Abstract
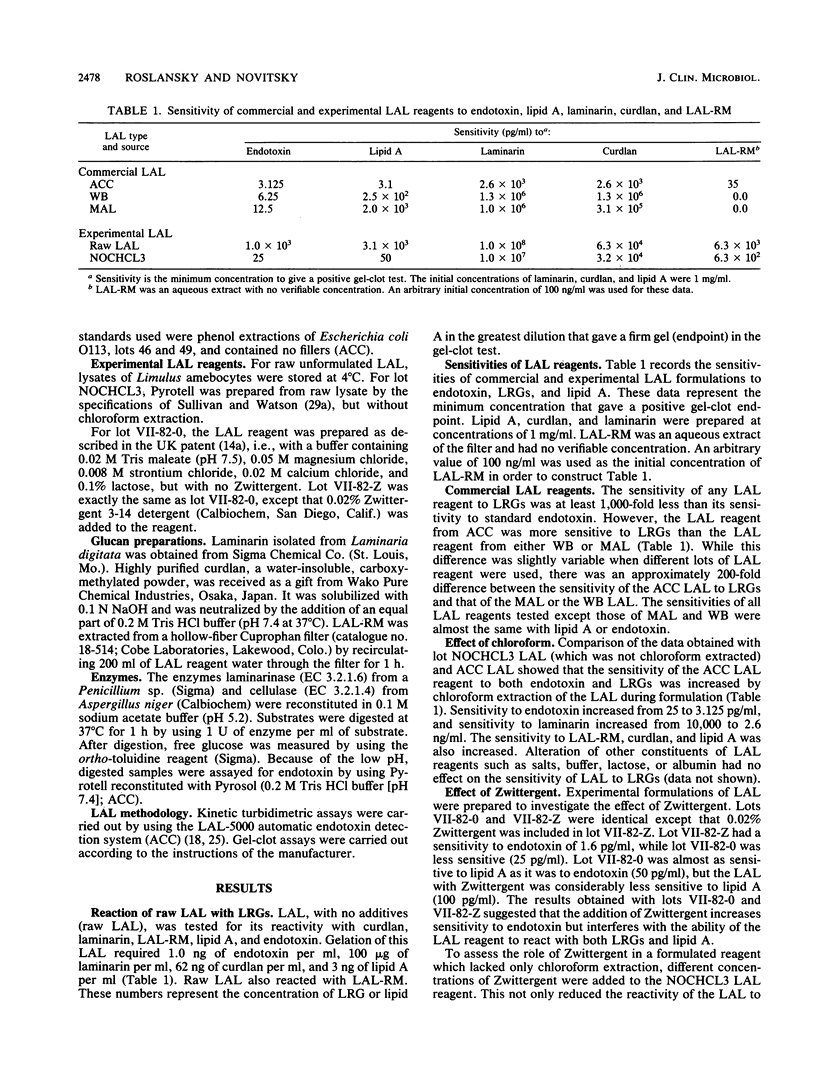
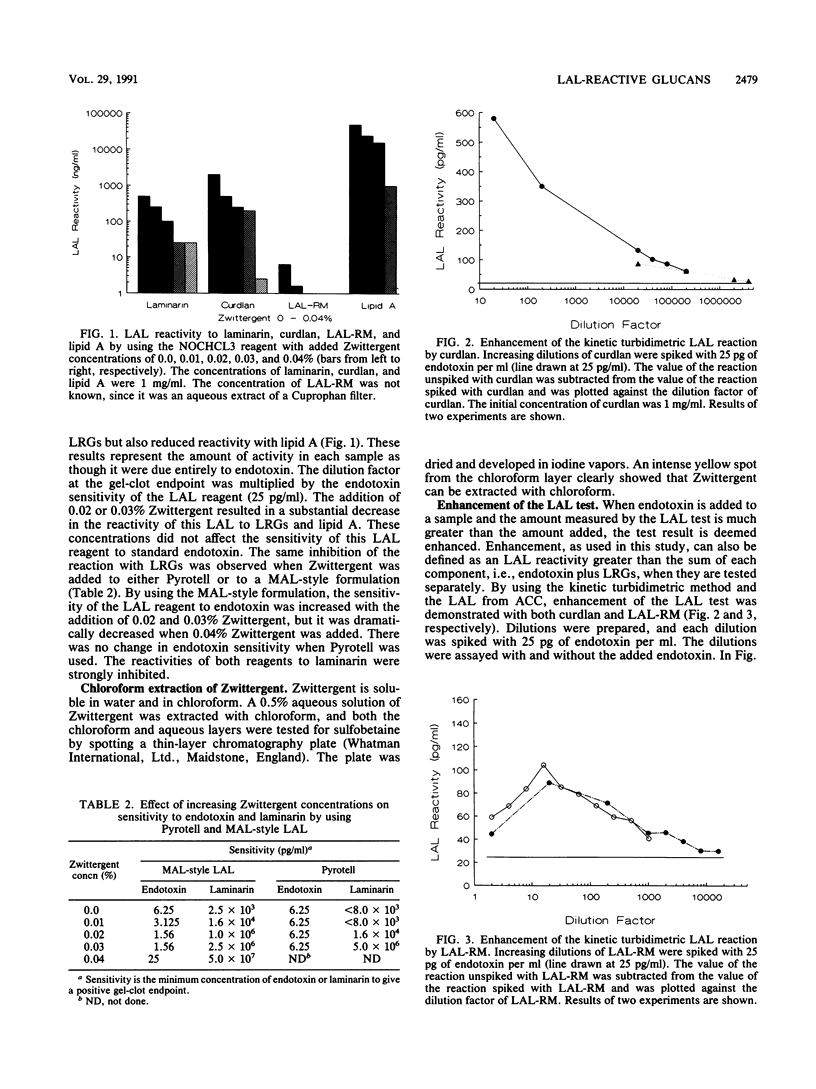
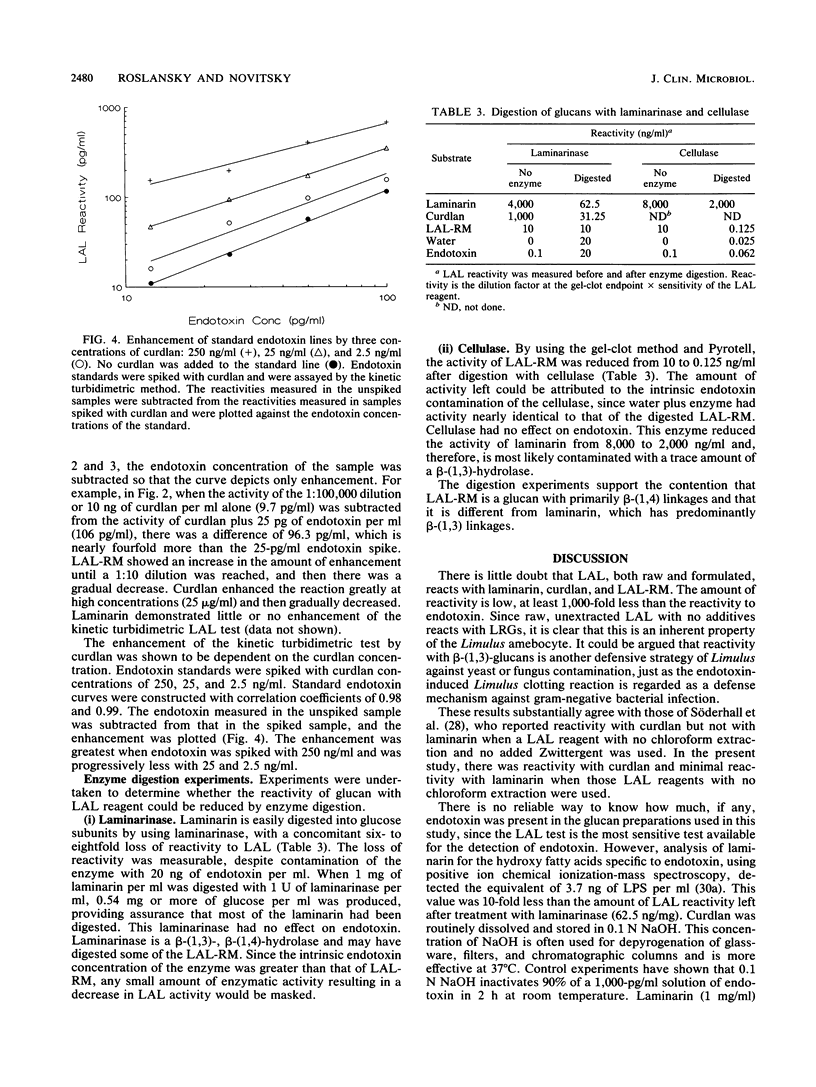
The sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans (LRGs) and lipid A was tested by using commercially available and experimentally formulated LAL reagents. The glucans included two kinds of beta-(1,3)-D-glucans, laminarin and curdlan, and cellulosic material, LAL-reactive material (LAL-RM), extracted from a hollow-fiber (Cuprophan) hemodialyzer. LAL-RM loses its LAL activity when it is digested with cellulase and thus appears to be a beta-(1,4)-D-glucan or a mixed glucan containing a substantial proportion of beta-(1,4) linkages. All LAL reagents tested were at least 1,000-fold more sensitive to endotoxin than to LRGs. The presence of the surfactant Zwittergent was shown to interfere with reactivity to LRGs; LAL reagents without added Zwittergent reacted more strongly to LRGs than did the same reagents containing Zwittergent. Chloroform extraction of LAL increased the reagents' sensitivity to both endotoxin and LRGs, but it was not responsible for LRG reactivity. The addition of Zwittergent significantly reduced the sensitivity of LAL reagents to lipid A. LAL without the surfactant was equally sensitive to endotoxin and lipid A. Both curdlan and LAL-RM amplified or enhanced the LAL response to endotoxin. Kinetic turbidimetric studies demonstrated that the enhancement was dependent on the glucan concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögwald J., Johnson E., Seljelid R. The cytotoxic effect of mouse macrophages stimulated in vitro by a beta-1,3-D-glucan from yeast cell walls. Scand J Immunol. 1982 Mar;15(3):297–304. doi: 10.1111/j.1365-3083.1982.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Carson L. A., Petersen N. J. LAL-reactive material associated with hemodialysis membranes. Prog Clin Biol Res. 1982;93:217–230. [PubMed] [Google Scholar]
- Cook J. A., Halushka P. V., Wise W. C. Modulation of macrophage arachidonic acid metabolism: potential role in the susceptibility of rats to endotoxic shock. Circ Shock. 1982;9(6):605–617. [PubMed] [Google Scholar]
- Di Luzio N. R., Williams D. L., McNamee R. B., Edwards B. F., Kitahama A. Comparative tumor-inhibitory and anti-bacterial activity of soluble and particulate glucan. Int J Cancer. 1979 Dec 15;24(6):773–779. doi: 10.1002/ijc.2910240613. [DOI] [PubMed] [Google Scholar]
- Hemmendinger S., Neumann M. R., Beretz A., Klein-Soyer C., Cazenave J. P., Rich A., Schohn D., Jahn H. Mitogenic activity on human arterial smooth muscle cells is increased in the plasma of patients undergoing hemodialysis with cuprophane membranes. Nephron. 1989;53(2):147–151. doi: 10.1159/000185728. [DOI] [PubMed] [Google Scholar]
- Ikegami K., Ikemura K., Shimazu T., Shibuya M., Sugimoto H., Yoshioka T., Sugimoto T. Early diagnosis of invasive candidiasis and rapid evaluation of antifungal therapy by combined use of conventional chromogenic limulus test and a newly developed endotoxin specific assay. J Trauma. 1988 Aug;28(8):1118–1126. doi: 10.1097/00005373-198808000-00003. [DOI] [PubMed] [Google Scholar]
- Ikemura K., Ikegami K., Shimazu T., Yoshioka T., Sugimoto T. False-positive result in Limulus test caused by Limulus amebocyte lysate-reactive material in immunoglobulin products. J Clin Microbiol. 1989 Sep;27(9):1965–1968. doi: 10.1128/jcm.27.9.1965-1968.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma A., Asano T., Torii H., Sugino Y. Gelation of Limulus amoebocyte lysate by an antitumor (1 leads to 3)-beta-D-glucan. Biochem Biophys Res Commun. 1981 Jul 30;101(2):434–439. doi: 10.1016/0006-291x(81)91278-x. [DOI] [PubMed] [Google Scholar]
- Kokoshis P. L., Williams D. L., Cook J. A., Di Luzio N. R. Increased resistance to Staphylococcus aureus infection and enhancement in serum lysozyme activity by glucan. Science. 1978 Mar 24;199(4335):1340–1342. doi: 10.1126/science.628841. [DOI] [PubMed] [Google Scholar]
- Lazar G., Agarwal M. K. Additive effect of glucan and streptozotocin on endotoxicosis in mice. Med Microbiol Immunol. 1982;171(3):179–186. doi: 10.1007/BF02123626. [DOI] [PubMed] [Google Scholar]
- Mikami T., Nagase T., Matsumoto, Suzuki S., Suzuki M. Gelatin of Limulus amoebocyte lysate by simple polysaccharides. Microbiol Immunol. 1982;26(5):403–409. doi: 10.1111/j.1348-0421.1982.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Novitsky T. J., Remillard J. F., Loy N. Design criteria and evaluation of the LAL-4000 for kinetic turbidimetric LAL assay. Prog Clin Biol Res. 1987;231:189–196. [PubMed] [Google Scholar]
- Obayashi T., Tamura H., Tanaka S., Ohki M., Takahashi S., Arai M., Masuda M., Kawai T. A new chromogenic endotoxin-specific assay using recombined limulus coagulation enzymes and its clinical applications. Clin Chim Acta. 1985 Jun 30;149(1):55–65. doi: 10.1016/0009-8981(85)90273-6. [DOI] [PubMed] [Google Scholar]
- Ohno N., Emori Y., Yadomae T., Saito K., Masuda A., Oikawa S. Reactivity of Limulus amoebocyte lysate towards (1----3)-beta-D-glucans. Carbohydr Res. 1990 Oct 25;207(2):311–318. doi: 10.1016/0008-6215(90)84058-3. [DOI] [PubMed] [Google Scholar]
- Parvin R., Goswami T., Pande S. V. Inhibition of mitochondrial carnitine-acylcarnitine translocase by sulfobetaines. Can J Biochem. 1980 Oct;58(10):822–830. doi: 10.1139/o80-115. [DOI] [PubMed] [Google Scholar]
- Patchen M. L., MacVittie T. J., Weiss J. F. Combined modality radioprotection: the use of glucan and selenium with WR-2721. Int J Radiat Oncol Biol Phys. 1990 May;18(5):1069–1075. doi: 10.1016/0360-3016(90)90442-m. [DOI] [PubMed] [Google Scholar]
- Pearson F. C., Bohon J., Lee W., Bruszer G., Sagona M., Dawe R., Jakubowski G., Morrison D., Dinarello C. Comparison of chemical analyses of hollow-fiber dialyzer extracts. Artif Organs. 1984 Aug;8(3):291–298. doi: 10.1111/j.1525-1594.1984.tb04293.x. [DOI] [PubMed] [Google Scholar]
- Pearson F. C., Caruana R., Burkart J., Katz D. V., Chenoweth D., Dubczak J., Bohon J., Weary M. The use of the Limulus amebocyte lysate assay to monitor hemodialyzer-associated soluble cellulosic material (LAL-reactive material). Prog Clin Biol Res. 1987;231:211–222. [PubMed] [Google Scholar]
- Remillard J. F., Gould M. C., Roslansky P. F., Novitsky T. J. Quantitation of endotoxin in products using the LAL kinetic turbidimetric assay. Prog Clin Biol Res. 1987;231:197–210. [PubMed] [Google Scholar]
- Roslansky P. F., Dawson M. E., Novitsky T. J. Plastics, endotoxins, and the Limulus amebocyte lysate test. J Parenter Sci Technol. 1991 Mar-Apr;45(2):83–87. [PubMed] [Google Scholar]
- Sasaki T., Abiko N., Nitta K., Takasuka N., Sugino Y. Antitumor activity of carboxymethylglucans obtained by carboxymethylation of (1 leads to 3)-beta-D-glucan from Alcaligenes faecalis var. myxogenes IFO 13140. Eur J Cancer. 1979 Feb;15(2):211–215. doi: 10.1016/0014-2964(79)90062-8. [DOI] [PubMed] [Google Scholar]
- Sullivan J. D., Jr, Watson S. W. Factors affecting the sensitivity of Limulus lysate. Appl Microbiol. 1974 Dec;28(6):1023–1026. doi: 10.1128/am.28.6.1023-1026.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Mikami T., Matsumoto T., Suzuki S. Gelation of Limulus lysate by synthetic dextran derivatives. Microbiol Immunol. 1977;21(8):419–425. doi: 10.1111/j.1348-0421.1977.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Yaeger R. G., Pretus H. A., Browder I. W., McNamee R. B., Jones E. L. Immunization against Trypanosoma cruzi: adjuvant effect of glucan. Int J Immunopharmacol. 1989;11(4):403–410. doi: 10.1016/0192-0561(89)90087-8. [DOI] [PubMed] [Google Scholar]
- Yamagami S., Yoshihara H., Kishimoto T., Sugimura T., Niwa M., Maekawa M. Cuprophan membrane induces interleukin-1 activity. ASAIO Trans. 1986 Jul-Sep;32(1):98–101. [PubMed] [Google Scholar]


