Abstract
The regulation of GnRH neurons by kisspeptin is critical for normal puberty onset in mammals. In the rodent the kisspeptin neurons innervating GnRH neurons are thought to reside in the rostral periventricular area of the third ventricle (RP3V). Using kisspeptin immunocytochemistry we show that kisspeptin peptide expression in the RP3V of female mice begins around postnatal d 15 (P15) and rapidly increases to achieve adult-like levels by P30, the time of puberty onset. Ovariectomy of female pups at P15 resulted in a 70–90% reduction (P < 0.01) in kisspeptin peptide expression within the RP3V of P30 or P60 mice. Replacement of 17-β-estradiol (E2) in P15-ovariectomized mice from P15–30 or P22–30 resulted in a complete restoration of kisspeptin peptide expression in the RP3V (P < 0.01). Kisspeptin-immunoreactive fibers throughout the hypothalamus, including the arcuate nucleus, followed the same pattern of estrogen-dependent expression. To test the absolute necessity of estrogen for kisspeptin expression in the RP3V, aromatase knockout mice were examined. Kisspeptin-immunoreactive cells were detected in the arcuate nucleus, but there was a complete absence of kisspeptin peptide in RP3V neurons of aromatase knockout adult females. These results demonstrate that E2 is essential for the prepubertal development of kisspeptin peptide within RP3V neurons and suggest that an E2-kisspeptin positive feedback mechanism exists before puberty. This implies that RP3V kisspeptin neurons are E2-dependent amplifiers of GnRH neuron activity in the prepubertal period.
Estradiol is responsible for initiating kisspeptin expression in periventricular hypothalamic neurons that are thought to activate gonadotropin-releasing hormone neurons controlling puberty onset.
Activation of the GnRH neuronal population is an essential primary event for puberty onset in all mammalian species. However, the mechanisms responsible for GnRH neuron activation remain unresolved (1), with key roles being proposed for specific neuronal inputs releasing amino acids and neuropeptides (2,3), as well as neuronal-glial interactions (4).
A major insight into GnRH neuron activation at puberty was obtained in 2003 when the kisspeptin receptor GPR54 was recognized to be essential for puberty onset in humans and mice (5,6,7). Investigations by many laboratories over the last 5 yr have led to the widely held concept that kisspeptin impacts upon reproductive functioning through hypothalamic kisspeptin neurons that project to and directly activate GnRH neurons (8,9,10). Nearly all GnRH neurons express GPR54 mRNA and are intensely activated by kisspeptin (11,12,13). Whereas the expression of GPR54 mRNA by GnRH neurons appears relatively stable over the postnatal period (12), kisspeptin peptide is only observed within fibers around GnRH neuron cell bodies just before the onset of puberty in mice (14). These findings have suggested that the primary developmental step in kisspeptin-GPR54-GnRH neuron signaling at puberty is the up-regulation of kisspeptin biosynthesis within neurons targeting the GnRH neuron cell body. A key role for kisspeptin release in initiating puberty is further evidenced by in vivo studies showing that late juvenile administration of exogenous kisspeptin advances the onset of puberty in primates (15) and rodents (16,17). Furthermore, an activating mutation of GPR54 has recently been found associated with precocious puberty in girls (18).
Because kisspeptin activation of GnRH neurons appears to be essential to evoke puberty onset, it is critical to understand the mechanisms underlying the late juvenile activation of kisspeptin neurons targeting GnRH neurons. Previous studies in the mouse have identified the kisspeptin neurons of the rostral periventricular area of the third ventricle (RP3V) as the primary kisspeptin neuron population innervating GnRH neuron cell bodies during development (14). Accordingly, we have performed experiments aimed at determining the mechanisms underlying the development of kisspeptin expression in RP3V neurons. We report here the unexpected finding that the postnatal expression of kisspeptin within the RP3V is entirely dependent upon 17β-estradiol (E2).
Materials and Methods
Animals
Female C57BL/6J or aromatase knockout (ArKO) and control wild-type mice (C57BL/6J/J129) were used. All mice were housed under conditions of 12-h light, 12-h dark cycles (lights on 0600 h) with food and water ad libitum. Mice younger than postnatal d 21 (P21) were housed with their dam. Upon weaning, mice were housed three to four per cage. The sex of P10 mice was confirmed with sry PCR, as detailed previously (19). All procedures were approved by the University of Otago Animal Ethics Committee or the Monash Medical Centre Animal Ethics Committee “B” under approval no. MMCB 2007/30.
Experiment 1: profile of postnatal kisspeptin peptide expression in RP3V
Our previous study had examined the developmental profile of kisspeptin expression in the RP3V of the female mouse at P10, P25, and P31 (14). To gain a more detailed temporal profile, we reexamined female mice at P10, P15, P20, P25, and P30, and as adults. Female mice at these ages were killed by a pentobarbital anesthetic overdose and perfused through the heart with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.6). The brains were removed and postfixed in the same fixative for 60 min, then transferred to a 30% sucrose/Tris-buffered saline (0.2 m Tris, 0.15 m sodium chloride) solution overnight. The following day, brains were frozen on the stage of a sliding microtome, and three sets of 30 μm thick coronal sections were cut from the level of the medial septum through to the end of the hypothalamus.
Two of the three sets of sections from each mouse were used for kisspeptin immunocytochemistry as reported previously (14). In brief, sections were incubated in a polyclonal rabbit kisspeptin-10 primary antiserum (1:10,000, no. 566; Dr. A. Caraty, INRA-Tours, Nouzilly, France) for 48 h at 4 C, followed by biotinylated goat-antirabbit secondary antibody (Vector Laboratories, Burlingame, CA) at 1:400 for 90 min at room temperature. Sections were then placed in Vector Elite avidin-peroxidase (Vector Laboratories) at 1:100 for 90 min at room temperature, and immunoreactivity revealed using glucose oxidase, nickel-enhanced diaminobenzidine hydrochloride. The sections were washed thoroughly in Tris-buffered saline, mounted onto gelatin-coated glass slides, air-dried, dehydrated in ethanol followed by xylene, and then coverslipped with DPX (Scharlau Chemie, Sentmenat, Spain). Controls consisted of experiments in which the primary antibody was omitted.
Experiment 2: effect of ovariectomy at P15 on kisspeptin peptide expression in the RP3V
To evaluate the role of ovarian steroids in the postnatal up-regulation of kisspeptin immunoreactivity in the RP3V, female mice were ovariectomized (OVX) on P15 and then killed at P30 or P60. P15 female mice were anesthetized with halothane inhalation (2%) and underwent either bilateral ovariectomy or sham operation. The mice were allowed to recover from the anesthetic on a heat pad before being returned to their dam. Mice were weaned at P21 and checked daily for vaginal opening (VO). On P30 (n = 4 per group) and P60 (n = 6 per group), mice were anesthetized, and the stage of the estrous cycle was determined using vaginal cytology (in mice that had exhibited VO). Then an atrial blood sample was collected for LH and mice perfusion fixed.
Experiment 3: effect of E2 replacement on kisspeptin peptide expression in P15 OVX mice
The role of E2 in the postnatal up-regulation of kisspeptin expression in the RP3V was examined by giving E2 to P15 OVX mice from P15–30 or P22–30. In the first group, P15 female mice were OVX with half receiving a sc E2-filled SILASTIC brand capsule (Dow Corning, Midland, MI) at surgery, while the other half received a vehicle capsule (n = 6–7 per group). Estradiol capsules were constructed as described previously (20) according to the method of Bronson (21). Briefly, SILASTIC brand tubing (1.0 mm internal, 2.1 mm external diameter) was filled with SILASTIC brand medical grade adhesive containing 0.1 mg E2/ml adhesive. Approximately 10 μl estradiol-adhesive mixture is required to fill 10 mm tubing, therefore, 10 μl × 0.1 mg/ml = 1 μg estradiol per 10 mm tubing. Vehicle capsules were constructed in the same manner except that E2 was omitted. Female mice grow to approximately 10 g by P30, therefore, capsules 5 mm in length (1 μg E2/20 g body weight) were implanted. This dose of E2 establishes estrogen-negative feedback in adult mice (20,22). To ensure that E2 was delivered consistently over the 15-d period, the E2 capsule was replaced with a fresh capsule on P22. Vehicle capsules were also replaced, and sham-OVX mice underwent incision and wound closure. The second experimental group received the same treatment as described previously, with the exception that it only received a single E2 capsule from P22 onwards. All mice were killed by anesthetic overdose and perfused as described previously.
Experiment 4: kisspeptin peptide expression in the RP3V of aromatase-null mice
To evaluate the development of RP3V kisspeptin expression in the complete absence of estrogens, female ArKO mice (23) and wild-type littermates (C57BL/6J/J129) were examined. These mice lack a functional aromatase enzyme and, consequently, are unable to produce estrogens. P60 ArKO and wild-type littermates (n = 6 each) were perfusion fixed, and immunocytochemistry for kisspeptin was undertaken as described previously.
Analysis
The number of kisspeptin-immunoreactive cell bodies located within the RP3V was counted using bright-field microscopy. As described previously (24), the RP3V is comprised of the anteroventral periventricular nucleus (AVPV), rostral preoptic periventricular nucleus (rPVpo), and caudal preoptic periventricular nucleus (cPVpo). Detailed topographical information on these brain areas in the C57BL/6J mouse brain has now been published (See Fig. 4) (24). Kisspeptin neurons were counted at three levels of the RP3V: the AVPV (Fig. 1A), rPVpo (Fig. 1B), and cPVpo (Fig. 1C) in the first and third set of sections from the 1:3 series. The distribution of kisspeptin neurons is such that they essentially define the AVPV and PVpo (Fig. 1). All kisspeptin-immunoreactive cell bodies at the level of the AVPV are confined to the AVPV. For the PVpo, all cells within 200 μm of the ventricle were counted. Two brain sections at each level of the RP3V were analyzed in each mouse, and the number of cells was counted bilaterally on an Olympus BZX51 microscope (Olympus, Hamburg, Germany) at ×60 objective power. A cell was considered positive if it exhibited a cytoplasmic immunoreactive profile with nuclear exclusion. Mean cell counts for each area in each mouse were determined and grouped to provide mean ± sem values for the experimental groups. Statistical analysis was undertaken using ANOVA with post hoc Student-Newman-Keuls tests. The analyzer was blind to the treatment of the animals.
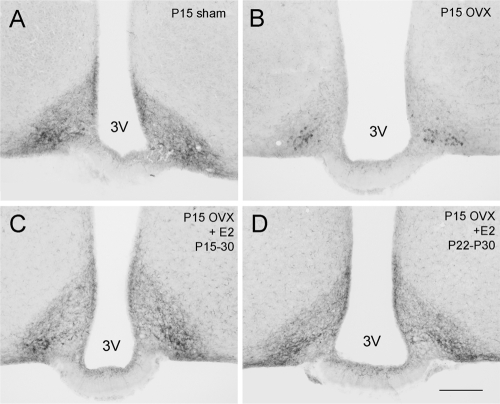
Figure 4.
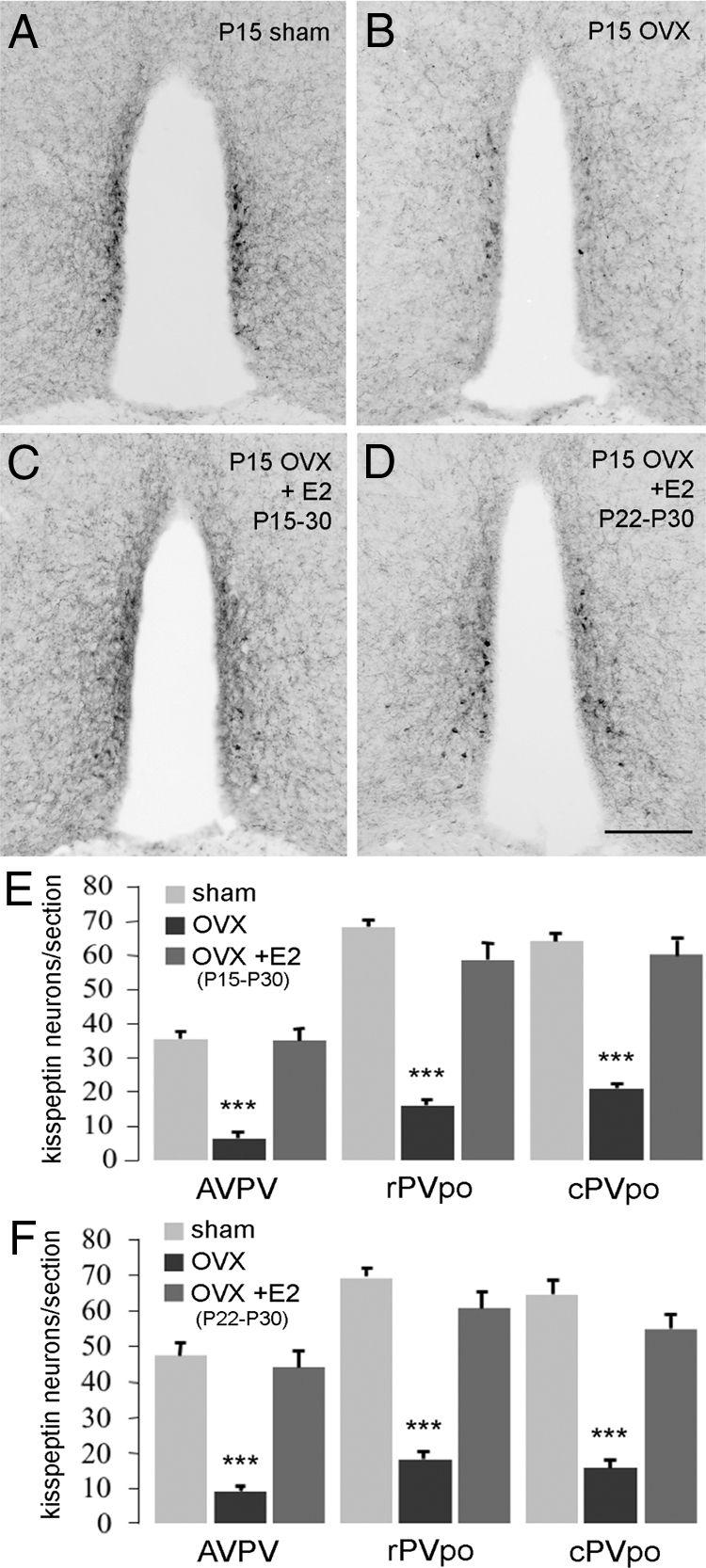
E2 involvement in the developmental increase in kisspeptin expression in the RP3V. A–D, Representative coronal sections through the rPVpo of P30 mice showing kisspeptin immunoreactivity in sham (A), P15 OVX plus vehicle capsule (B), P15 OVX plus E2 capsule from P15–30 (C), and P15 OVX plus E2 capsule from P22–30 (D) animals. E, Quantitative analysis of kisspeptin immunoreactivity in the AVPV, rPVpo, and cPVpo given as the mean (+sem) number of cell bodies detected per section in sham, P15 OVX, and P15 OVX plus E2 capsule from P15–30-treated mice. F, Same data from the experiment in which P15 OVX mice were given the E2 capsule for only the last 7 d (P22–30). ***, P < 0.001 compared with respective sham and E2-treated group.
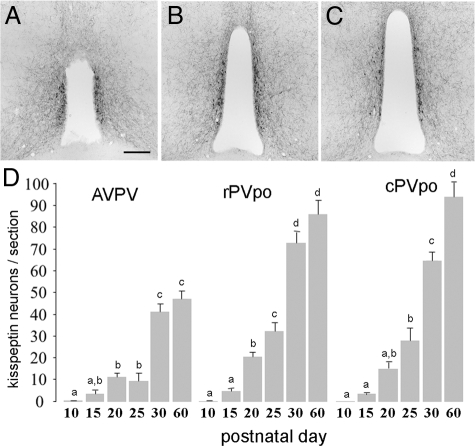
Figure 1.
Development of kisspeptin-10 immunoreactivity in the AVPV, rPVpo, and cPVpo of P10, P15, P20, P25, P30, and P60 female mice. A–C, Representative sections of kisspeptin immunoreactivity in the AVPV, rPVpo, and cPVpo. D, Histogram shows the mean (+sem) number of kisspeptin cell bodies per section at the three levels of the RP3V across postnatal development. Bars with different letters are significantly different from one another (P < 0.05; ANOVA with Student-Newman-Keuls post hoc test). Scale bar in A–C is 200 μm.
RIA for LH
LH levels were determined by RIA using the anti-rLH-S-11 antiserum and mLH-RP reference provided by Dr. A. F. Parlow (National Hormone and Pituitary Program, Torrance, CA). The sensitivity was 0.20 ng/ml and had an intraassay coefficient of variation of 4.03%. All plasma samples for this series of experiments were run in a single assay.
Results
Postnatal profile of kisspeptin-10-expression in the RP3V
As noted previously (14), kisspeptin-immunoreactive cell bodies existed as a periventricular continuum of cells within the AVPV and PVpo (RP3V), with no cell bodies detected elsewhere in the rostral hypothalamus (Fig. 1, A–C). Omission of the kisspeptin-10 primary antisera resulted in a complete absence of labeling.
At P10, no kisspeptin-immunoreactive cell bodies were detected in the RP3V. At P15, three to five immunoreactive cell bodies were detected per section throughout the RP3V, with numbers steadily increasing from P15–25 (P < 0.01; Fig. 1D). In all three RP3V subdivisions, there was then a doubling of kisspeptin cell numbers between P25 and P30 (P < 0.01), with P30 cell numbers resembling adult P60 values in the AVPv and rPVpo. These observations indicate that the major period of development of kisspeptin peptide expression in the RP3V occurs over a 15-d time period between P15 and P30.
The development of kisspeptin peptide expression in the RP3V depends on ovarian steroids
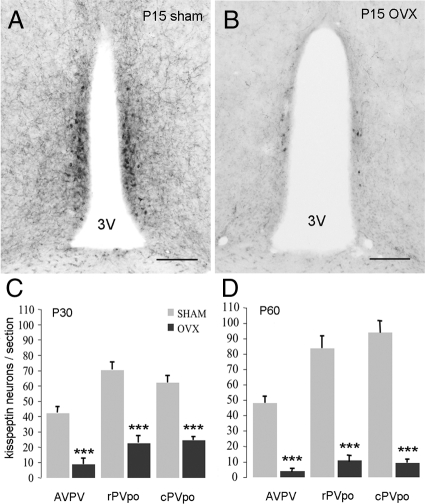
Ovariectomy at P15 resulted in a substantial 70–90% (P < 0.001) decrease in the number of kisspeptin-immunoreactive neurons (Fig. 2) detected throughout the RP3V in P30 (Fig. 2C) and P60 (Fig. 2D) female mice compared with sham-operated animals. Although kisspeptin-immunoreactive fiber density was not measured, we noted a similar marked reduction in kisspeptin fibers within the RP3V and adjacent nuclei in OVX mice (Fig. 2B). The number of kisspeptin-immunoreactive cell bodies in the arcuate nucleus (ARN) was not quantified due to the difficulty of discerning immunoreactive cell bodies from the heavy fiber labeling in this nucleus (Fig. 3A). However, qualitatively, a reduction in kisspeptin-immunoreactive fiber staining in the ARN was also noted in P30 and P60 mice OVX at P15 compared with controls (Fig. 3, A and B).
Figure 2.
Ovarian steroids control the developmental increase in kisspeptin immunoreactivity in the RP3V. A and B, Kisspeptin-10 immunoreactivity in the rPVpo of sham (A) and P15 OVX (B) mice killed at P30. C and D, Quantitative analysis of kisspeptin immunoreactive cell bodies in the AVPV, rPVpo, and cPVpo of the RP3V given as the mean (+sem) number of immunoreactive cell bodies detected per section in P15 OVX and sham-treated mice killed at P30 (n = 4 per group) (C) and P60 (n = 6 per group) (D). ***, P < 0.001 compared with respective sham. Scale bars, 100 μm. 3V, Third ventricle.
Figure 3.
Effects of P15 OVX and E2 replacement on kisspeptin immunoreactivity in the ARN. Kisspeptin immunoreactivity in the ARN at P30 in sham (A), P15 OVX (B), P15 OVX with E2 replacement from P15–30 (C), and P15 OVX with E2 replacement from P22–30 (D). Scale bar, 200 μm. 3V, Third ventricle.
None of the P30 mice exhibited VO at perfusion. In the P60 group, all of the sham mice, but none of the OVX mice, exhibited VO. LH levels were increased significantly in OVX mice killed at P30 (P < 0.05) or P60 (P < 0.01), compared with sham-treated mice (Table 1).
Table 1.
Mean (±sem) plasma LH levels in mice OVX or sham operated at P15, with or without estradiol replacement (E2)
| Treatment | LH (ng/ml) |
|---|---|
| P30, sham/OVX at P15 | |
| Sham | 0.32 ± 0.10 |
| OVX | 1.83 ± 0.40a |
| P60, sham/OVX at P15 | |
| Sham | 0.20 ± 0.01 |
| OVX | 4.07 ± 0.53b |
| P30, sham/OVX at P15 ± E2 P15–30 | |
| Sham | 0.29 ± 0.05 |
| OVX + vehicle | 1.50 ± 0.23a |
| OVX + E2 | 0.39 ± 0.13 |
| P30, sham/OVX at P15 ± E2 P22–30 | |
| Sham | 0.24 ± 0.02 |
| OVX + vehicle | 1.98 ± 0.26a |
| OVX + E2 | 0.77 ± 0.21 |
P < 0.05 compared with sham, and OVX plus E2 where included.
P < 0.01 compared with sham, and OVX plus E2 where included.
E2 regulates kisspeptin peptide expression in the RP3V during postnatal development
Because the number of kisspeptin neurons identified in the RP3V at P30 is essentially the same as at P60 (Fig. 1D), P30 was used subsequently as the single time point for examining the effects of steroid manipulations. The first set of experiments examined the effects on kisspeptin of replacing E2 from the time of ovariectomy at P15 until death at P30. As noted in the prior experiment, there was a substantial (P < 0.001) reduction in kisspeptin cell body numbers throughout the RP3V in OVX mice compared with sham-treated animals (Fig. 4, A, B, and E). Treatment with an E2 capsule from P15 resulted in a complete restoration of kisspeptin cell body numbers throughout the RP3V (Fig. 4, C and E). In the second set of experiments, mice were OVX at P15 but only given E2 from P22–30. This shorter 7-d E2 treatment also resulted in a complete restoration of kisspeptin cell body numbers throughout the RP3V (P < 0.001; Fig. 4, D and F). The P15–30 and P22–30 E2 treatments also increased kisspeptin fiber staining within the RP3V (Fig. 4, C and D) as well as within the ARN (Fig. 3, C and D).
Mice that were OVX and given E2 from P15–30 and P22–30 exhibited VO on P24.3 ± 0.2 and P25, respectively. Plasma LH levels were elevated significantly (P < 0.05) by ovariectomy and returned to basal levels by E2 treatment in both groups of mice (Table 1).
Complete dependence of RP3V kisspeptin peptide expression on estrogen
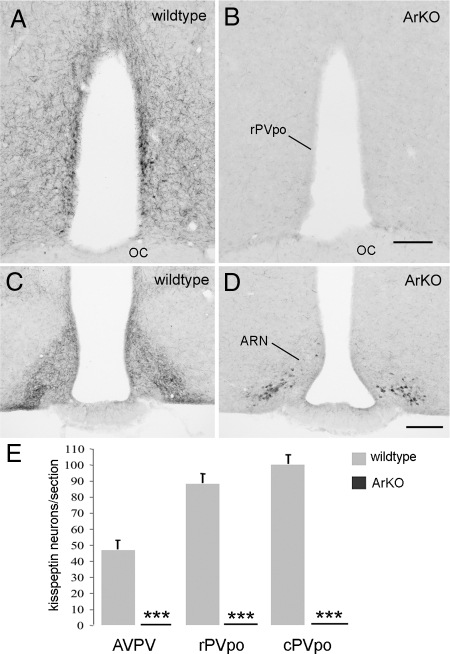
Kisspeptin immunoreactivity in wild-type littermates of ArKO mice (C57BL/6J/J129) was the same as that observed in C57BL/6J wild-type mice (Fig. 5A), with identical numbers of kisspeptin neurons/section throughout the RP3V (compare Figs. 2D and 5E). However, there were no kisspeptin-immunoreactive neurons in the RP3V of any of the six ArKO mice (Fig. 5, B and E). There was also a complete absence of kisspeptin fibers within the rostral hypothalamus (Fig. 5B). However, kisspeptin-immunoreactive cell bodies were detected in the ARN, but, again, a substantial reduction in kisspeptin fiber density was observed in all mice (Fig. 5D).
Figure 5.
Complete absence of RP3V kisspeptin expression in ArKO mice. A–D, Representative coronal sections throughout the rPVpo (A and B) and ARN (C and D) showing kisspeptin immunoreactivity in wild-type littermates (A and C) and ArKO (B and D) adult female mice. E, Quantitative analysis of kisspeptin immunoreactive cell bodies in the AVPV, rPVpo, and cPVpo given as the mean (+sem) number of cells detected per section in wild-type littermates and ArKO mice (n = 6 per group). ***, P < 0.001 compared with wild-type. Scale bars, 200 μm. OC, Optic chiasm.
Discussion
We show here that kisspeptin peptide begins to appear in RP3V neurons between P10 and P15, and thereafter, increases in an exponential-like manner to reach adult levels by the time of puberty onset around P30 in C57BL/6J mice. The increase in kisspeptin-immunoreactive cell bodies detected here very likely represents an increase in kisspeptin synthesis because Kiss1 mRNA expression follows the same developmental pattern (12). The expression of kisspeptin peptide in the RP3V was markedly reduced by ovariectomy at P15 and returned to normal by E2 administration, either from P15–30 or just in the week before puberty onset. In addition, using the ArKO mouse, we found that RP3V kisspeptin expression is totally dependent upon estrogens. Because ArKO mice exhibit a female-like pattern of sexual differentiation (25), it seems unlikely that the absence of RP3V kisspeptin neurons in ArKO female mice results from an absence of perinatal organizational E2 actions. Together, these findings demonstrate that E2 is essential for the postnatal increase in RP3V kisspeptin peptide expression leading up to puberty.
Whether E2 is permissive for this process or actually driving the increase in RP3V kisspeptin synthesis is not known at this time. The presence of estrogen response element motifs in the Kiss1 promotor and evidence for estrogen-dependent up-regulation of Kiss1 mRNA in the adult AVPV (26) would support the latter possibility.
Two main populations of kisspeptin neurons exist in the mammalian brain: one located in the ARN/infundibular nucleus; and one in the preoptic area, known as the RP3V in rodents. Several studies point to the RP3V kisspeptin neurons as being the population that directly innervates GnRH neuron cell bodies. As noted previously (14), a very close correlation exists between the number of kisspeptin cell bodies in the RP3V and kisspeptin fiber density around GnRH neuron cell bodies and dendrites, as well as the hypothalamus in general. Furthermore, neurons located in the RP3V provide one of the largest direct inputs to GnRH neurons (22), and RP3V kisspeptin neurons are activated coincident with GnRH neurons at the time of the GnRH/LH surge (27,28). In contrast, no correlation exists between the number of kisspeptin neurons in the ARN and fibers around GnRH neurons. Arcuate kisspeptin neurons can be identified as early as embryonic d 12 in the mouse (29) and show no developmental change across the postnatal period (12,30).
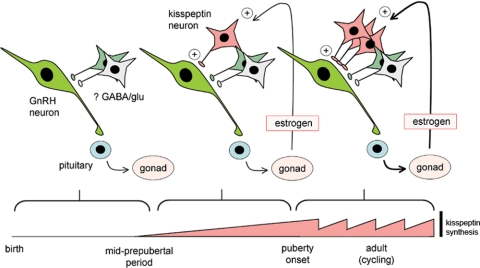
In both primates and rodents, gonadal steroids regulate circulating gonadotropin concentrations before puberty (3,31). In the rodent the ovaries are steroidogenically quiescent during the first week of life but thereafter, are subject to strong gonadotropin-dependent regulation involving both negative and positive feedback control mechanisms (31). Whereas estrogen negative feedback can be demonstrated to exist from P9 onwards (32,33), positive feedback, evident as afternoon mini surges of LH (34), is not apparent until around P20 in the rat (35). In the primate, estrogen negative feedback only exists during the juvenile, prepubertal period, whereas estrogen positive feedback is not evident until after menarche (2,3). In line with the dominant role of estrogen negative feedback both before and after puberty, we show here that ovariectomy of mice at P15 results in increased LH levels at P30. Although the role of varying estrogen negative feedback (the gonadostat hypothesis) in bringing about puberty in rodents is debatable (3,31), the gradual development of estrogen positive feedback nearer the time of puberty onset seems likely to represent the forerunner of the GnRH/LH surge mechanism (31).
To our knowledge the circulating levels of E2 have not been reported for mice at any prepubertal stage. Therefore, we have adapted (by body weight) an adult OVX plus E2 experimental protocol for use in the P15–30 mouse. Although it is not possible to determine how closely the replaced E2 approaches normal circulating concentrations, it is apparent that it returned LH levels to the normal suppressed state when measured at P30. It also resulted in VO at approximately P25, slightly in advance of approximately P28, when we would normally observe VO in our mice (20).
We report here that kisspeptin peptide is not present in the RP3V until P15, and thereafter is dependent upon circulating E2. Because gonadotrophic control of ovarian function occurs before P15 in the mouse (36), this suggests that RP3V kisspeptin neurons are not required for the low basal levels of activity in the hypothalamo-gonadal axis observed soon after birth. Evidence has accrued for important roles of γ-aminobutyric acid and glutamate in regulating GnRH neuron function throughout the embryonic and postnatal period (3,37,38), and we suggest that these, or other, neurotransmitters are responsible for the early activity of GnRH neurons. However, with increasing availability of E2 through enhanced ovarian production and/or reduced circulating α-fetoprotein levels (39,40), kisspeptin peptide expression in RP3V neurons begins in the third postnatal week and rapidly increases to adult levels over the next 15 d (Fig. 6). Although other sources of E2 exist, the 70–90% decrease in kisspeptin expression after ovariectomy at P15 suggests that the ovary is the principal source of E2 with respect to the RP3V kisspeptin neurons.
Figure 6.
Schematic diagram showing proposed activation of RP3V kisspeptin neurons by E2 from birth. In the initial phase up until the mid-prepubertal period, there is no kisspeptin expression (represented by pink bar at bottom). With increasing exposure to E2, kisspeptin expression begins and facilitates GnRH neuron activation. By puberty onset, the kisspeptin expression is fully developed and functioning to amplify GnRH neuron activity. After puberty, kisspeptin expression in the RP3V fluctuates with cyclical E2 levels where it facilitates the generation of the preovulatory GnRH/LH surge in each cycle. GABA, γ-Aminobutyric acid; glu, glutamate.
Although not quantified, we observed that the number of ARN kisspeptin neurons appears unchanged throughout the various manipulations performed. In contrast, the density of kisspeptin fibers within the ARN changes dramatically and in parallel with the number of RP3V kisspeptin cell bodies. This increase in ARN kisspeptin fiber density in the presence of E2 is at odds with the reported down-regulation of Kiss1 mRNA in the ARN of adult mice (26). One explanation for this apparent contradiction is that the great majority of kisspeptin fibers in the ARN originate from RP3V kisspeptin neurons.
A role for E2 in driving kisspeptin expression in the RP3V has been elucidated previously in the adult rodent. E2 increases RP3V Kiss1 mRNA expression through an estrogen receptor α-dependent mechanism (26), and this has been implicated in the estrogen positive feedback mechanism responsible for the preovulatory GnRH/LH surge in rats and mice (27,28,41). We show here that kisspeptin peptide expression is similarly regulated by E2 in the prepubertal period.
Brought together, we speculate that RP3V kisspeptin neurons are involved in a positive feedback circuit (E2→ kisspeptin→ GnRH neurons → gonadotropins → E2) that is essential for the full elaboration of puberty onset (Fig. 6). As such, the RP3V kisspeptin neurons would be best viewed as E2-dependent “amplifiers” of GnRH neuron activity. Humans and mice with mutated Grp54 or Kiss1 (5,42,43) fail to enter puberty, suggesting that, even though RP3V kisspeptin neurons are not required for baseline hypothalamo-gonadal activity, the proposed amplifying role of kisspeptin to enhance pulsatile gonadotropin secretion is crucial for puberty to proceed. This idea is supported further by humans with inactivating mutations of grp54 that, nevertheless, are able to exhibit small LH pulses (44,45).
In summary, we demonstrate that E2 is essential for the emergence of kisspeptin expression in RP3V neurons in the prepubertal period. We propose that the gradual development of an E2-kisspeptin positive feedback relationship provides a GnRH neuron amplification mechanism that is used to facilitate the emergence of pulsatile gonadotropin secretion necessary for puberty onset, as well as the preovulatory estrogen-dependent GnRH/LH surge (Fig. 6).
Acknowledgments
We thank R. Porteous for technical assistance, Drs. A. Caraty and F. Parlow for contributing valuable reagents, and Drs. R. Campbell and D. Grattan for helpful comments on the manuscript.
Footnotes
This work was supported by the Health Research Council of New Zealand, Department of Physiology, University of Otago, and a University of Otago Postgraduate Publishing Bursary.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 19, 2009
Abbreviations: ArKO, Aromatase knockout; ARN, arcuate nucleus; AVPV, anteroventral periventricular nucleus; cPVpo, caudal preoptic periventricular nucleus; E2, 17β-estradiol; OVX, ovariectomized; P21, postnatal d 21; RP3V, rostral periventricular area of the third ventricle; rPVpo, rostral preoptic periventricular nucleus; VO, vaginal opening.
References
- Plant TM 2008 Hypothalamic control of the pituitary-gonadal axis in higher primates: key advances over the last two decades. J Neuroendocrinol 20:719–726 [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF 2006 Puberty in nonhuman primates and humans. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. 3rd ed. Amsterdam: Elsevier; 2177–2230 [Google Scholar]
- Terasawa E, Fernandez DL 2001 Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Sandau US 2008 Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol 20:732–742 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL 2003 The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363 [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA 2007 Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci 30:504–511 [DOI] [PubMed] [Google Scholar]
- Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2008 New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol 29:48–69 [DOI] [PubMed] [Google Scholar]
- Caraty A, Franceschini I 2008 Basic aspects of the control of GnRH and LH secretions by kisspeptin: potential applications for better control of fertility in females. Reprod Domest Anim 43(Suppl 2):172–178 [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA 2004 Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone (GnRH) neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM 2008 Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM 2005 Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M 2004 Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol 561(Pt 2):379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T 2004 Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun 320:383–388 [DOI] [PubMed] [Google Scholar]
- Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC 2008 A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 358:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonian SX, Herbison AE 2001 Differing, spatially restricted roles of ionotropic glutamate receptors in regulating the migration of GnRH neurons during embryogenesis. J Neurosci 21:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR 2008 Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH 1981 The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology 108:506–516 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER 1998 Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95:6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE 2008 Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Pompolo S, Jones ME, Simpson ER, Boon WC 2004 Estrogen deficiency leads to apoptosis in dopaminergic neurons in the medial preoptic area and arcuate nucleus of male mice. Mol Cell Neurosci 27:466–476 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE 2008 Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll J, Clay C, Henion T, Schwarting GA, Tobet S: Sex differences in kisspeptin mRNA during murine development. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008 (Abstract P1-680) [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M 2007 Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Skinner MK 2006 Puberty in the rat. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. 3rd ed. Amsterdam: Elsevier; 2061–2126 [Google Scholar]
- Ojeda SR, Kalra PS, McCann SM 1975 Further studies on the maturation of the estrogen negative feedback on gonadotropin release in the female rat. Neuroendocrinology 18:242–255 [DOI] [PubMed] [Google Scholar]
- Goldman BD, Grazia YR, Kamberi IA, Porter JC 1971 Serum gonadotropin concentrations in intact and castrated neonatal rats. Endocrinology 88:771–776 [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Ojeda SR 1986 The development of afternoon minisurges of luteinizing hormone secretion in prepubertal female rats is ovary dependent. Endocrinology 118:1187–1193 [DOI] [PubMed] [Google Scholar]
- Andrews WW, Mizejewski GJ, Ojeda SR 1981 Development of estradiol-positive feedback on luteinizing hormone release in the female rat: a quantitative study. Endocrinology 109:1404–1413 [DOI] [PubMed] [Google Scholar]
- Halpin DM, Jones A, Fink G, Charlton HM 1986 Postnatal ovarian follicle development in hypogonadal (hpg) and normal mice and associated changes in the hypothalamic-pituitary ovarian axis. J Reprod Fertil 77:287–296 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol 254- 255:32–38 [DOI] [PubMed] [Google Scholar]
- Tobet SA, Bless EP, Schwarting GA 2001 Developmental aspect of the gonadotropin-releasing hormone system. Mol Cell Endocrinol 185:173–184 [DOI] [PubMed] [Google Scholar]
- Meijs-Roelofs HM, Kramer P 1979 Maturation of the inhibitory feedback action of oestrogen on follicle-stimulating hormone secretion in the immature female rat: a role for α-foetoprotein. J Endocrinol 81:199–208 [DOI] [PubMed] [Google Scholar]
- Germain BJ, Campbell PS, Anderson JN 1978 Role of the serum estrogen-binding protein in the control of tissue estradiol levels during postnatal development of the female rat. Endocrinology 103:1401–1410 [DOI] [PubMed] [Google Scholar]
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K 2007 Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH 2007 Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB 2007 Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N 2007 Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab 92:1137–1144 [DOI] [PubMed] [Google Scholar]
- Pallais JC, Bo-Abbas Y, Pitteloud N, Crowley Jr WF, Seminara SB 2006 Neuroendocrine, gonadal, placental, and obstetric phenotypes in patients with IHH and mutations in the G-protein coupled receptor, GPR54. Mol Cell Endocrinol 254–255:70–77 [DOI] [PubMed] [Google Scholar]