The physiological and endocrine homeostatic systems that regulate body weight are complex. Several peripheral systems need to be coordinated with central nervous system processes for proper energy balance regulation. Systems for energy storage (white adipose tissue), energy expenditure (skeletal muscle and brown adipose tissue), and energy absorption (gut) (1) inform the brain how much fuel is available short- and long-term. These systems coordinate with expected fuel demand, which is impacted by environment, temperature, season, reproduction and offspring care, food availability, and predation pressure. Whereas a handful of mainstream peptides receive ample of attention from investigators, other important signals are less recognized. A recent study by Peier et al. (2) highlights the importance of neuromedins U (NMU) and S (NMS) acting centrally on the NMU receptor 2 (NMUR2) in the regulation of body weight homeostasis.
So what are these neuromedins and why do they matter? NMU, first described in 1985 and named for its ability to stimulate smooth muscle contraction in the uterus (3), is a peptide synthesized in and secreted from several tissues, most notably (for our purposes), the gut and the brain (4) (Fig. 1). NMU acts on the brain to dampen energy intake and increase energy expenditure, partially through heightened physical activity (5,6,7,8,9). Thus, NMU follows a common pattern seen in hormones related to energy balance (10). Although NMU is found in the gut and stimulates smooth muscle contraction there (4), there is little evidence to suggest that it is released into the general circulation to act on the brain. Instead, it may be one mechanism through which leptin acts to exert its effects on energy balance and behavior (8,11,12). Also found in the brain is NMS, which is structurally similar to although not, as the authors point out, a splice variant of NMU (2,13,14). The S in NMS stands for the suprachiasmatic nucleus (SCN), the primary circadian clock in mammals or, as many circadian biologists consider it to be, the seat of the soul (take that, pineal gland). The precise nuclear locations of NMU and NMS within the brain are somewhat disputed because their sequence and structural similarities make interpretation of binding specificity problematic, but it is safe to say that NMU is synthesized in hypothalamic nuclei that are critical players in regulating energy homeostasis, and NMS is found predominantly in the SCN (13,14,15,16,17).
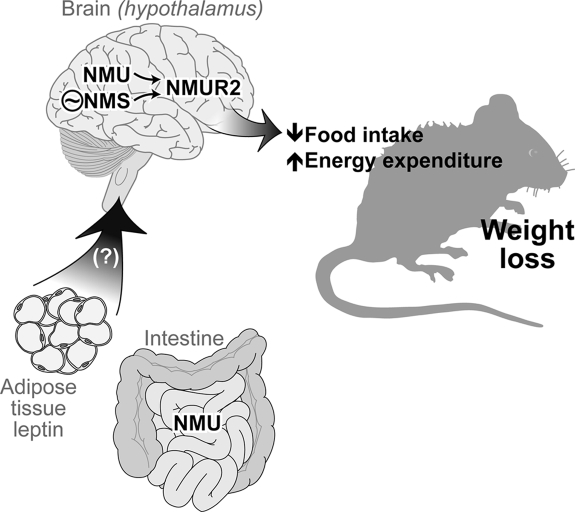
Figure 1.
A generalized schematic of the role of NMU and NMS in energy balance regulation. NMU is made in peripheral tissues, including the small intestine, but there is little evidence that it is released into the general circulation (4). Evidence suggests that circulating leptin induces NMU release (although NMU is not the only route through which leptin induces its central effects) (8,11,12). NMU is synthesized in hypothalamic brain regions important in energy balance regulation, and NMS is found in the SCN, the primary circadian clock in mammals (13,14,15,16,33,34). They both act centrally, primarily through the NMUR2, to decrease food intake and increase energy expenditure. This results in weight loss.
One take-home message from Peier et al. (2) is that chronic centrally applied NMU and NMS can decrease food intake and body weight (and, for NMU, alter body temperature) over 2 wk. The apparent potency did not diminish over time. Along with other studies, this hints that there may be individual differences in the NMU system that could be associated with obesity (18,19). Others, however, found that serial intra-paraventricular nucleus intraparaventricular (PVN) injections of NMU did not significantly alter body weight (20). Different methods and models can yield different results, but the data in Peier et al. were consistent: NMU and NMS induced weight loss in more than one study and mouse genotype. That these findings are inconsistent with previously reported results may suggest that whereas the PVN may play a role in NMU-induced physical activity and energy expenditure (5,6), other brain regions may be more important in the effects seen in the study by Peier et al. (2), who infused NMU intracerebroventricularly.
These two peptides, NMU and NMS, act on the same two receptors. As an oversimplification, NMUR1 is found in the predominantly in the periphery, and NMUR2 is found mostly in the brain (4,9,15,21,22). Here, the authors focus specifically the role of NMUR2 in the central actions of NMU and NMS. This leads to the second take-home message: the acute and chronic effects of central NMU and NMS, for the most part, appear to depend on NMUR2. NMU and NMS did not have the same effects in mice deficient in NMUR2 compared with wild types (WT), whereas NMUR1−/− mice were very similar to WT mice in their response to chronic central NMU. The authors, combining genetic and pharmacological manipulations, make a convincing argument for an important physiological role of NMU and NMS acting on NMUR2 in energy homeostasis.
Given the role of these neuromedins in energy balance regulation, we would predict that deficiencies in this system would result in an overweight mouse, prone to diet-induced obesity. Accordingly, over- vs. underexpression of NMU results in, respectively, lean and active vs. obese mice (11,23). Splice variants of NMU have been identified in some forms of familial obesity (19). Here, however, Peier et al. (2) report that deficiencies in NMUR2 resulted in smaller mice that were somewhat resistant to diet-induced obesity. This seems paradoxical and underscores the need to explore the role of NMU and NMS in metabolism using additional models and methods. One potential way of explaining this result may be to conceptualize NMU and/or NMU as a signal of energy surfeit, which may then modulate growth. The subtle effects on the growth curve evidenced here in NMUR2−/− could be due to differences in growth rather than adiposity given that both fat and lean mass were relatively lower in NMUR2−/− mice compared with WT. Whether or not the decreased food intake in NMUR2−/− mice is secondary to their smaller size is difficult to determine. Although NMUR2−/− mice consumed fewer calories, their food intake corrected to body weight did not differ from WT mice. When considering either caloric intake or expenditure, it is much more difficult to assign cause and effect when genetically altered and WT mice differ in body size.
Finally, the potential implications of the actions of NMS in particular should be highlighted (13,14). One topic of increasing interest of late is the interplay between circadian rhythms and energy balance homeostasis (24,25,26,27). NMS is a peptide synthesized in the SCN that acts on NMUR2, which are present in brain regions targeted by the SCN (13,28). NMU expression in the SCN follows a circadian rhythm, but there may be species differences with respect to whether these neuromedins reside in the SCN core or shell (29,30). Both NMU and NMS phase shift the circadian activity rhythm (the phase-response curve is of the nonphotic type, for you circadian people) (13,30), presumably by acting through NMUR2, but NMUR1 may also be involved (30). To my knowledge, nobody has yet explored the possibility that NMS or possibly NMU may be circadian output peptides that could potentially modulate rhythmic effects on energy balance through their influence on food intake, energy expenditure, and physical activity. In addition, if NMU and NMS signal metabolic state, then their actions on the SCN may be one route through which energy balance perturbations could directly affect the circadian clock. Because NMU also interacts with brain systems regulating the stress response, NMU is positioned at the interface between systems regulating energy balance and the stress response (31,32). Systematic studies such as these highlight the importance of peptides such as NMU and NMS for grasping the full complexity of energy balance and its interactions with other behavioral and regulatory systems.
Acknowledgments
We thank Dr. Antonio A. Nunez, Dr. James A. Levine, and Penny Hanson for critical reading of the manuscript.
Footnotes
Disclosure Summary: The author (C.M.N.) has nothing to disclose.
For article see page 3101
Abbreviations: NMS, Neuromedin S; NMU, neuromedin U; NMUR2, NMU receptor 2; SCN, suprachiasmatic nucleus; WT, wild type.
References
- Perez-Tilve D, Nogueiras R, Mallo F, Benoit SC, Tschoep M 2006 Gut hormones ghrelin, PYY, and GLP-1 in the regulation of energy balance [corrected] and metabolism. Endocrine [Erratum (2006) 29:383] 29:61–71 [DOI] [PubMed] [Google Scholar]
- Peier A, Kosinski J, Cox-York K, Qian Y, Desai K, Feng Y, Trivedi P, Hastings N, Marsh DJ 2009 The antiobesity effects of centrally administered Neuromedin U and Neuromedin S are mediated predominantly by the Neuromedin U receptor 2 (NMUR2). Endocrinology 150:3101–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Matsuo H 1985 Neuromedin U-8 and U-25: novel uterus stimulating and hypertensive peptides identified in porcine spinal cord. Biochem Biophys Res Commun 130:1078–1085 [DOI] [PubMed] [Google Scholar]
- Brighton PJ, Szekeres PG, Willars GB 2004 Neuromedin U and its receptors: structure, function, and physiological roles. Pharmacol Rev 56:231–248 [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, Murphy KG, Stanley SA, Zollner AN, Ghatei MA, Bloom SR 2002 Hypothalamic actions of neuromedin U. Endocrinology 143:4227–4234 [DOI] [PubMed] [Google Scholar]
- Novak CM, Zhang M, Levine JA 2006 Neuromedin U in the paraventricular and arcuate hypothalamic nuclei increases non-exercise activity thermogenesis. J Neuroendocrinol 18:594–601 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Hanada R, Murakami N, Date Y, Mondal MS, Kojima M, Yoshimatsu H, Kangawa K, Matsukura S 2000 Central effects of neuromedin U in the regulation of energy homeostasis. Biochem Biophys Res Commun 277:191–194 [DOI] [PubMed] [Google Scholar]
- Jethwa PH, Small CJ, Smith KL, Seth A, Darch SJ, Abbott CR, Murphy KG, Todd JF, Ghatei MA, Bloom SR 2005 Neuromedin U has a physiological role in the regulation of food intake and partially mediates the effects of leptin. Am J Physiol Endocrinol Metab 289:E301–E305 [DOI] [PubMed] [Google Scholar]
- Gartlon J, Szekeres P, Pullen M, Sarau HM, Aiyar N, Shabon U, Michalovich D, Steplewski K, Ellis C, Elshourbagy N, Duxon M, Ashmeade TE, Harrison DC, Murdock P, Wilson S, Ennaceur A, Atkins A, Heidbreder C, Hagan JJ, Hunter AJ, Jones DN 2004 Localisation of NMU1R and NMU2R in human and rat central nervous system and effects of neuromedin-U following central administration in rats. Psychopharmacology (Berl) 177:1–14 [DOI] [PubMed] [Google Scholar]
- Novak CM, Levine JA 2007 Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J Neuroendocrinol 19:923–940 [DOI] [PubMed] [Google Scholar]
- Hanada R, Teranishi H, Pearson JT, Kurokawa M, Hosoda H, Fukushima N, Fukue Y, Serino R, Fujihara H, Ueta Y, Ikawa M, Okabe M, Murakami N, Shirai M, Yoshimatsu H, Kangawa K, Kojima M 2004 Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat Med 10:1067–1073 [DOI] [PubMed] [Google Scholar]
- Jethwa PH, Smith KL, Small CJ, Abbott CR, Darch SJ, Murphy KG, Seth A, Semjonous NM, Patel SR, Todd JF, Ghatei MA, Bloom SR 2006 Neuromedin U partially mediates leptin-induced hypothalamo-pituitary adrenal (HPA) stimulation and has a physiological role in the regulation of the HPA axis in the rat. Endocrinology 147:2886–2892 [DOI] [PubMed] [Google Scholar]
- Mori K, Miyazato M, Ida T, Murakami N, Serino R, Ueta Y, Kojima M, Kangawa K 2005 Identification of neuromedin S and its possible role in the mammalian circadian oscillator system. Embo J 24:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Mori K, Miyazato M, Egi Y, Abe S, Nakahara K, Nishihara M, Kangawa K, Murakami N 2005 Neuromedin s is a novel anorexigenic hormone. Endocrinology 146:4217–4223 [DOI] [PubMed] [Google Scholar]
- Graham ES, Turnbull Y, Fotheringham P, Nilaweera K, Mercer JG, Morgan PJ, Barrett P 2003 Neuromedin U and Neuromedin U receptor-2 expression in the mouse and rat hypothalamus: effects of nutritional status. J Neurochem 87:1165–1173 [DOI] [PubMed] [Google Scholar]
- Ivanov TR, Lawrence CB, Stanley PJ, Luckman SM 2002 Evaluation of neuromedin U actions in energy homeostasis and pituitary function. Endocrinology 143:3813–3821 [DOI] [PubMed] [Google Scholar]
- Steel JH, Van Noorden S, Ballesta J, Gibson SJ, Ghatei MA, Burrin J, Leonhardt U, Domin J, Bloom SR, Polak JM 1988 Localization of 7B2, neuromedin B, and neuromedin U in specific cell types of rat, mouse, and human pituitary, in rat hypothalamus, and in 30 human pituitary and extrapituitary tumors. Endocrinology 122:270–282 [DOI] [PubMed] [Google Scholar]
- Novak CM, Zhang M, Levine JA 2007 Sensitivity of the hypothalamic paraventricular nucleus to the locomotor-activating effects of neuromedin U in obesity. Brain Res 1169:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainerová I, Torekov SS, Ek J, Finková M, Borch-Johnsen K, Jørgensen T, Madsen OD, Lebl J, Hansen T, Pedersen O 2006 Association between neuromedin U gene variants and overweight and obesity. J Clin Endocrinol Metab 91:5057–5063 [DOI] [PubMed] [Google Scholar]
- Thompson EL, Murphy KG, Todd JF, Martin NM, Small CJ, Ghatei MA, Bloom SR 2004 Chronic administration of NMU into the paraventricular nucleus stimulates the HPA axis but does not influence food intake or body weight. Biochem Biophys Res Commun 323:65–71 [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Jiang Q, Van Der Ploeg LH, Liu Q 2001 Distribution of neuromedin U receptor subtype 2 mRNA in the rat brain. Brain Res Gene Expr Patterns 1:1–4 [DOI] [PubMed] [Google Scholar]
- Shan L, Qiao X, Crona JH, Behan J, Wang S, Laz T, Bayne M, Gustafson EL, Monsma Jr FJ, Hedrick JA 2000 Identification of a novel neuromedin U receptor subtype expressed in the central nervous system. J Biol Chem 275:39482–39486 [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Spar BD, Markowitz L, Maguire M, Golovko A, Yang S, Farley C, Cook JA, Tetzloff G, Hoos L, Del Vecchio RA, Kazdoba TM, McCool MF, Hwa JJ, Hyde LA, Davis H, Vassileva G, Hedrick JA, Gustafson EL 2005 Transgenic overexpression of neuromedin U promotes leanness and hypophagia in mice. J Endocrinol 185:151–164 [DOI] [PubMed] [Google Scholar]
- Bray MS, Young ME 2007 Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes Rev 8:169–181 [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Bass J 2007 A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab 18:4–11 [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Kreier F, Fliers E, Sauerwein HP, Romijn JA, Buijs RM 2007 Circadian control of metabolism by the suprachiasmatic nuclei. Endocrinology 148:5635–5639 [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Marcheva B, Kohsaka A, Bass J 2007 The clockwork of metabolism. Annu Rev Nutr 27:219–240 [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY 2001 Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol 433:312–334 [DOI] [PubMed] [Google Scholar]
- Graham ES, Littlewood P, Turnbull Y, Mercer JG, Morgan PJ, Barrett P 2005 Neuromedin-U is regulated by the circadian clock in the SCN of the mouse. Eur J Neurosci 21:814–819 [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hanada R, Murakami N, Teranishi H, Ohgusu H, Fukushima N, Moriyama M, Ida T, Kangawa K, Kojima M 2004 The gut-brain peptide neuromedin U is involved in the mammalian circadian oscillator system. Biochem Biophys Res Commun 318:156–161 [DOI] [PubMed] [Google Scholar]
- Hanada T, Date Y, Shimbara T, Sakihara S, Murakami N, Hayashi Y, Kanai Y, Suda T, Kangawa K, Nakazato M 2003 Central actions of neuromedin U via corticotropin-releasing hormone. Biochem Biophys Res Commun 311:954–958 [DOI] [PubMed] [Google Scholar]
- Hanada R, Nakazato M, Murakami N, Sakihara S, Yoshimatsu H, Toshinai K, Hanada T, Suda T, Kangawa K, Matsukura S, Sakata T 2001 A role for neuromedin U in stress response. Biochem Biophys Res Commun 289:225–228 [DOI] [PubMed] [Google Scholar]
- Miyazato M, Mori K, Ida T, Kojima M, Murakami N, Kangawa K 2008 Identification and functional analysis of a novel ligand for G protein-coupled receptor, Neuromedin S. Regul Pept 145:37–41 [DOI] [PubMed] [Google Scholar]
- Mori K, Miyazato M, Kangawa K 2008 Neuromedin S: discovery and functions. Results Probl Cell Differ 46:201–212 [DOI] [PubMed] [Google Scholar]