Abstract
β-Cell apoptosis occurs in diabetes mellitus (DM). Heat shock protein (HSP) 27 (human homolog of rodent HSP25) mitigates stress-induced apoptosis but has not been studied in β-cells. We tested whether HSP27 overexpression attenuates streptozotocin (SZ)-induced DM in vivo and cytokine-induced islet apoptosis in vitro. DM was ascertained by ip glucose tolerance testing, and fasting serum insulin/glucose was measured. Pancreas was stained for insulin, HSP27, and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling, and insulin content was measured. HSP25/27 was measured by immunoblotting, isoelectric focusing, and RT-PCR. Islet HSP25/27 oligomerization and inhibitory κB protein kinase γ (nuclear factor κB essential modulator) binding were assessed by coimmunoprecipitation. HSP27 transgene (TG) in pancreas localized predominantly in β-cells. Baseline pancreatic insulin levels in wild-type (WT) and HSP27TG mice were similar, but lower in WT than HSP27TG after SZ (P < 0.01). Intraperitoneal glucose tolerance testing confirmed protection from SZ-DM in HSP27TG. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling and inducible nitric oxide synthase staining were increased in WT vs. HSP27TG islets (P < 0.05) after SZ. Caspase-3 activity was lower in islets from HSP27TG vs. WT mice after cytokine stress in vitro (P < 0.05). There was more HSP25 plus 27 protein from HSP27TG islets than HSP25 from WT (P < 0.01). HSP25 protein but not mRNA was increased in HSP27TG mice. Isoelectric focusing showed similar relative HSP phosphorylation in HSP27TG and WT (P > 0.05). HSP27 bound native HSP25 in TG islets; both bound to inhibitory κB protein kinase γ (nuclear factor κB essential modulator). These data show islet protection by HSP27 by mitigation of apoptosis, possibly through nuclear factor κB regulation.
HSP27 overexpression protects islets from cytokine-induced injury and streptozotocin-induced diabetes in vitro and in vivo, respectively, through a mechanism that is likely to involve modulation of NF-κB signaling.
Apoptosis is central to evolving islet and β-cell death in types 1 and 2 diabetes (DM), after multiple low doses of streptozotocin (SZ), and during isolation for transplantation (1,2,3,4). In cultured islets, cytokine exposure [IL-1β, TNFα, interferon (IFN)-γ] induces apoptosis (5,6,7,8,9,10) and is used to model autoimmune DM in vivo. Experiments to attenuate β-cell apoptosis after cytokines are used to model resolution of β-cell injury in vivo (11,12,13,14,15).
In rodents, high-single SZ dosing depletes cellular nicotinamide adenine dinucleotide and ATP, disrupts membrane integrity, and initiates β-cell necrosis. Multiple low SZ doses induce limited apoptosis (1,16), inciting autoimmunity to eliminate remaining cells. SZ is a methylating agent that enters β-cells via the glucose transporter 2, alkylates DNA, and induces poly ADP-ribosylation, and cellular nicotinamide adenine dinucleotide and ATP depletion (17). SZ liberates nitric oxide and inhibits aconitase activity, further damaging DNA (18). Cytokines, including inducible nitric oxide synthase (iNOS) that contribute to β-cell apoptosis, are released at least in part through the mediation of activated nuclear factor κB (NFκB). Mice harboring a mutation in NFκB(p50) are resistant to the development of multiple low-dose SZ-DM (19).
Heat shock protein (HSP) 27 (apparent molecular mass, 27 kDa) is the human homolog of rodent protein HSP25. The family of HSPs is up-regulated in response to cellular stressors such as temperature, hypoxia, ischemia, thrombin, growth factors, sodium arsenite, glutamate, osmolarity, heavy metals, and cytokines such as TNFα and IL-1β (20,21,22,23,24). HSP25/27 up-regulation mitigates apoptosis after numerous cellular challenges (25,26,27,28,29,30,31,32,33,34,35,36,37). HSP25/27 confers cytoprotection through various mechanisms. It is an antioxidant (38); it inhibits multiple steps in the intrinsic and extrinsic mitochondrial apoptotic pathways (39,40,41,42,43,44,45,46). It regulates Akt (47,48) and NFκB signaling (49,50). β-Cells express little HSP25 (51), so its potential for protection has not been directly studied. However, a recent microarray study reported HSP25 up-regulation in cytokine-exposed rat β-cells (14), identifying HSP25 as a potential stress modifier even in β-cells. Others showed that in cytokine- and isolation-stressed islets in vitro, the p38 MAPK inhibitor SB203580 impeded downstream HSP27 phosphorylation and improved islet survival, demonstrating an association between nonphosphorylated HSP27 and attenuation of cytokine-induced islet apoptosis (52,53). Although most antiapoptotic actions of HSP27 are phosphorylation independent, a few are phosphorylation dependent (49,54). Together, these data establish relevance for HSP27 in β-cell and islet protection, suggesting the potential for novel therapies. The studies described herein demonstrate for the first time that SZ-DM is attenuated in HSP27 transgenic (TG) mice. In vitro, mitigation of cytokine-induced β-cell apoptosis in HSP27TG islets implicates a mechanism involving the regulation of β-cell NFκB signaling.
Materials and Methods
Animals
Two HSP27TG mouse strains were used. Initial studies used mice expressing an HSP27TG containing human HSP27 cDNA with a chicken β-actin promoter and a cytomegalovirus enhancer bred onto a C57BL10xCBA/Ca background (27,28). To better evaluate HSP27 effects on SZ-DM and its complications, the initial hybrid strain was backcrossed to DBA2J mice (55) for at least five generations. Experiments were performed on male TG mice and wild-type (WT) male littermates. WT and TG mice were injected with either SZ in citrate buffer, 50 mg/kg ip daily for 5 d, or diluent. For the C57BL10xCBA/Ca strain, up to 12 SZ doses were required for SZ-DM induction, and DM was diagnosed by random blood glucose more than 200 mg/dl. For HSP27TGxDBA2J backcrossed mice, DM was diagnosed by ip glucose tolerance testing (GTT) (2 g/kg glucose) performed approximately 2 wk after the last SZ injection. Overnight (14–15 h) fasted mice received glucose 2.0 g/kg body weight, ip. Blood glucose was measured with an Elite Glucometer (Bayer, Mishawaka, IN) at fasting, and 60, 90, and 120 min after glucose challenge. Fasting serum insulin levels were measured with an ultrasensitive mouse insulin ELISA kit (ALPCO Diagnostics, Salem, NH). Characterization of untreated mice was also performed. In uninjected fasting TG and WT mice, glucose and insulin were measured. Pancreas was placed in radioimmunoprecipitation assay (RIPA) buffer with proteinase inhibitors for Western immunoblotting, in RNA STAT-60 (Tel-Test, Inc., Friendswood, TX) for RT-PCR, and fixed in buffered formalin, sectioned, and stained for insulin, HSP27, and/or the HA-tag. Pancreas total insulin content was measured with a mouse insulin ELISA kit (ALPCO Diagnostics) after extraction according to Mabley et al. (19), and expressed both as total insulin/pancreas and as insulin/pancreas protein content. Pancreas histology was reviewed by two pathologists (C.C.N. and Y.W.). HSP25 protein was also measured in β-galactosidase TG mice and WT littermate controls (n = 3 each). Experiments were approved by Los Angeles Biomedical Research Institute’s Animal Use and Care Committee.
HSP27 immunohistochemistry
Ten to 12-wk-old WT and HSP27TG mouse pancreas was harvested, fixed, embedded, sectioned, deparaffinized in xylene, and rehydrated with graded ethanol. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide in 0.1% Tween/1× Tris-buffered saline (TBST) and washed. Slides were incubated in 10% normal goat serum (Zymed Laboratories, Inc., San Francisco, CA) for protein blocking. Primary antibody was applied overnight at 4 C at 1:1000 dilution of rabbit anti-HSP27 antibody (Stressgen, Victoria, British Columbia, Canada), 1:100 dilution of rabbit anti-hydroxyapatite (HA) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or 1:200 dilution of normal rabbit Ig (Santa Cruz Biotechnology) in 3% BSA/TBST as a negative control. Slides were again TBST washed, incubated with 1:500 biotinylated goat-antirabbit secondary antibodies (DakoCytomation, Carpinteria, CA) for 30 min, rewashed, and counterstained with hematoxylin. Bound antibody was detected using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
Immunofluorescence staining
Tissue was deparaffinized, rehydrated, washed, and blocked with normal goat serum. After rinsing with TBST, tissue was incubated with both 1:100 dilution of guinea pig anti-insulin antibody (LINCO Research, Inc., St. Charles, MO) and either 1:1000 dilution of rabbit anti-HSP27 antibody or 1:100 dilution of rabbit anti-HA antibody in 3% BSA/TBST at 4 C overnight. Slides were washed in TBST three times; both orange-red Alexa Fluor 568 conjugated goat-antiguinea pig secondary antibody and green Alexa Fluor 488 conjugated goat-antirabbit secondary antibody (Molecular Probes, Inc., Eugene, OR) were incubated for 30 min. Slides were washed with TBST three times; nuclei were costained with 0.3 μm 4′, 6′-diamidino-2-phenylindole hydrochloride, and examined using an Olympus BX51 fluorescent microscope equipped with a Pixera 600 CCD camera (Olympus America, Inc., Melville, NY) and Bio-Rad 2000 confocal microscope.
Western immunoblotting
Immunoblotting was performed as described (56) using primary antibodies: polyclonal anti-HSP25 1:5000 (Stressgen); polyclonal anti-HSP27 1:20,000 (Stressgen); polyclonal anti-HA 1:1000; monoclonal anti-HSP25 plus HSP27 1:1000 (CHEMICON International, Inc., Temecula, CA); and monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 1:2000 (Fitzgerald, Concord, MA). Secondary horseradish peroxidase-conjugated antirabbit antibody was used for anti-HSP25 (1:10,000); anti-HA (1:2000); and anti-HSP27 (1:50,000). Secondary antimouse antibody was used for anti-HSP25 plus HSP27 (1:2000) and anti-GAPDH (1:50,000).
Relative quantitative RT-PCR for pancreas HSP25
Total RNA from pancreas of WT and HSP27 TG mice was isolated using RNA-STAT-60 reagent. Total RNA (2 μg) was used for first-strand cDNA synthesis using the Omniscript reverse transcriptase kit (QIAGEN, Inc., Valencia, CA). Pancreas HSP25 mRNA was quantified by relative quantitative RT-PCR using QuantumRNA Classic II 18S Internal Standard (Ambion, Inc., Austin, TX) according to the manufacturer. Primer sequences for mouse pancreas HSP25 are 5′-GAAGGAGATATAGACTGACCGAGC-3′ and 5′-GGCTTCTAGATGGCTCCAGACTGT-3′ as forward and reverse, respectively. Data were expressed as relative fold change of HSP25 mRNA in HSP27 TG mouse pancreas compared with WT.
Real-time PCR for islets HSP25
Total RNA was extracted from pooled islets isolated from groups of five mice using RNA-STAT-60. Contaminating genomic DNA was removed with ribonuclease-free deoxyribonuclease I (QIAGEN), followed by RNA cleanup with RNeasy kits (QIAGEN). A total of 0.5 μg total RNA was reverse transcribed in a reaction with random primer (Roche Diagnostics Corp., Indianapolis, IN) for 1 h at 37 C using the Omniscript reverse transcriptase kit. Real-time PCR was performed in an Applied Biosystems (Foster City, CA) instrument using SYBR green JumpStart Taq ReadyMix (Sigma-Aldrich Corp., St. Louis, MO). Primer sequences for mouse islets HSP25 were: forward, 5′-CAGGACGAACATGGCTACA-3′; and reverse, 5′-AGAGCGCACAGATTGACAG-3′. Sample values were generated against a standard curve created with the same gene primer pair and normalized to 18S values generated from the same cDNA samples. Data were expressed as relative fold change compared with control.
HSP25/27 immunoprecipitation
To examine HSP25-HSP27 interactions, isolated islets from 12 HSP27TG or WT mice were pooled separately. Islets were washed and resuspended in modified RIPA buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 20 mm sodium fluoride) with protease inhibitors. Islet disruption was accomplished by forcing lysate through a 25-gauge needle, freezing, and thawing. A total of 500 μg islet protein was immunoprecipitated (IP) with 5 μl polyclonal anti-HSP25 or anti-HSP27 antibody overnight at 4 C, then incubated for 2 h with protein A agarose, centrifuged, washed, resuspended in 2× loading buffer, and subjected to SDS-PAGE and immunoblotting with rabbit anti-HSP27 or anti-HSP25 primary antibody and peroxidase-conjugated goat-antirabbit IgG secondary antibody.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) and iNOS staining
To compare apoptosis in WT vs. TG mouse islets after SZ injection, TUNEL staining was performed on paraffin-embedded tissue sections. Eight to 12-wk-old HSP27TG and WT male littermates were injected with SZ ip at a concentration of 50 mg/kg for 5 consecutive days. Mice were killed on d 5 and 8–13 (d 1 corresponds to the day of first SZ injection). Mouse pancreas was isolated, fixed, sectioned, and TUNEL stained. The TUNEL assay was performed with the ApopTag plus Peroxidase In Situ Apoptosis Detection Kit (CHEMICON International) as per manufacturer’s instructions. The number of islets and apoptotic cells per section were quantified as per Stosic-Grujicic et al. (57). The apoptosis was scored as the average number of TUNEL-positive cells per islet. Scoring was performed by an observer masked to the mouse group and day of killing. The data were expressed as the relative fold change of TUNEL-positive cells per islet in the WT (n = 3) compared with the HSP27TG group (n = 5). iNOS was stained on d 5 tissue using polyclonal antibody (Assay Designs, Ann Arbor, MI). For each islet, the staining area was determined morphometrically using ImageJ software (National Institutes of Health, Bethesda, MD). INOS-positive cells were counted. Results are expressed as iNOS positive cells/1000 μm2 islet area.
Islet isolation
Mice were anesthetized, the abdominal cavity opened, and the common bile duct clamped, cannulated, and injected with 3 ml collagenase P 1 mg/ml (Roche Diagnostics) in Hanks’ balanced salt solution (HBSS) to inflate the pancreas. The pancreas was isolated, and fatty tissue, lymph nodes, and blood clots were removed, collected in two groups (WT and TG), and agitated for 18 min at 37 C. Preparations were homogenized, strained, washed with HBSS plus 2% fetal bovine serum, and passed through an 11–26% Ficoll gradient (Sigma-Aldrich) to purify islets. Islets were collected, washed three times with HBSS plus 10% fetal bovine serum, and used.
Islet incubation in cytokines
To assess differential responses to cytokine-induced apoptosis in cultured WT and HSP27TG islets, cytokine incubation was performed according to published studies (5,6,7,8,9). Islets were cultured in RPMI 1640 containing 25 mm glucose (high glucose, HG) for 24 h, followed by glucose 5.5 mm with or without cytokines for 48 h [recombinant human IL-1β 50 U/ml; recombinant mouse TNF-α 1000 U/ml (both Biosource, Camarillo, CA); and γ-IFN 1000 U/ml (Sigma-Aldrich)]. Harvested islets were resuspended in modified RIPA buffer without additional protease or phosphatase inhibitors.
Caspase-3 activity assay
Caspase-3 activity was performed in 96-well plates and measured using tetrapeptide p-nitroanilide (pNA) substrate in a colorimetric assay (58). Briefly, 50 μl tissue lysates (150 μg protein) were mixed with 50 μl assay buffer [250 mm HEPES, 1 mm diethylenetriamine, 50 mm KCl, 2 mm dithiothreitol, and 0.01% 3-[(3-chloroamidopropyl)dimethylammonio]-1-propane-sulfonate (pH 7.4)] for 5 min by shaking at room temperature. A total of 50 μl lysate buffer was used as a control. Five microliters of 1 mm substrate solution were added to each well and mixed again for 5 min at room temperature. The plate was incubated at 37 C for 60 min. Assay peptide substrate was Ac-Asp-Glu-Val-Asp-pNA (Ac-DEVD-pNA; Bachem California Inc., Torrance, CA). Absorbance was measured at 405 nm using a microtiter plate reader (Molecular Devices, Sunnyvale, CA). Results were expressed as the relative mean OD/150 μg protein fold changes of treated to untreated control samples.
Isoelectric focusing (IEF)
IEF was performed to assess HSP25/27 phosphorylation as previously described (56) using polyclonal anti-HSP25 (1:5000) and HSP27 (1:20,000) antibodies. Horseradish peroxidase-conjugated secondary antibody was used for anti-HSP25 (1:10,000) and anti-HSP27 (1:50,000).
Islet HSP25/HSP27 coprecipitates with inhibitory κB protein kinase (IKK) γ [nuclear factor κB essential modulator (NEMO)]
Islet lysates were prepared as described previously for HSP25/27 immunoprecipitation. A total of 500 μg WT or HSP27 TG islet protein was IP with 2.5 μl rabbit anti-HSP25 (Stressgen) and 2.5 μl rabbit anti-HSP27 antibodies overnight at 4 C, incubated for 2 h with protein A agarose, centrifuged, washed, resuspended in 2× loading buffer, subjected to SDS-PAGE, and immunoblotted with goat anti-IKKγ primary antibody (R&D Systems, Inc., Minneapolis, MN) and peroxidase-conjugated donkey antigoat IgG secondary antibody.
Statistics
Results are expressed as mean ± sd. Statistical analysis used SPSS for Windows Version 7.51 (SPSS, Inc., Chicago, IL). Results were analyzed using ANOVA, the Student’s t test, or χ2 analysis where appropriate.
Results
HSP27TG model characterization
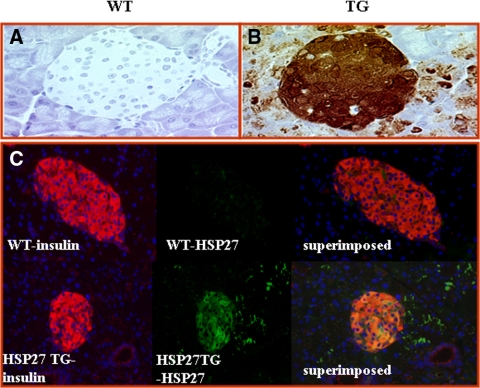
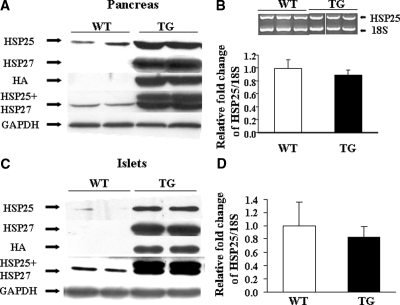
The exocrine pancreas and islets of WT and HSP27TG mice were of the same size, structure, and shape. Staining for HSP27 in WT mice (Fig. 1A) (and for the HA tag, data not shown) was absent. In TG mice, HSP27 was highly (but not exclusively) expressed in islets (Fig. 1B). Insulin and HSP27 double immunostaining shows the HSP27 expressed predominantly in pancreatic β-cells (Fig. 1C) (red = insulin; green = HSP27TG; orange confluence shows β-cells expressing both insulin and HSP27). HSP27 expression in the strain backcrossed to DBA2J was similar (data not shown). There were no significant differences in uninjected WT vs. HSP27TG mice in fasting glucose (65 ± 19 vs. 78 ± 20 mg/dl; P > 0.05), serum insulin to glucose ratio (2.1 ± 2.2 vs. 1.7 ± 1.8; P > 0.05), or pancreatic insulin content (119 ± 24 vs. 112 ± 12 ng insulin/mg protein; P = 0.6). To measure pancreatic insulin content after SZ injections, five male WT and four male HSP27 TG mice were injected with SZ at 50 mg/kg daily for 5 d and killed after 1 wk. The mean blood glucose was 97 ± 20 mg/dl in HSP27TG mice and 275 ± 20 mg/dl in WT (P < 10−5). The mean pancreas insulin content in HSP27 TG mice was 579 ± 148 vs. 393 ± 22 ng in WT (P = 0.02). The mean pancreas insulin to protein ratio was 44% higher in HSP27TG vs. WT mice (25.1 ± 3.6 vs. 14.0 ± 0.6 ng/mg; P = 0.01), respectively. These data demonstrate predominant localization of HSP27TG in β-cells. Baseline pancreas insulin content did not differ in the WT and TG mouse strains, but pancreas insulin content was lower in WT than HSP27TG after SZ injections. These data are consistent with the hypothesis that HSP27 may prevent SZ-DM by protecting β-cells.
Figure 1.
Localization of HSP27 in the pancreas from transgenic mice. A, Immunohistochemistry demonstrates no HSP27 staining in the WT islet or acinar cells. B, Heavy HSP27 staining (brown staining in a cytoplasmic pattern) in the pancreatic islet of the HSP27TG mouse. There was some additional staining in parenchymal cells. C, Immunofluorescence staining shows insulin (red) in pancreatic β-cells in the WT and HSP27TG mice. HSP27 stains (green) only in the HSP27TG mouse islet, and not the WT. Superimposition demonstrates confluence in the HSP27TG mouse islet, confirming the presence of the HSP27 transgene in β-cells in the HSP27TG mice.
HSP27TG mice are resistant to SZ-DM induction
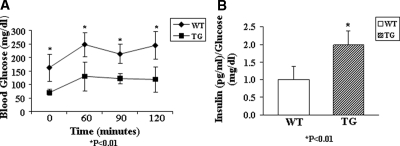
HSP27TG and WT control littermates received 50 mg/kg SZ ip daily to induce DM. In the C57BL10xCBA/Ca strain, DM diagnosis was based on a random glucose of 200 mg/dl or more. In WT mice, nine of 22 developed DM after five injections, eight of 22 developed DM after 12 injections, and five of 22 failed to develop DM even after 12 injections. In the HSP27TG mice, three of 18 developed DM after five injections, two of 18 developed DM after 12 injections, and 13 of 18 failed to develop DM even after 12 injections (χ2, P = 0.007). To evaluate HSP27 effects on SZ-DM in a more susceptible strain, and to develop a better model to study diabetic complications, the hybrid strain was backcrossed to DBA2J mice (38) and studied at the fifth generation or beyond. In these mice the DM diagnosis was based on ip GTT (2 g/kg glucose) performed 2 wk after the last of five SZ injections. There was dramatic DM attenuation in the HSP27TGxDBA2J mice (n = 5) vs. DBA2J WT littermates (n = 8) (mean ± sd, Fig. 2A). Transgenic mice also had significantly higher fasting serum insulin to glucose ratios compared with WT littermates (Fig. 2B). These data show robust protection from SZ-DM in two mouse strains that overexpress HSP27.
Figure 2.
HSP27TG mice are relatively resistant to the development of SZ-DM. A, In HSP27TG mice bred on a C57BL10/CBAxCa background backcrossed to DBA2J mice for five generations, SZ-DM was successfully induced with a lower dose and shorter duration of SZ injections than the parent HSP27TG strain. Intraperitoneal GTT (2 g/kg glucose) performed on the fifth generation (F5) backcrossed 2 wk after the last SZ injection showed dramatic attenuation of SZ-DM in the HSP27TG mice (n = 5) vs. DBA2J WT littermates (n = 8) (mean ± sd). *, P < 0.01. B, The transgenic mice showed significantly higher (∼2 fold) fasting insulin to glucose ratios.
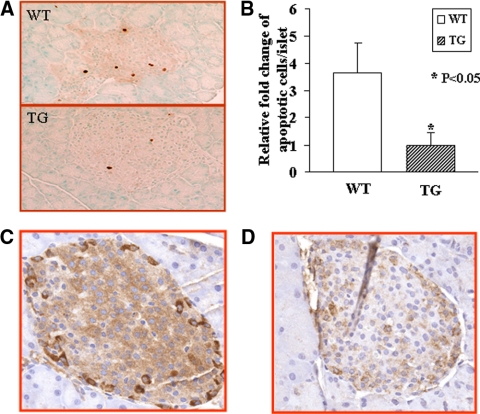
Islet apoptosis is attenuated in HSP27TG compared with WT mice. Pancreas TUNEL staining showed more apoptotic cells in WT islets compared with HSP27TG islets (3.7 fold; P < 0.05) 5–8 d after the first SZ injection (Fig. 3A). A total of 23% WT islets and 6% HSP27TG islets had at least one TUNEL-positive cell, although the number of cells per positive islet was low, averaging 3.0 in WT and 1.4 in HSP27TG at d 5–8. There was approximately 2-fold more islet iNOS staining in WT than HSP27TG (Fig. 3, C and D) immediately after the fifth SZ injection. Measured as iNOS positive cells/1000 μm2 islet area, there were 0.55 ± 0.47 cells in WT and 0.29 ± 0.35 in HSP27TG (P < 0.05). These data are consistent with a protective effect of HSP27 in attenuating islet apoptosis.
Figure 3.
Islet apoptotic cells and iNOS staining are more abundant in WT mice compared with TG mice after SZ injection. A, A representative photomicrograph of TUNEL staining demonstrated more islet apoptosis in WT than TG mice at 5–8 d after the first SZ injection. B, Quantification analysis of TUNEL staining showed that the apoptotic islet cells per islet in WT mice was approximately 3.6-fold of that in TG mice (P < 0.05). C, WT. There is low-level iNOS staining in the islet with numerous strongly staining cells. D, TG. All iNOS staining is significantly reduced (original magnification, ×400).
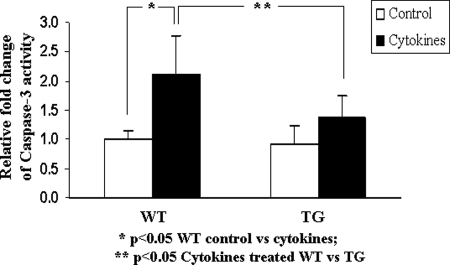
Caspase-3 activity is lower in islets from HSP27TG than WT mice after incubation with HG medium (25 mm) followed by glucose 5.5 mm plus cytokines in vitro
Figure 4 shows that in islets cultured for 24 h in HG, followed by 5.5 mm glucose plus cytokines (IL-1β 50 U/ml; TNF-α 1000 U/ml; γ-IFN 1000 U/ml) for 48 h, caspase-3 activity increases significantly in WT but not TG islets. These data demonstrate that HSP27 overexpression confers direct protection from cytokine-induced apoptotic injury to islets.
Figure 4.
Islets from HSP27TG mice experience less apoptosis from cytokines stress than WT. Isolated islets were incubated with 25 mm glucose medium for 24 h, followed by 5.5 mm glucose medium with cytokines (IL-1β 50 U/ml; TNF-α 1000 U/ml; γ-IFN 1000 U/ml) for 48 h. Cultured islets from WT mice showed higher caspase-3 activity than HSP27TG islets, demonstrating more apoptosis in WT than HSP27TG. The increment observed in cytokine-exposed HSP27TG islets compared with controls failed to reach statistical significance.
Both HSP27 and HSP25 protein levels are increased in pancreas and islets in HSP27TG mice; HSP25 mRNA is not increased
Immunoblotting demonstrated no HSP27 or HA in WT mice. The amount of pancreatic HSP27 in TG mice was approximately 6-fold higher than the amount of HSP25 in WT. Pancreatic HSP25 in the TG mice was also approximately 6.5-fold higher than pancreatic HSP25 in WT. Corrected to GAPDH, there was approximately 12.5-fold higher total pancreatic HSP25 plus 27 in TG mice compared with the amount of HSP25 in WT (P < 0.01) (Fig. 5A). Similar to pancreas, in isolated islets from HSP27TG mice, the amount of HSP25 and HSP27 was respectively 8.2- and 10.5-fold higher than the amount of HSP25 in WT islets. Together, HSP25 plus 27 in isolated TG mouse islets was increased approximately 18.7-fold compared with HSP25 in WT (P < 0.01) (Fig. 5C), consistent with preferential expression in islets. Relative quantitative RT-PCR on whole pancreas and real-time PCR on islets showed similar native HSP25 mRNA expression in WT and TG (Fig. 5, B and D). The increment in HSP25 in HSP27TG mice was unanticipated. Experiments to determine whether the HSP25 increase reflected a nonspecific injury signal due to the expression of nonmurine HSP27 protein in mouse cells did not show an increase in HSP25 (assessed by immunoblotting) in mice overexpressing bacterial β-galactosidase (data not shown). These data show a large increase in HSP25/27 in islets from HSP27TG mice, and reveal increased native HSP25 unaccompanied by an increment in HSP25 mRNA. The data suggest a posttranscriptional mechanism for the HSP25 increment.
Figure 5.
Relative amounts of HSP25 and HSP27 in both pancreas and isolated islets from WT and HSP27TG mice. A, In the pancreas of HSP27TG mice, there was a 6.5-fold increment in native HSP25 and a 6.0-fold increment in the HSP27 transgene. B, Quantified by relative RT-PCR, pancreatic mRNA levels for HSP25 corrected for ribosomal 18S mRNA in the WT and HSP27TG mice were similar. C, In islets of HSP27TG mice, the amount of HSP25 and HSP27 was approximately 8.2- and 10.5-fold the amount of HSP25 in WT, respectively. Total HSP25 plus 27 in the TG mouse islets was 18.7-fold the amount of HSP25 in WT (P < 0.01). D, Real-time PCR performed in islets showed similar native HSP25 mRNA expression in both WT and HSP27TG mice.
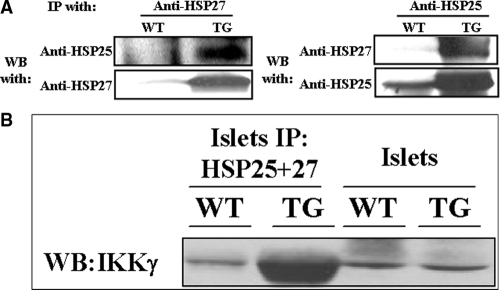
HSP27 oligomerizes with native HSP25
To define further a mechanism for high HSP25 protein levels in HSP27TG mice, immunoprecipitation experiments were performed in isolated islets from HSP27TG and WT mice (Fig. 6A). These experiments demonstrated that HSP27 oligomerizes with native HSP25, presumably inducing heterodimeric complexes that stabilize HSP25, increasing its cellular content.
Figure 6.
HSP27 oligomerizes with native HSP25 and coprecipitates with IKKγ (NEMO). A, WT and HSP27TG islet lysates were precipitated with either anti-HSP27 antibody (left panel) or anti-HSP25 antibody (right panel), and subsequently blotted with anti-HSP25 antibody (left panel) and anti-HSP27 antibody (right panel), respectively. Results show HSP25 and HSP27 coprecipitating in isolated islets from HSP27TG mice. B, WT and HSP27TG islet lysates were IP with anti-HSP25 and anti-HSP27 antibodies (HSP25 plus 27) simultaneously, and then immunoblotted with anti-IKKγ antibody. The left lane is the WT control showing that a small amount of IKKγ coprecipitates with native HSP25.The next lane indicates that dramatically more IKKγ is IP by overexpressed HSP27 together with native HSP25 in islets from the HSP27TG. The last two lanes are IKKγ controls from islet lysates. WB, Western blot.
Relative phosphorylation of HSP25/27 is similar in HSP27TG and WT mice
Whether protection from apoptosis is conferred by HSP25/27 quantity, oligomerization, or phosphorylation is controversial (54). In these studies, IEF of islet lysates showed no increase in the relative phosphorylation of HSP25/HSP27 in HSP27TG mice compared with WT (data not shown), although there was an absolute increase in phosphorylated HSP25/HSP27 attributable to the increase in total HSP25/HSP27. These data show that increased phosphorylated HSP25/HSP27 in TG mice was accounted for by the increase in total HSP25/HSP27. The data further suggest that relative phosphorylation of HSP27 is not a critical factor in determining the effect of HSP27 on β-cell apoptosis. It does not exclude a contribution from absolute increments in HSP27 phosphorylation.
Islet HSP27 coprecipitates with IKKγ (NEMO)
WT and HSP27TG islet lysates were IP with anti-HSP25 and anti-HSP27 antibodies (HSP25 plus 27) simultaneously, and then immunoblotted with anti-IKKγ antibody. These data show that in islets, both native HSP25 and constitutively overexpressed HSP27 bind to IKKγ (NEMO) (Fig. 6B).
Discussion
We report protection from SZ-DM in HSP27TG mice, whose pancreatic anatomy, morphology, and insulin content are otherwise indistinguishable from WT. Rodent and human β-cells are deficient in HSP25/27 relative to other cells (51). Nevertheless, HSP25 is induced in rat β-cells exposed to cytokines (14), suggesting an adaptive role, even in β-cells. Our data show that HSP27 overexpression confers robust protection from SZ-DM. There were fewer apoptotic cells and less iNOS staining in islets from HSP27TG mice vs. WT after SZ injections, at time points shown by others to be a peak for apoptosis in SZ-DM (1). After SZ, HSP27TG mice had more total pancreatic insulin and more insulin per mg pancreas protein compared with WT, consistent with a mechanism involving β-cell protection. TG mice had a 10.5-fold increase in islet total HSP27 relative to the amount of HSP25 in WT controls, and an unanticipated 8.2-fold increment in native HSP25, yielding approximately 18.7-fold increase in total HSP25 plus 27. Native HSP25 oligomerized with transgenic HSP27 in pancreatic islets from TG mice and HSP25 mRNA was not increased, implicating HSP25-HSP27 oligomerization as contributing to the HSP25 increment. Both HSP25 and HSP27 also co-IP with IKKγ (NEMO). In vitro, in cytokine-exposed isolated TG islets, caspase-3 activity was lower than in WT. Together, these data show for the first time that constitutive HSP27 overexpression protects against apoptotic cell death in cytokine-stressed islets and confers robust protection against SZ-DM, possibly through IKKγ (NEMO) mediated regulation of NFκB activation. Although much attention focuses on immunological strategies to mitigate cytokine-induced islet and β-cell injury and death, these data show mitigation through up-regulation of an endogenous protective pathway not dependent upon modulation of the immune system.
Abundant data show that HSP25/27 is antiapoptotic (25,26,27,28,29,30,31,32,33,34,35,36,37). Most of the antiapoptotic actions of HSP27 are phosphorylation independent (49,54). Homozygosity for an HSP25 knockout mutation is lethal due to apoptosis of embryonic stem cells (25,26). HSP27 inhibits apoptosis in motor neurons subjected to mechanical injury (26,28) and inhibits kainite-induced seizures by protection from neuronal apoptosis (27,28,29). In a different strain of HSP27TG mice than the ones used herein, in which the HSP27TG was expressed abundantly in pancreatic acinar cells, HSP27 overexpression attenuated cerulean-induced pancreatitis (30,31). HSP27 reduces apoptosis in breast cancer cells (59), retinal neurons (31), bronchial epithelial and endothelial cells (33,34), and myeloma cells exposed to corticosteroids (35). HSP27 overexpression in myocardium protected against infarction after reperfusion/ischemia (36). Overexpression of a phosphorylation-defective mutant HSP27 in LLC-PK1 renal tubular epithelial cells exposed to a chemical toxin accelerated the onset of apoptosis, presumably by acting as a dominant negative (37).
The attenuation of apoptosis by HSP25/27 is at least partially understood. HSP25/27 inhibits the intrinsic/mitochondrial apoptotic pathway at multiple steps. HSP25/27 inhibits the initiation of mitochondrial outer membrane permeability by participating in a multimolecular complex that includes pAkt and HSP90, which is upstream to and activates the phosphorylated Bad/14-3-3 complex, thereby stabilizing the Bax pore (60). The interaction between HSP25/27 and pAkt confers resistance to apoptosis in cells with high HSP25/27 content (47,61). In neutrophils, HSP27 forms a trimolecular complex with Akt and p38MAPK. HSP27 phosphorylation by Akt dissociates HSP27 from the complex, initiating apoptosis (48).
HSP25/27 also inhibits the intrinsic/mitochondrial apoptotic pathway downstream of mitochondrial outer membrane permeability by binding to and inactivating cytochrome c (42,43,45). One report (62), but not another (39), showed that HSP27 blocks cytochrome c release. HSP27 glyoxalation, which facilitates HSP27 oligomerization, inhibits cytochrome c-dependent apoptosis, demonstrating the importance of HSP27 oligomers for antiapoptosis (63). HSP25/27 also impedes apoptosis by binding to and inactivating procaspase-3 (42,45).
HSP25/27 further inhibits apoptosis mediated by death-domain pathways by preventing DAXX binding to the Fas-Fas ligand death domain, a required step in noncaspase mediated Fas-Fas ligand death domain activation (64). An HSP25/27-DAXX interaction may also prevent DAXX translocation from the nucleus (41,64). As part of a trimolecular complex with pAkt and HSP90, early reports suggested that HSP27 inhibited TNFα-induced apoptosis (25,39,41,62,64).
Recently, the antiapoptotic activity of HSP27 in cells exposed to TNFα has been ascribed to enhanced IKKγ proteasomal degradation, which increases NFκB activity in some cells (50), whereas potentially decreasing it in others (49). Islet susceptibility to iNOS, TNFα, NFκB activation, and apoptosis differs among insulinoma cell lines and islets across species. Most (14,65,66,67,68), but not all (69,70), data show that activated NFκB is apoptotic in islets and β-cells. The data in islets presented herein show that HSP25 and constitutively overexpressed HSP27 interact with their physiological target, IKKγ (NEMO), as reported by Parcellier (50). Our studies demonstrating binding of HSP25/27 to IKKγ (NEMO), a key regulator of NFκB activation, suggest a mechanism whereby overexpressed HSP27 may modulate cytokine-mediated apoptotic pathways in islets. These data suggest that the antiapoptotic effect of HSP27 in islets may be mediated, at least in part, by upstream regulatory effects on NFκB signaling.
Oligomers of HSP25/27 mediate many of its antiapoptotic and cell-cycle regulatory functions. The study reported herein shows oligomerization of HSP27 with native HSP25, increasing total HSP25/27 content by approximately 18-fold. Moreover, as validation of these findings, HSP25 protein overexpression in another HSP27TG mouse line is suggested by the published results of others (71). In the latter work, which used a different HSP27 overexpressing mouse line than the ones studied herein, HSP25 was increased visually approximately 2-fold (by Western blotting) in the cardiac tissue of HSP27TG mice compared with WT, but the investigators comment little upon this increment. In contrast to our report, in which the increment in HSP25 was approximately equivalent to the increment in HSP27, the relative increase in HSP25 in cardiac tissue was substantially less than the increase in HSP27, but the Western blot was not subjected to densitometry, nor were studies of oligomerization performed. Cardiac mRNA levels for HSP25 visually appeared to be little if at all increased in the WT compared with HSP27TG mice, but densitometry of the PCR experiments were also not reported. Our experimental results are similar to the cardiac study in direction, if not magnitude, for HSP25 protein, and identical for mRNA, but our experiments formally analyzed mRNA levels and protein oligomerization. In so doing, our experiments suggest that a posttranscriptional change may contribute to the overall increment in total HSP25 and 27 protein, thereby further contributing to the overall effect.
The interpretation of our data is subject to some limitations. The β-actin promoter used to generate the HSP27TG mouse line induces random expression in tissues. Overexpression in cells besides β-cells may contribute to protection from SZ-DM. However, the in vitro protection from apoptosis in cytokine-stressed islets isolated from the TG mice demonstrated herein directly implicates islet HSP27 overexpression in the protection observed.
These studies are the first to demonstrate that constitutive HSP27 overexpression protects islets from cytokine-induced injury in vitro and attenuates SZ-DM in vivo by inhibiting apoptosis. This antiapoptotic effect may be mediated, at least in part, by direct interactions between HSP25/27 and IKKγ (NEMO), the latter a critical upstream regulator of NFκB activation. The small difference in pancreatic insulin content between HSP27TG and WT mice after SZ, coupled to the robust difference in DM induction, suggests either a threshold effect or the possibility of an additional peripheral contribution to the observed protection in islets. A number of compounds increase endogenous HSP25/27 levels in vitro and in vivo, including estradiol (52), curcumin (53), and the proteasomal inhibitors lactacystin and MG132 (54,55). Our data are consistent with the large body of evidence showing that HSP25/27 uniformly and robustly protects from apoptosis, suggesting a novel method of islet protection based on this approach.
Acknowledgments
We thank Dr. Luo Xunrong at Northwestern University Medical School (Chicago, IL) for teaching islet isolation, and Donna Isbel at Beckman Research Institute of City of Hope (Duarte, CA) for outstanding mouse backcross services.
Footnotes
This work was supported by grants from the American Diabetes Association (to S.G.A.), the Juvenile Diabetes Research Foundation (to M.P.-C.), National Institutes of Health Grant AT002945 (to S.G.A.), and the University of California Los Angeles Diabetes and Endocrine Research Center.
Disclosure Summary: The authors have nothing to declare.
First Published Online March 26, 2009
Abbreviations: DM, Diabetes mellitus; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GTT, glucose tolerance testing; HA, hydroxyapatite; HBSS, Hanks’ balanced salt solution; HG, high glucose; HSP, heat shock protein; IEF, isoelectric focusing; IFN, interferon; IKK, inhibitory κB protein kinase; iNOS, inducible nitric oxide synthase; IP, immunoprecipitated; NEMO, nuclear factor κB essential modulator; NFκB, nuclear factor κB; pNA, p-nitroanilide; RIPA, radioimmunoprecipitation assay; SZ, streptozotocin; TG, transgene/transgenic; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling; WT, wild type.
References
- O'Brien BA, Harmon BV, Cameron DP, Allan DJ 1996 β-Cell apoptosis is responsible for the development of IDDM in the multiple low-dose streptozotocin model. J Pathol 178:176–181 [DOI] [PubMed] [Google Scholar]
- Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L 2000 Cell loss in isolated human islets occurs by apoptosis. Pancreas 20:270–276 [DOI] [PubMed] [Google Scholar]
- Mathis D, Vence L, Benoist C 2001 β-Cell death during progression to diabetes. Nature 414:792–798 [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Gysemans CA, Ladrière L, Callewaert H, Rasschaert J, Flamez D, Levy DE, Matthys P, Eizirik DL, Mathieu C 2005 Disruption of the γ-interferon signaling pathway at the level of signal transducer and activator of transcription-1 prevents immune destruction of β-cells. Diabetes 54:2396–2403 [DOI] [PubMed] [Google Scholar]
- Liu D, Pavlovic D, Chen M, Flodström M, Sandler S, Eizirik D 2000 Cytokines induce apoptosis in β-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS−/−). Diabetes 49:1116–1122 [DOI] [PubMed] [Google Scholar]
- Cetkovic-Cvrlje M, Eizirik DL 1994 TNF-α and IFN-γ potentiate the deleterious effects of IL-1β on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine 6:399–406 [DOI] [PubMed] [Google Scholar]
- Mellado-Gil JM, Aguilar-Diosdado M 2004 High glucose potentiates cytokine- and streptozotocin-induced apoptosis of rat islet cells: effect on apoptosis-related genes. J Endocrinol 183:155–162 [DOI] [PubMed] [Google Scholar]
- Mellado-Gil JM, Aguilar-Diosdado M 2005 Assay for high glucose-mediated islet cell sensitization to apoptosis induced by streptozotocin and cytokines. Biol Proced Online 7:162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup-Poulsen T, Bendtzen K, Dinarello CA, Nerup J 1987 Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic β-cell cytotoxicity. J Immunol 139:4077–4082 [PubMed] [Google Scholar]
- Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M 1986 Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science 232:1545–1547 [DOI] [PubMed] [Google Scholar]
- Rabinovitch A, Suarez-Pinzon WL 1998 Cytokines and their roles in pancreatic islet β-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol 55:1139–1149 [DOI] [PubMed] [Google Scholar]
- Suk K, Kim S, Kim YH, Kim KA, Chang I, Yagita H, Shong M, Lee MS 2001 IFN-γ/TNF-α synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic β cell death. J Immunol 166:4481–4489 [DOI] [PubMed] [Google Scholar]
- Cardozo AK, Heimberg H, Heremans Y, Leeman R, Kutlu B, Kruhøffer M, Ørntoft T, Eizirik DL 2001 A comprehensive analysis of cytokine-induced and nuclear factor-κ B-dependent genes in primary rat pancreatic β-cells. J Biol Chem 276:48879–48886 [DOI] [PubMed] [Google Scholar]
- Karlsen AE, Rønn SG, Lindberg K, Johannesen J, Galsgaard ED, Pociot F, Nielsen JH, Mandrup-Poulsen T, Nerup J, Billestrup N 2001 Suppressor of cytokine signaling 3 (SOCS-3) protects β-cells against interleukin-1β- and interferon-γ-mediated toxicity. Proc Natl Acad Sci USA 98:12191–12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini KS, Thompson C, Winterford CM, Walker NI, Cameron DP 1996 Streptozotocin at low doses induces apoptosis and at high doses causes necrosis in a murine pancreatic β cell line, INS-1. Biochem Mol Biol Int 39:1229–1236 [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Eisenbarth GS 2001 Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358:221–229 [DOI] [PubMed] [Google Scholar]
- Szkudelski T 2001 The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:536–546 [PubMed] [Google Scholar]
- Mabley JG, Haskó G, Liaudet L, Soriano F, Southan GJ, Salzman AL, Szabó C 2002 NFκB1 (p50)-deficient mice are not susceptible to multiple low-dose streptozotocin-induced diabetes. J Endocrinol 173:457–464 [DOI] [PubMed] [Google Scholar]
- Satoh J, Kim SU 1995 Cytokine and growth factors induce HSP27 phosphorylation in human astrocytes. J Neuropathol Exp Neurol 54:504–512 [DOI] [PubMed] [Google Scholar]
- Bongalon S, Dai YP, Singer CA, Yamboliev IA 2004 PDGF and IL-1β upregulate cofilin and LIMK2 in canine cultured pulmonary artery smooth muscle cells. J Vasc Res 41:412–421 [DOI] [PubMed] [Google Scholar]
- Radloff M, Delling M, Marti T, Gercken G 1998 Protein phosphorylation in alveolar macrophages after stimulation with heavy metal-coated silica particles. Biochem Biophys Res Commun 96- 97:69–75 [DOI] [PubMed] [Google Scholar]
- Smoyer WE, Ransom R, Harris RC, Welsh MJ, Lutsch G, Benndorf R 2000 Ischemic acute renal failure induces differential expression of small heat shock proteins. J Am Soc Nephrol 11:211–221 [DOI] [PubMed] [Google Scholar]
- Brophy CM, Woodrum D, Dickinson M, Beall A 1998 Thrombin activates MAPKAP2 kinase in vascular smooth muscle. J Vasc Surg 27:963–969 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mehlen A, Godet J, Arrigo AP 1997 hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem 272:31657–31665 [DOI] [PubMed] [Google Scholar]
- Benn SC, Perrelet D, Kato AC, Scholz J, Decosterd I, Mannion RJ, Bakowska JC, Woolf CJ 2002 Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron 36:45–56 [DOI] [PubMed] [Google Scholar]
- Akbar MT, Lundberg AM, Liu K, Vidyadaran S, Wells KE, Dolatshad H, Wynn S, Wells DJ, Latchman DS, de Belleroche J 2003 The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J Biol Chem 278:19956–19965 [DOI] [PubMed] [Google Scholar]
- Akbar MT, Wells DJ, Latchman DS, de Belleroche J 2001 Heat shock protein 27 shows a distinctive widespread spatial and temporal pattern of induction in CNS glial and neuronal cells compared to heat shock protein 70 and caspase 3 following kainate administration. Brain Res Mol Brain Res 93:148–163 [DOI] [PubMed] [Google Scholar]
- Kalwy SA, Akbar MT, Coffin RS, de Belleroche J, Latchman DS 2003 Heat shock protein 27 delivered via a herpes simplex virus vector can protect neurons of the hippocampus against kainic-acid-induced cell loss. Brain Res Mol Brain Res 111:91–103 [DOI] [PubMed] [Google Scholar]
- Kubisch C, Dimagno MJ, Tietz AB, Welsh MJ, Ernst SA, Brandt-Nedelev B, Diebold J, Wagner AC, Göke B, Williams JA, Schäfer C 2004 Overexpression of heat shock protein Hsp27 protects against cerulein-induced pancreatitis. Gastroenterology 127:275–286 [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB 2000 The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells. J Neurosci 20:3552–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LF, Tiffee JC, Griffin JP, Hamano H, Guo Z 2000 Estrogen-induced resistance to osteoblast apoptosis is associated with increased hsp27 expression. J Cell Physiol 185:401–407 [DOI] [PubMed] [Google Scholar]
- Merendino AM, Paul C, Vignola AM, Costa MA, Melis M, Chiappara G, Izzo V, Bousquet J, Arrigo AP 2002 Heat shock protein-27 protects human bronchial epithelial cells against oxidative stress-mediated apoptosis: possible implication in asthma. Cell Stress Chaperones 7:269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabakov AE, Budagova KR, Bryantsev AL, Latchman DS 2003 Heat shock protein 70 or heat shock protein 27 overexpressed in human endothelial cells during posthypoxic reoxygenation can protect from delayed apoptosis. Cell Stress Chaperones 8:335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Li G, Auclair D, Hideshima T, Podar K, Mitsiades N, Mitsiades C, Chen LB, Munshi N, Saxena S, Anderson KC 2004 2-Methoxyestardiol and bortezomib/proteasome-inhibitor overcome dexamethasone-resistance in multiple myeloma cells by modulating heat shock protein-27. Apoptosis 9:149–155 [DOI] [PubMed] [Google Scholar]
- Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM 2004 Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol 99:392–394 [DOI] [PubMed] [Google Scholar]
- de Graauw M, Tijdens I, Cramer R, Corless S, Timms JF, van de Water B 2005 Heat shock protein 27 is the major differentially phosphorylated protein involved in renal epithelial cellular stress response and controls focal adhesion organization and apoptosis. J Biol Chem 280:29885–29898 [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C 2005 Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal 7:414–422 [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C 2000. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2:645–652 [DOI] [PubMed] [Google Scholar]
- Charette SJ, Landry J 2000 The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann NY Acad Sci 926:126–131 [DOI] [PubMed] [Google Scholar]
- Charette SJ, Lavoie JN, Lambert H, Landry J 2000 Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 20:7602–7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon CG, Orrenius S, Samali A 2001 Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expr 9:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E 1999 HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J 13:2061–2070 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo AP 1996 Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem 271:16510–16514 [DOI] [PubMed] [Google Scholar]
- Pandey P, Farber R, Nakazawa A, Kumar S, Bharti A, Nalin C, Weichselbaum R, Kufe D, Kharbanda S 2000 Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 19:1975–1981 [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP 2002 Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol 22:816–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U 1997 Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett 410:493–498 [DOI] [PubMed] [Google Scholar]
- Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB 2003 Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 278:27828–27835 [DOI] [PubMed] [Google Scholar]
- Park KJ, Gaynor RB, Kwak YT 2003 Heat shock protein 27 association with the I κ B kinase complex regulates tumor necrosis factor α-induced NF-κ B activation. J Biol Chem 278:35272–35278 [DOI] [PubMed] [Google Scholar]
- Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantôme A, Plenchette S, Khochbin S, Solary E, Garrido C 2003 HSP27 is a ubiquitin-binding protein involved in I-κBα proteasomal degradation. Mol Cell Biol 23:5790–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T, Iseki S 1998 Expression and cellular localization of the mRNA for the 25-kDa heat-shock protein in the mouse. Cell Biol Int 22:295–304 [DOI] [PubMed] [Google Scholar]
- Matsuda T, Omori K, Vuong T, Pascual M, Valiente L, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F, Mullen Y 2005 Inhibition of p38 pathway suppresses human islet production of pro-inflammatory cytokines and improves islet graft function. Am J Transplant 5:484–493 [DOI] [PubMed] [Google Scholar]
- Omori K, Valiente L, Orr C, Rawson J, Ferreri K, Todorov I, Al-Abdullah IH, Medicherla S, Potter AA, Schreiner GF, Kandeel F, Mullen Y 2007 Improvement of human islet cryopreservation by a p38 MAPK inhibitor. Am J Transplant 7:1224–1232 [DOI] [PubMed] [Google Scholar]
- Garrido C 2002 Size matters: of the small HSP27 and its large oligomers. Cell Death Differ 9:483–485 [DOI] [PubMed] [Google Scholar]
- Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD 2005. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54:2628–2637 [DOI] [PubMed] [Google Scholar]
- Dai T, Natarajan R, Nast CC, LaPage J, Chuang P, Sim J, Tong L, Chamberlin M, Wang S, Adler SG 2006 Glucose and diabetes: effects on podocyte and glomerular p38MAPK, heat shock protein 25, and actin cytoskeleton. Kidney Int 69:806–814 [DOI] [PubMed] [Google Scholar]
- Stosic-Grujicic S, Stojanovic I, Maksimovic-Ivanic D, Momcilovic M, Popadic D, Harhaji L, Miljkovic D, Metz C, Mangano K, Papaccio G, Al-Abed Y, Nicoletti F 2008 Macrophage migration inhibitory factor (MIF) is necessary for progression of autoimmune diabetes mellitus. J Cell Physiol 215:665–675 [DOI] [PubMed] [Google Scholar]
- Datta R, Banach D, Kojima H, Talanian RV, Alnemri ES, Wong WW, Kufe DW 1996 Activation of the CPP32 protease in apoptosis induced by 1-β-D-arabinofuranosylcytosine and other DNA-damaging agents. Blood 88:1936–1943 [PubMed] [Google Scholar]
- Hansen RK, Parra I, Lemieux P, Oesterreich S, Hilsenbeck SG, Fuqua SA 1999 Hsp27 overexpression inhibits doxorubicin-induced apoptosis in human breast cancer cells. Breast Cancer Res Treat 56:187–196 [DOI] [PubMed] [Google Scholar]
- Beere HM 2005 Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest 115:2633–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G 1997 Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385:544–548 [DOI] [PubMed] [Google Scholar]
- Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, Cotgreave IA, Arrigo AP, Orrenius S 2001 Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones 6:49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Mashima T, Yamamoto K, Tsuruo T 2002 Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J Biol Chem 277:45770–45775 [DOI] [PubMed] [Google Scholar]
- Yang X, Khosravi-Far R, Chang HY, Baltimore D 1997 Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MS, Chen X, Cao XC, Kaufman DB 2001 Expression of a dominant negative inhibitor of NF-κB protects MIN6 β-cells from cytokine-induced apoptosis. J Surg Res 97:117–122 [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Rudert WA, Trucco M, Robbins PD 2000 Protection of human islets from the effects of interleukin-1β by adenoviral gene transfer of an Iκ B repressor. J Biol Chem 275:36509–36513 [DOI] [PubMed] [Google Scholar]
- Quan N, Ho E, La W, Tsai YH, Bray T 2001 Administration of NF-κB decoy inhibits pancreatic activation of NF-κB and prevents diabetogenesis by alloxan in mice. FASEB J 15:1616–1618 [DOI] [PubMed] [Google Scholar]
- Heimberg H, Heremans Y, Jobin C, Leemans R, Cardozo AK, Darville M, Eizirik DL 2001 Inhibition of cytokine-induced NF-κB activation by adenovirus-mediated expression of a NF-κB super-repressor prevents β-cell apoptosis. Diabetes 50:2219–2224 [DOI] [PubMed] [Google Scholar]
- Chang I, Kim S, Kim JY, Cho N, Kim YH, Kim HS, Lee MK, Kim KW, Lee MS 2003 Nuclear factor κB protects pancreatic β-cells from tumor necrosis factor-α-mediated apoptosis. Diabetes 52:1169–1175 [DOI] [PubMed] [Google Scholar]
- Stephens LA, Thomas HE, Ming L, Grell M, Darwiche R, Volodin L, Kay TW 1999 Tumor necrosis factor-α-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic β cells. Endocrinology 140:3219–3227 [DOI] [PubMed] [Google Scholar]
- Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH 2004 Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation 110:3544–3552 [DOI] [PubMed] [Google Scholar]