (red) (Molecular Probes, Eugene, OR). Slides were mounted onto coverslips using ProLong Gold antifade reagent with 4′,6′-diamino-2-phenylindole (DAPI) (a nuclear blue stain) (Invitrogen). Negative controls were also performed using rabbit or mouse IgG instead of the primary antibody. For cell staining, Sertoli cells were plated on Matrigel-coated coverslips at 0.05 × 106 cells per cm2 and subjected to hypotonic treatment approximately 48 h thereafter, as described (17), to lyse residual germ cells. On d 4, cells were incubated with either vehicle (0.9% NaCl) or CdCl2 (3 μm) for 0, 3, 6, and 9 h at 35 C, and immunofluorescent staining was performed as described. For actin staining, cells were processed as described above, and rhodamine-conjugated phalloidin (Invitrogen) was used instead of antibodies. At least 500 cells from nine to 12 randomly selected fields of an experiment were photographed and scored, and each experiment was repeated at least three to four times using different batches of Sertoli cell cultures.
Immunoblot analysis and co-immunoprecipitation (Co-IP)
Immunoblot analysis was performed as described (23,24). The source of primary and secondary antibodies and their working dilutions are listed in supplemental Table 1. Co-IP was performed as described (24). In brief, adult rat testis lysates were prepared in immunoprecipitation buffer [50 mm Tris, 0.15 m NaCl, 1% (vol/vol) Nonidet P-40, 1 mm EGTA, 2 mm N-ethylmaleimide, 10% glycerol (vol/vol), 1 mm phenylmethylsulfonylfluoride, 15 μl/ ml phosphatase inhibitor cocktail 1 (Sigma-Aldrich), 15 μl/ml phosphatase inhibitor cocktail 2 (Sigma-Aldrich), and 15 μl/ml protease inhibitor cocktail (Sigma-Aldrich) (pH 7.4) at 22 C]. About 700 μg protein in approximately 300 μl was used per sample tube for Co-IP.
Statistical analyses
All in vitro culture experiments reported herein were repeated at least three to four times with duplicate or triplicate dishes or bicameral units using different batches of Sertoli cells. For in vivo experiments, at least four rats for each time point were used. Statistical analyses were performed by two-way ANOVA using the repeated measures model followed by Dunnett’s test to compare changes between treatment groups and their corresponding controls using the GB-STAT statistical analysis software package (version 7; Dynamic Microsystems, Silver Spring, MD). Thus, we considered changes in protein levels (or TER) in treatment groups vs. the corresponding controls (first variable) and also as a function of time (second variable) in these analyses.
Results
Stage-specific expression of FAK at the BTB and its interaction with occludin to form an occludin-FAK protein complex
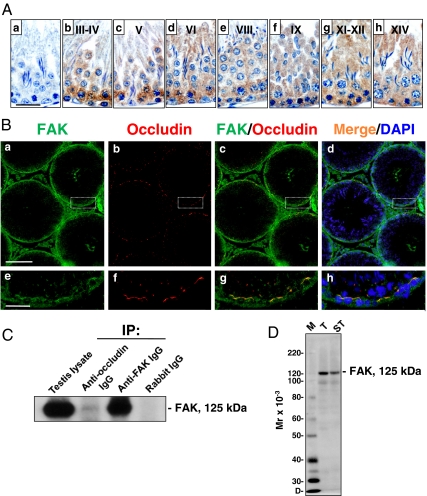
Figure 1A, a–h, shows the stage-specific expression of FAK in the seminiferous epithelium of adult rat testes. Immunoreactive FAK was predominantly found near the basal compartment, consistent with its localization at the BTB. FAK was expressed at high levels at the BTB during all stages of the seminiferous epithelial cycle, suggesting its importance in the maintenance of the BTB. However, its staining was considerably diminished at stages VIII–IX vs. other stages (Fig. 1A, e and f vs. b–d, g, and h; it is noted that Fig. 1Aa is a control in which the primary antibody was substituted with normal rabbit IgG, illustrating that staining shown in b–h was specific to FAK), when the BTB underwent restructuring at these stages to facilitate the transit of primary leptotene spermatocytes. To confirm further that FAK was indeed a component of the BTB, its colocalization with occludin, a TJ-integral membrane protein, was examined. In fact, FAK colocalized with occludin to the same site at the BTB (Fig. 1B, a–h), illustrating that FAK is likely a regulatory component of the BTB. In addition, FAK also appeared to be localized to the cellular zone of the tunica propria, such as myoid cells and/or lymphatic endothelium, and the Leydig cells in the interstitium (Fig. 1B).
Figure 1.
Stage-specific localization of FAK in the seminiferous epithelium of adult rat testes and its interaction with occludin at the BTB. Aa, cross-sections of normal testes from adult rats (∼300 g body weight) incubated with normal rabbit IgG for immunohistochemistry. A, b–h, FAK, which appears as reddish-brown precipitates, was localized to the basal compartment in the epithelium, consistent with its localization at the BTB. A, e and f, In late stage VIII and IX tubules, the localization of FAK at the BTB was considerably diminished (see e and f vs. b–d, g and h). B, In these fluorescence micrographs, FAK (green) colocalized with occludin (a TJ protein) (red) at the BTB (see merged images in c–d, g, and h). Some FAK staining was also detected in the interstitium, likely to be Leydig cells, and peritubular myoid cells and/or lymphatic endothelium in tunica propria (a–h). The boxed area in a–d was magnified and shown in e–h, respectively. C, Co-IP was performed using testis lysate (∼700 μg protein) from adult rats, and occludin was shown to interact structurally with FAK. Testis lysate (50 μg protein, without Co-IP) or Co-IP with normal rabbit IgG served as positive and negative controls, respectively. D, Immunoblot analysis using lysates (∼50 μg protein) of testes (T) and seminiferous tubules (ST) illustrating the specificity of the antibody. Scale bars in Aa is 30 μm, which applies to A, a–h, in Ba is 200 μm, which applies to B, b–d, and in Be is 40 μm, which applies to B, f–h. Nuclei were stained with DAPI (blue). Results shown in A and B are representative data from four independent experiments. Co-IP data shown in C are representative data of 10 independent experiments. IP, Immunoprecipitation; D, dye-front; M, protein markers.
Because FAK was expressed in the testis stage specifically, similar to that observed for occludin (25), with both proteins colocalized to the BTB (Fig. 1B) and also diminished considerably at the BTB in stage VIII tubules (Fig. 1A) (25), it was postulated that FAK could structurally interact with occludin at the BTB to form a regulatory protein complex. To verify this hypothesis, Co-IP of occludin and FAK was performed using testis lysates vs. controls. Results of the Co-IP experiment are shown in Fig. 1C, demonstrating that FAK structurally interacted with occludin, but not JAM-A and claudin-11 in normal rat testes (data not shown). To illustrate the specificity of the anti-FAK antibody used in these experiments, Fig. 1D is an immunoblot using lysates of testes and seminiferous tubules that yielded a prominent protein band of approximately 125 kDa, consistent with the apparent molecular weight of FAK.
Assembly of Sertoli cell TJ-permeability barrier, and the expression and cellular distribution of FAK in Sertoli cells vs. other BTB-associated proteins in vitro
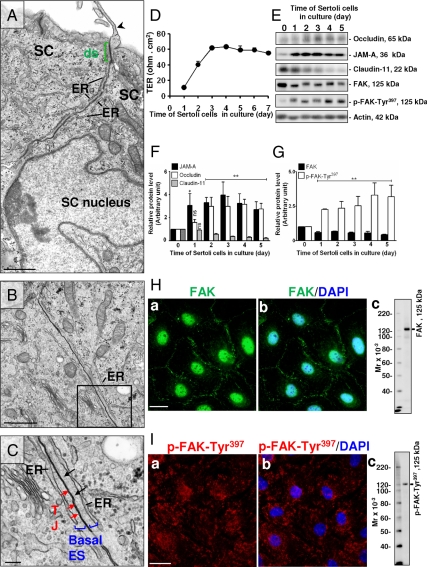
We next examined if FAK is functionally related to the assembly of the Sertoli cell TJ barrier in vitro. When Sertoli cells were cultured for 6 d in vitro (see the microvillus indicated by the black arrowhead in Fig. 2A, which is typical of Sertoli cells cultured in vitro), ultrastructures that mimicked the BTB in vivo were detected such as the basal ES and the coexisting TJ (Fig. 2, A–C) and the desmosome (ds) (green, see the electron dense substances typical of ds in Fig. 2A). For instance, two adjacent Sertoli cells cultured in vitro displayed the basal ES ultrastructure (blue), which is typified by the presence of actin filaments (black arrows in Fig. 2C, even though these actin filaments were less developed than those found in vivo, possibly because of the embedding procedures) sandwiched between the endoplasmic reticulum (ER) and the plasma membrane of the Sertoli cell (Fig. 2, A–C), with the TJ found between the two Sertoli cells (see red arrows) in the magnified area of Fig. 2B and shown in Fig. 2C. These electron microscopy data, coupled with the functional data by assessing the TER across the Sertoli cell epithelium illustrating an increase in TER from d 1–3, have conclusively demonstrated that these cultured cells were assembling the functional TJ barrier, with a stable TER reached by d 4, which persisted till the end of this experiment by d 7 (Fig. 2D).
Figure 2.
The steady-state levels and cellular localization of FAK vs. selected BTB-associated proteins during the assembly of the Sertoli cell (SC) TJ-permeability barrier. A–C, Sertoli cells cultured in vitro displayed the typical finger-like microvillus (arrowhead, A) in the apical compartment. ds-like junction (A) (green bracket), typified by the presence of electron-dense substances in the apposing Sertoli cell plasma membranes, was detected; basal ES (the boxed area in B and magnified in C) typified by the presence of actin filaments (black arrows) sandwiched between the Sertoli cell plasma membrane and the endoplasmic reticulum (ER) (C). Coexisting basal ES (blue bracket) and TJ (red arrows) that constitute the BTB are shown in C. D, The establishment of the TJ barrier in these cells (1.2 × 106 cells per cm2) was confirmed by quantifying TER across the cell epithelium. E, Immunoblot analysis to assess changes in the steady-state levels of proteins at the BTB. F and G, Histograms of composite data, such as those shown in E, and normalized against actin. The relative protein level of a target protein on d 0 was arbitrarily set at one, against which statistical analysis was performed. Each bar is the mean ± sd of three experiments. *, P < 0.05; **, P < 0.01. H and I, Sertoli cells plated at 0.05 × 106 cells per cm2 onto Matrigel-coated coverslips and cultured for 4 d were stained for FAK (H, a and b) and p-FAK-Tyr397 (I, a and b). Fluorescence microscopy shows the localization of FAK (H, a and b) mostly at the cell-cell interface and cell nuclei with little staining in the cytosol. In contrast, p-FAK-Tyr397 (I, a and b) was mostly restricted to the cytosol with considerably less staining at the cell-cell interface and nuclei. Specificity of the antibodies was confirmed by immunoblotting using approximately 30 μg protein of Sertoli cell lysates (Hc and Ic). Scale bars in A and B are 1 μm, in C are 200 nm, and in Ha and Ia are 20 μm, which applies to Hb and Ib. Nuclei were stained with DAPI (blue).
During the assembly of this TJ-permeability barrier, an induction in the steady-state levels of the TJ integral membrane proteins occludin and JAM-A was observed (Fig. 2, E and F), showing the increase in the TJ-building blocks for forming the BTB in vitro. However, claudin-11, also a TJ-integral membrane protein, was present at high levels at the beginning of the TJ-barrier assembly but significantly diminished when the TJ barrier was formed (Fig. 2, E and F), illustrating that this protein might not be needed for BTB maintenance, consistent with an earlier report that claudin-11 was greatly diminished at the BTB in adult rats vs. immature rats (26).
Interestingly, the steady-state level of FAK declined considerably after cell plating, but it still remained at a relative stable level throughout the culture, whereas the p-FAK-Tyr397 level increased significantly (Fig. 2, E and G), suggesting that the relative ratio of FAK to p-FAK-Tyr397 may be important for the assembly and maintenance of the BTB in vitro.
To validate further that the FAK is indeed a component of BTB, fluorescence microscopy was used to confirm the localization of both FAK and pFAK-Tyr397 in cultured Sertoli cells. Most of the FAK staining was localized at the Sertoli-Sertoli cell interface and also nuclei, with relatively little FAK staining detected in cell cytosol (Fig. 2H). In contrast, considerably less p-FAK-Tyr397 was detected at the cell-cell interface, but the majority of it was found in cell cytosol, and some staining was noted in the nucleus (Fig. 2). The specificity of these two antibodies used for immunofluorescence microscopy was confirmed by immunoblotting using Sertoli cell lysates, and is shown in Fig. 2, Hc and Ic.
Effects of CdCl2 on Sertoli cell cytoskeletal F-actin, the TJ-barrier function, and steady-state levels of BTB-associated proteins in vitro
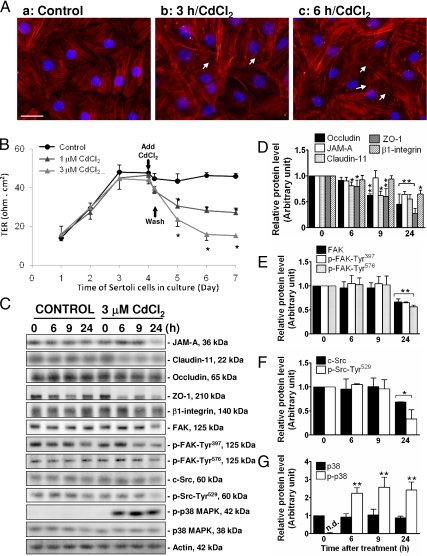
Subsequently, we used this Sertoli cell culture system as a model to study the role of the occludin/FAK complex on the BTB function in vitro. Because occludin is an actin-based TJ protein at the BTB, we first assessed if cadmium would induce alteration of the actin cytoskeleton, which is used by occludin for attachment. The distribution of F-actin in Sertoli cells was monitored using rhodamine-conjugated phalloidin after treatment of cells with 3 μm CdCl2. Figure 3Aa illustrates the typical F-actin filaments and their organization and arrangement in Sertoli cells cultured in vitro for 4 d. After 3 h of CdCl2 exposure, disorganization of F-actin filaments was noted (Fig. 3Ab), which was worsened by 6 h (Fig. 3Ac). Besides the noticeable disorganization of the actin filaments in the two treatment groups vs. controls (Fig. 3A, b and c vs. a), the actin filaments appeared to be defragmented (white arrows in Fig. 3A, b and c). Thus, these effects of CdCl2 on the cytoskeleton F-actin filaments in Sertoli cells demonstrate that at these time points, CdCl2 would have affected the Sertoli cell TJ barrier. The functional TJ barrier was shown to be assembled in these cultures by d 4, which was confirmed by a stable TER across the cell epithelium (Fig. 3B). At this time, cells were treated with CdCl2, 1 and 3 μm, for 8 h; cells were then washed to remove CdCl2, and TER across the epithelium was monitored every 24 h for the next 3 d. CdCl2 was shown to disrupt the Sertoli cell TJ barrier dose dependently, as demonstrated by a significant and dose-dependent decline in the TER when compared with control (Fig. 3B). This disruptive effect of CdCl2 to the Sertoli cell TJ barrier was irreversible because the damaged BTB could not be reestablished, even after the toxicant was removed from the media. It is noted that these concentrations were noncytotoxic to Sertoli cells as earlier reported (13,14).
Figure 3.
A study to assess the effects of CdCl2 on Sertoli cell cytoskeletal F-actin, the TJ-barrier function, and steady-state levels of BTB-associated proteins in vitro. A, Sertoli cells plated at 0.05 × 106 cells per cm2 onto Matrigel-coated coverslips and cultured for 4 d were incubated with 3 μm CdCl2 for 0, 3, and 6 h. Thereafter, cells were stained for F-actin using rhodamine-conjugated phalloidin (red) and DAPI (nuclei staining, blue). Aa, Normal Sertoli cells displayed typical organization and arrangement of F-actin filaments. After 3 h CdCl2 exposure, disorganization and defragmentation of F-actin filaments were observed (Ab); after 6 h, these changes were clearly noted (Ac) (see white arrows in b and c vs. a). Scale bar in Aa is 30 μm, which applies to A, b and c. Sertoli cells plated at 0.5 × 106 cells per cm2 onto Matrigel-coated bicameral units (B) or 12-well dishes (C–G) were allowed to assemble a functional TJ barrier, and on d 4, CdCl2 at specified concentrations was included in the F12/DMEM in these cultures. B, TER across the Sertoli cell epithelium in cultures exposed to CdCl2 (8 h) was shown to decline dose dependently, illustrating a CdCl2-induced disruption in the TJ barrier. C–G, In these cultures, Sertoli cells on d 4 were treated with vehicle (0.9% NaCl, control) or CdCl2 (3 μm), and terminated at 0, 6, 9, and 24 h thereafter to assess changes in the steady-state levels of different proteins at the BTB. Because the steady-state levels of the target proteins at 0, 6, 9, and 24 h in all the control groups were not significantly different, we only compared protein levels by 6, 9, and 24 h after CdCl2 treatment vs. time zero. Histograms shown in D–G were results of the data such as those shown in C, normalized against actin. Relative level of a target protein at time zero in the treatment group was arbitrarily set at one, against which statistical analysis was performed. Because p-p38 MAPK was not detectable (n.d.) in control samples and at time zero of the treatment group, p-p38 MAPK at 6, 9, and 24 h was compared with total p38 MAPK at respective time points (note: no differences on total p38 MAPK levels were detected), which was arbitrarily set at one. Each bar is the mean ± sd of at least three different experiments. *, P < 0.05; **, P < 0.001.
We next evaluated the effects of CdCl2 exposure to Sertoli cells on the steady-state levels of proteins associated with TJs (e.g. JAM-A, claudin-11, occludin, and ZO-1), hemidesmosome (β1-integrin), as well as some signaling molecules that have already been reported to regulate junction restructuring in other epithelia and/or testis (e.g. FAK, c-Src, and p38 MAPK, and their respective phosphorylated forms, namely p-FAK-Tyr397, p-FAK-Tyr576, p-Src-Tyr529, and p-p38 MAPK). Cells were incubated with vehicle (0.9% NaCl) or 3 μm CdCl2, and terminated after 0, 6, 9, and 24 h. It was noted that occludin and ZO-1, a TJ integral membrane protein and an adaptor, respectively, and also claudin-11 were apparently more sensitive to Cd than other proteins analyzed because their levels were reduced after approximately 6–9 h CdCl2 exposure, whereas most of the proteins studied were reduced only after 24 h (Fig. 3, C–G). Among the signaling molecules investigated, p-p38 MAPK was significantly induced after 6 h treatment with CdCl2 and remained elevated thereafter, but the steady-state level of the nonphosphorylated form, p38, was not responsive to CdCl2 treatment (Fig. 3, C and G).
Effects of CdCl2 on the occludin/ZO-1/FAK protein complex and its cellular localization in Sertoli cells in vitro
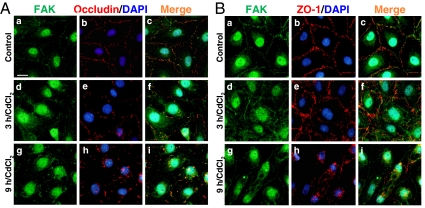
Because it was shown that FAK structurally interacted with occludin, but occludin is also known to form a protein complex with ZO-1 at a stoichiometric ratio of 1:1 (27,28), we sought to investigate the distribution of FAK with occludin (Fig. 4A) and ZO-1 (Fig. 4B) after CdCl2 treatment. FAK and occludin were colocalized to the same site in control Sertoli cells (Fig. 4A, a–c) and CdCl2-treated cells (Fig. 4A, d–i), as observed in the merged images (Fig. 4A, c, f, and i). However, after CdCl2 exposure both proteins were redistributed from the cell-cell interface into the cytosol, as early as 3 h exposure (Fig. 4A, d–f) and more evident by 9 h (Fig. 4A, g–i). Interestingly, FAK also colocalized with ZO-1 to the cell-cell interface in control cells (Fig. 4B, a–c), illustrating that FAK is indeed a component of the occludin/ZO-1 protein complex. In addition, a similar pattern of protein redistribution after CdCl2 treatment observed for occludin and FAK was noticed for ZO-1 and FAK (Fig. 4, B vs. A). These results suggest that FAK may be involved in CdCl2-induced occludin and ZO-1 redistribution because integral membrane protein phosphorylation at both TJ and AJ is known to affect its cellular distribution (for review, see Refs. 29 and 30), and it is likely that occludin and/or ZO-1 is a substrate of FAK.
Figure 4.
Colocalization of FAK with occludin or ZO-1 at the Sertoli-Sertoli cell interface and their redistribution after CdCl2 treatment. Sertoli cells plated at 0.05 × 106 cells per cm2 onto Matrigel-coated coverslips and cultured for 4 d, forming an intact epithelium, were incubated with 3 μm CdCl2 for 0, 3, and 9 h. FAK (green) was shown to colocalize with occludin (red; A, a–i) and ZO-1 (red; B, a–i) both in control, at the Sertoli-Sertoli cell interface (A, a–c, and B, a–c), and CdCl2-treated cells (A, d–i, and B, d–i) as observed in the merged images (A, c, f, and i, and B, c, f, and i). These findings not only illustrate that FAK is a component of the Sertoli cell BTB in vitro, consistent with the in vivo findings (see Fig. 1), but also that FAK is a component of the occludin-ZO-1 protein complex at the BTB. After CdCl2 exposure, proteins were mislocalized, redistributed from the cell-cell interface into the cytoplasm, as early as 3 h (A, d–f, and B, d–f), which became more evident after 9 h (A, g–i, and B, g–i). DAPI (blue) was used for nucleus staining. Scale bar in Aa is 20 μm, which applies to A, b–i, and B, a–i.
CdCl2-induced redistribution of occludin in Sertoli cells is apparently mediated via an endocytic vesicle-mediated process
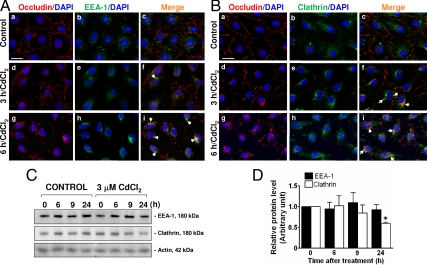
Because previous studies have shown that endocytosis and/or recycling of testicular cell junction proteins is an important mechanism to induce BTB restructuring to facilitate the transit of primary leptotene spermatocytes at the BTB (23,31), and CdCl2 was shown to induce redistribution of occludin from the Sertoli-Sertoli cell interface to the cytoplasm, we envisioned that the toxicant-induced disruption of the BTB may be mediated via such pathways. Dual-labeled immunofluorescence microscopy was performed to assess changes in the colocalization of occludin and an early endosome marker, early endosome antigen-1 (EEA-1; Fig. 5A), and clathrin (Fig. 5B), an endocytic protein known to be involved in protein endocytosis at the BTB (23,31), after CdCl2 treatment vs. controls. In control cells, relatively little colocalization of occludin and EEA-1 (Fig. 5A, a–c), as well as between occludin and clathrin (Fig. 5B, a–c), was detected. Nonetheless, 3 h after incubation with CdCl2, occludin was shown to be colocalized with EEA-1 in the cytoplasm (Fig. 5A, d–f) as well as with clathrin (Fig. 5B, d–f), and after 9 h such association became even more evident (Fig. 5, Ag–i and Bg–i), suggesting that the mislocalization of occludin upon CdCl2 treatment was due to an internalization of the TJ-integral membrane protein at the BTB. Moreover, colocalization of ZO-1 and EEA-1 after CdCl2 treatment was also detected in a similar pattern (supplemental Fig. 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). The specificity of the antibodies against EEA-1 and clathrin used in this laboratory has been previously reported (23). Although the immunofluorescence microscopy illustrated an increase in colocalization between occludin and EEA-1, as well as between occludin and clathrin, no changes in the steady-state levels of EEA-1 and clathrin were observed by immunoblotting 6 h after treatment; only a mild decrease for clathrin was detected 24 h after treatment (Fig. 5, C and D).
Figure 5.
CdCl2-induced changes in the distribution of occludin in Sertoli cells and the association of occludin with early endosomes marker (EEA-1) and endocytic vesicles (clathrin). Sertoli cells plated at 0.05 × 106 cells per cm2 on Matrigel-coated coverslips and cultured for 4 d were incubated with 3 μm CdCl2 for 0, 3, and 6 h. In controls, most occludin was detected at the cell-cell interface, and virtually no colocalization of occludin (red) and EEA-1 (green) (A, a–c), as well as occludin and clathrin (green, B, a–c) was detected. However, 3 h after CdCl2 treatment, occludin redistributed from the cell-cell interface into the cell cytosol, colocalized with EEA-1 (A, d–f) and clathrin (B, d–f) (white arrowheads), and after 9 h these changes were more evident (white arrowheads, A, g–i, and B, g–i), suggesting that the mislocalization of occludin after CdCl2 treatment might be the result of an increase in protein endocytosis. No changes in the steady-state levels of EEA-1 or clathrin were detected by immunoblotting (∼30 μg protein per lane) between treatment and control groups, except for a mild decline in clathrin after 24 h CdCl2 treatment (C and D). Histogram shown in D corresponding to the data such as those shown in C, normalized against actin. Because the levels of target proteins at 0, 6, 9, and 24 h in controls and at time zero of the treatment group were not significantly different, we only compared protein levels by 6, 9, and 24 h after CdCl2 treatment vs. time zero, which was arbitrarily set at one, against which statistical analysis was performed. Each bar is the mean ± sd of three experiments. *, P < 0.05. Scale bars in Aa and Ba are 20 μm, which applies to A, b–i, and B, b–i. DAPI (blue) stained for cell nuclei.
Discussion
The role of occludin/ZO-1 at the BTB in the testis
The BTB in the seminiferous epithelium of adult mammalian testes is pivotal to spermatogenesis. Besides its role to confer cell polarity, it creates a unique immunological barrier to segregate post-meiotic germ cell development from the systemic circulation (4,32,33). However, the BTB, unlike other blood-tissue barriers such as the blood-brain barrier, is a highly dynamic structure as it undergoes extensive restructuring at stages VIII–IX of the epithelial cycle to facilitate the transit of primary leptotene spermatocytes (1). Thus, it is envisioned that the BTB must be tightly regulated to coordinate this cellular event during spermatogenesis. In order to perform functional studies to understand the biology and regulation of BTB dynamics, its composition must be fully elucidated. To date, the integral membrane proteins that are known to constitute the BTB between adjacent Sertoli cells in rodent testes include: TJ proteins (e.g. occludin, JAM-A, and claudins); basal ES proteins (e.g. N-cadherin, nectin-2); and gap junction proteins (e.g. connexin43) (4,29). However, the peripheral regulators (e.g. protein kinases and phosphatases) that are structurally associated with these integral membrane proteins are virtually unknown. Based on studies in other epithelia that regulate TJ-barrier function, it is likely that protein kinases that alter the phosphorylation status of integral membrane proteins at the BTB can affect the cell adhesion status (29,30) at stages VIII–IX of the epithelial cycle, such as by dissociating from the underlying cytoskeletal network, thereby “destabilizing” and/or “opening” the TJ barrier to facilitate the passage of primary spermatocytes at the BTB. For instance, occludin that is found at the TJ fibrils in most epithelia is mostly phosphorylated at the Ser/Thr and Tyr residues (34,35), whereas the less phosphorylated occludin is not assembled into TJ fibrils but restricted to the basolateral membrane (34,36).
Occludin/ZO-1/FAK is a novel regulatory protein complex at the BTB
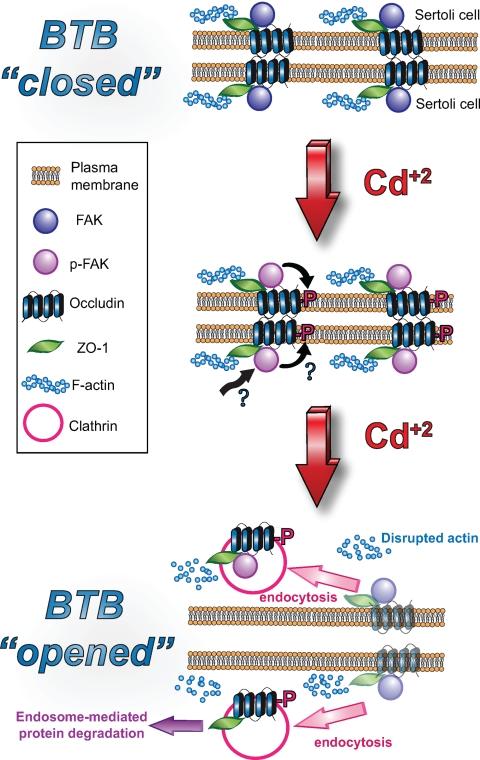
FAK, a nonreceptor protein tyrosine kinase, is a known modulator in the integrin-mediated signal transduction pathway (8,9,10,11,37,38,39), which regulates an array of cellular events, including cell movement, viral transfection, apoptosis, cell differentiation, cell adhesion, junction dynamics, and others. Most of the studies in the literature have shown that FAK is a modulator of the integrin-based signaling pathway that works in concert with Src and ILK to regulate cellular function at focal contacts (40). However, earlier studies in adult rat testes have shown that two activated forms of FAK, namely p-FAK-Tyr397 and p-FAK-Tyr576, were predominantly localized to the apical ES and are components of the apical ES (5), which is a testis-specific atypical AJ type restricted to the Sertoli cell-spermatid (step 8 and beyond) (41). At the apical ES, p-FAK-Tyr397 forms a bona fide complex with the α6β1-integrin-laminin-α3β3γ3 (42,43,44,45) adhesion complex (5), regulating adhesion between developing spermatids and Sertoli cells. Indeed, subsequent studies have shown that this p-FAK/β1-integrin complex persists at the apical ES until spermiation (6). Interestingly, FAK was also detected at the BTB in an earlier study (5). However, its stage specificity, its binding partner(s), nor its function at the BTB is known. Herein, FAK was shown to be a component of the BTB, consistent with this earlier report (5). Most importantly, this protein was shown to display a stage-specific localization at the BTB with its expression considerably lowered at stages VIII–IX of the epithelial cycle, at the time primary leptotene spermatocytes are in transit at the BTB (1), which also coincides with the stage-specific expression pattern of occludin at the BTB as reported earlier (25). In our initial survey to examine the binding partner of FAK at the BTB vs. p-FAK-Tyr397 and p-FAK-Tyr576 at the apical ES, which were shown to interact with β1-integrin, we thought FAK would form a complex with integrins at the BTB as well because the BTB is composed of coexisting TJ, basal ES, gap junction, and ds-like junction in rodent testes. However, virtually all the β1-integrin staining was detected at the apical ES (44,46), consistent with an earlier report (43), and for the β1-integrin that was detected in the basal compartment, subsequent studies have demonstrated that it is a component of the hemidesmosome, colocalizing with α2-laminin (46), which is a component of the basement membrane in rodent testes (47). Although α6β4-integrin has been postulated to be a possible component at the basal ES (48), we failed to coimmunoprecipitate β4-integrin with FAK. Furthermore, neither JAM-A nor claudin-11 (both are putative TJ-integral membrane proteins at the BTB in rat testes) was associated with FAK (our unpublished observations). Interestingly, occludin was shown to interact structurally with FAK, as demonstrated by Co-IP, consistent with results of dual-labeled immunofluorescence microscopy. The concept of the presence of an occludin/ZO-1/FAK protein complex at the BTB was further validated by using an in vitro model involving treatment of Sertoli cells with CdCl2 in the presence of a functional BTB that mimics the in vivo barrier. These findings illustrate that the internalization of occludin and ZO-1 in Sertoli cells after CdCl2-induced TJ-barrier disruption was also accompanied by FAK, demonstrating unequivocally for the presence of a novel occludin/ZO-1/FAK complex. However, it remains to be determined whether FAK modulates occludin adhesion function and if occludin can also mediate signaling function via FAK at the TJ. In addition, it remains to be determined whether the FAK at the BTB is activated at Tyr residues other than Tyr-397 and Tyr-576, which are the two activated forms of FAK that are the integrated structural components of the β1-integrin-based adhesion complex at the apical ES (5,6). In this context, it is of interest to note that in Sertoli cells cultured in vitro with established BTB ultrastructures, FAK was localized to the cell-cell interface, but most of the p-FAK-Tyr397 was detected in Sertoli cell cytosol instead of the cell-cell interface, possibly because these cultures were devoid of apical ES (note: germ cells were lysed in the hypotonic treatment step). Thus, the p-FAK-Tyr397 was not able to localize at the apical ES, so that most of its staining was found in cell cytosol. As such, these observations further validate the notion that p-FAK-Tyr397 is a component of apical ES (5). Based on the findings reported herein, we postulate that the unusual sensitivity of the BTB to cadmium toxicity perhaps is mediated by its effects on FAK; this, in turn, alters the phosphorylation status of the occludin/ZO-1 adhesion complex that confers TJ-permeability barrier function at the BTB (Fig. 6). The net result of this interaction induces accelerated endocytosis of the occludin/ZO-1/FAK complex, which destabilizes the BTB and leads to its disruption. However, this scheme is likely used by the testis to induce BTB restructuring at stage VIII of the epithelial cycle to facilitate the transit of primary leptotene spermatocytes at the BTB. In this context it is of interest to note that the BTB integrity is known to be regulated by cytokines (e.g. TGF-β3, TNFα) and testosterone, wherein cytokines perturb (19,21,25) and testosterone promotes (26) the Sertoli cell TJ-permeability barrier function, possibly mediated by their differential effects on protein endocytosis, recycling, and/or transcytosis at the BTB (23). However, we did not include these reagents to avoid additional parameters in the cell cultures that would affect and/or complicate the experimental outcomes in the cadmium model; as such, the observed effects we reported herein can be attributed entirely to the effects of cadmium instead of the net result of interactions of multiple factors.
Figure 6.
A schematic drawing illustrating that the occludin/ZO-1/FAK is a regulatory protein complex that mediates CdCl2-induced BTB disruption, which is likely used by the testis to restructure BTB at stages VIII–IX of the epithelial cycle to facilitate the passage of primary leptotene spermatocytes. Top panel, The BTB “closed” wherein the occludin/ZO-1/FAK protein complexes between apposing Sertoli cells at the site they interact with each other to confer the TJ barrier. Middle panel, After treatment of Sertoli cells with CdCl2, FAK alters the phosphorylation status of occludin (and/or ZO-1), causing the mislocalization of the complex from the cell-cell interface, likely via a clathrin-mediated endocytic pathway, perhaps targeted to endosome-mediated intracellular degradation, destabilizing the BTB; thereby the BTB “opened” as shown in the lower panel. CdCl2 also induced disruption of actin filaments as reported herein.
In summary, we have identified a novel occludin/ZO-1/FAK protein complex at the BTB in adult rat testes. This finding provides the basis to design functional experiments to study its regulatory role in BTB dynamics.
Supplementary Material
Acknowledgments
We thank Ms. Eleana Sphicas at the Rockefeller University Bio-Imaging Resource Center for her excellent technical assistance in studies by electron microscopy.
Footnotes
This work was supported by grants from the National Institutes of Health (National Institute of Child Health and Human Development, R01 HD056034, and R03 HD051512), (to C.Y.C.). Studies conducted by E.R.S. were performed in the Laboratory of C.Y.C. in New York. E.R.S. was supported in part by a Predoctoral Fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (06/51281-6). Some of the data reported herein were submitted by E.R.S. to the Universidade Federal de Sao Paulo for the partial fulfillment for the requirements of a Doctor of Philosophy.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 12, 2009
Abbreviations: AJ, Adherens junction; BTB, blood-testis barrier; Co-IP, co-immunoprecipitation; DAPI, 4′,6′-diamino-2-phenylindole; ds, desmosome; EEA-1, early endosome antigen-1; ER, endoplasmic reticulum; ES, ectoplasmic specialization; FAK, focal adhesion kinase; JAM-A, junctional adhesion molecule-A; TER, transepithelial electrical resistance; TJ, tight junction ZO-1, zonula occludens-1.
References
- Russell LD 1977 Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat 148:313–328 [DOI] [PubMed] [Google Scholar]
- de Kretser D, Kerr J 1988 The cytology of the testis. In: Knobil E, Neill J, Ewing L, Greenwald G, Markert C, Pfaff D, eds. The physiology of reproduction. New York: Raven Press; 837–932 [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED 1990 Histological and histopathological evaluation of the testis. Clearwater, FL: Cache River Press [Google Scholar]
- Wong CH, Cheng CY 2005 The blood-testis barrier: its biology, regulation and physiological role in spermatogenesis. Curr Top Dev Biol 71:263–296 [DOI] [PubMed] [Google Scholar]
- Siu MK, Mruk DD, Lee WM, Cheng CY 2003 Adhering junction dynamics in the testis are regulated by an interplay of β 1-integrin and focal adhesion complex-associated proteins. Endocrinology 144:2141–2163 [DOI] [PubMed] [Google Scholar]
- Beardsley A, Robertson DM, O'Donnell L 2006 A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol 190:759–770 [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, Parinandi NL, Natarajan V 2006 Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem 281:35554–35566 [DOI] [PubMed] [Google Scholar]
- Galen B, Cheshenko N, Tuyama A, Ramratnam B, Herold BC 2006 Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J Virol 80:12209–12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M, Ogita H, Takai Y 2007 Involvement of integrin-induced activation of protein kinase C in the formation of adherens junctions. Genes Cells 12:651–662 [DOI] [PubMed] [Google Scholar]
- Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J 2007 Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87:545–564 [DOI] [PubMed] [Google Scholar]
- Parsons JT 2003 Focal adhesion kinase: the first ten years. J Cell Sci 116:1409–1416 [DOI] [PubMed] [Google Scholar]
- Vandenbroucke E, Mehta D, Minshall R, Malik AB 2008 Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123:134–145 [DOI] [PubMed] [Google Scholar]
- Janecki A, Jakubowiak A, Steinberger A 1992 Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures—a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol 112:51–57 [DOI] [PubMed] [Google Scholar]
- Chung NP, Cheng CY 2001 Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology 142:1878–1888 [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mather JP, Byer AL, Bardin CW 1986 Identification of hormonally responsive proteins in primary Sertoli cell culture medium by anion-exchange high performance liquid chromatography. Endocrinology 118:480–488 [DOI] [PubMed] [Google Scholar]
- Siu MK, Wong CH, Lee WM, Cheng CY 2005 Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem 280:25029–25047 [DOI] [PubMed] [Google Scholar]
- Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M 1981 Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl 2:249–254 [Google Scholar]
- Lee NP, Mruk DD, Conway AM, Cheng CY 2004 Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl 25:200–215 [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY 2003 Transforming growth factor-β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod 68:1597–1612 [DOI] [PubMed] [Google Scholar]
- Li JC, Lee TW, Mruk TD, Cheng CY 2001 Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl 22:266–277 [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY 2001 Transforming growth factor-β 3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology 142:1865–1877 [DOI] [PubMed] [Google Scholar]
- Lee NP, Cheng CY 2003 Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology 144:3114–3129 [DOI] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Lee WM, Cheng CY 2008 Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J 22:1945–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Cheng CY 2005 Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci USA 102:11722–11727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, Lui WY, Lee WM, Cheng CY 2006 TNF α reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol 190:313–329 [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE 2005 Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA 102:16696–16700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S 1994 Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol 127:1617–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Tsukita S 1999 Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 147:891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD 2002 Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 82:825–874 [DOI] [PubMed] [Google Scholar]
- Gumbiner B 2000 Regulation of cadherin adhesive activity. J Cell Biol 148:399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Wong EWP, Mruk DD, Cheng CY 2009 TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol 327:48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell BP 2008 Blood-testis barrier, junctional and transport proteins and spermatogenesis. In: Cheng CY, ed. Molecular mechanisms in spermatogenesis. Austin, TX: Landes Bioscience/Springer Science; 212–233 [DOI] [PubMed] [Google Scholar]
- Pelletier RM, Byers SW 1992 The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech 20:3–33 [DOI] [PubMed] [Google Scholar]
- Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S 1997 Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol 137:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Nigam S 1999 Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol 276(5 Pt 2):F737–F750 [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Mazzon E, Derigo L, Baraldo S, Meggio F, Citi S 1997 Occludin dephosphorylation in early development of Xenopus laevis. J Cell Sci 110:3131–3139 [DOI] [PubMed] [Google Scholar]
- Reyes CD, Petrie TA, García AJ 2008 Mixed extracellular matrix ligands synergistically modulate integrin adhesin and signaling. J Cell Physiol 217:450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger DA, Ammer H 2008 δ-Opioid receptors activate ERK/MAP kinase via integrin-stimulated receptor tyrosine kinases. Cell Signal 20:2324–2331 [DOI] [PubMed] [Google Scholar]
- Bouchard V, Harnois C, Demers MJ, Thibodeau S, Laquerre V, Gauthier R, Vézina A, Noël D, Fujita N, Tsuruo T, Arguin M, Vachon PH 2008 β1-integrin/FAK/Src signaling in intestinal epithelial crypt cell survival: integration of complex regulatory mechanisms. Apoptosis 13:531–542 [DOI] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P 2008 Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev 60:261–310 [DOI] [PubMed] [Google Scholar]
- Wong EW, Mruk DD, Cheng CY 2008 Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta 1778:692–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova M, Ricci G, Boitani C, Stefanini M, De Grossi S, Palombi F 1998 Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain α6β1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol Reprod 58:371–378 [DOI] [PubMed] [Google Scholar]
- Palombi F, Salanova M, Tarone G, Farini D, Stefanini M 1992 Distribution of β1 integrin subunit in rat seminiferous epithelium. Biol Reprod 47:1173–1182 [DOI] [PubMed] [Google Scholar]
- Yan HH, Cheng CY 2006 Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem 281:17286–17303 [DOI] [PubMed] [Google Scholar]
- Siu MK, Cheng CY 2004 Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod 70:945–964 [DOI] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Wong EW, Lee WM, Cheng CY 2008 An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 105:8950–8955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Giltay R, Talts U, Timpl R, Talts JF 2002 Expression and distribution of laminin α1 and α2 chains in embryonic and adult mouse tissues: an immunochemical approach. Exp Cell Res 275:185–199 [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Vogl AW 2001 Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod 64:396–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.