Abstract
Research has shown that animals and humans habituate on a variety of behavioral and physiological responses to repeated presentations of food cues, and habituation is related to amount of food consumed and cessation of eating. The purpose of this article is to provide an overview of experimental paradigms used to study habituation, integrate a theoretical approach to habituation to food based on memory and associative conditioning models, and review research on factors that influence habituation. Individual differences in habituation as they related to obesity and eating disorders are reviewed, along with research on how individual differences in memory can influence habituation. Other associative conditioning approaches to ingestive behavior are reviewed, as well as how habituation provides novel approaches to preventing or treating obesity. Finally, new directions for habituation research are presented. Habituation provides a novel theoretical framework from which to understand factors that regulate ingestive behavior.
Keywords: Habituation, food intake, ingestive behavior, Eating behavior, obesity, sensory specific satiety, energy intake
Habituation as a determinant of human food intake
Eating involves the repeated presentation of visual, olfactory and gustatory cues as a meal or snack is consumed. One effect of repeated stimulus presentations is habituation to those stimuli. Habituation represents a general model of how repeated stimulus presentations influence responding and is ubiquitous across response systems (Groves & Thompson, 1970). Habituation describes reductions in both physiological and behavioral responses to eating that occur as an eating episode progresses, and may provide a model to understand factors that are important for the cessation of eating, or satiation, within a meal. After the response rate to food has decreased, presentation of a new stimulus will result in recovery of responding to the new stimulus as well as recovery of responding, or dishabituation, to the habituated food stimulus (Epstein, Rodefer, Wisniewski, & Caggiula, 1992). The recovery of appetite or the motivation to eat is apparent to anyone who has consumed a large meal, and is quite full, and does not require additional energy or nutrients to meet their daily needs, but decides to consume additional calories after seeing the dessert cart.
It is common to consider the influence of sensory characteristics of food as being important guides to what foods to eat, and important determinants of the pleasure derived from eating (Bartoshuk, 1991; Cabanac, 1990), but habituation goes beyond this to explain factors that are important in regulating the amount of food consumed (Swithers, 1996). Habituation studies provide a framework to understand how sensory stimuli influence not only choice of food, but the amount of food consumed. One purpose of this paper is to present different experimental paradigms for studying habituation, along with consideration of sensitization, an increase in responding that often precedes the reduction observed in habituation. This overview is followed by presentation of a connectionist, memory-based associative conditioning theory developed by Wagner (Wagner, 1989; Wagner & Brandon, 2001) that we are adapting to ingestive behavior. There are several ways in which application of this model to eating behavior is unique. First, although it has been important for understanding animal learning and associative processes, it has not been extended to human behavior. An early version of Wagner’s model (Wagner, 1978) was used in an influential theoretical account of how learning might be involved in drug tolerance (Baker & Tiffany, 1985), but to our knowledge Wagner’s models have never been extended to human eating behavior. Second, extending the model to eating behavior provides a new opportunity to integrate associate conditioning and habituation research. Early versions of Wagner’s models were designed to integrate habituation and associative learning, and this paper extends this tradition by further integrating habituation with conditioning and motivation processes (Wagner, 1989; Wagner & Brandon, 2001). Third, because Wagner’s conditioning model is a memory-based model (Wagner, 1989; Wagner & Brandon, 2001), it makes further suggestions about how memory might related to habituation and improve our understanding of habituation. For example, individual differences in memory may provide clues into how habituation is related to normal or abnormal eating patterns.
A brief overview of animal research on habituation and food intake is presented to show generalization of the basic principles across species. Habituation is related to consumption of food in situations in which either single or multiple foods are consumed. Habituation is used as a model to understand the effects of food variety on eating, and eating that occurs in combination with other behaviors, such as watching television, or in response to stress. Research on sensitization and ingestive behavior is reviewed. The role of energy intake in habituation is discussed to demonstrate that habituation provides a model that does not depend on energy consumption and does not reduce intake by increasing energy repletion. The relationship between individual differences in habituation and obesity and bulimia nervosa are explored, followed by a discussion of how individual difference in memory could influence habituation. Habituation is related to another model designed to understand the influence of sensory influences rather than energy depletion on eating, sensory specific satiety. An overview of associative conditioning approaches to ingestive behavior, along with other approaches to cessation of eating are briefly presented. The paper concludes with ideas on how habituation can be related to preventing or treating obesity, and on gaps in the habituation and eating literature.
Habituation paradigms
The following section presents an overview of paradigms used to study habituation. Research on habituation to food has been studied across a broad range of subjects, from rodents (Swithers, 1996; Swithers & Hall, 1994) to non-human primates (Critchley & Rolls, 1996; Rolls, Murzi, Yaxley, Thorpe, & Simpson, 1986; Rolls, Sienkiewicz, & Yaxley, 1989), and humans (Epstein, Robinson et al., 2008; Temple, Giacomelli, Roemmich, & Epstein, 2008a), and research is presented that includes both animal and human studies. In addition, a broad range of responses have been studied in habituation research, ranging from reflexive responses such as acoustic startle (Geyer, Swerdlow, Mansbach, & Braff, 1990), muscle firing (Epstein & Paluch, 1997; Swithers, Westneat, & Hall, 1998), or salivation (Epstein et al., 1992), to eating behaviors (Swithers, 1996; Swithers & Hall, 1994) and motivated behavior (McSweeney & Swindell, 1999). This introduction to habituation paradigms provides a flavor for these areas which will be discussed in greater depth later in the manuscript.
Dishabituation
The dishabituation paradigm is to repeatedly present one habituating stimulus, present a new dishabituting stimulus, and then represent the initial habituating stimulus. Responding to the habituating stimulus must decrease during the initial series of stimulus presentations and responding recovered after presenting the dishabituating stimulus before the label habituation can be applied. The disruption of habituation by the novel dishabituating stimulus shows that habituation is the mechanism that causes the decrease in responding. Response decrement to repeated stimulus presentation may be due to factors other than habituation, such as receptor or effector fatigue (Thompson & Spencer, 1966; Thorpe, 1966), but the demonstration that a dishabituator can result in recovery of responses to an habituated stimulus is unique to habituation theory. There is no reason to believe that a novel stimulus should disrupt receptor or effector fatigue.
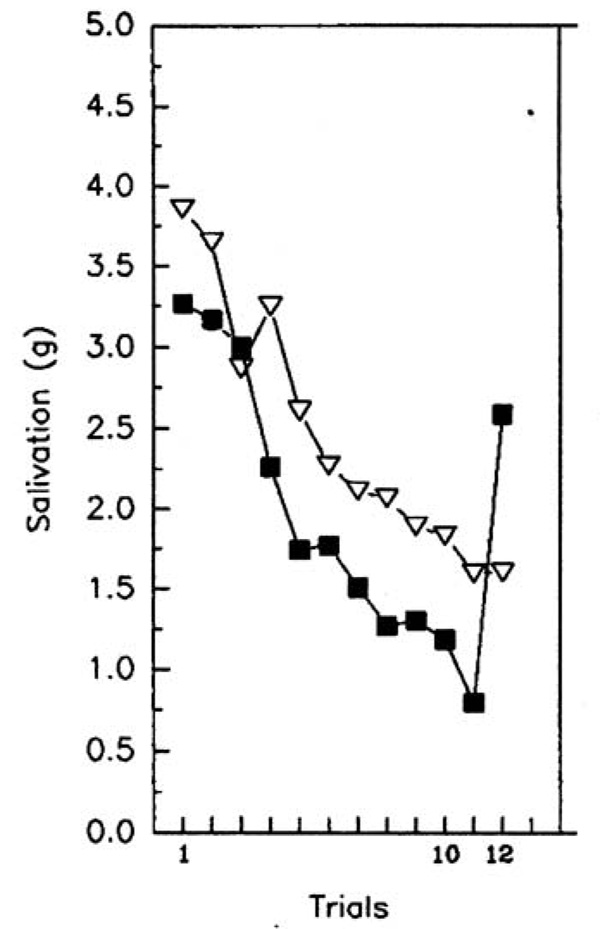
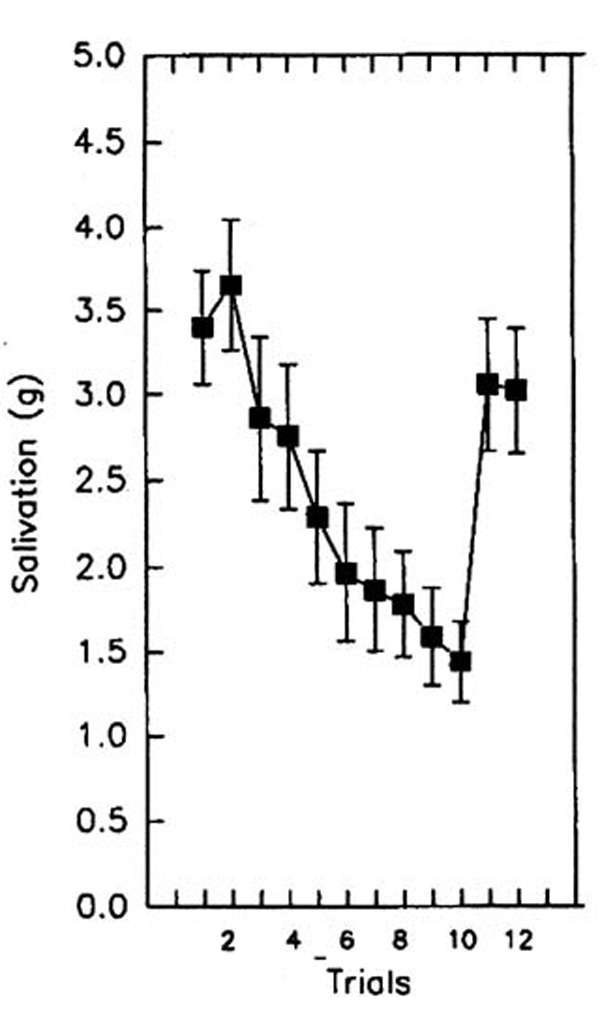
In paradigms designed to study food intake, the dishabituating stimulus can be a food (Epstein et al., 1992) or non-food (Epstein, Mitchell, & Caggiula, 1993) stimulus. For example, Epstein and colleagues (Epstein et al., 1992) studied salivary habituation to repeated presentations (trials) of a small amount of lemon or lime juice, with the alternative juice as the dishabituating stimulus. As shown in Figure 1, salivation increased slightly after the first presentation, followed by a reliable decrease in salivation through trial 10. On trial 11 the dishabituating juice was presented, which resulted in an increase in salivation, and on trial 12 a recovery of responding to the initial habituating stimulus was observed, with the recovery back to the level of initial responding. The dishabituating stimulus need not stimulate responding to be effective. To demonstrate this, bitter chocolate, which does not produce salivation, was used as a dishabituating stimulus. As shown in Figure 2, a reliable decrease in salivation was shown for the repeated lemon juice condition through trial 10, followed by the bitter chocolate dishabituator on trial 11, followed by recovery of responding to the lemon juice habituating stimulus (Epstein et al., 1992). A control condition that repeated lemon juice resulted in continuation of the decrease in salivation throughout all the trials. The dishabituation paradigm provides a methodology to test one novel aspect of applying habituation theory to eating, which is that non-food environmental or sensory stimuli can serve as dishabituators (Epstein, Mitchell et al., 1993).
Figure 1.
Salivation (mean ± SEM) for subjects who received lemon or lime juice as the habituating stimulus in trials 1–10, the other juice as the dishabituator in trial 11, and presentation of the habituating stimulus in trial 12. Adapted from (Epstein et al., 1992). Copyright 1992 by Pergamon Press. Reprinted by permission.
Figure 2.
Salivation (mean ± SEM) for subjects who received lemon juice as the habituating stimulus in trials 1–10, bitter chocolate as the dishabituator in trial 11, and presentation of the habituating stimulus in trial 12. Control subjects received 12 trials of lemon juice. Adapted from (Epstein et al., 1992). Copyright 1992 by Pergamon Press. Reprinted by permission.
Stimulus specificity
The most basic habituation paradigm is stimulus specificity. In this paradigm one stimulus is repeatedly presented, followed by presentation of a novel stimulus, testing whether the response recovers when a new stimulus is presented. The stimulus specificity paradigm does not require demonstration of recovery of responding to the previously habituated stimulus, but rather tests whether responding is recovered when a novel stimulus is presented. The stimulus specificity paradigm (McSweeney & Swindell, 1999) tests whether the decrease in responding is specific to the habituating stimulus. An elegant demonstration of stimulus specificity is provided by Rolls and colleagues, who repeatedly presented blackcurrant juice to non-human primates, and observed a simultaneous reduction in activation of neurons in the orbitofrontal cortex and behavioral responding to obtain blackcurrant juice. Presentation of a new stimulus, such as banana or apple flavor, resulted in a recovery of responding in those specific neurons (Critchley & Rolls, 1996). An example of habituation of salivation (left graphs) and motivated responding for food (right graphs) in children is presented in Figure 3 (Epstein et al., 2003). abituation of salivation was measured to repeated visual and olfactory cues of cheeseburgers, and habituation of motivated behavior was then measured to repeated opportunities to obtain portions of cheeseburgers based on operant responding for food. The motivated responding task involved responding to a computer generated variable interval 120 second schedule of reinforcement, with the first response after an average of 120 seconds earning portions of cheeseburger. In the next phase, children participating in the salivation task were presented visual and olfactory cues of apple pie, a novel food, while children participating in the motivated responding task were provided the opportunity to obtain apple pie. The groups differed by when the novel food was presented, with Group 2 experiencing the novel food one trial delayed from Group 1. Results showed a similar pattern of reduction in reflexive or motivated responding across trials until the novel food was presented, which was associated with an increase in responding. The responding in both groups did not increase until the novel food was presented (Epstein et al., 2003).
Figure 3.
Salivation (left graphs, mean ± SEM) and motivation (right graphs, mean ± SEM) for subjects who were presented cheeseburgers followed by apple pie as the new food. The introduction of the new food was delayed one trial for Group 2 in relationship to Group 1 test whether the recovery of responding occurred after presentation of the new food. Adapted from (Epstein et al., 2003). Copyright 2003 by Elsevier Ltd. Reprinted by permission.
Variety
The variety paradigm varies the stimulus characteristics over repeated trials in comparison to a group in which the same stimulus is repeatedly presented. An example might be random presentations of three different types of food versus repeated presentations of the same food. The intertrial intervals and all other aspects of the experimental paradigms are kept constant between groups to isolate the effects of food variety. The expectation is that the rate of decrease in responding to the same repeated food presentation will be slower if there is a varied presentation of foods. One explanation for this phenomenon may be that the varied foods act as novel stimuli or as dishabituators. The foods can be different foods (Myers Ernst & Epstein, 2002), or the same type of food with a different flavor (Epstein & Paluch, 1997). The sequential presentation of a variety of foods either prevents habituation from occurring, or slows down the rate of habituation. As shown in Figure 4, children presented with a variety of either low or high energy dense foods continued to respond at a high rate for foods in comparison to children who were provided only their same favorite low or high energy dense food, with the energy content of the foods for the same and variety conditions similar (Temple et al., 2008a). No differences were observed in responding as a function of the energy density of the food. Presenting a variety of foods is equivalent to a number of stimulus specificity or dishabituation paradigms. As shown in the later section of the habituation curve, when foods in the variety condition have been presented several times, habituation does occur, as also shown in another study on the effects of variety on motivated responding for food (Myers Ernst & Epstein, 2002). This is consistent with habituation theory, as habituation should eventually occur to dishabituators (Thompson & Spencer, 1966).
Figure 4.
Motivated responding (mean ± SEM) for a variety of either low or high energy dense foods or the same favorite low or high energy dense food. Adapted from (Temple et al., 2008a). Copyright 2008 by the American Psychological Association. Reprinted by permission.
Distractor
The distractor paradigm presents the same food stimulus throughout for subjects in both the distractor and control groups, but the distractor group is presented a novel stimulus during intertrial intervals. For example, Epstein and colleagues repeatedly presented lemon juice, and some adult subjects played a computer game during intertrial intervals, while other subjects had no stimulus presented during the intertrial intervals. As shown in Figure 5, presentation of the distractor slowed the rate of decrease in salivation respective to the control group. When the distractor was presented to the control group between trial 10 and 11, salivation was reinstated, consistent with dishabituation (Epstein et al., 1992).
Figure 5.
Salivation (mean ± SEM) for subjects who were received lemon juice with or without presentation of a video game distractor between trials. For subjects who were not presented the distractor between trials, the distractor was presented after trial 11, and recovery of salivation was observed. Adapted from (Epstein et al., 1992). Copyright 1992 by Pergamon Press. Reprinted by permission.
The distractor paradigm differs from dishabituation in the timing of when the distractor is presented. In dishabituation, the habituating stimulus is repeatedly presented until a response decrement is observed, and the dishabituator restores responding to the habituating stimulus. In the distractor paradigm the distractor is presented independent of response decrement, and can prevent the development of a response decrement.
The distractor effects are maximized when attentional or working memory processes are engaged. Epstein and colleagues compared an automatic search task that did not require continual utilization of working memory versus a controlled search task, that required utilizing working memory. Both tasks were presented during the intertrial intervals, and only the controlled search task slowed the rate of response decrement (Epstein, Paluch, Smith, & Sayette, 1997). The distractor paradigm may be particularly useful for studying the effects of environmental stimuli on eating, since many people simultaneously engage in eating along with other activities.
Long-term habituation
Habituation of changes in physiological or behavioral responding is usually studied during one eating session; however, it is also possible to consider that the effects of habituation on one meal extend to subsequent eating situations. The basic paradigm for long-term habituation is to observe short-term habituation and then to retest responding in a new session, usually after an extended interval in which the subject has not had contact with the habituating stimulus. To our knowledge, there is no research on long-term habituation for food cues in humans, but research has shown long-term habituation of the acoustic startle (Frings et al., 2006; Ornitz & Guthrie, 1989), sexual arousal (O'Donohue & Plaud, 1991; Plaud, Gaither, Henderson, & Devitt, 1997), and skin conductance responses (Churchill, Remington, & Siddle, 1987). For example, the paradigm used by Frings and colleagues to test long-term habituation of the acoustic startle response was to provide a series of 42 acoustic startle stimuli in daily sessions over 5 days. Results showed a decrement in startle response within each session, indicating short-term habituation, as well as a general reduction in responding over days, with generally lower initial responses and lower average startle responses over days (Frings et al., 2006). Similarly, Plaud and colleagues assessed physiologically measured sexual arousal after presentation of either a variety of erotic stimuli or the same erotic stimulus for 15 trials over 3 sessions, separated by 2–4 days. Each subject served in both conditions in a counterbalanced order. Results showed a reduction in arousal within the session if the same stimulus was presented repeatedly, as well as a general reduction in arousal across days for the same stimulus. Presenting a variety of sexual stimuli maintained responding both within and across sessions (Plaud et al., 1997).
Sensitization
Sensitization does not refer to a paradigm for studying habituation, but rather a pattern of responding. A common pattern in habituation curves is an increase in responding prior to the reduction in responding (Figure 1 and right graphs, Figure 3) (Groves & Thompson, 1970). This increase in responding prior to the reduction in responding characteristic of habituation is called sensitization. Sensitization has been observed for salivary responses to olfactory cues (Wisniewski, Epstein, & Caggiula, 1992), salivary responses to gustatory cues (Epstein et al., 1992; Wisniewski, Epstein, Marcus, & Kaye, 1997), and facial muscle responses to gustatory cues (Epstein & Paluch, 1997). There is considerably less work on the sensitization part of the response curve than on the habituation curve in relationship to eating, and it is possible that the processes responsible for the sensitization and habituation components of the response curves are different. For example, Swithers showed that administration of dopamine antagonists produced a reduction in the sensitization component of the habituation/sensitization curve for mouthing behaviors (Swithers, 1996), but the dopamine antagonists did not disrupt the habituation of mouthing behaviors. We have shown in two studies that participants who show a greater sustained increase in responding when first presented food cues consume more energy than those who begin to habituate sooner (Epstein, Robinson et al., 2008; Epstein, Temple, Robinson, Roemmich, & Marusewski, 2008).
Integrating Wagner’s SOP model to habituation research
Habituation is a fertile field of study, and a variety of theoretical models have been developed to account for the phenomena of habituation. The theoretical approach that we have used to guide our research was based initially on Wagner’s early priming model, which provided an influential approach to understanding habituation (Wagner, 1976, 1978). That model has been updated and revised to Wagner’s SOP (Standard Operating Procedure) model (Wagner, 1989) and extended to include emotional as well as sensory inputs (affective extension of SOP or AESOP) (Wagner & Brandon, 1989).
The priming model and SOP
The general idea behind the initial priming model (Wagner, 1976, 1978) was that habituation occurs when stimulus presentations are no longer surprising. Information is temporarily stored in short term memory, and when the habituating stimulus matches the information already in short term memory, a reduction in stimulus processing occurs, along with a reduction in the response. Thus, if you taste a food, it is stored briefly in short term memory, and if a second taste of that food matches the information in short term memory, then a reduction in stimulus processing and the response magnitude would take place. Variables that remove the stimulus from short term memory would slow or prevent habituation, and variables that prime the recall of the original habituating stimulus, would initiate habituation. Because short-term memory has a limited capacity, attending to a new food stimulus would remove the information about the habituating stimulus from short term memory. This would result in reactivation of responding when the habituating stimulus was represented. The same prediction would occur if a non-food environmental stimulus required reallocation of attention, and thus occupied short term memory, while eating. Shifting attention from food to the environmental stimulus would maintain responding to the food longer than if the food alone was presented.
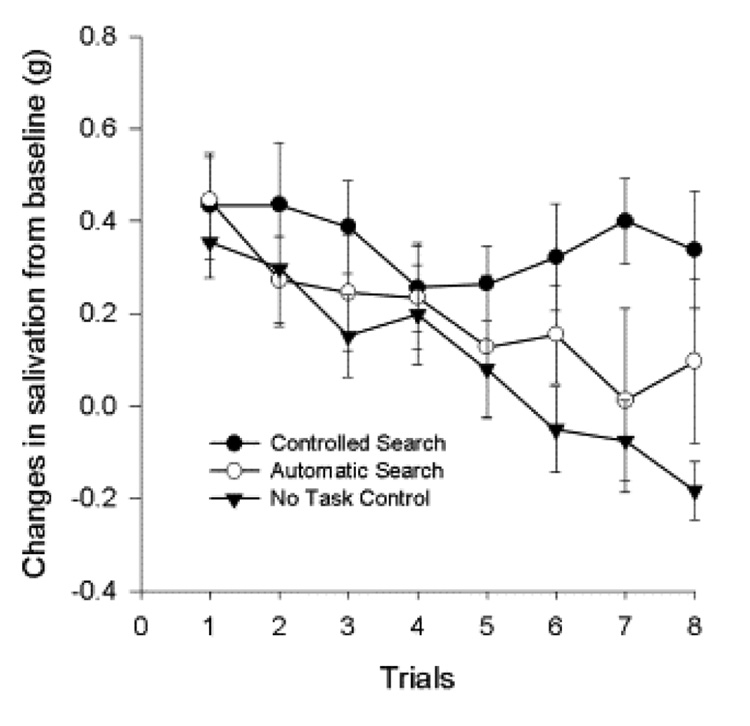
The role of allocation of attention to irrelevant stimuli as a disruptor of habituation has been studied in adults and children in distractor paradigms. In the first experiments with adults (Epstein et al., 1997), the salivary response to 10 presentations of lemon yogurt was assessed while subjects engaged in a controlled cognitive search task (demanding attentional resources), an automatic search task that matched the response requirements of the controlled cognitive task but which needed fewer attentional resources, or no task. In Experiment 1, the controlled and automatic search tasks differed in the number of memory set items. In Experiment 2, the size of the memory sets was held constant, and individuals were provided practice to stabilize the different search strategies in the task. In both experiments, the automatic search and no task groups habituated to the repeated presentation of food cues, but the controlled search group did not. The basic paradigm was studied in children who were presented a series of 8 hamburger food stimulus presentations and during each intertrial interval, participants completed the controlled or automatic visual memory task, or no task. As with adults, children in the controlled task did not habituate to repeated food cues, while children in the automatic or no task groups decreased responding over time (Figure 6) (Epstein, Saad, Giacomelli, & Roemmich, 2005).
Figure 6.
Changes (mean ± SEM) in salivation from baseline for participants in the controlled search, automatic search and no task groups. Reprinted from (Epstein et al., 2005). Copyright 2005 by Elsevier Inc. Reprinted by permission.
The priming model fits many observations within the habituation literature, but the model has been revised and expanded as new models of memory and associative learning have evolved. The first generation of Wagner’s newer models is called SOP, which stands for either Standard Operating Procedure or Sometimes Opponent Process (Wagner, 1989). This model builds on the earlier priming model, but uses a connectionist approach to memory (in which short-term memory is represented by activity in memory “nodes” embedded in an associative structure representing many nodes and their interconnections) rather than the standard information-processing model (with separate hypothetical boxes corresponding to short-term memory and long-term memory). A core principle of the SOP model is that when a stimulus is presented, a representation of that stimulus in the form of a memory node is activated to high state of activity (the A1 state), which decays over time to a lower level of activity (the A2 state). From there it decays and becomes inactive (the I state). When a node is in the A1 state, it is maximally active. In contrast, when it is in the A2 state, the processing is more peripheral. The flow of information is also unidirectional, always from A1 to A2 to I. Thus, processing cannot go from A2 to A1. A visual representation of the timing and differences in response strength of how stimuli activate the A1 and A2 states is shown in Figure 7. The figure illustrates another important assumption of SOP, namely, that the decay from A2 to Inactivity is much slower than the decay from A1 to A2.
Figure 7.
Activation of a memory node in SOP theory. (A) When the stimulus is presented, the node goes into A!, decays to A2, and then becomes inactive again. (B) Activation of the node actually depends on the proportion of elements within the node that individually go from A1, to A2, and then inactive. Some elements decay more quickly than others; activation of the node really reflects the proportion of elements in A! or A2 at any given time. Reprinted from (Bouton, 2007). Copyright 2007 by Sinauer Aoociates, Inc. Reprinted by permission.
As noted above, the A1 and A2 states correspond to different levels of nodal activation. One of the main behavioral consequences of this is that strong responding to a stimulus mainly occurs when the node is activated to A1. Under ordinary conditions, when the stimulus processing goes to A2, it does not have as strong an influence on responding (A2 is assumed to elicit its own type of behavior, which can sometimes be opposite to that controlled by A1; hence the label “sometimes opponent-process” theory). The inactive state is present when there is no stimulus processing for a memory node or connection of nodes. Application to the habituation paradigm (Jordan, Strasser, & McHale, 2000) begins as follows. At the onset of the first habituating stimulus, the node representing this stimulus is activated to the A1 state and then quickly decays to the A2 state. At presentation of the second and subsequent habituating stimuli the memory node may already be in the A2 state, which would prevent this stimulus from commanding full behavioral potential because activation cannot go from A2 to A1. In contrast, if the second presentation of the stimulus is delayed enough so that the node has gone from A2 to I, then responding can occur again. Presentation of a new stimulus activates its own, new, node to the A1 state. There are constraints on how many nodes in the system can be active at one time (a constraint that corresponds to short-term memory’s limited capacity), so presentation of a new stimulus would cause the representation of the habituating stimulus in the A2 state to go to inactive, resulting in recovery of responding (return to the A1 state) when the habituating stimulus is presented again. Thus, in the dishabituation, variety, or distractor paradigms described above, responding to the habituating stimulus recovers because a recent different stimulus has hastened return of processing of the target stimulus to the Inactive state. In the Stimulus Specificity paradigm, presentation of a novel food stimulus activates its own new node to A1. Responding will be high to the new stimulus (and appear to recover) because only the memory node associated with the first stimulus has shifted to the A2 state.
Another application of the model is to conceptualize how non-food stimuli such as television watching can disrupt habituation. If someone is dividing their attention between watching television and eating, television watching will influence the rate of habituation. In the theory’s terms, television stimuli will activate their own set of nodes, which would hasten the decay of the habituating food stimulus from A2 to Inactivity; the television stimulus serves to remove information about food stored in short term memory, thus slowing down the rate of habituation.
For example, children were presented with 10 presentations of a pizza food stimulus and either listened to an interesting, novel audiobook during the intertrial intervals or no audiobook control (Epstein et al., 2005). As predicted, children in the no audiobook group habituated while children in the audiobook group did not habituate (Figure 8). Allocation of attention to the interesting and constantly changing audiobook required activation of new A1 states, serving to dishabituate responding to food, because they take up limited short-term memory space. These ideas about the distractor effects of attending to nonfood stimuli also apply to habituation of motivated behavior. In one experiment children worked for access to cheeseburgers as the habituating stimulus in trials 1–7, and in trials 8–10, children in the control group continued to work for cheeseburgers without any dishabituating stimuli, whereas children in the other groups received either a novel food (French fries) or watched television as dishabituating stimuli. Both the novel food and the television watching groups recovered their responding for cheeseburger and increased the amount of energy earned above the level of children in the control group, with no differences between groups. In a second experiment, children had access to 1000 kcal of a preferred snack food. One group watched a continuous television show, and the control groups either watched no television or watched a repeated segment of a television show. No new information is presented if the children watch a repeated segment of a show, which should reduce its processing in A1. Results showed the continuous television group spent more time eating and consumed more energy than the no television and the repeated segment group (Temple, Giacomelli, Kent, Roemmich, & Epstein, 2007). Changes in the rate of habituation when attending to non-food stimuli while eating may be a mechanism for increasing energy intake (Epstein et al., 2005). These results support the hypothesis that distractors can influence the processing of food cues, and dishabituate eating or disrupt the development of habituation by requiring stimulus processing and activation of new memory nodes, which may provide a mechanism for increasing energy intake associated with watching television or engaging in other behaviors while eating (Epstein et al., 1997; Epstein et al., 2005; Temple, Giacomelli, Kent et al., 2007). Such results may be unique to the habituation perspective.
Figure 8.
Changes (mean ± SEM) in salivation for participants in the continuous audio group and no audio group. Reprinted from (Epstein et al., 2005). Copyright 2005 by Elsevier Inc. Reprinted by permission.
We have also shown that stressful stimuli can influence habituation and that the intensity of the affective stimuli can differentially influence habituation. Thirty women were provided a preload of a lemon yogurt milkshake to equate immediate experience with lemon flavor and habituated to seven presentations of lemon juice. Prior to the eighth presentation of juice, subjects were presented an engaging video game designed to produce subjective arousal, but no autonomic arousal (LO); a video game plus mental arithmetic stressor, designed to produce both subjective and physiological arousal (HI); or a no stimuli (REST) control. As shown in Figure 9, both heart rate and subjective arousal were greatest during the HI condition, followed by the LO condition, which was greater than the REST condition. Dishabituation of salivation followed the same pattern as subjective and physiological arousal. These results show salivation can be differentially dishabituated by nonfood stimuli, and these stimuli influence salivation without influencing subjective hunger or hedonics (Epstein, Mitchell et al., 1993). Furthermore, stressful stimuli can serve to disrupt habituation, and the disruption is greater based on the strength of the subjective and physiological arousal, defined in accord with Duffy’s model of arousal and activation (Duffy, 1972). In this regard, it would be interesting to test some of the predictions of the AESOP model that differentiates between the sensory and affective qualities of a stimulus (Wagner & Brandon, 1989). As described below, the effect of the affective qualities of a stimulus would be predicted to be longer lasting, and may influence habituation over a greater time interval and may make more associations than purely sensory stimuli.
Figure 9.
Changes in salivation, subjective arousal and heart rate (mean ± SEM) after presentation of the dishabituators for subjects in the control (REST), video game (LO) and video game plus mental arithmetic (HI) conditions. Reprinted from (Epstein, Mitchell et al., 1993). Copyright 1993 by Pergamon Press Ltd. Reprinted by permission.
Associative learning and habituation
One potentially important extension of Wagner’s model to new behavioral paradigms has been to understand how habituation theory can apply to eating over time, or long-term habituation, rather than only habituation within a meal. Understanding how habituation can influence responding over multiple meals requires a mechanism to explain how stimuli related to intake at one meal can influence intake in a second meal. More than just a theory of habituation, SOP is a general theory of conditioning and learning that successfully assimilates the phenomena of habituation with associative learning. It importantly assumes that when nodes corresponding to two stimuli are in the A1 at the same time (and thus receive maximal processing simultaneously), they will be associated. This is how the model explains conditioning; when a conditioned stimulus (CS) is activated to the A1 state at the same time as a US, there will be a strengthening of the association between them. As the association grows over repeated pairings, activation of the CS to the A1 state will increasingly activate the US node through the learned association. However, a crucial assumption is that the CS will activate the US node to the A2 rather than the A1 state. This is consistent with the idea that memories of events (activated by an associated cue) are less vivid or intense than the experience of the real event. Notice that one consequence is that a strongly-conditioned CS will fully activate the associated US node to the A2 state, and that this will prevent presentation of the US from activating its node to A1 (because a node cannot move from A2 to A1 without first going to I). This state of affairs, described in terms of the conditioning experiment in which a CS and US are paired, applies equally to the habituation experiment in which habituating stimuli are repeatedly paired with the context in which they are presented. Thus, if a stimulus is presented repeatedly in a context, the context will be associated with it. And as a consequence, the presence of the context will now activate the habituating stimulus to A2–preventing its full activation to A1 and thus decreasing responding. Thus, associative learning provides a second mechanism for habituation. The model predicts that long-term habituation will be specific to the context where habituation has occurred.
The prediction that habituation will be context-specific has been tested, and the results have not always been consistent (Bouton, 1993; Hall, 1991). Nonetheless, the prediction does work well in some systems, potentially those dealing with motivationally significant stimuli. For example, drug tolerance, the habituation that occurs to the unconditional effects of or responses to drugs such as morphine as a function of drug exposure, is strongly context-specific; after tolerance has developed, presenting the drug in a different context causes a loss of tolerance and a return of responding (Siegel, 1989). Similarly, although habituation of startle responses to a noise may transfer well across contexts, habituation of the noise’s suppression of a baseline activity may not (Jordan et al., 2000). The context specificity of long-term habituation might thus depend on the response that is studied. As noted, habituation of a variety of responses has been observed, and it is possible that some of these responses may show long-term habituation, while others may not. Research is needed to assess differences across responses to identify which responses can be used to take advantage of how long-term habituation could influence energy intake over meals.
The prediction of SOP for understanding long-term habituation, then, is that the context will be critical to habituation to meals over days. As food is associated with available contextual cues (e.g., the particular time of day, the setting, perhaps the people present when food is ingested), habituation within the meal might appear to occur faster and faster over days. We would further predict that habituation to the same food stimulus across meals and/or across days would be faster if presented in the same context than if the context was changing. These predictions will need further analysis and testing for a complete understanding of habituation and learning processes in the control of food intake.
Affective Extension of SOP (AESOP)
Motivationally-significant stimuli, like foods, have motivational as well as sensory properties. The dual nature of motivationally charged stimuli has been recognized in an affective extension of the SOP model (Wagner & Brandon, 1989). In this expansion of the theory, stimuli are explicitly recognized as activating separate nodes corresponding to their emotional (an emotive node) and sensory (a sensory node) properties. The presentation of a shock to the rabbit’s eye can activate fear (through an emotive node) and also a response corresponding more closely to the shock’s sensory properties (the sensory node; the specific left or right eye might blink). The two types of nodes have the same general characteristics; both are activated to A1, A2, and then become Inactive again. One difference between emotional and sensory responses is that emotional responses unfold more slowly in time. This feature of behavior is captured in the model by assuming that the speed of transition between A1 and A2 and then A2 and I is much slower in the emotive node than in the sensory node. Conditioning occurs in parallel in the two nodes. That is, when a CS is paired with a motivationally-significant CS, it is separately associated with both the emotive and the sensory node. Associative activation of the nodes (to A2, as usual) has different behavioral consequences. A crucial feature of the emotive node activated to A2 is that it invigorates behavior that is otherwise initiated. Thus, the fear evoked by a CS that predicts shock increases URs, CRs, and startle responses evoked by presentation of USs, CSs, and startling stimuli. Consistent with this analysis, in startle habituation with rats, presentations of the startle stimulus can condition fear of the context, which then slows the rate of habituation of the startle response (Borszcz, Cranney, & Leaton, 1989; Leaton & Cranney, 1990).
To our knowledge, AESOP has never been applied to responding to foods, or, in fact, to habituation itself. However, the scheme is compatible with earlier theorists who have claimed that conditioning with food events can excite both “preparatory” (motivational) and “consummatory” behaviors (Konorski, 1967). Thus, the theory implies that the presentation of pizza will excite both consumption and also a more diffuse motivational state. Up to now, our focus has been the dynamics of the consummatory (sensory) node. But at the same time activation and habituation of this node is occuring, we should expect motivational activation and conditioning as well. Thus, at the same time consumption of a food is habituating within the meal (following the short-term dynamics of the consummatory node), the motivational node is exciting appetite and invigorating consumption behavior. This somewhat paradoxical state of affairs potentially explains why habituation might occur within and across meals, yet the presence of a CS or situation associated with food will also excite and initiate feeding behavior (Johnson, McPhee, & Birch, 1991; Weingarten, 1983, 1984; Woods & Strubbe, 1991). Moreover, since different stimuli will excite the same motivational node (they differ in their sensory but not motivational attributes), the presentation of an alcoholic drink at the start of a meal may excite appetizer effects and stimulate intake beyond what would have been consumed without the drink (Tremblay & St-Pierre, 1996; Westerterp-Plantenga & Verwegen, 1999).
One implication of the parallel activation of motivational and consummatory nodes is that it might begin to account for sensitization effects that are often seen at the beginning of a meal (e.g., Figure 1 and Figure 3). As just noted, the first few bites of a food will excite both the motivational and the consummatory node. Since the motivational node decays more slowly over time, with intermediate interstimulus intervals, one would expect the lingering motivational activation to invigorate consummatory responding initiated by the next presentation of food. When the interstimulus interval is long enough to allow the motivational node to return to the Inactivity state, sensitization is less likely to occur. To our knowledge, this prediction has not been evaluated experimentally.
The activation—and conditioning—of motivational nodes and responses introduces a new, perhaps realistic, level of complexity to understanding food intake. Somewhat paradoxically, the presentation of a food CS or US may excite both the motivational node and the sensory/consummatory node to A2. The first effect will tend to invigorate consummatory responding, whereas the second one will tend to decrease it. Which effect will win and control behavior? Perhaps the most important thing to recognize is that activation of the motivational node cannot invigorate food consumption (consummatory responding) if the consummatory node fails to reach A1. Therefore, the dynamics of habituation of the consummatory node described above will tend to override the consequences of motivational conditioning and responding. Thus, although the motivating effects of encountering a situation previously associated with food might well enhance consumption at the start of a meal, this invigorating tendency would rapidly decline as the behavior elicited by the consummatory node habituates. It would also be offset by any tendency for the context to prime the consummatory node to A2. As a consequence of these short-term (nonassociative) or long-term (associative) processes, there would be less consummatory behavior available to motivate and invigorate. Although there is evidence for each of these effects and processes, the rules that govern their interaction will require more careful analysis and investigation.
Non-human animal habituation research
Habituation for food has been studied in a number of non-human animal models. In both juvenile and adult rats, mouthing and eating decrease with successive presentations of the same food stimulus and recover when a new flavor is presented (Swithers-Mulvey, Miller, & Hall, 1991; Swithers & Martinson, 1998). This can be shown with both behavioral observations as well as with electromyographic recordings of the mouth muscles (Swithers-Mulvey et al., 1991; Swithers et al., 1998). These behaviors are influenced by the deprivation state of the animal, with increases in hunger resulting in simultaneous decreases in the rate of habituation and increased energy consumption (Swithers, 1995). Oral stimulation, with sweet solutions such as Kool Aid™ or sucrose result in habituation of mouthing responses in young rats, even if the amount of stimulation is too small to cause significant gastric filling (Swithers-Mulvey et al., 1991; Swithers & Martinson, 1998). By contrast, bypassing oral stimulation and directly filling the stomach via a gastric cannulae, results in no decrease in food consumption (Kissileff & Van Itallie, 1982). Habituation is observed for oral stimulation alone, but habituation is more rapid for the combination of oral experience plus gastric fill (Swithers-Mulvey & Hall, 1993). These experiments highlight the important contribution of oral cues to the regulation of feeding behavior and suggest that a complex interplay of pre-cephalic and post-ingestive cues may influence termination of an eating bout, or satiation.
Rolls and colleagues have conducted a series of elegant experiments in non-human primates examining changes in neuronal responses to presentation of food stimuli and how this relates to motivation to eat. They have utilized electrophysiology to record from neurons in the nucleus of the solitary tract (NTS) in the brainstem, which receives first-order afferents from visceral organs and the gustatory and olfactory systems (Yaxley, Rolls, Sienkiewicz, & Scott, 1985), to the orbitofrontal cortex, a cortical center where multiple sensory inputs converge to modulate perception of complex processes, such as satiety and satiation (Rolls & Bayliss, 1994). In addition to mapping out these complex pathways, Rolls and colleagues have shown that neurons in the NTS respond to food stimuli regardless of the state of food deprivation of the animal and do not habituate to taste stimuli (Yaxley et al., 1985). Neurons that fire in response to food stimuli in the lateral hypothalamus and the orbitofrontal cortex habituate and are sensitive to food deprivation, showing a delay in habituation to food stimuli when the animal is fasted, relative to the fed state (Critchley & Rolls, 1996; Rolls et al., 1986; Rolls et al., 1989). This suggests that the “higher order” neurons that are integrating multiple signals are able to use information about nutritional status to adjust the rate of habituation and, thus, the rate of responding to food stimuli (Rolls et al., 1989). This is further supported by studies showing that the decrease in responding of orbitofrontal cortical neurons is associated with a shift in behavior from avid acceptance to rejection of food (Rolls et al., 1989). Examination of the receptive fields of orbitofrontal cortex neurons revealed that they are multimodal in their response characteristics; responding to taste, olfactory, and visual stimuli (Rolls & Bayliss, 1994). This provides support for the theory that integration of sensory cues at the level of the cortex is an important factor in meal initiation and meal termination and recovery of eating when new stimuli are presented.
The types of responses that are often measured in habituation paradigms are reflexive, physiological responses, such as acoustic startle (Geyer et al., 1990), muscle firing (Epstein & Paluch, 1997; Swithers et al., 1998), or salivation (Epstein et al., 1992). More complex behavioral responses, such as operant responding for food, which we describe as motivated responding for food, can also be conceptualized from the perspective of habituation (McSweeney & Swindell, 1999). For example, in rats and pigeons trained to press a lever or peck a key for access to a high rate of food presentation, high levels of responding are observed in the initial part of a session, followed by a decrease in responding during later parts of the session (McSweeney, Murphy, & Kowal, 2001; McSweeney, Swindell, & Weatherly, 1996). These data on changes in operant responding with repeated presentations of food suggest that habituation theory can be applied to the study of motivated responding for food (McSweeney, Hinson, & Cannon, 1996; McSweeney & Swindell, 1999; McSweeney, Weatherly, & Swindell, 1996). McSweeney has provided a thorough test of the points of fit between changes in motivated behavior observed in dishabituation, stimulus specificity, variety and distractor and classical habituation theory, and has outlined areas of study to confirm the majority of these predictions of habituation theory (McSweeney, Hinson et al., 1996; McSweeney & Swindell, 1999). Based on the consistency of these results, we consider the demonstration that motivated behavior habituates in animals to support the hypothesis that habituation influences eating in humans (Epstein et al., 2003; Myers Ernst & Epstein, 2002).
Applying habituation to within meal eating
Single food meals
Many meals consist of single foods, such as pizza, or macaroni and cheese, and/or single food snacks, such as ice cream, cookies, chips, etc. The habituation model fits these situations very well, and based on research using both the dishabituation and stimulus specificity paradigms it can be argued that the termination of consumption occurs when the subject habituates to food. Habituation of the motivation to eat may provide one mechanism for satiation or the termination of eating. Many people describe the cessation of eating in ways consistent with habituation, such as the food no longer tasting good, or being tired of eating.
Dishabituation and distractor paradigms provide a model for exposure to environmental stimuli during eating a meal or snack of a single food. A good example of how distractors may influence intake is popcorn consumption during a movie. Many people purchase large buckets of popcorn to eat during a movie, larger amounts than they would be likely to consume if they were just eating popcorn without watching a movie. The context of the movie theater might excite the motivational US node to eat through its prior association with popcorn eating, and the movie itself may further serve as a dishabituater for popcorn eating. Using the SOP framework, at the initiation of popcorn consumption a memory node for popcorn is activated to A1. When attention is allocated to the movie, then a new A1 node is activated, and the node that was activated to register popcorn is reactivated before it shifts to A2 status, thus maintaining the motivation to eat. It might be expected that popcorn intake will continue until either the bucket is empty, or signals that serve to signal meal termination, such as gastric distention (Cecil, 2001), occur (Swithers-Mulvey & Hall, 1993). As described above, research suggests that watching television shows or listening to audiobooks (Epstein et al., 2005; Temple, Giacomelli, Kent et al., 2007) increases the amount of food consumed in comparison to eating the food in the absence of environmental distractors.
Meals or snacks with single foods may include simple foods or combination foods. A simple food item could be an apple, or a glass of milk, while a combination food could be a pizza or a soup or stew, which involves combining several foods into a final food product. It is likely that habituation would be faster to simple than combination foods, as combination foods will have more complex sensory qualities that may require habituation to more stimuli than when foods with fewer or less intense sensory stimulation are consumed.
Multiple food meals
The majority of meals are not consumed with a single food, but rather involve multiple foods. Multiple foods can be presented in a sequential fashion, as may occur in multiple courses. It is also possible for several foods to be presented concurrently, which would be the case for many meals. The stimulus specificity paradigm provides a model in which foods are presented sequentially, for example a salad, followed by macaroni and cheese, followed by a dish of ice cream. In this example the amount consumed during each course could be regulated in part by habituation, as well as by portion size, with the introduction of the new food leading to recovery of responding for food and consumption of more food.
The presentation of a meal with a combination of foods, such as an entrée of meat, potatoes and a vegetable, with the diner consuming the foods concurrently, such as taking a bite of steak, followed by a bite of potato, followed by a bite of vegetable, would maintain responding for each food by the variety effect. Of course it is also possible that someone presented with the same meal may eat it in sequential fashion. For example, first eat all of the meat, followed by the potato and then the vegetable. To our knowledge, there is no research on which approach to consumption would lead to greater energy intake. This would be an interesting study that could lead to simple manipulations for obesity treatment.
It is straightforward to extend SOP theory to either simultaneous or concurrent intake of different foods. In the simultaneous situation, people eat one food and then after completing that food, begin to eat another food. This is similar to the stimulus specificity paradigm. Thus, a memory node for the first food is activated to the A1 state, which decays to the A2 state and results in a reduction in responding to that food. A new food is then consumed, which activates a new memory node to the A1 state, and a renewal of responding. This pattern can continue over several different types of foods. The concurrent presentation of foods represents dishabituation, in which a memory node for the first food is activated to the A1 state, which shifts to the A2 state. After a new food is consumed, activating a new memory node, and restoring the memory node for the first food to the inactive state, so that when that food is consumed again, a new memory node will be activated, reestablishing responding for the first food.
Contribution of beverages
The research to date has focused on habituation to foods, but in a usual eating situation people consume both foods and drinks, and usually in a combination fashion, with a bite or two of food followed by a sip of drink, etc. It is unknown what effect adding the drink to the sequence of foods and tastes would have on eating regulation. Based on the research on habituation to foods it would be predicted that drinking between bites might serve to recover responding for food leading to greater eating. This may be an even bigger issue if the drinks are changed during meal courses, with for example a different wine with the appetizer and main courses, followed by a different drink with dessert. Not only could the introduction of new foods lead to recovery, but the changing of the drinks could amplify this effect. It might be predicted that the dishabituation effect of drinking would be greater for more complex beverages that require habituation to different components of the beverage than unflavored beverages, perhaps with water as the least dishabituating drink. There may be physiological interactions between feeding and drinking that influence the rate of habituation, or that influence energy or fluid consumption beyond habituation. This research has obvious implications for the amount of food consumed during a meal.
Relationship between habituation and energy intake
If energy consumption was the primary factor that leads to habituation, then no habituation would be expected without energy consumption. On the contrary, it is not necessary to consume food to show habituation of a reflexive response to food cues, since salivary habituation can be observed for olfactory cues (Epstein et al., 2003). Similarly, salivary habituation can be observed for small presentations of lemon or lime juice, which have no energy and very limited fluid volume (Epstein et al., 1992). Even motivated responding for food will show habituation to repeated presentation of visual stimuli without food consumption (Temple, Giacomelli, Roemmich, & Epstein, 2008b).
In some habituation paradigms subjects consume food. One paradigm is to provide repeated trials of a food to consume until subjects eat as much food as desired (reach satiation), and then they are provided a new food. The recovery of responding after a new food is presented is a strong argument against energy intake being the primary factor that determines a reduction in responding to food or the motivation to eat. This has been tested for salivary habituation using the stimulus specificity paradigm. Salivation was measured as adults consumed portions of cheeseburger or pizza until full (Wisniewski et al., 1992). The number of trials varied based on how many trials were needed to reach at least fullness based on subjective ratings. Salivation to a new food or another portion of the same food was measured, and ad libitum access to the new food was provided. Results showed a recovery of salivation when a new food was presented, and greater energy intake for the new food than another serving of the same food (Wisniewski et al., 1992).
If habituation is a mechanism for cessation of eating, then a slower rate of habituation (increased responding over longer duration) should predict greater energy intake. This has been demonstrated for paradigms studying habituation of motivated behavior. For example, variety slows the rate of habituation of motivated behavior, and greater energy intake is consumed when a variety of foods, rather the same food, is repeatedly presented (Temple et al., 2008a). In addition, a slower rate of habituation of motivated responding for food is related to greater energy intake (Temple, Giacomelli, Roemmich, & Epstein, 2007).
An important methodological consideration for paradigms that have studied habituation of operant behavior and energy intake is that these paradigms use variable interval schedules of reinforcement to present food availability. In variable interval schedules food is available for the first response after the interval has timed out, and thus food availability is based on time, and not directly on the rate of responding. Availability of reinforcement depends primarily on the passage of time, not on the number of responses, so the measure of habituation (responses) has no necessary relationship to consumption (reinforcers). In ratio schedules, which are used to study reinforcing value of food (Epstein, Leddy, Temple, & Faith, 2007), an increased rate of responding would lead to more food earned and thus more food consumption, and measures of responses and consumption (obtained reinforcers) are linked together by the paradigm.
Research has also varied food energy, but equated the sensory experience to study the extent to which habituation depends on energy intake or on sensory changes that occur with repeated food intake. We compared salivary habituation for sucrose- and artificially-sweetened gelatin, and showed no differences in the rate of hunger, fullness or habituation, despite a difference in energy of 300 kilocalories (Epstein, Caggiula, Rodefer, Wisniewski, & Mitchell, 1993). We also compared habituation for lemon yogurts that differed in both their dietary fat and carbohydrate content. Subjects could not detect differences in the yogurts, and showed similar changes in appetite and hedonic rating of the food. Consistent with the previous study, there were no differences in the rate of habituation as a function of low and high carbohydrate yogurts. However, the high-fat yogurts were associated with a more rapid habituation than the low-fat yogurts (Myers & Epstein, 1997). This may have been due to differences in how fat versus carbohydrate influence habituation. It is possible that the greater nutrient density of dietary fat speeded up habituation or the sensory experience changed more rapidly for the higher fat yogurts, leading to more rapid habituation. More research is needed on differences in habituation in relation to macronutrient content of food.
Another test of the impact of energy intake on habituation would be to vary the energy content or portion size of the food. If satiation is the mechanism for the reduction in responding, then more rapid habituation would be expected after more energy dense portions of food. Melville and colleagues (Melville, Rue, Rybiski, & Weatherly, 1997) varied the energy density and intensity of food by changing the concentration of a sucrose solution. They found steeper late-session decreases in operant responding in rats for food when less concentrated sucrose solutions rather than when more concentrated sucrose solutions served as the reinforcer, which is the opposite of the prediction that greater energy intake would increase the rate of habituation. When the effect of portion size was tested in children, we found no differences in the rate of habituation of motivated responding for small or large portions of food (Temple et al., 2008b). We also found in that study that habituation occurred in children provided repeated visual presentations of the food, without any consumption. The rate of habituation was more rapid for children who were provided food than only visual stimuli. Differences in the rate of habituation to visual versus the combination of visual plus olfactory and gustatory cues that are presented when eating may be due to differences in the rate of habituation for different types of stimuli, or that consumption can provide post-ingestive stimulation that may interact with sensory stimuli to influence habituation and intake. Animal research suggests that habituation occurs with oral experience without gastric fill (Swithers-Mulvey et al., 1991), but more rapid habituation is observed for the combination of oral experience plus gastric fill (Swithers-Mulvey & Hall, 1993), supporting the idea that habituation may be a model for integrating multiple behavioral and physiological signals to influence eating (Swithers & Hall, 1994). Based on the composite of these data, it is hard to argue that the reduction in salivary responding or motivated responding for food depends on energy consumption, or that the reduction and cessation of eating in a usual meal (satiation) is due entirely to people being full or energy repleted.
Factors that influence habituation
Food variety
Understanding how variety influences energy intake may be important in understanding how the variety of foods is related to the increasing prevalence of obesity (McCrory et al., 1999). Overweight people consume a greater variety of foods than lean people (McCrory et al., 1999), and overweight people who enter weight control programs are more successful if they reduce the variety of high energy density foods they consume (Raynor, Jeffery, Phelan, Hill, & Wing, 2005; Raynor, Jeffery, Tate, & Wing, 2004).
If presentation of a novel food is associated with recovery of responding for food after habituation has occurred, then presentation of novel foods may disrupt the process of habituation, and slow down the rate of habituation. This is likely what happens when one consumes a variety of food during a meal. When consuming a variety of foods, the food stimuli are changing, which would suggest the rate of habituation for a variety of foods is slower than that for the same food. This phenomenon has been demonstrated for physiological (Epstein & Paluch, 1997) responses as well as motivated responding (Myers Ernst & Epstein, 2002). These results are consistent with the results of a large number of eating experiments in which subjects consume significantly more energy when given a variety of food than when given the same food (Clifton, Burton, & Sharp, 1987; Raynor & Epstein, 2001; Rolls et al., 1981; Rolls, van Duijvenvoorde, & Rolls, 1984), even when the source of the variety is small, such as different shaped pasta or different flavors of yogurt (Rolls & McDermott, 1991; Rolls, Rowe, & Rolls, 1982). This propensity to seek out and respond to food variety may be an evolutionarily advantageous phenomenon that may have arisen to ensure a balanced nutrient intake (Raynor & Epstein, 2001).
Food variety has consistently been shown in animals and humans to increase energy intake (Raynor & Epstein, 2001). For example, Melville and colleagues (Melville et al., 1997) have shown in animals that operant responding for grape-flavored liquid reinforcers decreases within sessions, but operant responding is maintained when grape reinforcers are sometimes replaced with one of the three types of solid pellets. In humans, presenting a variety of yogurt flavors at the same intertrial intervals slowed the rate of habituation of electrophysiologically measured mouth movements versus presentation of the same flavor (Epstein & Paluch, 1997). Presenting a variety of foods slows down the rate of habituation of motivated responding in adults (Myers Ernst & Epstein, 2002).
While food variety is often conceptualized as contributing to overconsumption of food, this is due in part to the fact that variety is often observed for less healthy, high-nutrient dense foods. A cursory exploration of foods in a grocery store would reveal multiple formats and flavors of salty snacks, cookies, candies, ice cream, sodas, and so on, each of which would lead to greater intake and, if consumed regularly, poorer health. If variety increases intake of less healthy foods, it may also increase intake of healthier foods. We examined this issue in a sample of children who were provided either a variety of healthier or less healthy foods. As predicted, variety increased energy intake for both healthy and less healthy foods (Temple et al., 2008a). Thus, it may be possible to take advantage of food variety to improve healthy eating by increasing access to a variety of healthy foods while simultaneously reducing access to a variety of less healthy alternatives.
Non-food novel stimuli and distractors
Habituation theory suggests that a wide variety of novel stimuli can lead to disruption of habituation, and they should not be limited to food stimuli. There are a number of ways in which the use of non-food stimuli can be used to understand factors that influence eating. We will focus on two factors, environmental distractors and stress. Many people engage in alternative activities as they eat, including watching television or movies or reading. Based on habituation theory, the presentation of alternative stimuli would serve as dishabituators or distractors to slow down the rate of habituation and increase energy intake. In our first demonstration of non-food stimuli to disrupt salivary habituation we repeatedly presented lemon juice, but for one group had them play a computer game during the intertrial interval. Playing the video game slowed down the rate of habituation (Epstein et al., 1992), showing that the disruption of habituation is not specific to food distractors.
Watching television is one behavior that has been related to obesity, and one way in which watching television may influence obesity is by slowing down the rate of habituation to foods that are consumed concurrently with watching television. As described earlier, we have shown that watching television programs disrupts habituation of motivation to eat, and people consume more food when watching television than if eating alone (Temple, Giacomelli, Kent et al., 2007). Similarly, we have shown that listening to audiobooks also can disrupt salivary habituation (Epstein et al., 2005). There are presumably a wide variety of environmental stimuli that can lead to disruption of habituation. For example, it is possible that attending to social stimuli during eating could serve to dishabituate eating, which could lead to greater eating during social situations, which is commonly called social facilitation of eating (de Castro, 1990, 1994; de Castro, Brewer, Elmore, & Orozco, 1990; de Castro & de Castro, 1989), though complex social situations also provide for the opportunity for social comparison of eating, which could have independent effects on eating (Herman, Roth, & Polivy, 2003). Social cues may also act as conditioned stimuli for eating, as eating often occurs in social situations and eating is thus paired with social cues. Over repeated pairings, social cues may become conditioned cues for eating.
The effects of distractors fit very well into a memory-based theory that accounts for how presentation of environmental stimuli that require allocation of attention will lead to a disruption of habituation, and a restoration of physiological processes stimulated by eating as well as recovery of responding for food (Wagner, 1989; Wagner & Brandon, 2001). Distractors shift allocation of memory resources from the A2 state to the inactive state, which could result in recovery of responding when a new stimulus that reactivates the memory node that is now in the inactive state. The strength of the distractor may be relevant to the degree of dishabituation, and stronger distractors may shift a memory node from the A1 to A2 to inactive state faster than a weaker distractor. Given the potential of a distractor to influence the rate of habituation and energy intake, research is needed to understand the characteristics of effective distractors beyond the fact that they require allocation of memory resources or attention. It is of course possible that repeated presentation of a distractor would result in habituation to that distractor, reducing its effects on habituation. However, most distractors do not present a common, static stimulus, but rather involve continuously changing stimuli. Watching television involves the possibility of different shows all the time, but if the same exact television show were watched repeatedly, it would lose the power to act as a distractor (Temple, Giacomelli, Kent et al., 2007).
A second way in which non-food stimuli may influence habituation is by presenting stressful stimuli. Stressful stimuli may disrupt habituation and increase eating, and as we have mentioned, the dishabituating effects of stress may be due to shifts in allocation of attention. For example, we presented a series of lemon juice stimuli, and prior to the final presentation, participants were given access to an engaging video game designed to produce subjective but no autonomic arousal, a video game plus mental arithmetic stressor, designed to produce both subjective and physiological arousal, or a no task control. Significant dishabituating effects of the video game conditions were observed, with the greatest dishabituation for the subjective and subjective plus physiological arousal condition (Epstein, Mitchell et al., 1993).
As noted previously, the AESOP model pays particular attention to affective or emotive components of stimuli. AESOP hypothesizes that stressful stimuli would result in longer activation of a memory node in the A1 state than sensory stimulation. An emotional stimulus would be predicted to be longer lasting than a purely sensory stimulus, and thus may play a bigger role as a distractor (Wagner & Brandon, 1989), as well as influence habituation over a greater time interval and may make more associations than purely sensory stimuli. It is possible that pairing an emotional distractor with neutral stimuli could also result in the neutral stimulus acquiring some of the properties of the distractor and influencing habituation. As proposed by the theory, the combination of two stimuli in the A1 state allows an excitatory association to form between them, as might be the case for food paired with particular social situations, which could increase responding as well as form the basis for a conditioned association. For example, a person may find particular social situations stressful, and consequently not habituate to food cues in this situation. There may be a particular person who often attends these social situations, and characteristics of the social situation will be associated with this person, so that eating with this person in the absence of the usual social situation may itself disrupt habituation to food, and increase energy intake. Social situations require a lot of allocation of attention, and they may disrupt habituation and lead to greater intake, consistent with the observation of social facilitation of eating (Herman et al., 2003). In addition, social situations also provide the opportunity for emotional distress that could lead to cues associated with the social situation as emotive cues that could influence habituation.
Individual differences in habituation and obesity/bulimia nervosa
If habituation is related to eating, then habituation could serve as a model for disorders that involve eating. This has been studied for both obesity and bulimia nervosa. Obesity represents a disorder that involves eating in excess of energy expenditure. Since slower habituation would be related to greater intake, then obesity may be a disorder related to slower habituation and thus greater energy intake. This has been studied in both children and adults. For example, obese children show slower rates of habituation of motivated behavior than leaner peers (Figure 10) (Epstein, Robinson et al., 2008), and adults show slower rates of salivary habituation then leaner adults (Figure 11) (Epstein, Paluch, & Coleman, 1996). Thus, a slower rate of habituation in overweight persons may maintain the motivation to eat more than for leaner persons.
Figure 10.
The mean ± SEM number of responses made on each trial for cheeseburger trials (1–10) and French fries (trials 11–13) in children who are below the 85th BMI percentile (open circles) or at or above the 85th BMI percentile (filled circles). Reprinted from (Temple, Giacomelli, Roemmich et al., 2007). Copyright 2007 by Elsevier Inc. Reprinted by permission.
Figure 11.
Salivary responses to lemon yogurt in obese and nonobese subjects across five blocks of two trials. Reprinted from (Epstein et al., 1996). Copyright 1996 by the American Psychosomatic Society. Reprinted by permission.
Bulimia nervosa is characterized by prolonged periods of food deprivation to maintain reduced body weight, and bouts of uncontrolled eating when food is consumed after deprivation. In the only study done on habituation to food in bulimia nervosa patients (Wisniewski et al., 1997), significant differences in salivary habituation were observed as normal non-bulimic women showed the decrement in responding usually observed in habituation experiments, but the women with bulimia nervosa showed no evidence of habituation, and even slight evidence for sensitization over trials. In addition, the bulimics reported a desire to vomit as they were required to taste foods over repeated trials. The failure of the bulimics to habituate is consistent with idea of a binge, as bulimic patients face a challenge in terminating a binge episode after it has begun. It is unknown how many trials would be required before the bulimic patients would shift the pattern of responding from an increase in salivation to a decrease associated with habituation, but it is clear that the pattern is very different than what is observed in obese or non-bulimic individuals.
To our knowledge there have not been any studies on habituation in patients with binge eating disorder. Binge eating disorder is characterized by periods of binge eating that is not compensated for by vomiting or pharmacological means as is the case with bulimic patients. It would be predicted that binge eaters would also show variations in habitation from non-binge eating normal subjects. It is possible that the pattern of delayed habituation for the obese compared to lean participants involves some obese binge eaters, since binge eating disorder patients represent a proportion of obese patients (Yanovski, 2003). Research is needed to examine the relationship between binge eating disorder and habituation, and future research on habituation and obesity should assess the contribution of binge eating to aberrant patterns of habituation to repeated food cues.
Obesity, eating disorders and memory
As the SOP model is a memory-based model, it is possible that differences in energy intake that are related to habituation may also be related in part to individual differences in memory processes between obese and lean subjects, or between bulimia nervosa and non-bulimia nervosa subjects. There is substantial empirical and theoretical work relevant to how memory is related to obesity and bulimia. For example, research has shown that obese adults (King, Polivy, & Herman, 1991) and children (Soetens & Braet, 2007) have an explicit memory bias for food stimuli when compared to non-obese peers, as assessed by free recall tests. This bias could relate to habituation as it could strengthen responding and increase the duration of processing in the A1 state in the memory node, which would slow down the rate of habituation, consistent with slower habituation in obese compared to lean persons (Epstein et al., 1996; Temple, Giacomelli, Roemmich et al., 2007). If the bias were also reflected in heightened activity in the motivational node, it could create more motivational conditioning. In addition, obese persons have reduced memory for the body shape of others, perhaps to avoid social comparisons made on the basis of body shape, as predicted by social comparison theory (Wegener et al., 2008). Persons with dietary restraint (King et al., 1991), which may be a risk factor for eating disorders, as well as patients with eating disorders (Sebastian, Williamson, & Blouin, 1996), have a bias towards remembering more words associated with weight, food and fatness.
Relevant to understanding how memory may be related to obesity are fMRI studies that assess activation of brain sites as a function of food stimuli. For example, Holsen and colleagues studied whether hunger modulated activation (differences in activation between deprived and fed states) in the hippocampus, orbitofrontal cortex, and medial frontal cortex and the amygdala (Holsen et al., 2005). As the authors note, activation of the hippocampus might implicate memory in the processing of food stimuli. A stronger test of the role of the hippocampus in obesity is provided by DelParigi and colleagues (DelParigi et al., 2004), who studied a sample of obese and normal weight individuals before and after tasting a liquid meal before and after weight loss. Differences in activation of the posterior hippocampus were observed in response to the meal for obese and lean subjects, and these differences did not change after weight loss, suggesting they were not a result of the weight gain, and thus may be involved in the pathophysiology of obesity.
Obesity may also decrease memory performance, but this effect may be due in part to the effect of the comorbidities of obesity that can influence memory, such as hypertension or diabetes. For example, obese Zucker rats have shown a deficit in memory in a variable interval alternation test that is hippocampus dependent when the tasks require memory over long intervals, with the deficits being related to central nervous system insulin receptor signaling (Winocur et al., 2005). Both obesity and hypertension in men are independently associated with deficits in logical memory, and the effects of obesity and hypertension on memory were additive, with performance most impaired for obese men with hypertension (Elias, Elias, Sullivan, Wolf, & D'Agostino, 2003).
It is not surprising that memory is involved in the regulation of eating. Without memory of eating, there is a reduced inhibition to eat. In the classic case of a patient who had experienced removal of most of his hippocampus, he always rated himself as moderately hungry, independent of when he ate, and he would consume a second meal right after consuming a meal (Hebben, Corkin, Eichenbaum, & Shedlack, 1985). Rozin showed a similar pattern in two amnesic patients who would consume multiple meals within 10–30 minutes of the previous meal since they did not remember they had eaten (Rozin, Dow, Moscovitch, & Rajaram, 1998). The most comprehensive approach to obesity and memory is empirical and theoretical work by Davidson and colleagues (Davidson, Kanoski, Walls, & Jarrard, 2005). Their theoretical approach is based on the idea that the hippocampus is critical to behavioral inhibition, and hippocampal memory problems will disrupt the development of appropriate inhibition of the drive to eat. When subjects eat in the absence of satiety cues, associations regarding the positive ingestive consequences of eating are stored in memory, while if people eat while sated, or in the presence of satiety cues, food cues are not followed by positive postingestive consequences, and inhibitory associations between food and eating are formed. These inhibitory mechanisms provide a brake on eating. The failure to develop these inhibitory associations can lead to overeating. Research suggests that the hippocampus is involved in the ability to inhibit inappropriate memories (Anderson et al., 2004), and animal research shows that removal of the hippocampus is related to more greater energy intake and weight gain than control animals (Davidson & Jarrard, 1993, 2004; Tracy, Jarrard, & Davidson, 2001). In addition, hippocampal damage disrupts interoceptive discrimination learning in which the animal has to utilize information about their current state of hunger or thirst to solve discriminations problems for food or water rewards (Kennedy & Shapiro, 2004). There are also data to suggest that high saturated fat diets may interfere with learning and memory by reducing levels of brain-derived neurotrophic factor (BDNF), which is important for appropriate hippocampal function and activity dependent long-term potentiation, which is a potential mechanism for memory formation (Molteni, Barnard, Ying, Roberts, & Gomez-Pinilla, 2002; Molteni et al., 2004). This model focuses on the hippocampus as important for the inhibition of eating in the presence of a toxic environment, and the inability to inhibit eating in these environments could lead to overeating and obesity.
Davidson and colleagues (Davidson et al., 2005) do not mention habituation in their model, but our model of short term habituation also makes predictions about the termination or inhibition of meals. It is easy to conceptualize that memory problems alter the processing of food cues in the SOP model (Wagner & Brandon, 1989), which could result in changes in the ability to habituate to food cues, which could lead to overeating and obesity. The inability to use memories of eating while full to inhibit eating when sated could be related to the failure to activate A1 memory nodes after eating, or the more rapid decay from A1 state to A2 and then the inactive state, which would reduce the rate of habituation. Relating habituation to Davidson and colleagues model of how memory may influence eating (Davidson et al., 2005) may produce an interesting set of new hypotheses about how memory is related to habituation and obesity. The current research on memory includes paradigms that involve explicit memory, using recall methods (King et al., 1991; Soetens & Braet, 2007), as well as studies that focus on memory processes (Davidson et al., 2005). It is worth noting that the SOP model is based on memory processes, but the type of memory evoked by SOP is not necessarily conscious or explicit memory, but is implicit memory that can occur in the absence of awareness.
Sensitization
Individual differences in sensitization may also influence energy intake. While it is common to show an increase in responding (Figure 1 and right graphs, Figure 3) prior to a reduction as part of the usual habituation curve (Groves & Thompson, 1970), there are considerable individual differences in this pattern of responding. This increase in responding prior to the reduction in responding characteristics of habituation is called sensitization. Sensitization has been observed for salivary responses to olfactory cues (Wisniewski et al., 1992), salivary responses to gustatory cues (Epstein et al., 1992; Wisniewski et al., 1997), and facial muscle responses to gustatory cues (Epstein & Paluch, 1997). To our knowledge there has been little work on the conditions that promote sensitization, or differences in eating as a function of conditions that promote or do not promote sensitization. Earlier we noted that AESOP might account for sensitization if food also activates a motivational node that tends to exaggerate consummatory responding. On this account, individuals with higher motivational node activity would be expected to sensitize more readily. We have found individual differences in sensitization in two studies, such that some participants show an increase in responding when first presented food cues, and others begin the deceleration in responding typical of habituation (Epstein, Robinson et al., 2008; Epstein, Temple et al., 2008). Interestingly, overweight participants who sensitized, or showed an initial increase in responding, habituated at a slower rate, a finding that is consistent with higher motivational node activity in obese individuals. All subjects who sensitized, whether overweight or not, consumed more food than those who did not sensitize. In addition, sensitization was not related to the increased eating that was a result of providing a variety of high energy dense foods. Research is needed to identify predictors of who sensitizes and who may be at greater risk for overconsumption in a meal.
It is thus possible that the processes responsible for the sensitization and habituation components of the response curves are different. As noted earlier, Swithers showed that dopamine antagonists were associated with a reduction in the sensitization component of the habituation/sensitization curve (Swithers, 1996), but the dopamine antagonists did not disrupt the habituation component. Geyer and associates have shown that serotonin antagonists (Geyer et al., 1990; Geyer & Tapson, 1988) speed up the rate of habituation, but have no influence on sensitization. It would be interesting to further explore the neurobiology of the sensitization and habituation components of the habituation curve. If the sensitization component of the curve is related to dopaminergic activity, it may thus be possible to increase motivation for food by enhancing the sensitization to repeated food cues, perhaps by presenting dopamine agonists paired with food cues, since dopaminergic activity is one factor that influences food reward (Berridge, 1996). What would happen if someone is presented a reinforcing food, and provided enough exposure to produce sensitization, but are not presented additional trials that lead to habituation? Would this lead to longer term sensitization, so that the next time they are presented with this food they sensitize even faster, or show greater amounts of responding? This could then be a strategy for enhancing food reward, particularly in healthy foods that people may not generally find that reinforcing. People who regularly consume a favorite food but do not show habituation for that food may only consume small amounts of the food, and they have calibrated their exposure to maximize their responsivity to the food and minimize habituation. As described previously, AESOP theory provides the basis for extending conditioning theory to sensitization and habituation, which may be useful in understanding the motivational conditions under which food tends to activate behavior, as opposed to conditions during which continued consumption leads to a reduction in eating behavior.
Other theoretical approaches: Sensory specific satiety
Sensory specific satiety represents another approach that deals with changes in eating as a function of repeated exposure to food. Sensory specific satiety is defined as a decrease in hedonics, or the subjective liking for the food that is consumed, with little change in the hedonics of uneaten food (Hetherington & Rolls, 1996). The most common sensory specific satiety paradigm is to assess hedonics of a variety of foods at baseline, have subjects consume one of the foods to satiation, and then re-measure hedonics of the food consumed as well as other foods. Results generally show that there is a greater decline in hedonics for the food that is consumed in relationship to foods that are not consumed. In addition, some studies have measured food intake over multiple courses of food presentation, and these studies show less consumption in each course of food, and that presenting multiple foods will increase intake relative to presenting only one food (Rolls et al., 1981; Rolls et al., 1984).
These changes in hedonic ratings occur within minutes of eating the food, suggesting that they are specific to pre-cephalic cues, such as flavor, texture, or odor, as opposed to post-ingestive changes in hormones or peptides associated with satiation (Rolls, 1986). This claim is further substantiated by studies showing that hedonic ratings for food decline when foods are tasted, but not swallowed (Rolls & Rolls, 1997). In fact, over the time course with which digestion occurs, there is a recovery of hedonic ratings (Rolls, 1986).
The core principle of sensory specific satiety is that the shift in hedonic ratings is specific to the characteristics of the food that are consumed. For example, a study using chocolate candies varied only food color and showed that hedonic ratings decreased more for the eaten color than for the uneaten color (Rolls et al., 1982). When actual consumption is measured, small changes in the sensory properties of foods are sufficient to increase energy intake. For example, presentation of different shaped pasta led to increased hedonic ratings and increased energy consumption relative to subjects eating only a single shape of pasta (Rolls et al., 1982). As such, variations in these properties within meals may contribute to increases in energy intake relative to a monotonous diet. However, other studies have shown that hedonic ratings for uneaten foods with similar characteristics to the eaten food will decrease, as subjects eating cheese and crackers as a meal also decreased hedonic ratings for sausages and potato chips, while ratings for bananas and yogurt remained high (Rolls et al., 1984), perhaps due to stimulus generalization to the sausage and potato chips and a failure to generalize to bananas and yogurt.
If sensory specific satiety were related to post-ingestive factors, then changes in hedonics of food or energy consumption would vary based on the energy density of the foods consumed. However, when meals of low energy density or high energy density soup or gelatin were presented, there were no differences in the change in hedonic ratings by energy density, although there were large differences in energy intake between the groups (Rolls, Hetherington, & Burley, 1988a, 1988b). This same effect was also shown for high and low calorie puddings (Birch & Deysher, 1986). These studies suggest that postingestive feedback had limited effect on changes in hedonics of foods that were consumed. Instead, factors such as flavor, texture, and color play a more important roll in the hedonic ratings of eaten and uneaten foods.
Sensory specific satiety has been a very influential approach to ingestive behavior that has guided a large body of research (Hetherington & Rolls, 1996; Raynor & Epstein, 2001). The terms sensory specific satiety and habituation are often used interchangeably. Both deal with changes in factors that influence eating based on repeated exposure to food. Indeed, both approaches predict that there will be a decrease in responding and consumption of food that occurs with repeated presentations of the same food cues and greater intake if a variety of foods is presented rather than one food. To complicate matters further, data can be interpreted either within the framework of sensory specific satiety or habituation theory. For example, a reduction in the responding of neurons in the lateral hypothalamus and orbitofrontal cortex to repeated food presentations is recovered when a new food is presented (Rolls, Critchley, Browning, Hernadi, & Lenard, 1999; Rolls et al., 1986; Rolls et al., 1989). These data have been presented in support of sensory specific satiety, but they also support the neurobiology of habituation.
Sensory specific satiety involves changes in evaluation of repeated food stimuli, not necessarily in the reduction in the intensity of the experience of the stimulus. Research with non-human primates has shown neurons in the NTS and gustatory cortex continue to respond to food after repeated presentations (Yaxley et al., 1985), while neurons in the lateral hypothalamus and the orbitofrontal cortex are reduced (habituate) to repeated presentations of food (Critchley & Rolls, 1996; Rolls et al., 1986; Rolls et al., 1989). Similarly, humans who show a reduction in liking for repeated presentation of a food do not show a reduction in subjective intensity of these foods (Rolls, Rolls, & Rowe, 1983). Changes in intensity of lemon or lime juice has been tested in an habituation paradigm, with considerable variability in the experience of intensity over 10 trials, with a pattern that was not consistent with the linear decrease in salivation (Epstein et al., 1992), which provided partial support for the independence of salivation and experience of the intensity of lemon or lime juice stimulation.
There are several important differences in the two theoretical approaches. First, a challenge in comparing the two theoretical approaches is that the methods to study habituation or sensory specific satiety are different. For example, habituation repeatedly presents food stimuli, with the expectation that responding to these stimuli will decrease, and then presentation of a new stimulus will result in recovery of responding. Representation of the habituating stimulus will result in dishabituation. In the sensory specific paradigm subjects are presented with one food to eat, just as in habituation. However, during the test for sensory specific satiety subjects are presented with a number of foods to rate for hedonics. If the first food that is tested after repeated food consumption is the same food that was consumed it is possible to show habituation, and if the subsequent food is a different food, then stimulus specificity of responding is similar to habituation paradigms. However, it is common to randomize or counterbalance the order of foods presented during the tests for sensory specific satiety, so that data needed to test habituation and stimulus specificity of hedonics are not available. Changes in the usual sensory specific paradigm so that the consumed food is always presented first after satiation, followed by foods that were not consumed, would provide data needed to generalize the results on hedonics across the two paradigms, however, this change in the usual research design may compromise the study of sensory specific satiety.
Second, sensory specific satiety refers to the influence of food consumption on hedonics of other foods. To our knowledge, this model is silent in regard to the influence of non-food environmental factors or stressors on eating. In contrast, habituation theory incorporates mechanisms to understand how attending to environmental stimuli or stressors can influence eating, providing a rich theoretical framework and body of research that can be used to generate new hypotheses on conditions or factors that influence eating. Third, sensory specific satiety does not deal with the effect of dishabituators on responding to the original food, which is a core principle of habituation theory, and part of the rich empirical tradition of habituation. Fourth, habituation is a general property of the nervous system, and can be used to understand changes in responding for a wide variety of physiological and behavioral responses, ranging from reflexive physiological responses (Groves & Thompson, 1970) to feeding (Swithers, 1996; Swithers & Hall, 1994) to drug consumption (McSweeney, Murphy, & Kowal, 2005). Sensory specific satiety applies only to feeding.
Fifth, the cardinal effect in sensory specific satiety is hedonics or liking, while habituation can be shown for a wide variety of physiological and behavioral responses, including food ingestion and the motivation to eat. Liking represents a potential determinant of what foods are consumed and how much food is consumed, but liking may not be the major determinant of food intake (Berridge, 1996; Epstein, Temple et al., 2007; Epstein et al., 2004). There are advantages to focusing more on objective measures of eating or biological or behavioral variables that are related to eating rather than to subjective factors related to food hedonics. Many sensory specific satiety studies measure eating or energy intake in addition to changes in hedonics, but the primary definition of sensory specific satiety is based on the pattern of changes in hedonics.
Liking has been measured in a number of habituation experiments, so it is possible to explore how well changes in liking are related to changes in responding to repeated food cues, and if shifts in responding after dishabituation or stimulus specificity paradigms are related to changes in liking. Liking ratings have been shown to dishabituate after presentation of a novel food in two studies (Epstein et al., 1992; Myers & Epstein, 1997), as liking for the habituated food decreased over trials, with liking showing a subsequent increase after presentation of the dishabituator. Similarly, when a new food was provided in a stimulus specificity paradigm, subjects showed an increase in liking of the new food in the experimental group compared to a reduction in liking of the same food in the control group (Wisniewski et al., 1992).
The relationship between change in habituation and change in liking during presentation of repeated food stimuli does not support change in liking as the basis for habituation. For example, in a test of differences in energy density of gelatins controlling for taste, subjects showed reliable reductions in salivation and in food hedonics, but these two measures were not related (Epstein, Caggiula et al., 1993). When dietary fat was manipulated in a series of habituation trials, differences in the rate of salivary habituation by amounts of dietary fat by group were observed. Hedonics also decreased, but with no differences in the rate of change in hedonics between groups (Myers & Epstein, 1997). In a study on the effects of food variety on habituation, differences in habituation were observed for the variety versus the same food condition, but liking of foods was not concordant. Four foods were included in the variety condition, and a difference in the rate of change in hedonics was only shown for one of the four foods (Myers Ernst & Epstein, 2002). The other three foods showed reductions in liking, but no differential changes by group that could be related to the between group differences in habituation. Finally, liking was studied when assessing differences in salivary habituation between bulimia nervosa patients and controls (Wisniewski et al., 1997). While differences in the rate of habituation were observed between groups, liking ratings decreased for all subjects, independent of group. Taken together, these studies are consistent with the idea that liking ratings change after repeated presentations of food, consistent with sensory specific satiety, and interestingly, that liking ratings to a food that has been reduced after repeated exposure can be dishabituated after presentation of a novel food (Epstein et al., 1992; Myers & Epstein, 1997). However, there is not a significant relationship between reductions in responding observed during habituated responding and liking as food stimuli (Epstein, Caggiula et al., 1993), and the two measures do not change at the same rate in response to different experimental conditions (Myers Ernst & Epstein, 2002; Wisniewski et al., 1997).
Both theoretical models have provided important insights into variables that influence eating. One way to integrate the two models may be to assume that sensory specific satiety represents a special case of habituation theory. If the data can be generalized across the paradigms based on the order of presentation of foods during testing for sensory specific satiety, the SOP model can be used as a model to understand changes in sensory specific satiety. For example, consumption of a food should result in activation of a memory node for that food in the A1 state, and repeated consumption of that food should further shift activation to the A2 state, resulting in a reduction in response to repeated presentations of that food relative to other foods. Since habituation patterns may differ across response systems (Jordan et al., 2000), research is needed to examine relationships among changes in hedonic responses, which are the basis for sensory specific satiety, with behavioral or physiological responses that are usually used to study habituation.
Other associative conditioning approaches to eating
There are other approaches that also use associative conditioning to understand different aspects of eating. For example, research has shown conditioned eating, such that environmental cues paired with eating can stimulate eating, even after satiation (Weingarten, 1983). Similarly, investigators have shown conditioned satiety, such that flavors paired with satiation can lead to a reduction of eating when presented in association with meals (Booth, 1980; Booth, Mather, & Fuller, 1974). The role of associative processes can span the range of eating situations from stimulating to suppressing eating. As noted before, AESOP theory predicts that cues associated with food may stimulate eating via conditioned activation of the motivational node. It would also accommodate conditioned satiety if satiety at the end of a meal constitutes a US that supports the conditioning of satiety responses. As noted previously, the adaptation of the SOP and AESOP models (Wagner, 1989; Wagner & Brandon, 2001) to habituation may provide a unified theoretical approach to understand how early presentations of food may stimulate eating while continued food presentations may begin to suppress it..
Other theoretical approaches to cessation of eating
Habituation represents only one theoretical approach to or cause of meal cessation. There are multiple subjective and physiological changes that may predict meal cessation, or satiation (De Graaf, Blom, Smeets, Stafleu, & Hendriks, 2004). For example, cessation of eating is associated with perception of fullness or absence of hunger (Mook & Votaw, 1992). Stomach distension is related to food intake (Cecil, Francis, & Read, 1999; Geliebter et al., 1992; Geliebter, Westreich, & Gage, 1988), and subjects report an increase in fullness as stomach distension increases. A variety of hormonal changes are associated with satiation, including cholecystokinin (Kissileff, Pi-Sunyer, Thornton, & Smith, 1981; MacIntosh et al., 2001; Schick et al., 1991), glucagon-like peptide 1 (Verdich et al., 2001), and bombesin (Lieverse, Jansen, Masclee, & Lamers, 1994; Lieverse et al., 1993; Lieverse, Masclee, Jansen, Lam, & Lamers, 1998). Peptide YY (Batterham et al., 2003; Batterham et al., 2002), which inhibits the release of neuropeptide Y, an appetite stimulant (Batterham et al., 2002) may be a reliable biomarker for satiation. In addition to these subjective and physiological changes, some people who are on a calorically restricted diet may limit energy intake as a function of daily energy goals, and learn to ignore or override physiological hunger signals. Diets and long-term changes in energy intake may influence physiological factors that influence satiation. For example, dieting can reduce stomach capacity (Geliebter, Schachter, Lohmann-Walter, Feldman, & Hashim, 1996), making you feel full on less food, and dieting can increase ghrelin concentrations (Cummings et al., 2002), which could influence satiation. Animal models provide clues for central signals associated with satiation, but there is less consistency about brain imaging and satiation in humans, due in part to challenges in adapting methods for studying satiation and brain imaging (De Graaf et al., 2004).
Many of these factors provide alternative mechanisms for satiation. However, it is possible that some of the mechanisms may influence satiation by habituation, or may interact with habituation to influence satiation. Humans report a reduction in reward value of food as a reason to stop eating (Mook & Votaw, 1992), which is very similar to the changes in motivation to eat that are observed in habituation paradigms (Myers Ernst & Epstein, 2002). Rolls has shown reliable changes in specific neuronal activity that are associated with a reduction in eating, and recovery of responding when a new food is presented (Critchley & Rolls, 1996; Rolls et al., 1986; Rolls et al., 1989), which provides ideas about brain processes relevant to habituation and satiation. Habituation can occur to repeated presentation of sensory stimulation with little or no energy intake, but stimulating gastric fill may speed up the rate of habituation (Swithers-Mulvey & Hall, 1993). It is possible that other factors that influence satiation may work in part by habituation or that habituation may interact with these factors to influence satiation.
Applications of habituation theory to eating and obesity treatment
One goal in investigating factors that regulate habituation to food and eating is to apply that knowledge to the improvement of obesity treatment. One approach is to further examine individual differences in habituation that may relate to obesity. Data show that obese children (Temple, Giacomelli, Roemmich et al., 2007) and adults (Epstein et al., 1996) habituate at slower rates to repeated food cues than leaner peers. It is possible that this difference is due to the chronic overeating that typically accompanies obesity, but it is also possible that these individual differences precede the development of obesity and could either be considered a risk factor or cause of the obesity. This would need to be tested using a prospective design in which non-obese children that differ in their rate of habituation are followed over time. If those lean children who habituate at a slower rate are more likely to increase their body weight or become obese then habituation could be a risk factor for the development of obesity.
One of the most important findings in habituation of eating research is that habituation helps to explain eating that is not regulated by energy deficit. Habituation thus provides insight into behavioral processes and interventions that will modify eating that is not controlled by an energy deficit. There are a number of habituation findings that can be applied to interventions to prevent or treat obesity. These include the reliable observation that introducing a novel food, even after satiation is reached, will result in an increase in physiological variables related to eating or in the motivation to eat. This may provide an explanation of how desserts lead to overconsumption. While this hypothesis is by no means novel, habituation provides a theoretical explanation of this phenomenon, and perhaps an approach to introducing desserts that mimic the flavors in the meals that may enhance habituation to the dessert, or perhaps the identification of desserts that are themselves associated with rapid habituation, on the basis of their composition or their taste.
Habituation is slower when a variety of foods is presented. The effect of variety occurs for both low and high energy dense foods (Temple et al., 2008a), which would lead to the dual recommendation that weight loss programs (1.) increase access to a variety of low energy density foods, to decrease habituation to them, and (2.) decrease access to a variety of high energy density foods, which would facilitate habituation to them. It would be beneficial to provide a wide variety of healthy foods, to minimize any generalization across tastes or smells that might facilitate habituation across the foods. Similarly, if high energy dense foods are available, it would be advantageous to have them as similar as possible to facilitate generalization and maximize habituation.
Another reliable finding in the habituation literature is the effect of environmental distractors on eating (Epstein et al., 1997; Epstein et al., 1992; Epstein et al., 2005; Temple, Giacomelli, Kent et al., 2007). This leads to the obvious recommendation to limit distracting stimuli during eating, such as television or reading the newspaper, may also improve weight loss outcomes, given that distracting stimuli disrupt the habituation process and lead to increased energy intake. Stressful stimuli may also represent a type of event that disrupts habituation and can lead to excess energy intake. Since basic research shows that affective memories may be longer lasting than more sensory stimuli, it may be particularly important to avoid pairing of stressful stimuli with contexts that disrupt habituation. It would be interesting to assess whether clinical interventions that alter the association between stress and contexts associated with eating would result in a reduction in eating and weight loss.
New Directions for Habituation Research
Habituation provides a theoretical approach to studying ingestive behaviors that can be applied to a broad array of dependent variables, and can be used to model a number of ways in which eating may be influenced. While there have been consistent demonstrations of habituation across animal and human subjects, and the habituation model may be useful to describe important eating phenomena, the research remains in the initial stage of development. In this section we will highlight gaps in the literature and new directions for human habituation research.
One major gap in the literature is the lack of research on long-term habituation. Research on within-session or within-meal habituation is consistent, but it is likely that there is also long-term habituation. According to the approach taken here, long-term habituation will occur if activation of a contextual memory node leads (as a consequence of conditioning) to activation of the memory of the food stimulus. Casually speaking, retrieval of a memory that the food was eaten could lead to more rapid habituation during a second set of food presentations than was observed during the initial set of food presentations. This would provide a model for studying how variety can influence eating over multiple meals, and could lead to testable hypotheses regarding conditions that would facilitate or impair the effect of reducing the number of foods consumed on long-term energy intake. Thus, while short term habituation is an important aspect of energy intake, long-term habituation may provide another, complementary mechanism that adds to the knowledge of how habituation may influence eating, in particular chronic patterns of eating.
Another gap in the literature is an understanding of the subtle interplay between sensitization and habituation processes involved in eating. For example, we have noted AESOP’s possible account of sensitization, in which activation of the motivational node on early trials may exaggerate consummatory responding on subsequent trials. Motivational nodes are assumed to decay relatively slowly over time. As a consequence, sensitized responding is predicted to be most likely if the interval between food presentations is short enough so that the motivational node has not become inactive before the next food presentation. We know of no research that has considered the influence of inter-stimulus interval on sensitization. In addition, the model acknowledges conditioned motivational effects wherein a context previously associated with food will tend to enhance responding to food until sensory habituation processes come into play. New research on long-term habituation may thus help us understand the paradoxical potential for both long-term habituation (through conditioned priming of the sensory node) as well as invigoration of eating (through conditioned priming of the motivational node).
Habituation to food cues has been studied for a number of different responses. There are several research questions that relate to habituation across multiple responses. First, it is important to determine how these measures are interrelated, and if there are some determinants of eating that do not habituate, and if there are differences between these variables in the rate of habituation. Second, it is important to determine whether habituation to food extends to habituation to other types of responses. Since habituation is a general property of the nervous system, it may be that slow habituators to food are also slow habituators in other systems. This covariation in response systems may suggest that correlated responses are also important. Since habituation to food can involve responding to external stimuli as dishabituators or distractors, it is important to learn whether those individuals who show greater effects of distractors experience the distractors differently and may habituate at a slower rate to these types of stimuli than other people. Similarly, there are individual differences in the habituation to stressors, and it is possible that those who habituate slower to stressful stimuli show greater disruption of habituation than those habituate more rapidly.
Another question to be addressed by assessing multiple response systems is whether there is habituation across modalities. One important consideration for food is that eating involves multiple sensory modalities, including taste and smell. While they are complementary in their influence on eating (Bartoshuk, 1991; Frank & Byram, 1988; Warwick, Hall, Pappas, & Schiffman, 1993), research has shown effects of olfactory (Epstein et al., 2005) and taste (Epstein, Caggiula et al., 1993) stimuli on habituation. Research has shown an interaction between smell and taste (Small & Prescott, 2005; Stevenson & Boakes, 2003), and the perception of flavor that results after the pairing of smell and taste can be conceptualized in terms of associative conditioning (Small & Prescott, 2005; Stevenson & Boakes, 2003). This suggests that there may be interplay between smell and taste such that habituation of a person to the smell of food may result in an earlier termination of an eating bout than if the person did not habituate to the smell prior to initiating eating. To our knowledge, there is no research that examines whether there is any transfer of habituation across the senses.
The breakdown of food into different stimulus elements fits well with the SOP model (Wagner, 2003), and one prediction would be that after habituation to a compound stimulus that includes both smell and taste, then presenting just smell will active part of a memory node, which could lead to faster habituation when the person is exposed to taste cues. Elemental theory makes important distinctions between the patterning of stimuli that are presented during learning versus testing. Responding is maximal when the stimulus conditions for conditioning match those in the testing situation, but the magnitude of the response decreases when the conditions of training and testing vary. For example, consider a conditioning paradigm in which a compound stimulus that includes stimulation from visual, olfactory and gustatory senses is used as the conditioned stimulus. If the same compound stimulus is used during testing, then a response close in magnitude to the original stimulus will be observed, but if testing only involves one of the three senses that comprised the initial compound training stimulus, a suppression of the conditioned response will be observed (Wagner, 2003). Given that habituation may involve visual, olfactory and gustatory cues, variations in the presentation of stimuli that signal food availability may predictably evoke different degrees of responding and habituation.
Very little is known about the exact specificity of habituation, and how different stimuli have to be to lead to dishabituation. While it is obvious that cheeseburgers and French fries are different from apple pie, what about cheese and pepperoni pizza, or macaroni and cheese in different shapes, or even chicken fingers with different types of sauces or spices? It is probable that if the food items can be discriminated, they may be sufficiently novel to lead to dishabituation. This research is important both to determine how to present foods in a meal, as well as providing a theoretical approach to differentiating foods in terms of variety. One hypothesis is if there is not dishabituation with a novel food, then that food will not lead to the variety effect. Thus, if pepperoni pizza did not lead to dishabitution of responding to cheese pizza, then these two varieties of pizza could be presented in the same meal without the risk of overconsumption relative to one varieity of pizza.
Important developmental studies need to be conducted to understand the development of habituation. When does habituation develop? Is it present at birth, unfolds developmentally, or learned after contact with the infant/child’s environment. There is one interesting developmental question that may shed light on how habituation is related to intake in infants. Many nutritionists assume that the purpose of variety is to ensure that people obtain a balance of nutrients from their diet (Raynor & Epstein, 2001), and habituation research suggests that variety in eating increases intake, potentially allowing people to maintain healthy nutrition. If variety does not lead to an increase in intake, then people might stop eating after consuming one food, which could result in inadequate nutritional balance. Infants don’t consume a variety of foods, but rather consume either breast milk, formula or some combination of these up to weaning. It would be disadvantageous for infants to habituate to the one food that meets their nutritional needs up to weaning, but after weaning it would be worthwhile for habituation to develop to ensure that the child does not focus their intake on only one food, but rather gets a variety of foods for a balanced diet. Thus, understanding when habituation develops, and how it develops may shed some light on intake during the first years of life, and also may help understand factors that are at play in normal and excessive intake during weaning.
The pattern of eating may be important for facilitating or inhibiting habituation during a meal, or across meals. For example, in a meal in which multiple foods are consumed, they can be eaten sequentially, so that one is completed before beginning to eat the next food, or in combination so that foods are consumed together, with a bite of one food followed by a bite of a new food. Research is needed to examine whether these patterns influence habituation. Similarly, food is usually consumed in combination with drinks, and it is unknown whether drinks can serve to dishabituate eating so that more food is consumed in combination with drinks than if consuming only food. Likewise, eating faster or slower will vary the rate of stimulus presentation, which may influence the rate of habituation.
Research is needed to better understand the brain processes that regulate habituation. Research has shown a reduction in responding to olfactory stimulation in the orbitofrontal cortex in humans, which is restored after presentation of a food that was not consumed (O'Doherty et al., 2000). In addition, distinct activation of the medial and lateral cerebellum is involved in short and long-term habituation of the acoustic startle response in humans (Frings et al., 2006). It may be useful to use pharmacological probes to examine the neurobiological basis for habituation. If habituation provides a model for understanding ingestive behaviors, then pharmacological probes that influence neurobiological activity that is presumed to be related to eating may provide insights both into physiological factors that influence habituation. For example, the extension of habituation to motivated behavior (McSweeney et al., 2005; McSweeney & Swindell, 1999) would suggest that factors that influence the motivation to obtain food may work by modifying habituation. One neurotransmitter that is thought to be involved in eating is dopamine (Berridge, 1996), and it would be interesting to use dopamine agonists and antagonists to manipulate brain dopamine levels and examine their effect on habituation and eating. If pharmacological probes can be identified to manipulate brain dopamine levels, then these drugs could be used to modify individual differences in habituation that may be central to the development of obesity or in binge eating.
Finally, it is likely that habituation interacts with nutritional factors to influence eating. As we have noted, gastric filling (Swithers-Mulvey & Hall, 1993) and macronutrients (Myers & Epstein, 1997) may influence habituation. Even in the prototypical habituation paradigm, in which introducing a new food produces recovery of eating for that food, it would not be expected that this would go on indefinitely, but rather that there are limits to intake that would be governed by stomach volume and gastric filling. It may be possible to take advantage of the relationship between nutritional factors and habituation to either speed up or slow down habituation. It is also possible that based on macronutrients or sensory characteristics of food, there are foods that seem resistant to habituation, that people would eat as often as possible without getting tired of that food. Many chocolate lovers would surely agree.
In summary, we have provided data to make a case for the use of habituation model to study eating. This research is in its infancy, and may help to understand some aspects of eating, and may be able to provide insight into factors responsible for obesity and eating disorders. To this point the research has focused on demonstrating paradigms that show how habituation may be used to understand within meal eating. It is hoped that the next generation of habituation research can move in two distinct, but related branches. One set of experiments should explore whether habituation provides an explanation for obesity or eating disorders, and if habituation theory can be used in the development of novel and innovative treatments for obesity. The second set of studies should be theory driven, to explore the basic mechanism for how habituation influences eating, and if habituation can be extended beyond a meal to long-term habituation. In the ideal world, the applied and basic research would interact and inform each other to simultaneously increase the utility of the research and provide the best science base to move the field forward.
Acknowledgments
Appreciation is expressed to Tony Caggiula, who helped develop the initial research program on habituation at the University of Pittsburgh, to Fran McSweeeny, who stimulated the study of habituation of motivated behavior, and who provided comments on an earlier version of this manuscript, and to Brian Wrotniak and Jodie Robinson, for comments on an earlier version of the manuscript. The development of this article was funded in part by Grant R01 HD 44725 to Dr. Leonard H. Epstein from the National Institute of Child Health and Human Development.
Contributor Information
Leonard H. Epstein, Department of Pediatrics, University at Buffalo
Jennifer L. Temple, Department of Exercise and Nutrition Sciences, University at Buffalo
James N. Roemmich, Department of Pediatrics, University at Buffalo
Mark E. Bouton, Department of Psychology, University of Vermont
References
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Baker TB, Tiffany ST. Morphine tolerance as habituation. Psychological Review. 1985;92:78–108. [PubMed] [Google Scholar]
- Bartoshuk LM. Taste, smell and pleasure. In: Bolles RC, editor. The hedonics of taste. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1991. pp. 15–28. [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3–36. New England Journal of Medicine. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Birch LL, Deysher M. Caloric compensation and sensory specific satiety: Evidence for self regulation of food intake in young children. Appetite. 1986;7:323–331. doi: 10.1016/s0195-6663(86)80001-0. [DOI] [PubMed] [Google Scholar]
- Booth DA. Acquired behavior controlling energy intake and output. In: Stunkard AJ, editor. Obesity. Philadelphia: W.B. Saunders; 1980. pp. 101–143. [Google Scholar]
- Booth DA, Mather P, Fuller J. Starch content of ordinary foods associatively conditions human appetite and satiation, indexed by eating and eating pleasantness of starch-paired flavours. Appetite. 1974;2:313–319. doi: 10.1016/s0195-6663(82)80009-3. [DOI] [PubMed] [Google Scholar]
- Borszcz GS, Cranney J, Leaton RN. Influence of long-term sensitization on long-term habituation of the acoustic startle response in rats: central gray lesions, preexposure, and extinction. Journal of Experimetal Psychology: Animal Behavior Processes. 1989;15:54–64. [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retreival in the interference paradigms of Pavlovian learning. Pscyhological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Learning and behavior: A contemporary synthesis. Sunderland, MA: Sinauer Associates, Inc; 2007. [Google Scholar]
- Cabanac M. Taste: The maximization of multidimensional pleasure. In: Capaldi ED, Powley TL, editors. Taste, experience, and feeding. Washington, D.C.: American Psychological Association; 1990. pp. 28–42. [Google Scholar]
- Cecil JE. Oral, gastric and intestinal influences on the control of appetite and feeding in humans. Appetite. 2001;36:235–236. doi: 10.1006/appe.2001.0397. [DOI] [PubMed] [Google Scholar]
- Cecil JE, Francis J, Read NW. Comparison of the effects of a high-fat and high-carbohydrate soup delivered orally and intragastrically on gastric emptying, appetite, and eating behaviour. Physiology and Behavior. 1999;67:299–306. doi: 10.1016/s0031-9384(99)00069-4. [DOI] [PubMed] [Google Scholar]
- Churchill M, Remington B, Siddle DAT. The effects of context change on long-term habituation of the orienting response in humans. Quarterly Journal of Experimental Psychology B. 1987;39:315–338. [Google Scholar]
- Clifton PG, Burton MJ, Sharp C. Rapid loss of stimulus-specific satiety after consumption of a second food. Appetite. 1987;9:149–156. doi: 10.1016/0195-6663(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. Journal of Neurophysiology. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. New England Journal of Medicine. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behavioral and Neural Biology. 1993;59:167–171. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. The hippocampus and inhibitory learning: a 'Gray' area? Neuroscience and Biobehavioral Reviews. 2004;28:261–271. doi: 10.1016/j.neubiorev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiology and Behavior. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- de Castro JM. Social facilitation of duration and size but not rate of the spontaneous meal intake of humans. Physiology and Behavior. 1990;47:1129–1135. doi: 10.1016/0031-9384(90)90363-9. [DOI] [PubMed] [Google Scholar]
- de Castro JM. Family and friends produce greater social facilitation of food intake than other companions. Physiology and Behavior. 1994;56:445–455. doi: 10.1016/0031-9384(94)90286-0. [DOI] [PubMed] [Google Scholar]
- de Castro JM, Brewer ME, Elmore DK, Orozco S. Social facilitation of the spontaneous meal size of humans occurs regardless of time, place, alcohol or snacks. Appetite. 1990;15:89–101. doi: 10.1016/0195-6663(90)90042-7. [DOI] [PubMed] [Google Scholar]
- de Castro JM, de Castro ES. Spontaneous meal patterns of humans: Influence of the presence of other people. American Journal of Clinical Nutrition. 1989;50:237–247. doi: 10.1093/ajcn/50.2.237. [DOI] [PubMed] [Google Scholar]
- De Graaf C, Blom WA, Smeets PA, Stafleu A, Hendriks HF. Biomarkers of satiation and satiety. Am J Clin Nutr. 2004;79:946–961. doi: 10.1093/ajcn/79.6.946. [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Persistence of abnormal neural responses to a meal in postobese individuals. International Journal of Obesity and Related Metabolic Disorders. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- Duffy E. Activation. In: Greenfield NS, Sternbach RA, editors. Handbook of Psychophysiology. New York: Holt, Rinehart and Winston, Inc; 1972. pp. 577–622. [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D′Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. International Journal of Obesity and Related Metabolic Disorders. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Caggiula AR, Rodefer JS, Wisniewski L, Mitchell SL. The effects of calories and taste on habituation of the human salivary response. Addictive Behaviors. 1993;18:179–185. doi: 10.1016/0306-4603(93)90048-e. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychological Bulletin. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Mitchell SL, Caggiula AR. The effect of subjective and physiological arousal on dishabituation of salivation. Physiology and Behavior. 1993;53:593–597. doi: 10.1016/0031-9384(93)90158-c. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Paluch R, Coleman KJ. Differences in salivation to repeated food cues in obese and nonobese women. Psychosomatic Medicine. 1996;58:160–164. doi: 10.1097/00006842-199603000-00011. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Paluch R, Smith JD, Sayette M. Allocation of attentional resources during habituation to food cues. Psychophysiology. 1997;34:59–64. doi: 10.1111/j.1469-8986.1997.tb02416.x. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA. Habituation of facial muscle responses to repeated food stimuli. Appetite. 1997;29:213–224. doi: 10.1006/appe.1997.0102. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Robinson JL, Temple JL, Roemmich JN, Marusewski A, Nadbrzuch R. Sensitization and habituation of motivated behavior in overweight and non-overweight children. Learning and Motivation. 2008;39:243–255. doi: 10.1016/j.lmot.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Rodefer JS, Wisniewski L, Caggiula AR. Habituation and dishabituation of human salivary response. Physiology and Behavior. 1992;51:945–950. doi: 10.1016/0031-9384(92)90075-d. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saad FG, Giacomelli AM, Roemmich JN. Effects of allocation of attention on habituation to olfactory and visual food stimuli in children. Physiology and Behavior. 2005;84:313–319. doi: 10.1016/j.physbeh.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saad FG, Handley EA, Roemmich JN, Hawk LW, McSweeney FK. Habituation of salivation and motivated responding for food in children. Appetite. 2003;41:283–289. doi: 10.1016/s0195-6663(03)00106-5. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype and energy intake in obese and non-obese humans. Behavioral Neuroscience. 2007;121:877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Robinson JL, Roemmich JN, Marusewski A. Variety influences habituation of motivated behavior for food and energy intake in children. Manuscript submitted for publication. 2008 doi: 10.3945/ajcn.2008.26911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy J, Hawk LW, Jaroni JL, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiology and Behavior. 2004;81:511–517. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Frank RA, Byram J. Taste-smell interactions are tastant and odorant dependent. Chemical Senses. 1988;13:445–455. [Google Scholar]
- Frings M, Awad N, Jentzen W, Dimitrova A, Kolb FP, Diener HC, et al. Involvement of the human cerebellum in short-term and long-term habituation of the acoustic startle response: a serial PET study. Clinical Neurophysiology. 2006;117:1290–1300. doi: 10.1016/j.clinph.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Melton PM, McCray RS, Gallagher DR, Gage D, Hashim SA. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. American Journal of Clinical Nutrition. 1992;56:656–661. doi: 10.1093/ajcn/56.4.656. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Schachter S, Lohmann-Walter C, Feldman H, Hashim SA. Reduced stomach capacity in obese subjects after dieting. American Journal of Clinical Nutrition. 1996;63:170–173. doi: 10.1093/ajcn/63.2.170. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. American Journal of Clinical Nutrition. 1988;48:592–594. doi: 10.1093/ajcn/48.3.592. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Research Bulletin. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Tapson GS. Habituation of tactile startle is altered by drugs acting on serotonin-2 receptors. Neuropsychopharmacology. 1988;1:135–147. doi: 10.1016/0893-133x(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hall G. Perceptual and associative learning. Oxford: Clarendon Press; 1991. [Google Scholar]
- Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behavioral Neuroscience. 1985;99:1031–1039. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- Herman CP, Roth DA, Polivy J. Effects of the presence of others on food intake: a normative interpretation. Psychological Bulletin. 2003;129:873–886. doi: 10.1037/0033-2909.129.6.873. [DOI] [PubMed] [Google Scholar]
- Hetherington MM, Rolls BJ. Sensory-specific satiety: Theoretical frameworks and central characteristics. In: Capaldi ED, editor. Why we eat what we eat: The psychology of eating. Washington, D.C.: American Psychological Association; 1996. pp. 267–290. [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, et al. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, McPhee L, Birch LL. Conditioned preferences: Young children prefer flavors associated with high dietary fat. Physiology and Behavior. 1991;50:1245–1251. doi: 10.1016/0031-9384(91)90590-k. [DOI] [PubMed] [Google Scholar]
- Jordan WP, Strasser HC, McHale L. Contextual control of long-term habituation in rats. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:323–339. doi: 10.1037//0097-7403.26.3.323. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. Journal of Neuroscience. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GA, Polivy J, Herman CP. Cognitive aspects of dietary restraint: Effects on person memory. International Journal of Eating Disorders. 1991;10:313–321. [Google Scholar]
- Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. American Journal of Clinical Nutrition. 1981;34:154–160. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- Kissileff HR, Van Itallie TB. Physiology of the control of food intake. Annual Review of Nutrition. 1982;2:371–418. doi: 10.1146/annurev.nu.02.070182.002103. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain: An interdiscilpinary approach. Chicago, Ill: University of Chicago Press; 1967. [Google Scholar]
- Leaton RN, Cranney J. Potentiation of the acoustic startle response by a conditioned stimulus paired with acoustic startle stimulus in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:279–287. [PubMed] [Google Scholar]
- Lieverse RJ, Jansen JB, Masclee AA, Lamers CB. Significant satiety effect of bombesin in lean but not in obese subjects. International Journal of Obesity and Related Metabolic Disorders. 1994;18:579–583. [PubMed] [Google Scholar]
- Lieverse RJ, Jansen JB, van de Zwan A, Samson L, Masclee AA, Rovati LC, et al. Bombesin reduces food intake in lean man by a cholecystokinin-independent mechanism. Journal of Clinical Endocrinology and Metabolism. 1993;76:1495–1498. doi: 10.1210/jcem.76.6.8501156. [DOI] [PubMed] [Google Scholar]
- Lieverse RJ, Masclee AA, Jansen JB, Lam WF, Lamers CB. Obese women are less sensitive for the satiety effects of bombesin than lean women. European Journal of Clinical Nutrition. 1998;52:207–212. doi: 10.1038/sj.ejcn.1600541. [DOI] [PubMed] [Google Scholar]
- MacIntosh CG, Morley JE, Wishart J, Morris H, Jansen JB, Horowitz M, et al. Effect of exogenous cholecystokinin (CCK)-8 on food intake and plasma CCK, leptin, and insulin concentrations in older and young adults: evidence for increased CCK activity as a cause of the anorexia of aging. Journal of Clinical Endocrinology and Metabolism. 2001;86:5830–5837. doi: 10.1210/jcem.86.12.8107. [DOI] [PubMed] [Google Scholar]
- McCrory MA, Fuss PJ, McCallum JE, Yao M, Vinken AG, Hays NP, et al. Dietary variety within food groups: association with energy intake and body fatness in men and women. American Journal of Clinical Nutrition. 1999;69:440–447. doi: 10.1093/ajcn/69.3.440. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Hinson JM, Cannon CB. Sensitization-habituation may occur during operant conditioning. Psychological Bulletin. 1996;120:256–271. [Google Scholar]
- McSweeney FK, Murphy ES, Kowal BP. Within-session changes in responding during concurrent variable interval variable ratio schedules. Behavioral Processes. 2001;55:163–179. doi: 10.1016/s0376-6357(01)00179-6. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Murphy ES, Kowal BP. Regulation of drug taking by sensitization and habituation. Experimental and Clinical Psychopharmacology. 2005;13:163–184. doi: 10.1037/1064-1297.13.3.163. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S. General-process theories of motivation revisited: The role of habituation. Psychological Bulletin. 1999;125:437–457. [Google Scholar]
- McSweeney FK, Swindell S, Weatherly JN. Within-session changes in responding during concurrent schedules with different reinforcers in the components. Journal of the Experimental Analysis of Behavior. 1996;66:369–390. doi: 10.1901/jeab.1996.66-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, Weatherly JN, Swindell S. Reinforcer value may change within experimental sessions. Psychonomic Bulletin & Review. 1996;3:372–375. doi: 10.3758/BF03210763. [DOI] [PubMed] [Google Scholar]
- Melville CL, Rue HC, Rybiski LR, Weatherly JN. Altering reinforcer variety or intensity changes the within-session decrease in responding. Learning and Motivation. 1997;28:609–621. [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Mook DG, Votaw MC. How important is hedonism? Reasons given by college students for ending a meal. Appetite. 1992;18:69–75. doi: 10.1016/0195-6663(92)90211-n. [DOI] [PubMed] [Google Scholar]
- Myers Ernst M, Epstein LH. Habituation of responding for food in humans. Appetite. 2002;38:224–234. doi: 10.1006/appe.2001.0484. [DOI] [PubMed] [Google Scholar]
- Myers MD, Epstein LH. The effect of dietary fat on salivary habituation and satiation. Physiology and Behavior. 1997;62:155–161. doi: 10.1016/s0031-9384(97)00028-0. [DOI] [PubMed] [Google Scholar]
- O′Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11:893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- O'Donohue W, Plaud JJ. The long-term habituation of sexual arousal in the human male. Journal of Behavior Therapy and Experimental Psychiatry. 1991;22:87–96. doi: 10.1016/0005-7916(91)90003-n. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D. Long-term habituation and sensitization of the acoustic startle response in the normal adult human. Psychophysiology. 1989;26:166–173. doi: 10.1111/j.1469-8986.1989.tb03149.x. [DOI] [PubMed] [Google Scholar]
- Plaud JJ, Gaither GA, Henderson SA, Devitt MK. The long-term habituation of sexual arousal in human males: A crossover design. Psychological Record. 1997;47:385–398. [Google Scholar]
- Raynor HA, Epstein LH. Dietary variety, energy regulation, and obesity. Psychological Bulletin. 2001;127:325–341. doi: 10.1037/0033-2909.127.3.325. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Jeffery RW, Phelan S, Hill JO, Wing RR. Amount of food group variety consumed in the diet and long-term weight loss maintenance. Obesity Research. 2005;13:883–890. doi: 10.1038/oby.2005.102. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Jeffery RW, Tate DF, Wing RR. Relationship between changes in food group variety, dietary intake, and weight during obesity treatment. International Journal of Obesity and Related Metabolic Disorders. 2004;28:813–820. doi: 10.1038/sj.ijo.0802612. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. Sensory-specific satiety. Nutrition Reviews. 1986;44:93–101. doi: 10.1111/j.1753-4887.1986.tb07593.x. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Hetherington M, Burley VJ. Sensory stimulation and energy density in the development of satiety. Physiology and Behavior. 1988a;44:727–733. doi: 10.1016/0031-9384(88)90053-4. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Hetherington M, Burley VJ. The specificity of satiety: The influence of foods of different macronutrient content on the development of satiety. Physiology and Behavior. 1988b;43:145–153. doi: 10.1016/0031-9384(88)90230-2. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, McDermott TM. Effects of age on sensory-specific satiety. American Journal of Clinical Nutrition. 1991;54:988–996. doi: 10.1093/ajcn/54.6.988. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Rowe EA, Rolls ET. How sensory properties of foods affect human feeding behavior. Physiology and Behavior. 1982;29:409–417. doi: 10.1016/0031-9384(82)90259-1. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Rowe EA, Rolls ET, Kingston B, Megson A, Gunary R. Variety in a meal enhances food intake in man. Physiology and Behavior. 1981;26:215–221. doi: 10.1016/0031-9384(81)90014-7. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, van Duijvenvoorde PM, Rolls ET. Pleasantness changes and food intake in a varied four course meal. Appetite. 1984;5:337–348. doi: 10.1016/s0195-6663(84)80006-9. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Bayliss LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. Journal of Neuroscience. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning AS, Hernadi I, Lenard L. Responses to the sensory properties of fat of neurons in the primate orbitofrontal cortex. Journal of Neuroscience. 1999;19:1532–1540. doi: 10.1523/JNEUROSCI.19-04-01532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Murzi S, Yaxley S, Thorpe SJ, Simpson SJ. Sensory-specific satiety: Food-specific reduction in responsiveness of ventral forebrain neurons after feeding in the monkey. Brain Research. 1986;368:79–86. doi: 10.1016/0006-8993(86)91044-9. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Rolls BJ, Rowe EA. Sensory-specific satiety and motivation specific satiety for the sight and taste of food and water in man. Physiology and Behavior. 1983;30:185–192. doi: 10.1016/0031-9384(83)90003-3. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Rolls JH. Olfactory sensory-specific satiety in humans. Physiology and Behavior. 1997;61:461–473. doi: 10.1016/s0031-9384(96)00464-7. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. European Journal of Neuroscience. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Rozin P, Dow S, Moscovitch M, Rajaram S. What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychological Science. 1998;9:392–396. [Google Scholar]
- Schick RR, Schusdziarra V, Mossner J, Neuberger J, Schroder B, Segmuller R, et al. Effect of CCK on food intake in man: physiological or pharmacological effect? Z Gastroenterology. 1991;29:53–58. [PubMed] [Google Scholar]
- Sebastian SB, Williamson DA, Blouin DC. Memory bias for fatness stimuli in the eating disorders. Cognitive Therapy and Research. 1996;20:275–286. [Google Scholar]
- Siegel S. Pharmacological conditioning and drug effects. In: Goudie AJ, Emmett-Oglesby MW, editors. Psychoactive drugs: Tolerance and sensitization: Contemporary neuroscience. Clinton, N.J.: Humana Press; 1989. pp. 115–180. [Google Scholar]
- Small DM, Prescott J. Odor/taste integration and the perception of flavor. Experimental Brain Research. 2005;166:345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- Soetens B, Braet C. Information processing of food cues in overweight and normal weight adolescents. British Journal of Health Psychology. 2007;12:285–304. doi: 10.1348/135910706X107604. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Boakes RA. A mnemonic theory of odor perception. Psychol Rev. 2003;110:340–364. doi: 10.1037/0033-295x.110.2.340. [DOI] [PubMed] [Google Scholar]
- Swithers-Mulvey SE, Hall WG. Integration of oral habituation and gastric signals in decerebrate rat pups. American Journal of Physiology. 1993;34:R216–R219. doi: 10.1152/ajpregu.1993.265.1.R216. [DOI] [PubMed] [Google Scholar]
- Swithers-Mulvey SE, Miller GL, Hall WG. Habituation of oromotor responding to oral infusions in rat pups. Appetite. 1991;17:55–67. doi: 10.1016/0195-6663(91)90084-6. [DOI] [PubMed] [Google Scholar]
- Swithers SE. Effects of physiological state on oral habituation in developing rats: cellular and extracellular dehydration. Developmental Psychobiology. 1995;28:131–145. doi: 10.1002/dev.420280302. [DOI] [PubMed] [Google Scholar]
- Swithers SE. Effects of oral experience on rewarding properties of oral stimulation. Neuroscience and Biobehavioral Reviews. 1996;20:27–32. doi: 10.1016/0149-7634(95)00031-9. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Hall WG. Does oral experience terminate ingestion? Appetite. 1994;23:113–138. doi: 10.1006/appe.1994.1041. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Martinson FA. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Behavioral Neuroscience. 1998;112:213–224. [Google Scholar]
- Swithers SE, Westneat MW, Hall WG. Electromyographic analysis of oral habituation in rat pups. Physiology and Behavior. 1998;63:197–203. doi: 10.1016/s0031-9384(97)00421-6. [DOI] [PubMed] [Google Scholar]
- Temple JL, Giacomelli AM, Kent KM, Roemmich JN, Epstein LH. Television watching increases motivated responding for food and energy intake in children. American Journal of Clinical Nutrition. 2007;85:355–361. doi: 10.1093/ajcn/85.2.355. [DOI] [PubMed] [Google Scholar]
- Temple JL, Giacomelli AM, Roemmich JN, Epstein LH. Overweight children habituate slower than non-overweight children to food. Physiology and Behavior. 2007;91:250–254. doi: 10.1016/j.physbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Giacomelli AM, Roemmich JN, Epstein LH. Dietary variety impairs habituation in youth. Health Psychology. 2008a;27:S10–S19. doi: 10.1037/0278-6133.27.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Giacomelli AM, Roemmich JN, Epstein LH. Habituation and within-session changes in motivated responding for food in children. Appetite. 2008b;50:390–396. doi: 10.1016/j.appet.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Thorpe WH. Learning and instinct in animals. Cambridge, MA: Harvard University Press; 1966. [Google Scholar]
- Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity. Behavioural Brain Research. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- Tremblay A, St-Pierre S. The hyperphagic effect of a high-fat diet and alcohol intake persists after control for energy density. American Journal of Clinical Nutrition. 1996;63:479–482. doi: 10.1093/ajcn/63.4.479. [DOI] [PubMed] [Google Scholar]
- Verdich C, Flint A, Gutzwiller JP, Naslund E, Beglinger C, Hellstrom PM, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. Journal of Clinical Endocrinology and Metabolism. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- Wagner AR. Priming in STM: An information-processing mechanism for self-generated or retrieval-generated depression in performance. In: Tighe TJ, Leaton RN, editors. Habituation. Hillsdale, NJ: Lawrence Erlbaum Associates; 1976. pp. 95–128. [Google Scholar]
- Wagner AR. Expectancies and the priming of STM. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive Process in Animal Behavior. Hillsdale, NJ: Lawrence Erlbaum Associates; 1978. pp. 177–209. [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1989. pp. 5–47. [Google Scholar]
- Wagner AR. Context-sensitive elemental theory. Quarterly Journal of Experimental Psychology Section B: Comparative and Physiological Psychology. 2003;56:7–29. doi: 10.1080/02724990244000133. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Brandon SE. Evolution of a structured connectionist model of Pavlovian conditioning (AESOP) In: Klein SB, Mowrer RR, editors. Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1989. pp. 149–189. [Google Scholar]
- Wagner AR, Brandon SE. A componential theory of Pavlovian conditioning. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 2001. pp. 23–64. [Google Scholar]
- Warwick ZS, Hall WG, Pappas TN, Schiffman SS. Taste and smell sensations enhance the satiating effect of both a high-carbohydrate and a high-fat meal in humans. Physiology and Behavior. 1993;53:553–563. doi: 10.1016/0031-9384(93)90153-7. [DOI] [PubMed] [Google Scholar]
- Wegener I, De Beer K, Schilling G, Conrad R, Imbierowicz K, Geiser F, et al. Patients with obesity show reduced memory for others' body shape. Appetite. 2008;50:359–366. doi: 10.1016/j.appet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Meal initiation controlled by learned cues: Effects of peripheral cholinergic blockade and cholecystokin. Physiology and Behavior. 1984;32:403–408. doi: 10.1016/0031-9384(84)90254-3. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS, Verwegen CR. The appetizing effect of an aperitif in overweight and normal-weight humans. American Journal of Clinical Nutrition. 1999;69:205–212. doi: 10.1093/ajcn/69.2.205. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, et al. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behavioral Neuroscience. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- Wisniewski L, Epstein LH, Caggiula AR. Effect of food change on consumption, hedonics, and salivation. Physiology and Behavior. 1992;52:21–26. doi: 10.1016/0031-9384(92)90428-5. [DOI] [PubMed] [Google Scholar]
- Wisniewski L, Epstein LH, Marcus MD, Kaye W. Differences in salivary habituation to palatable foods in bulimia nervosa patients and controls. Psychosomatic Medicine. 1997;59:427–433. doi: 10.1097/00006842-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Woods SC, Strubbe JH. The psychobiology of meals. Psychonomic Bulletin and Review. 1991;1:141–155. doi: 10.3758/BF03200770. [DOI] [PubMed] [Google Scholar]
- Yanovski SZ. Binge eating disorder and obesity in 2003: could treating an eating disorder have a positive effect on the obesity epidemic? Int J Eat Disord. 2003;34 Suppl:S117–S120. doi: 10.1002/eat.10211. [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls ET, Sienkiewicz ZJ, Scott TR. Satiety does not affect gustatory activity in the nucleus of the solitary tract of the alert monkey. Brain Research. 1985;347:85–93. doi: 10.1016/0006-8993(85)90891-1. [DOI] [PubMed] [Google Scholar]