Abstract
Ovary-specific acidic protein (OSAP) is a novel molecule discovered from a genomic project designed to identify ovary-selective genes in mice. Whereas public databases suggest extraovarian expression of OSAP, its tissue distribution has not yet been well documented. Thus, the expression profile of mouse and human OSAP was determined by quantitative real-time RT-PCR using RNAs isolated from various tissues. The results demonstrate that the human and mouse OSAP expression profiles are similar; OSAP is prominently expressed in steroidogenic tissues with the highest level of expression observed in the adrenal gland. Placenta served as an exception and possessed minimal level of OSAP mRNA. Immunohistochemical studies show that mouse OSAP localizes almost exclusively to the steroid-producing cells of the ovary, adrenal gland, and testis. Consistent with predictions made by several subcellular localization algorithms, dual labeling studies in Y-1 mouse adrenocortical cells indicate OSAP resides in the mitochondria. Because of its abundant expression in steroidogenic cells and mitochondrial localization, a role for OSAP in steroidogenesis was determined. OSAP silencing by specific small interfering RNAs significantly inhibits 8-bromoadenosine-cAMP-induced progesterone production in Y-1 cells. Reduction in OSAP levels results in mitochondrial fragmentation and a decrease in the cellular content of mitochondrial DNA, indicative of decreased mitochondrial abundance. Lastly, 8-bromoadenosine-cAMP does not regulate OSAP protein expression in Y-1 cells as is the case for other steroidogenic components known to be induced by cAMP. Collectively these results suggest that OSAP is involved in steroidogenesis, potentially through its ability to maintain mitochondrial abundance and morphology.
The results suggest that ovary-specific acidic protein is involved in steroidogenesis, potentially through its ability to maintain mitochondrial abundance and morphology.
Genes expressed selectively or specifically in a certain organ may play crucial physiological and/or functional roles in that particular tissue. Recently we systemically isolated and cloned genes expressed in an ovary-selective/specific manner. Such was accomplished using suppression subtractive hybridization, resulting in the identification of a total of 83 novel mouse cDNAs (1,2). Those cDNAs were subjected to further determination of ovarian and phase specificity by Northern blot analysis. Among them was ovary-specific acidic protein (OSAP); its name was based on the restricted expression to the ovary and its highly acidic nature (theoretical isoelectric point of 3.9) due to the large number of glutamic acid residues.
OSAP-driven BLASTp analysis of the National Center for Biotechnology Information database (http://blast.ncbi.nlm.nih. gov/blast.cgi) demonstrates orthologs in mammalian species such as the rat, rabbit, cow, and human but thus far fails to detect an ortholog in nonmammalian species. The expression of the OSAP gene, originally determined by Northern blot analysis, proved negative for all the mouse nonovarian samples studied (brain, heart, kidney, spleen, thymus, liver, stomach, small intestine, skeletal muscle, lung, testis, and skin) (1). Thus, the previous Northern blot analysis suggested, subject to the tissue selection and the sensitivity of the approach, that the tissue expression pattern of OSAP is ovary specific. However, a search of the mouse expressed sequence tag database (http://www.ncbi. nlm.nih.gov/dbEST/) discloses that expressed sequence tags derived from tissues other than the ovary exist.
Therefore, we set out to revisit this issue and investigated the expression profile of OSAP in murine and human tissues. Abundant OSAP expression in steroidogenic tissues such as the adrenal gland and ovary, as well as its mitochondrial subcellular localization, prompted us to examine whether OSAP is involved in steroidogenesis.
Materials and Methods
Reagents and animals
Pregnant mare serum gonadotropin (PMSG) was purchased from Calbiochem (San Diego, CA). Human chorionic gonadotropin (hCG) and 8-bromoadenosine cAMP (8-Br-cAMP) were from Sigma (St. Louis, MO). Female and male C57BL/6J mice were obtained from a local breeder and maintained under specific pathogen-free conditions with free access to food and water. All animal experiments were performed in compliance with the relevant laws and institutional guidelines.
RNA isolation and quantitative real-time RT (qRT)-PCR
Total RNA from cultured cells was isolated and purified as described previously (3). Samples of total RNA derived from human and mouse tissues were purchased from CLONTECH Laboratories (Mountain View, CA), Zyagen (San Diego, CA), and BioChain (Hayward, CA). Reverse transcription reactions with random primers were carried out with Omniscript reverse transcriptase (QIAGEN, Valencia, CA) using conditions recommended by the supplier. Real-time RT-PCR assay, based on TaqMan technology previously described (4,5), was adapted to the LightCycler (LC) instrument with a LC FastStart DNA master hybridization probe kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s directions. All reactions were performed in glass capillaries with a final reaction volume of 20 μl. PCR was conducted using the following cycle parameters: 95 C for 10 min and 40 cycles at 95 C for 10 sec, 60 C for 30 sec. The levels of expression of mouse or human OSAP were normalized against glyceraldehyde-3 phosphate dehydrogenase (GAPDH) or β-glucuronidase mRNA levels as previously described (5,6). Sequences for the PCR primers and TaqMan probe for human GAPDH were described previously (4). Primer and probe concentrations of 500 and 50 nm, respectively, were used for human GAPDH. The sets of primers and TaqMan fluorogenic probes for mouse GAPDH, β-glucuronidase, and OSAP and human OSAP were proprietary to Applied Biosystems (Foster City, CA) and were used according to instructions provided by the manufacturer.
Antibody generation against mouse OSAP
An antimouse OSAP serum was raised in rabbits against a 30-mer peptide from the C terminus of OSAP: GSEPPAQPESQEE ETQVTEETASPQG (amino acids 254-283, GenBank no. NP_080634). The generation and affinity purification of the antibodies was carried out by Bethyl Laboratories Inc. (Montgomery, TX).
Immunohistochemistry
Superovulation was induced by gonadotrophin treatment in 4-wk-old female mice. An ip injection of PMSG was given at a dose of 10 IU per mouse. An ip administration of hCG (10 IU per mouse) was given to PMSG-primed mice at 48 h after the PMSG injection. Mice were killed, and tissues were collected and fixed for 4 h in 10% neutral formalin in PBS (pH 7.4) at room temperature, embedded in paraffin, sectioned at 6 μm. Immunostaining was performed using the Ventana automated immunohistochemistry staining system (Ventana Medical Systems, Tucson, AZ) with a diaminobenzidine kit. After incubation with an avidin-biotin blocker for 8 min, tissue sections were incubated overnight at room temperature with 0.6 μg/ml of the affinity-purified primary (rabbit) antibody against OSAP. After washing, the slides were reincubated with 1:750 dilution of a biotinylated goat antirabbit IgG (Sigma). The sections were counterstained with hematoxylin. Negative controls included the use of a nonspecific rabbit IgG and the omission of a secondary antibody. Background staining using these controls under the conditions described above was minimal.
Y-1 cell culture
Murine Y-1 adrenocortical tumor cells, obtained from the Health Science Research Resources Bank of Japan (Tokyo, Japan), were cultured in Ham’s F10 (Invitrogen, Carlsbad, CA) supplemented with 15% horse serum, 2.5% fetal bovine serum, and antibiotics as described previously (7). The cell line was kept at 37 C in a 5% CO2 humidified atmosphere.
Transfection of small interfering RNA (siRNA)
Silencing of OSAP expression in Y-1 cells was achieved using siRNA. One day before transfection, Y-1 cells were seeded in poly-d-lysine-coated 24-well plates (BD Biosciences, San Jose, CA), similarly coated coverslips, or BIOCOAT culture slides (BD Biosciences) such that they were 30% confluent the next day. Cells were transfected with siRNA constructs (100 nm) targeting mouse OSAP (SMART pool reagent; Dharmacon, Lafayette, CO) or nontargeting control siRNA constructs (siControl nontargeting pool; Dharmacon) using DharmaFECT transfection reagent (Dharmacon) in Opti-MEM I medium (Invitrogen) as per the manufacturer’s instructions. Cells were kept at 37 C in a 5% CO2 humidified atmosphere for 48 h. In some experiments, the medium was replaced and 8-Br-cAMP was added at the doses indicated in the figures.
Progesterone measurements
Y-1 cell-conditioned medium samples were assayed for progesterone using an electrochemiluminescence immunoassay (Roche Diagnostics) (8).
Western blotting
Total protein was isolated from the ovaries of 4-wk-old female mice undergoing the above-described superovulation protocol. The ovaries were collected and homogenized in protein extraction buffer [50 mm Tris-HCl (pH 7.5), 0.5 m urea, 2% Nonidet-P40, 1% protease inhibitor cocktail, 1% phosphatase inhibitor cocktail, 5% 2-mercaptoethanol]. The lysate was centrifuged at 3000 × g for 10 min and supernatant was collected. The supernatant (20 μg of protein) was resolved on 12% SDS-PAGE gels and blotted onto nitrocellulose membranes. Proteins were also isolated from cultured Y-1 cells treated with or without 8-Br-cAMP (0.1 or 1 mm) for 48 h, separated by electrophoresis on a 4–12% gradient gel under reducing conditions, and transferred onto polyvinylidene difluoride membranes as described previously (3). After blocking, the membranes were probed with the primary anti-OSAP antibody (1:1000) for 1 h, followed by a secondary antibody (goat antirabbit IgG horseradish peroxidase conjugated antibody, 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA). Protein expression was detected with an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ). Protein extracts (20 μg of protein) from Y-1 cells were also subjected to enzymatic deglycosylation using a Glycopro kit (ProZyme, San Leandro, CA) according to the manufacturer’s protocol. Aliquots of fetuin (20 μg) were incubated under the same conditions and served as reaction controls. After enzymatic deglycosylation, the samples were analyzed by Western blotting as described above.
Immunofluorescence studies
Cells grown on poly-d-lysine-coated coverslips or BIOCOAT culture slides (BD Bioscience) were incubated with 25 nm of MitoTracker Red CMXRos dye (Molecular Probes, Eugene, OR) for 30 min at 37 C and fixed for 20 min at room temperature in a freshly prepared solution of 2% paraformaldehyde in PBS (pH 7.4). The slides were then incubated for 1 h at room temperature in a blocking solution (0.01 m PBS, 10% goat serum, and 0.5% Triton X-100). The fixed/blocked cells were then incubated for 1 h at room temperature with the affinity-purified primary OSAP antibody (0.6 μg/ml) in a dilution buffer solution (0.01 m PBS and 0.5% Triton X-100). Thereafter the slides were washed three times for 15 min in 0.01 m PBS, and reincubated for 1 h at room temperature with a 1:1000 diluted Alexa Fluor 488 goat antirabbit IgG conjugate (Molecular Probes). After washing, slides were mounted with VectorShield mounting medium with 4, 6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Samples were examined using an AX70 microscope (Olympus, Tokyo, Japan) equipped for epifluorescence with appropriate filter sets. Confocal images were acquired using a TCS SP2 confocal microscopy system (Leica Microsystems, Wetzlar, Germany) with a Leica DMIRE2 inverted microscope (Leica).
Transient transfection of the OSAP-enhanced green fluorescent protein (EGFP) fusion construct
A full-length 848-bp clone of the OSAP was PCR amplified (exclusive of the translation termination codon) with the primers (ATGTATCTCAGCAGGGCTGTGTCCAAGACT and CCTTGGGGTGATGCTGTTTCCTCTGTGACC) that correspond to nucleotides 2-31 and 849-820 (GenBank no. NM_ 026358). The amplified product was subcloned into pGEM-T Easy cloning vector (Promega, Madison, WI) and then subcloned as an EcoRI-EcoRI fragment into the pEGFP-N2 vector (CLONTECH Laboratories) and selected for the correct direction. The fusion protein contained the EGFP in frame at the C terminus of OSAP.
NIH/3T3 cells were cultured in DMEM supplemented with 10% fetal calf serum and antibiotics. Cells were plated on polylysine-coated 35-mm glass-bottom dishes (Iwaki Glass, Tokyo, Japan) at a density of 2 × 105 cells. Transfection with Lipofectamine 2000 (Invitrogen) was performed according to the manufacturer’s protocol. Mitochondria were further stained with 200 nm of MitoTracker Red (Molecular Probes) by way of its addition to OSAP-EGFP-transfected mouse NIH/3T3 cells followed by a 30-min incubation at 37 C. Localization of OSAP-EGFP and of MitoTracker Red (Molecular Probes) was accomplished with an LSM510 laser confocal microscope (Carl Zeiss, Jena, Germany).
Isolation and quantitative real-time PCR for mitochondrial DNA
Relative amounts of nuclear DNA and mitochondrial DNA were determined by qRT-PCR. The ratio of mitochondrial DNA to nuclear DNA reflects the tissue concentration of mitochondria per cell (9). Total DNA was extracted from Y-1 cells with a DNeasy kit (QIAGEN). PCR was run on a LightCycler instrument with a LC FastStart DNA master hybridization probe kit (Roche Diagnostics), according to the manufacturer’s directions. To quantitate mitochondrial DNA copy number per nuclear genome, the well-conserved nuclear and mitochondrial genes, β-actin, and cytochrome b, were used as markers for nuclear DNA and mitochondrial DNA, respectively, as previously described (10). Sequences for the PCR primers and TaqMan probe for β-actin and cytochrome b were described previously (11). PCR was conducted using the following cycle parameters: 95 C for 10 min and 40 cycles at 95 C for 10 sec, 60 C for 30 sec.
Statistical analysis
Data are presented as means ± se. Data were analyzed by ANOVA, followed by Tukey’s test for multiple comparisons or Student’s t test, where appropriate. Differences were considered significant at P < 0.05.
Results
Tissue-specific abundance of mouse and human OSAP mRNA
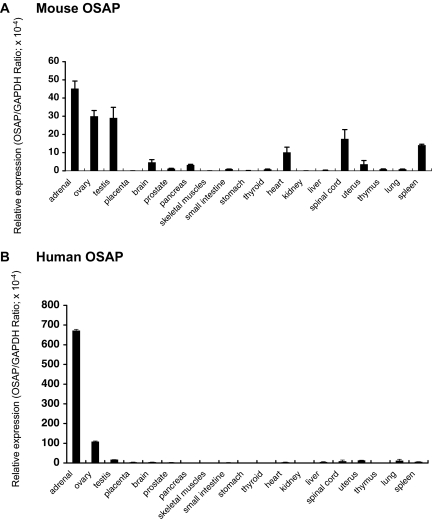
To gain insight into the functions of OSAP, we quantitatively determined mRNA levels of OSAP in various mouse and human tissues. With the exception of the placenta, qRT-PCR revealed that abundant OSAP mRNA expression was detected in steroidogenic tissues. Of the tissues analyzed, the highest level of expression was noted in the adrenal gland in both mouse (Fig. 1A) and human (Fig. 1B). Relative to the pattern of expression in the mouse, human OSAP mRNA was primarily detected in adrenal and ovary (Fig. 1B).
Figure 1.
Expression of OSAP mRNA in different mouse (A) and human (B) tissues. OSAP mRNA levels were analyzed by qRT-PCR as described in Materials and Methods. GAPDH-normalized data are shown. Each bar represents the mean ± se of four determinations.
Cellular localization of OSAP
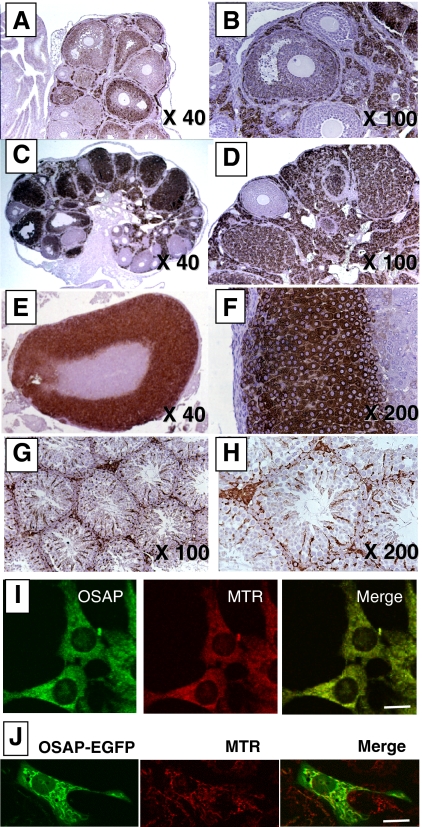
We further localized OSAP protein in mouse steroidogenic organs by immunohistochemistry. In PMSG-stimulated ovaries, immunoreactive OSAP localized primarily to the theca-interstitial compartment and to some degree granulosa cells of antral follicles (Fig. 2, A and B). Modest immunoreactive OSAP was detected in granulosa (but not cumulus) cells of antral follicles (Fig. 2B). In contrast, granulosa cells of preantral follicles were devoid of immunoreactive OSAP (Fig. 2B). After the administration of an ovulatory bolus of hCG in PMSG-primed animals, OSAP immunoreactivity was abundant in corpora lutea and the theca-interstitial compartment but not preantral follicles (Fig. 2, C and D). In the mouse adrenal gland, intense immunoreactive OSAP staining was observed in the adrenal cortex encompassing the zona glomerulosa, zona fasciculata, and zona reticularis (Fig. 2, E and F). Immunoreactive OSAP protein was not detected in the adrenal medulla. In the testis, immunoreactive OSAP was readily detectable in interstitial Leydig cells as well as, albeit less prominently, in Sertoli cells within the seminiferous tubules (Fig. 2, G and H).
Figure 2.
Cellular and subcellular localization of OSAP protein in mice. Immunohistochemical studies were carried out as described in Materials and Methods using ovaries collected 48 h after the administration of PMSG (A and B); ovaries collected 48 h after the administration of hCG to PMSG-primed mice (C and D); adrenal gland (E and F); and testis (G and H). Anti-OSAP antibody immunostaining (left panel) and Mitotracker Red CMX Ros (MTR; a mitochondrial marker) fluorescence (middle panel) show colocalization in Y-1 cells when merged (right panel) (I). Green fluorescence (left panel) generated by a OSAP-EGFP-transfected mouse NIH/3T3 cell and MitoTracker red (MTR; a mitochondrial marker) fluorescence (middle panel) show colocalization when merged (right panel) (J). Magnification bar, 10 μm.
Mitochondrial localization of OSAP
The PSORT II subcellular localization prediction algorithm (http://psort.ims.u-tokyo.ac.jp/) suggests that OSAP may reside in the mitochondria. Dual staining of cultured Y-1 cells with both a mitochondrial marker (MitoTracker Red CMXRos; Molecular Probes) and the rabbit anti-OSAP antibody revealed overlapping subcellular distribution (Fig. 2I). Similar results were observed in cultured dispersates of whole ovaries obtained from immature mice (data not shown). Furthermore, confocal immunofluorescence imaging revealed that the signal generated by OSAP-EGFP-transfected mouse NIH/3T3 cells colocalized with that of a mitochondrial marker (MitoTracker red) (Fig. 2J).
Regulation of OSAP expression
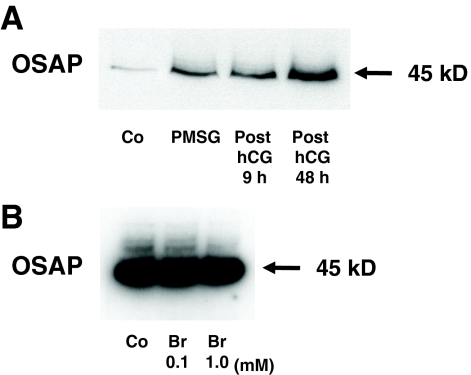
Western blot analysis of the mouse ovarian OSAP protein disclosed a single 45-kDa band (Fig. 3A). Similarly, the mouse OSAP protein of Y-1 cells migrated at 45 kDa (Fig. 3B). Enzymatic deglycosylation of Y-1 cell protein extracts, performed as described in Materials and Methods, did not cause any change in OSAP protein mobility as analyzed by Western blot (data not shown). Thus, the higher molecular mass of OSAP (higher than the 30 kDa predicted from the amino acid sequence) may be attributed to gel retardation, a phenomenon often observed for an extremely acidic protein (calculated pI = 3.95) or one featuring an unusual amino acid composition (12). Moreover, in vivo administration of PMSG and hCG increased OSAP protein levels in the mouse ovary (Fig. 3B). In contrast, mouse Y-1 adrenocortical cells treated with 8-Br-cAMP (0.1–1 mm) for 48 h did not alter abundance of OSAP protein (Fig. 3B), whereas the 8-Br-cAMP treatment increased progesterone secretion in the culture medium as expected (data not shown).
Figure 3.
Effects of PMSG and hCG on OSAP protein abundance in lysates of the mouse ovary (A), as analyzed by Western blotting (20 μg protein per lane). Effects of 8-Br-cAMP (Br) on OSAP protein abundance in lysates of Y-1 cells (B) (20 μg protein per lane). Co, Control: ovaries from untreated immature mice or Y-1 cells incubated with media alone.
Effects of OSAP silencing
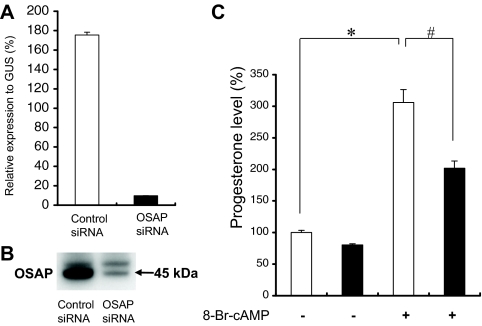
To assess its role in cellular activities, mouse adrenocortical Y-1 cells were transfected with siRNA directed against OSAP. As shown in Fig. 4, A and B, the siRNA transfection led to a marked suppression in the levels of OSAP mRNA and protein. Furthermore, Y-1 cells transfected with control or OSAP siRNA for 48 h were incubated in the presence or absence of 1 mm 8-Br-cAMP for 60 min. OSAP silencing significantly inhibited 8-Br-cAMP-induced progesterone secretion (Fig. 4C). However, in the absence of 8-Br-cAMP, progesterone levels did not differ significantly between control and OSAP siRNA-transfected cells.
Figure 4.
Effects of OSAP siRNA on progesterone production. Y-1 cells were transfected with siRNA targeted against OSAP or a scrambled control siRNA as described in Materials and Methods. qRT-PCR (A) and Western blot (B) analyses show a marked reduction (<90%) in OSAP expression at the mRNA and protein levels, respectively, in Y-1 cells transfected with OSAP siRNA. C, Progesterone production in the cell culture medium after 60 min of treatment. Y-1 cells transfected with control (white bars) or OSAP (black bars) siRNA were incubated in the presence or absence of 1 mm 8-Br-cAMP. For comparison, progesterone levels are presented as percentage of values from control siRNA cells and are expressed as the mean ± se. Data shown are from one representative experiment performed in triplicate. *, P < 0.001 by Tukey’s test (vs. untreated control siRNA cells); #, P < 0.001 by Tukey’s test (vs. 8-Br-cAMP-treated control siRNA cells).
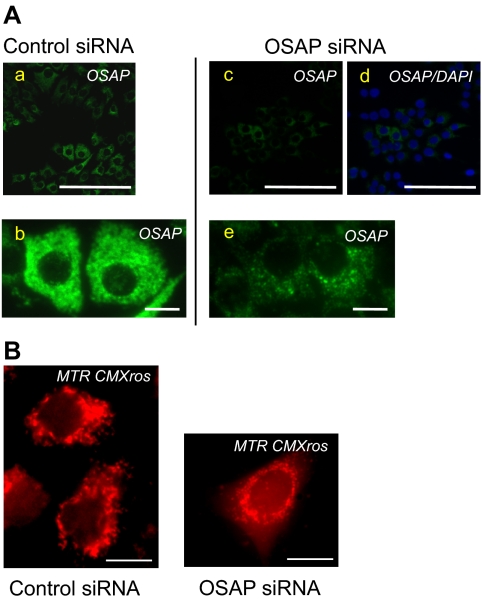
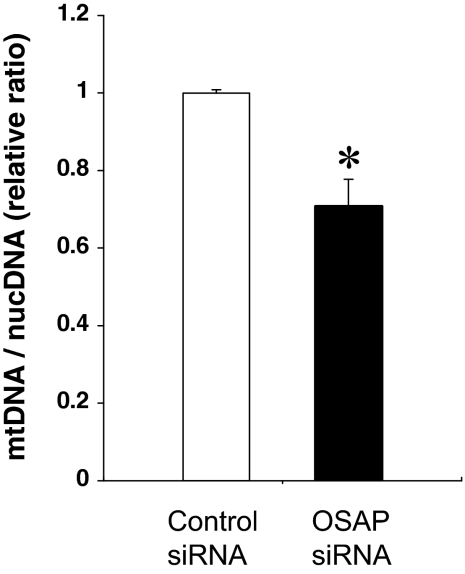
We further investigated the effects of reduced OSAP protein expression on mitochondrial morphology and abundance. OSAP immunoreactivity was markedly reduced in cells transfected with OSAP siRNA (Fig. 5A). As shown in Fig. 5B, OSAP siRNA-treated cells exhibited fragmentation of the filamentous mitochondrial network, indicative of mitochondrial aggregation and perinuclear mitochondrial rearrangement. Similar morphological changes and distribution of mitochondria were also observed in Fig. 5A. OSAP siRNA transfection did not change the number of Y-1 cells. The cellular content of mitochondria was estimated by PCR using oligonucleotide primers specific for nuclear (β-actin) and mitochondrial (cytochrome b) genes. OSAP silencing led to a decrease in mitochondrial DNA compared with nuclear DNA, indicating a reduced cellular abundance of mitochondria (Fig. 6).
Figure 5.
Y-1 cells transfected with OSAP siRNA show reduced immunoreactive OSAP (A). Markedly decreased OSAP immunoreactivity in cells transfected with OSAP siRNA (c and e) compared with cells transfected with control siRNA (a and b). d, OSAP immunostaining performed in parallel with nuclear staining using 4, 6-diamidino-2-phenylindole (DAPI). Magnification bar, 100 μm (a, c, and d) and 10 μm (b and e). OSAP knockdown alters mitochondrial morphology (B). Fluorescence microscopy was performed on Y-1 cells using a mitochondrial marker Mitotracker Red CMX Ros 48 h after transfection with control or OSAP siRNA. OSAP siRNA-treated cells show a change in mitochondrial morphology from filamentous and elongated to fragmented structures relative to control siRNA-treated cells. Magnification bar, 10 μm.
Figure 6.
OSAP knockdown decreases mitochondrial DNA abundance per cell. Relative amounts of mitochondrial DNA (mtDNA) and nuclear DNA (nucDNA) in Y-1 cells, 48 h after transfection with control or OSAP siRNA, were determined by qRT-PCR as described in Materials and Methods. The mtDNA to nucDNA ratio, an index of mtDNA concentration per cell, was significantly decreased in OSAP siRNA cells compared with control siRNA cells. For comparison, the relative mtDNA to nucDNA ratio of the control is arbitrarily presented as 1. Data shown are the mean ± se of four determinations from one representative experiment. *, P < 0.01 by Student’s t test (vs. control siRNA cells).
Discussion
In identifying biologically important genes and their products, an increasing focus has been on genes whose expression is enriched or selective to a small number of tissue types, or exclusive to only one particular tissue type. OSAP is such an example. Thus, as this study reveals, OSAP is abundantly expressed in steroidogenic tissues. Here we also provide evidence that OSAP is a mitochondrial protein that maintains mitochondrial abundance and morphology, thereby allowing for optimal steroidogenesis.
We present quantitative data on OSAP expression in a variety of murine and human tissues using qRT-PCR. The results demonstrate that OSAP expression is greatest in steroidogenic organs such as the adrenal gland, ovary, and testis. The expression profile of OSAP is quite similar in both mouse and human tissues. Immunohistochemical studies of the mouse ovary, adrenal gland, and testis demonstrate that OSAP protein is generally positive in steroid-producing cells. However, we also localized OSAP to nonsteroid-producing cells such as Sertoli cells. In this context, recent studies of Yamagami and coauthors (13) are of particular interest. They constructed a human corneal endothelial cDNA library and identified a novel gene, GS3582. They later named the protein from this gene as human corneal endothelium-specific protein (CESP)-1. The N-terminal 238-amino acid sequence of CESP-1 is identical with human OSAP that consists of 268 amino acids (13), and mouse CESP-1 is entirely consistent with mouse OSAP (14). Human CESP-1 expression was the highest in the ovary among the tissues examined other than the human corneal endothelium (14). Based on such a unique pattern of expression, it is possible that OSAP may be involved in processes that require significant mitochondrial-dependent activities including steroidogenesis and energy production. With regard to the latter, the OSAP-expressing nonsteroidogenic tissues such as the heart and spleen could require OSAP for active mitochondrial energy production and metabolism. As for the central nervous system (brain and spinal cord), mounting evidence indicates that it is a steroidogenic tissue as well (15,16). Thus, the function of OSAP in the nervous system might be similar to those in classic steroidogenic cells and tissues. At any rate, the earlier presumption that OSAP may constitute an ovary-specific gene has now been disproved.
Our immunofluorescence studies demonstrate that OSAP is a mitochondrial protein. MitoTracker CMXRos (Molecular Probes), a dye selectively taken up by mitochondria, colocalizes with immunoreactive OSAP in Y-1 mouse adrenocortical cells. Moreover, transient transfection of NIH/3T3 cells with the OSAP-EGFP fusion construct indicates mitochondrial localization of OSAP as well. As for the submitochondrial localization of OSAP, mitochondrial fractionation and digestion studies are currently underway.
Mitochondria are a key control point for the regulation of steroidogenesis (17). Thus, we questioned whether OSAP is involved in steroidogenesis as our results strongly suggest that OSAP is a mitochondrial protein abundantly expressed in steroidogenic cells. In this study, silencing OSAP in Y-1 murine adrenocortical cells inhibited cAMP-stimulated progesterone production, indicating a role for OSAP in steroidogenesis. The magnitude of decrease in cAMP-stimulated progesterone production by OSAP silencing was moderate, compared with the magnitude of down-regulation of OSAP expression (<90%). One possibility for this discrepancy is that the residual OSAP expression may be critical for maintaining steroid production. Alternatively, there could be a compensatory change in another component in the steroid-producing machinery that can partially rescue OSAP depletion.
OSAP knockdown also induced a decrease in mitochondrial abundance as evidenced by reduced cellular mtDNA content. Several previous studies indicate correlations between mitochondrial abundance and steroidogenic capacity. For example, ACTH, the principal positive regulator of adrenal steroidogenesis, induces an increase in mitochondrial abundance in adult human adrenocortical cells (18). In contrast, mice lacking CRH receptor-1 display a decrease in the amount of mitochondria in adrenocortical cells, consistent with decreased corticosterone production in these animals (19). Thus, the inhibition of 8-Br-cAMP-stimulated progesterone production in Y-1 cells transfected with OSAP siRNA may be secondary to the reduced cellular mitochondrial content, suggesting a role for OSAP in maintaining mitochondrial abundance. Down-regulation of OSAP also induced alterations in the mitochondrial network (e.g. mitochondrial aggregation) in Y-1 cells. Mitochondria exist as small isolated particles or extended filaments or clusters that can convert from one form to the other. The predominance of either form shapes the mitochondrial network and is determined by the dynamic balance between mitochondrial fission and fusion (20). Thus, one possibility is that the loss of OSAP may have changed the mitochondrial fusion-fission balance toward fission through an unknown mechanism. Alternatively, the observed perinuclear mitochondrial aggregation may represent a preapoptotic state (20) although the perinuclear mitochondrial distribution might simply reflect a denser aggregation of mitochondria in perinuclear positions commonly observed in a variety of cells (21). In our preliminary experiments, silencing OSAP by siRNA in Y-1 cells induced mitochondrial release of cytochrome c into the cytosol, a key initial step in the apoptotic process (22,23), but OSAP siRNA-treated cells did not display classic signs of apoptosis (data not shown). Of interest, Waterhouse et al. (24) indicated that before or in the absence of caspase activation, mitochondria can maintain several functions, including the generation of ATP, and may contribute to survival of the cells for prolonged periods even after cytochrome c release. Thus, further detailed investigations, including assays to establish the presence or absence of the apoptotic program, should be performed before drawing a conclusion on the possibility of an antiapoptotic role for OSAP.
Regulation of OSAP expression remains unclear. In the present study, in vivo administration of PMSG- and hCG-induced OSAP protein in the mouse ovary as assessed by Western blotting. The result may be a reflection of an increased number of OSAP-positive cells within the ovary. PMSG increases the number of growing follicles per ovary, thereby increasing the number of OSAP-positive granulosa cells. On the other hand, hCG induces ovulation with the subsequent formation of corpus lutea, thereby increasing the number of luteal cells that are OSAP positive. Therefore, PMSG and/or hCG may not directly regulate expression of OSAP. Supporting this interpretation, our current in vitro experiments demonstrate that cAMP, a major second messenger of the gonadotropins and ACTH, does not regulate abundance of OSAP protein in Y-1 cells. These findings are in contrast to results obtained from studies using adrenocortical cells, whereby expression of the low-density lipoprotein receptors, steroidogenic acute regulatory protein (StAR), and steroidogenic enzymes increase in response to ACTH or its downstream signaling molecule cAMP (25,26). Further studies that include a variety of steroidogenic cells are needed to investigate regulation of OSAP.
It is interesting to note that no apparent OSAP ortholog could be identified in nonmammalian species to date. Mammals, with few exceptions (e.g. monotremes), give birth to live young (viviparity) instead of laying eggs. In viviparous animals, progesterone is involved in implantation and maintenance of pregnancy (27). Thus, it is tempting to speculate that the mitochondrial protein OSAP may be a mammalian-specific invention to meet the increase in demand for steroidogenesis including production of progesterone. Alternatively, OSAP may have evolved, however, a yet-unknown function that is not shared with nonmammalian species. Another intriguing finding of the present study is the similarity in expression pattern between OSAP and the mitochondrial protein StAR. StAR is expressed in steroidogenic tissues exhibiting an acute induction in steroid synthesis in response to endocrine mediators but not in tissues (i.e. placenta) that do not respond in such a manner (28). Therefore, the possibility arises that OSAP could also be associated with the acute response or that OSAP might play a role in cholesterol transport in concert with StAR.
Collectively these results indicate that the mitochondrial protein OSAP is abundantly expressed in steroidogenic tissues with a few exceptions. The results also suggest that OSAP is involved in steroidogenesis. However, our findings point to a permissive role for OSAP in steroidogenesis rather than a regulatory role because OSAP expression was not stimulated by a cAMP analog. Thus, we speculate that this protein could be involved in insuring optimal steroidogenesis through maintaining mitochondrial abundance and/or function. Further studies are warranted to substantiate this postulated role for OSAP in steroidogenic cells.
Acknowledgments
We thank SRL Inc. (Tokyo, Japan) for performing the electrochemiluminescence immunoassays.
Footnotes
This work was supported by grants-in-aid from the Japan Society for the Promotion of Science (to T.M., H.I., K.M., T.H., and Y.Y.). It was presented, in part, at the 87th Annual Meeting of The Endocrine Society, June 2005, San Diego, CA.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 26, 2009
Abbreviations: 8-Br-cAMP, 8-Bromoadenosine cAMP; CESP, corneal endothelium-specific protein; EGFP, enhanced green fluorescent protein; GAPDH, glyceraldehyde-3 phosphate dehydrogenase; hCG, human chorionic gonadotropin; LC, LightCycler; OSAP, ovary-specific acidic protein; PMSG, pregnant mare serum gonadotropin; qRT-PCR, quantitative real-time RT-PCR; siRNA, small interfering RNA; StAR, steroidogenic acute regulatory protein.
References
- Hennebold JD, Tanaka M, Saito J, Hanson BR, Adashi EY 2000 Ovary-selective genes I: the generation and characterization of an ovary-selective complementary deoxyribonucleic acid library. Endocrinology 141:2725–2734 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Hennebold JD, Miyakoshi K, Teranishi T, Ueno K, Adashi EY 2003 The generation and characterization of an ovary-selective cDNA library. Mol Cell Endocrinol 202:67–69 [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Ginzinger DG, Jaffe RB 2006 Adrenocorticotropin preferentially up-regulates angiopoietin 2 in the human fetal adrenal gland: implications for coordinated adrenal organ growth and angiogenesis. J Clin Endocrinol Metab 91:1909–1915 [DOI] [PubMed] [Google Scholar]
- Muench MO, Ratcliffe JV, Nakanishi M, Ishimoto H, Jaffe RB 2003 Isolation of definitive zone and chromaffin cells based upon expression of CD56 (neural cell adhesion molecule) in the human fetal adrenal gland. J Clin Endocrinol Metab 88:3921–3930 [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Muench MO, Higuchi T, Minegishi K, Tanaka M, Yoshimura Y, Jaffe RB 2006 Midkine, a heparin-binding growth factor, selectively stimulates proliferation of definitive zone cells of the human fetal adrenal gland. J Clin Endocrinol Metab 91:4050–4056 [DOI] [PubMed] [Google Scholar]
- Ginzinger DG 2002 Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol 30:503–512 [DOI] [PubMed] [Google Scholar]
- Schimmer BP 1979 Adrenocortical Y1 cells. Methods Enzymol 58:570–574 [DOI] [PubMed] [Google Scholar]
- Blackburn GF, Shah HP, Kenten JH, Leland J, Kamin RA, Link J, Peterman J, Powell MJ, Shah A, Talley DB, Tyagi SK, Wilkins E, Wu T, Massey RJ 1991 Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin Chem 37:1534–1539 [PubMed] [Google Scholar]
- Bogacka I, Ukropcova B, McNeil M, Gimble JM, Smith SR 2005 Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. J Clin Endocrinol Metab 90:6650–6656 [DOI] [PubMed] [Google Scholar]
- Berthiaume J, Wallace KB 2002 Perfluorooctanoate, perflourooctanesulfonate, and N-ethyl perfluorooctanesulfonamido ethanol; peroxisome proliferation and mitochondrial biogenesis. Toxicol Lett 129:23–32 [DOI] [PubMed] [Google Scholar]
- Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce Jr WM, Klein JB, Epstein PN 2004 Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab 287:E896–E905 [DOI] [PubMed] [Google Scholar]
- Zirwes RF, Schmidt-Zachmann MS, Franke WW 1997 Identification of a small, very acidic constitutive nucleolar protein (NO29) as a member of the nucleoplasmin family. Proc Natl Acad Sci USA 94:11387–11392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R, Kinouchi T, Kawamoto S, Dana MR, Hamamoto T, Tsuru T, Okubo K, Yamagami S 2002 Construction of human corneal endothelial cDNA library and identification of novel active genes. Invest Ophthalmol Vis Sci 43:1749–1756 [PubMed] [Google Scholar]
- Kinouchi R, Kinouchi T, Hamamoto T, Saito T, Tavares A, Tsuru T, Yamagami S 2006 Distribution of CESP-1 protein in the corneal endothelium and other tissues. Invest Ophthalmol Vis Sci 47:1397–1403 [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD 2002 Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab 13:35–43 [DOI] [PubMed] [Google Scholar]
- Mellon SH 2007 Neurosteroid regulation of central nervous system development. Pharmacol Ther 116:107–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL 1995 Mitochondrial specificity of the early steps in steroidogenesis. J Steroid Biochem Mol Biol 55:607–616 [DOI] [PubMed] [Google Scholar]
- Nussdorfer GG, Armato U, Mazzocchi G, Andreis PG, Draghi E 1977 Investigations on the turnover of adrenocortical mitochondria. IX. A stereological study of the effects of chronic treatment with ACTH on the size and number of mitochondria from adult human adrenocortical cells cultured in vitro. Cell Tissue Res 182:145–150 [DOI] [PubMed] [Google Scholar]
- Yoshida-Hiroi M, Bradbury MJ, Eisenhofer G, Hiroi N, Vale WW, Novotny GE, Hartwig HG, Scherbaum WA, Bornstein SR 2002 Chromaffin cell function and structure is impaired in corticotropin-releasing hormone receptor type 1-null mice. Mol Psychiatry 7:967–974 [DOI] [PubMed] [Google Scholar]
- Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H 2007 Regulation of mitochondrial fusion and division. Trends Cell Biol 17:563–569 [DOI] [PubMed] [Google Scholar]
- Collins TJ, Berridge MJ, Lipp P, Bootman MD 2002 Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J 21:1616–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S 2002 Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci USA 99:1259–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler M, Kroemer G 2000 The mitochondrion in cell death control: certainties and incognita. Exp Cell Res 256:19–26 [DOI] [PubMed] [Google Scholar]
- Waterhouse NJ, Goldstein JC, von Ahsen O, Schuler M, Newmeyer DD, Green DR 2001 Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol 153:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB 1997 Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 18:378–403 [DOI] [PubMed] [Google Scholar]
- Miller WL 2007 StAR search—what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol 21:589–601 [DOI] [PubMed] [Google Scholar]
- Callard IP, Fileti LA, Perez LE, Sorbera LA, Giannoukos G, Klosterman LL, Paul T, Mccracken JA 1992 Role of the corpus luteum and progesterone in the evolution of vertebrate viviparity. Am Zoologist 32:264–275 [Google Scholar]
- Miller WL 2007 Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta 1771:663–676 [DOI] [PubMed] [Google Scholar]