Summary
An important aspect of the aging process in Drosophila melanogaster is the natural loss of antennae, legs, bristles, and parts of wings with age. These injuries lead to a loss of hemolymph, which contains water and nutrients. Stress-resistant lines of D. melanogaster are sometimes longer-lived than the populations from which they are derived. One hypothesis tested here is that increased stress resistance fosters longevity because it allows fruit flies to cope with the loss of hemolymph due to injury to the aging fly. We tested the effects of surgically induced injury on the aging and reproduction of five replicate populations. We then tested the effects of injury on populations that had been selected for different levels of stress resistance and on control populations. Injury affected aging more in males than in females, in part because of a counter-balancing reduction in female reproduction brought about by injury. More specifically, injury reduced female fecundity and male virility. Injury significantly reduced the starvation resistance in some groups of flies, but not in others. These findings undermine any simple interpretation of the interactions between injury, reproduction, and aging based on stress resistance. But they do indicate the existence of significant interactions between these biological processes, interactions that should be resolved in greater mechanistic detail than has been managed here.
Keywords: Aging, Injury, Stress Resistance, Reproduction, Drosophila
Introduction
An important aspect of the aging process in insects is the accumulation of injuries that lead to loss of physical function (Burkhard et al., 2002; Carey, 2003; Carey et al., 2007; Finch, 1990; Hayes and Wall, 1999). In the laboratory fruit fly, Drosophila melanogaster, these injuries include the loss of antennae, legs, bristles, and parts of wings with age (Burkhard et al., 2002), leading to a loss of hemolymph, which contains water and nutrients that may be essential to a fly’s survival (Folk et al., 2001).
Resistance to stresses such as starvation and desiccation is determined by the amount of water and nutrients in an insect’s body, as well as their rate of loss (Djawdan et al., 1998; Gibbs et al., 1997; Williams and Bradley, 1998, reviewed in Hoffman and Harshman, 1999). Djawdan et al. (1998) found a strong correlation when total energy content of both lipid and carbohydrate stores was regressed against starvation resistance in D. melanogaster. Gibbs et al. (1997) demonstrated that water content and water loss rates were sufficient to explain almost all of the variability in desiccation resistance in D. melanogaster lines. Desiccation resistant lines of D. melanogaster also show genetically based differences in respiratory pattern that may result in reductions in rates of respiratory water loss (Williams et al., 1997). Longer-lived populations of D. melanogaster have evolved reduced cuticular water permeability and increased whole-body water content, but no change in water content at death (Nghien et al., 2000).
In a study of the effects of induced injury to D. melanogaster, Carey et al. (2007) saw a decrease in life expectancy in deliberately injured flies, suggesting that the losses incurred via injury have a survival cost. Given these results, it is conceivable that reductions in available water and nutrients as a result of injury to the exoskeleton of insects might decrease resistance to starvation and desiccation stresses, and thereby negatively affect survival. Thus it is natural to suppose that the key to the effects of injury on adult survival are mediated by the effects of injury on stress resistance.
Several studies have shown a positive relationship between resistances to different types of stresses in insects. Drosophila populations that are selected for increased desiccation resistance often show increased resistance to starvation, ethanol vapors, heat, acetic acid vapors and gamma radiation (Hoffman and Parsons 1989a, 1989b; Hoffman and Parsons, 1993; Lamb and McDonald, 1973; Parsons, 1970), and to ambient alcohol (Rose et al., 1990). Hoffman and Parsons (1991) proposed that a reduction in mass-specific metabolic rate might be a general mechanism of resistance to a variety of stresses, but Djawdan et al. (1997) found that reductions in mass-specific metabolic rates in stress resistant D. melanogaster populations can be accounted for by increases in weight arising from lipid and carbohydrate accumulation. These studies do not establish general mechanism for stress resistance. Therefore, the effects of injury on different types of stress resistance do not have to be ubiquitous.
Life span and early reproduction are negatively correlated in insects. Selection for high fecundity at early ages in Tribolium correlates with a reduction in adult life span (Mertz, 1975; Sokal, 1970). Conversely, selection for high fertility at later ages results in increased longevity and reduced early fecundity (Luckinbill et al., 1984; Rose, 1984; Rose and Charlesworth, 1981; Wattiaux, 1968a, 1968b). Thus, if injury to the exoskeleton of flies causes a decrease in reproductive activity, this could lead to a decrease in mortality rates, consequently counteracting the increase in mortality due to hemolymph loss. In other words, injury might increase the risk of some hazards, such as hemolymph loss, but at the same time decrease the risk of other hazards, such as the energetic costs of reproduction. It has been shown that injury affects mating patterns in insects. Injured medflies will mate less frequently than intact medflies (Javois and Tammaru, 2004).
The specific interactions among injury, stress resistance, reproduction and aging in insects have not been worked out in detail. Laboratory-selected fruit fly populations allow comparisons of injury effects among populations that have different aging, reproduction, and stress-resistance patterns, and thus provide a good model system for studies of the interactions among these characters. Specifically, laboratory selection for postponed reproduction in D. melanogaster has produced populations that have postponed aging (Luckinbill et al., 1984; Rose, 1984; Rose and Charlesworth, 1980) and lowered reproduction (Luckinbill et al., 1984; Rose, 1984; Service, 1989, 1993; Service and Fales, 1993; Service and Vossbrink, 1996). Such fruit flies sometimes show a trade-off between stress resistance and early reproduction (Chippindale et al., 1993; Leroi et al., 1994; Rose et al., 1992; Service and Rose, 1985), suggesting a trade-off of energetic reserves between survival and reproduction (Chippindale et al., 1993;Chippindale, 1994; Graves et al., 1992). Furthermore, selection for stress resistance has been shown to increase longevity (Rose et al., 1992; Rose et al., 2004), suggesting that stress resistance is a causal contributor to longevity and postponed senescence.
This array of findings motivated us to test experimentally the relationship between injury, adult survival, and stress resistance in laboratory D. melanogaster. Specifically, we test the hypothesis that increased stress resistance allows fruit flies to cope better with the loss of hemolymph that results from injury to the exoskeleton of the aging fly.
Our experiments are based on surgically inducing injury to fruit flies and then monitoring the effects of injury on mortality, reproduction, and stress resistance. We begin with an analysis of the effects of injury on adult survival (experiment 1), followed by an examination of the effects of injury on fecundity and virility, two aspects of reproduction (experiments 2–5). Lastly, we test the stress resistance of injured flies among populations selected for resistance to different stresses (experiment 6).
Materials and Methods
Stocks
In experiments 1–5, we used the IV population of Drosophila melanogaster (Rose, 1984). The IV population had been selected for more than 700 generations for early reproduction prior to the start of our experiments. This population is well adapted to laboratory conditions and has been used previously as the founding population for numerous studies of mortality, reproduction and stress resistance (reviewed in Rose et al., 2004).
In experiment 6 we used the following five-fold replicated stocks: “D” selected for desiccation resistance; “C” selected for starvation resistance, and “CO” as controls (Rose et al., 1992). The D, C, and CO lines of flies were derived from the long-lived O populations in 1989. The CO populations are the controls for starvation resistant SO populations, but themselves have not been selected for stress resistance. The D and C populations are on three-week reproductive cycles, and the CO’s are on four-week reproductive cycles. When handling genetically differentiated populations, we eliminated parental effects by rearing experimental populations through two generations of common IV-type conditions (eg. Rose et al., 2004).
Experiment 1: Aging patterns of injured flies
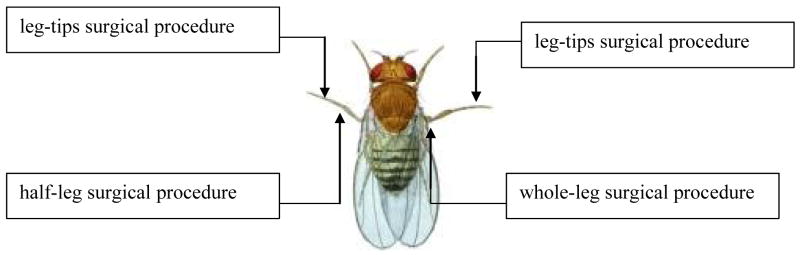
In this experiment we tested the effects of injury on aging patterns and established a usable injury protocol for subsequent experiments. We operationalized injury as surgical damage to legs. A total of 1280 IV flies (equal numbers of males and females) were kept in 8-dram vials with 4 male and 4 female flies per vial, and divided equally among the following treatments: (1) “whole-leg,” in which one entire middle leg was removed; (2) “half-leg,” in which half of one middle leg was removed; (3) “leg-tips,” in which the tips of both middle legs were removed; and (4) controls (Figure 1). We anesthetized flies with CO2 when injuring them. Control flies underwent similar handling with no surgical removal. We transferred flies to fresh banana-agar media (Rose 1984) on alternate days, and recorded the numbers that died for 28 days from the start of the transfers, in order to obtain an estimate of adult survival rates. During each transfer, dead flies were removed and live flies were recombined within groups to keep a constant density in each vial for the duration of the experiment.
Fig. 1.
The different surgical treatments assayed in experiment 1. Only the female fruit fly is depicted, but the surgical treatments are exactly the same for the male fruit fly. For the half-leg and whole-leg procedures, the selection of which middle leg (right or left) to remove was a random process. In the leg-tips procedure, the tips of both middle legs were removed.
Experiment 2: Aging patterns of injured flies with varied mating status
Here we tested the effects of mating status among injured flies on their aging patterns. The “leg-tips” procedure of experiment 1 was used. A total of 3200 IV flies were kept in vials with 8 flies per vial (4 males and 4 females whenever mated) among the following seven treatments: (1) 80 control vials, containing intact males mated to intact females; (2) 80 vials of injured males mated to intact females; (3) 80 vials of injured females mated to intact males; (4) 40 vials of injured virgin males; (5) 40 vials of intact virgin males; (6) 40 vials of injured virgin females; and (7) 40 vials of intact virgin females. Procedures for transfers and mortality counts were identical to experiment 1.
Experiment 3: Fecundity of injured females
1760 IV flies were kept in vials with 8 flies per vial (4 males and 4 females when mated) among the following five treatments: (1) controls, which contained intact males and intact females; (2) injured males and injured females; (3) injured males and intact females; (4) intact males and injured females; and (5) injured virgin females. Fecundity was measured by counting the number of eggs laid on yeasted charcoal media each day (Rose, 1984) in each vial for 10 consecutive days at the start of adult life.
Experiment 4: Mating success of injured males in competition with intact males
363 IV flies were placed in vials containing banana-agar media with three flies per vial: one injured male, one intact male, and one intact female. All flies were virgins at the start of the experiment. Markings on the wings of one of the male flies allowed us to see which of the males mated first with the female. To correct for possible effects of the mark’s odor and appearance, we marked the wing of the injured male in half of the mating vials and the wing of the intact male in the other half. For all vials, we recorded which male mated first with the female. The experiment was terminated after three hours of observation.
Experiment 5: Time to mate of injured males
This experiment consisted of four treatments, each with 40–50 vials and two flies per vial (one male and one female, both virgins): (1) controls, which contained an intact male and an intact female; (2) injured male and injured female; (3) injured male and intact female; and (4) intact male and injured female. We recorded the time of the first mating event in each of the vials. The experiment was terminated one hour after 80% of the controls had mated.
Experiment 6: Effects of injury on populations differentiated for stress resistance
We tested the effects of injury on starvation resistance in 15 populations with different levels of stress resistance (as described above). Individual replicates of the five-fold replicated D, C, and CO populations were staggered three days apart for the major events of egg collection, surgery, and set-up with the replicates labeled as 1’s three days apart from the 2’s, which were three days apart from the 3’s, etc.
Virgin flies (~10 days from egg) were injured and exposed to starvation stress using the same procedures as those of Service et al. (1985). Four flies, either males or females, and either all injured or all intact, were placed in each of the starvation vials, which contained absorbent rayon wetted with 5 ml of distilled water and a sponge separating the wet rayon from the flies. Vials were sealed with parafilm to block transfer of moisture. The number of dead flies in each vial was recorded every four hours. Death was indicated by a fly’s inability to move when the vial was tapped.
Statistical analysis
The effects of different injury protocols in experiment 1, and the effects of injury on populations with different injury treatments in experiment 2, were compared with respect to age-specific mortality. The dynamics of mortality were modeled by the Gompertz equation. Specifically the observed number of survivors in a population at each age, p̂t, were compared to the numbers predicted from the Gompertz equation (Mueller et al., 1995),
| (1) |
where A is the age-independent mortality rate and α is the age-dependent parameter that indicates the rate of senescence. The parameters of the Gompertz were estimated for each population using standard techniques of non-linear regression as implemented by the NLME package of R (www.r-project.org). The effects of injury on the two parameters of the Gompertz equation were tested with a mixed effects model (Pinheiro and Bates, 2000). This model assumed that A and α were affected by the fixed treatment effects (control vs. injury) and random effects within and between populations.
In these experiments the numbers that died at the first census age were omitted because of the characteristically high number of deaths that arise from handling effects, including the surgery. Thus our data reflect the chronic effects of injury, not the immediate deaths that occur, the latter possibly involving surgical errors. Survival was not followed until every fly died, rather mortality in the first four weeks of adult life only were used. It has been established experimentally that the aging phase ends after four to five weeks of adult life in IV-type populations (Rose et al., 2002), such that deaths after 28 days typically involve “post-aging” mortality patterns. Thus our characterization of adult survival does not include effects on “late life.” As we have argued in detail elsewhere, confounding mortality rates during the aging phase with mortality rates measured during late life is likely to generate errors of scientific inference (Rose et al., 2005). Thus we truncated our adult survival assays prior to the onset of late life. This protocol has been shown to yield excellent estimates of the Gompertz model parameters (Mueller et al., 1995).
In experiment 3, differences in fecundity due to injury treatment were determined by a one-analysis of variance. Pairs that show mean differences that are significantly different than zero were determined by Tukey’s “honestly significant” difference method (pg. 92 Miller, 1966).
Hierarchical log linear models were used to evaluate the effects of injury and marking status on mating success that were measured in experiment 4 (Bishop et al., 1975). Experiment 5 looks at a different aspect of mating, which we call male mating kinetics. The speed of mating, rather than male competition, is the focus of this experiment. The experiment starts with 40–50 virgin females and the number of mated females in this group increases with time. We modeled the number of mated females (y(t)) with the following two parameter model,
| (2) |
In this model a0 represents the asymptotic number of females mated while a1 measures the rate of increase of this function with time. Larger values of a1 imply more rapid mating. We used non-linear regression techniques to estimate a0 and a1 for each population of injured and control flies. We calculated the difference between the control population parameter estimate and the estimate for a given injury treatment. Using the estimated variances of these parameters we calculated a 95% confidence interval on these differences.
The results of experiment 6 were analyzed with a linear mixed effects model. The random effects of this experiment were the five temporal blocks and the vial effects that were nested within blocks. We represent the measured starvation resistance of an individual fly as yijklmn, where i represents either population C, CO or D, j indicates whether the fly was injured or not, k is the sex of the fly, l is the block of the experimental fly, m is the particular vial the fly was raised in, and n is the replicate number. The linear model of starvation time is then,
| (3) |
The random factors a, b, and c are assumed to all have zero mean but different variances, σ2a, σ2b, and σ2c respectively. The significance of the population, sex, and injury were assessed by t-tests of the magnitude of the parameters β, λ, and γ. Interactions are assessed by the terms in parentheses, e.g (βγ)ik, etc.
Results
Experiment 1: Effects of injury on aging patterns
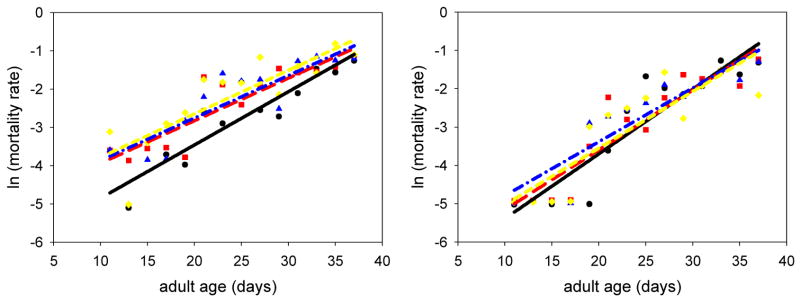
Each of the three surgical procedures tested (leg-tips, whole-leg, and half-leg) led to a significant increase in the background mortality in males but not in females (Figure 2). Injury had no significant effect on the rate of change of age-dependent mortality (the α parameter in the Gompertz model) in either males or females (Figure 2). The y-intercept in the mortality curves represents the background mortality (including environmental effects on mortality) and the slope of the lines indicates the acceleration of the mortality rate.
Fig. 2.
Results of experiment 1. The graph on the left depicts the mortality data for the males in experiment 1. The background mortality of the half-leg treatment ( ; line: red, long dash; p=0.0010), the whole-leg treatment (
; line: red, long dash; p=0.0010), the whole-leg treatment ( ; line: blue, dash-dot; p=0.0022), and the leg-tips treatment (
; line: blue, dash-dot; p=0.0022), and the leg-tips treatment ( ; line: yellow, short dash; p=0.0239) were all significantly higher than that of the controls (●; line: black, solid). The graph on the right depicts the mortality data for the females from experiment 1. Neither the background mortality nor the mortality rate was significantly different from the controls (all p-values > 0.05). The y-intercept in each of the mortality curves represents the background mortality, and the slope portrays the natural rate of aging.
; line: yellow, short dash; p=0.0239) were all significantly higher than that of the controls (●; line: black, solid). The graph on the right depicts the mortality data for the females from experiment 1. Neither the background mortality nor the mortality rate was significantly different from the controls (all p-values > 0.05). The y-intercept in each of the mortality curves represents the background mortality, and the slope portrays the natural rate of aging.
Experiment 2: Effects of injury on aging patterns in varied mating conditions
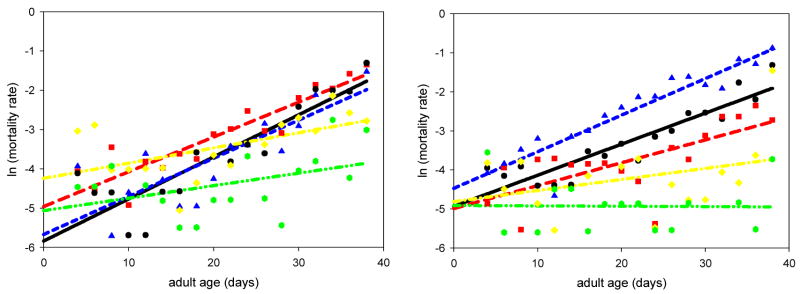
Virgins of both sexes showed a slowed mortality rate acceleration compared to mated flies regardless of injury status (Figure 3). Injured virgin males had higher background mortalities than that of the controls (intact mated flies). Injured males that were mated with intact females had lower rates of mortality acceleration and higher background mortalities than the control males. Intact males that were mated with injured females had lower rates of mortality acceleration and unaffected background mortalities. Injured females mated with intact males had higher rates of mortality acceleration and higher background mortalities than the controls. Intact females mated with injured males had lower rates of mortality acceleration and unaffected background mortalities. These results are summarized in Table 1.
Fig. 3.
Results of experiment 2. The graph on the left depicts the mortality data for the males from experiment 2; all p-values are listed in Table 1. The virgin treatments had the most significant differences in their mortality rate and background mortality. The graph on the right depicts the mortality data for the females from experiment 2, and all p-values are also given in Table 1. Again, the virgin treatments had the most significant differences in their mortality rate and background mortality. Controls (●; line: black, solid); injured males with intact females ( ; line: red, long dash); intact males with injured females (
; line: red, long dash); intact males with injured females ( ; line: blue, short dash); injured virgins (
; line: blue, short dash); injured virgins ( ; line: yellow, dash-dot); intact virgins (
; line: yellow, dash-dot); intact virgins ( ; line: green, dash-dot-dot)
; line: green, dash-dot-dot)
Tab. 1.
This table lists all the p-values for the mortality rate and background mortality data for the males (top) and females (bottom) from experiment 2.
| MALES | ||||
|---|---|---|---|---|
| treatment | mortality rate (α) | p-value for test involving a comparison with the controls | background mortality (A) | p-value for test involving a comparison with the controls |
| controls | 0.126 | n/a | 0.001 | n/a |
| injured males with intact females | 0.097 | <0.001 | 0.003 | <0.001 |
| injured females with intact males | 0.114 | 0.038 | 0.001 | 0.160 |
| virgin injured males | 0.038 | <0.001 | 0.008 | <0.001 |
| virgin intact males | 0.051 | <0.001 | 0.002 | <0.001 |
| FEMALES | ||||
| treatment | mortality rate (α) | p-value for test involving a comparison with the controls | background mortality (A) | p-value for test involving a comparison with the controls |
|
| ||||
| controls | 0.093 | n/a | 0.003 | n/a |
| injured males with intact females | 0.064 | <0.001 | 0.004 | 0.16 |
| injured females with intact males | 0.103 | 0.034 | 0.006 | <0.001 |
| virgin injured females | 0.052 | 0.003 | 0.003 | 0.77 |
| virgin intact females | −0.021 | <0.001 | 0.005 | 0.013 |
Experiment 3: Effects of injury on fecundity in females
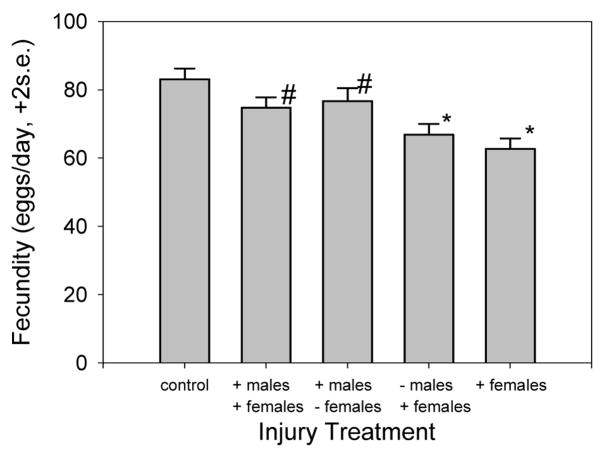
Female fecundity is significantly greater when neither fly is injured, compared to all other treatments (Figure 4), indicating that the presence of injury in either or both sexes hinders fecundity (Table 2).
Fig. 4.
Experiment 3: female fecundity averaged over 10 days for each injury treatment. A “+” before the name of a sex indicates injured flies, a “−” indicates intact flies. The symbols (# and *) identify groups with statistically similar means. All other pairs show mean differences that are statistically significant by Tukey’s honestly significant difference method (pg. 92 Miller, 1966).
Tab. 2.
The effects of injury on female fecundity over a ten day period. Injury is treated as fixed effect in a one way ANOVA.
| Source | Degrees of freedom | Sum of squares | Mean Square | F value | Pr(>F) |
|---|---|---|---|---|---|
| Injury treatment | 4 | 59021 | 14755 | 24.58 | <2 × 10−16 |
| Residual | 1099 | 659733 | 600 |
Experiment 4: Effects of injury on the mating success of injured males in competition for a virgin female
Both marking status and injury affected success at mating (p<0.00001) intact males mated more than 90% of the time (Table 3) Thus, injury appears to decrease the mating success of fruit flies.
Tab. 3.
Probability and 95% confidence interval of an intact male mating when competing against an injured male.
| Male | Probability of mating | 95% confidence interval* |
|---|---|---|
| Intact marked | 0.92 | (0.81, 0.97) |
| Injured unmarked | 0.08 | (0.023, 0.19) |
| Intact unmarked | 0.98 | (0.91, 0.9996) |
|
| ||
| Injured marked | 0.016 | (0.0004, 0.087) |
computed from the binomial distribution
Experiment 5: Effects of injury on the time to mate of injured males
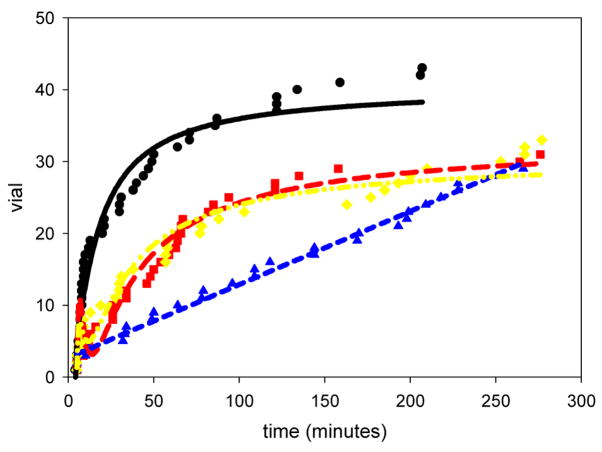
Injuries to both males and females slowed down the rate of mating (Figure 5). All injuries resulted in a significant decrease in the parameter a1, which determines the rate of mating (Figure 6).
Fig. 5.
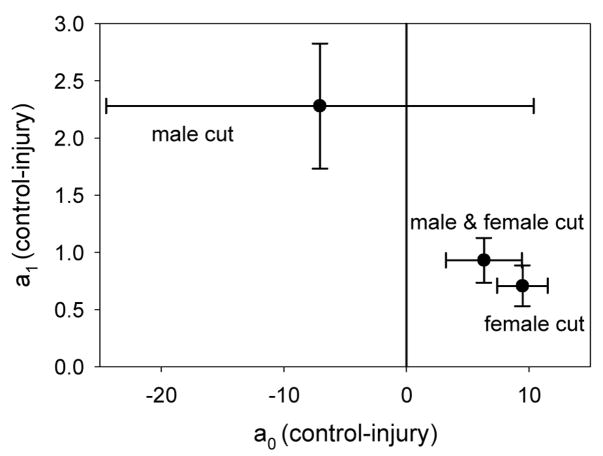
Experiment 5: parameter estimates of the mating kinetics model (equation 2) from the control and three injury treatments are used to construct each point and 95% confidence interval in this figure. Each point is the control parameter estimate minus the indicated injury treatment parameter estimate. A 95% confidence interval has been constructed on this difference. If the interval does not intersect the a0=0 or the a1=0 line the injury parameter estimate is considered significantly different from the control.
Fig. 6.
The virility data from experiment 5. The rate of mating for the injured males with injured females (95% confidence interval = 0.931 ± 0.197), the injured males with intact females (95% confidence interval = 2.28 ± 0.547), and the intact males with the injured females (95% confidence interval = 0.708 ± 0.178) treatments were all significantly lower than the controls. Controls (●; line: black, solid); injured males with injured females ( ; line: red, long dash); injured males with intact females (
; line: red, long dash); injured males with intact females ( ; line: blue, short dash); intact males with injured females (
; line: blue, short dash); intact males with injured females ( ; line: yellow, dash-dot-dot)
; line: yellow, dash-dot-dot)
Experiment 6: Effects of injury on stress resistant populations
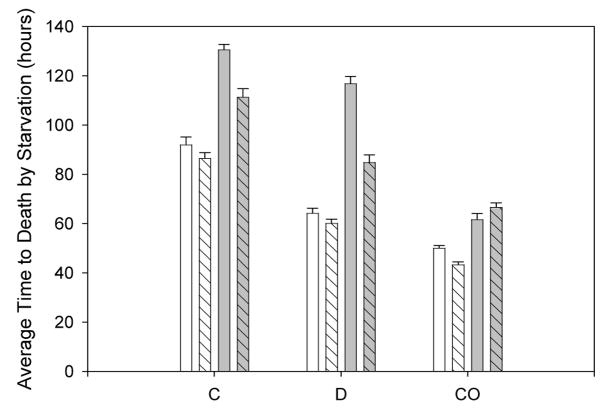
Injured flies, were significantly less resistant to starvation stress than intact flies by about 23 hours (Table 4, λinjured). The C flies were significantly more resistant to starvation than the D flies (by 11.9 hours, Table 4), which were in turn more resistant than the CO flies (by 54.9 hours, Table 4). On average, females resisted starvation better than males over all populations (by 40.6 hours, Table 4). There were statistically significant population by sex interactions, sex by treatment interactions, and population by sex by treatment interactions (Figure 7, Table 4).
Tab. 4.
The estimated parameters and their significance from the mixed effects linear model (eq. 3) of starvation resistance.
| Value | Std. Error | DF | t-value | p-value | |
|---|---|---|---|---|---|
| Intercept (α) | 129 | 4.67 | 1487 | 27.7 | <0.0001 |
| CO population (βCO) | −66.8 | 3.09 | 1487 | −21.6 | <0.0001 |
| D population (βD) | −11.9 | 3.24 | 1487 | −3.67 | 0.0003 |
| Males (γmale) | −40.6 | 3.09 | 1487 | −13.1 | <0.0001 |
| Injured (λinjured) | −23.2 | 3.07 | 1487 | −7.57 | <0.0001 |
| CO× male (βγ)CO,male | 28.7 | 4.36 | 1487 | 6.60 | <0.0001 |
| D × male (βγ)D,male | −12.6 | 4.55 | 1487 | −2.76 | 0.0058 |
| CO × injury (βγ)CO,injured | 28.5 | 4.36 | 1487 | 6.53 | <0.0001 |
| D × injury (βλ)D,injured | −8.90 | 4.61 | 1487 | −1.93 | 0.054 |
| Male × injury (γλ) male,injured | 17.7 | 4.36 | 1487 | 4.05 | 0.0001 |
| CO × male × injury (βγλ) CO,male,injured | −28.1 | 6.10 | 1487 | −4.60 | <0.0001 |
|
| |||||
| D × male × injury (βγλ) D,male,injured | 11.6 | 6.36 | 1487 | 1.82 | 0.069 |
Fig. 7.
Experiment 6: effects of injury on flies already selected for a particular type of stress resistance: desiccation resistant (D), starvation resistant (C), and controls (CO). The bars represent the average time to death for each specific category of the combined replicate data. Bars with grey shading represent females, and stripes indicate the injury treatments.
Discussion
We posited that loss of hemolymph due to age-specific injury to the exoskeleton in Drosophila melanogaster is one physiological mechanism underlying the association between aging and stress resistance observed in our long-lived and stress-resistant lines of flies. The first five experiments established the existence of effects of injury both on adult survival during aging and on reproductive patterns, and the sixth experiment revealed the effects of injury among differentially selected lines of fruit flies. We now enumerate our specific conclusions seriatim.
Experiment 1: Injury had a significant effect on male aging patterns but not on female aging patterns
All injury protocols (the cutting of two leg tips, half of one middle leg, and one entire middle leg) led to significant increases in the background mortality of males but not females. These results partially corroborate Carey et al. (2007), who found a stronger effect of injury on males than on females. However, we recorded mortality rates, while Carey et al. (2007) recorded life expectancies, so the results are not directly comparable. Injury did not affect the rate of aging (i.e. acceleration of mortality rate) in either sex. These results are depicted in Figure 2.
Before deciding among injury protocols for this experiment, we explored different potential procedures, such as ones involving the amputation of wings or antennae. However, these procedures resulted in acute effects on mortality, in that the flies died within the first few days, and sometimes within only the first few hours. We avoided removal of the front legs, which is a procedure that Carey et al. (2007) included in their study, because of the role of the front legs in mating: males use the sex combs on their front legs to mount the female. We also avoided removal of the back legs in order to avoid a radical effect on the mobility of the injured flies. Since the three injury protocols tested gave qualitatively similar results in this experiment, the leg-tips procedure, which was the easiest to perform, was retained as our injury protocol for all subsequent experiments.
Experiment 2: Mating influences the observed effects of injury on aging patterns
The results of experiment 1 were unexpected in that injury did not seem to affect the aging patterns of females. This led us to explore the consequences of injury for mating, in view of previous studies in which a decrease in female fecundity was correlated to reduced mortality (Luckinbill et al., 1984; Rose, 1984; Rose and Charlesworth, 1981). If injured females had reduced reproduction, this might explain the lack of an effect on mortality after injury, because the benefits of reduced reproduction might counterbalance the directly deleterious effects of injury.
We recorded the effects of a variety of injury and mating treatments on aging patterns. Similar to the results in experiment 1, the background mortality of males was significantly increased when injured, regardless of mating status. More importantly, when males (either injured or intact) were mated to injured females, the males had significantly lowered mortality rates, suggesting that males mate less when they are paired with injured females. This could be because of an injured female’s inability to carry the male during mating, or her unwillingness to do so. Interestingly, there was a significant increase in the mortality rates of injured females when mated to intact males, compared to the case in which they were mated to injured males. The harassment that injured females received from the efforts of intact males to mate with them may have led to this result. Mating is harmful to both genders; when the male injects the female with sperm, the male introduces a foreign substance to the female’s body which elicits an immune response. Thus, in addition to making sperm, the male makes enzymes that protect the sperm from the female’s immune response (Rolff and Siva-Jothy, 2002).
Females showed a reduction in the rate of aging when virgin regardless of injury status, and when paired with injured males. The obvious interpretation is that the injured males were less successful at mating with the females, reducing the deleterious effects of reproduction on the latter. We did observe directly that injured male flies would sometimes slide off the female fly’s back when attempting to mate her.
Our results to this point supported the hypothesis that injury has an effect on reproduction, which is manifested as a change in aging patterns. In the next three experiments, we examined the effects of injury specifically on female fecundity and male virility.
Experiment 3: Injury to either sex reduces female fecundity
Injury to either one or both of the sexes significantly reduced the number of eggs laid in the ten days of the experiment compared to the controls. These results corroborate the results of experiment 2 in showing that injury has a negative effect on female reproduction.
Experiment 4: Intact males out compete injured males for access to females
Injured males are less successful at mating when in direct competition with intact males. Thus, injury negatively affects male virility, supporting the interpretation that we have already offered for the results of experiment 2. There is however, another aspect to male virility, namely, the rate or frequency of mating, which was examined in experiment 5.
Experiment 5: Injury to one or both sexes reduces the rate of mating
The rate of mating was significantly reduced when either or both partners were injured. Coupled with the results of experiment 4, this constitutes evidence that injury hinders male virility. The results of experiments 2–5 allow us to explain the curious mortality patterns observed in experiment 1 in terms of the effect of injury first on reproduction and then consequently on aging rates. Specifically, injury tends to increase mortality rates directly but injury also decreases reproduction by females, this second effect benefiting female survival rates.
The experiments that we have summarized so far showed that injury affects both the rate of aging and reproductive activity in flies. In the next experiment, we examined the effects of injury on starvation resistance.
Experiment 6: Injury reduces starvation resistance
In this experiment we used populations of flies that had been specifically selected for starvation (C1–5) and desiccation (D1–5), as well as control populations (CO1–5). Not surprisingly, the C populations were the most starvation resistant overall, followed by the D populations, and then CO populations, with statistically significant differences among all three types (Figure 7). Females were on average more resistant to starvation than males, corroborating the earlier findings of Service et al. (1985).
Several studies have shown that selection for stress resistance fosters longevity (e.g. Rose et al., 1992, Harshman et al. 1999) and that selection for postponed aging increases stress resistance (Service et al., 1985). If the physiological changes that sustain increased stress resistance are helpful in withstanding the negative effects of age-related injury, such as hemolymph loss, then stress resistant stocks of flies should be less affected by injury than control populations. For example, flies are known to deplete lipid reserves under starvation conditions (Wigglesworth, 1949), which could potentially worsen the effects of injury under starvation conditions compared to normal conditions. But because starvation-selected lines have more lipid reserves (Djawdan et al., 1998), the effect of injury on such stress-resistant lines might be expected to be less than the effect of injury on control populations. Our results do not support these hypotheses. In our experiment, the stress-resistant females were significantly more affected by injury than the control flies (Figure 7), even though the stress-selected stocks were generally more resistant to starvation than the controls. This in turn suggests that the improved adult survival that is produced by selection for increased stress resistance (e.g. Rose et al., 2002) may in fact not be mediated by an improved capacity to cope with injury, given that we have found the opposite effect in our experiments.
Several factors may have led to the observed reductions in starvation resistance among some types of injured flies. Loss of nutrients in the hemolymph as a result of injury might be one causal factor. Following injury, the flies may have higher energy needs in order to recover. In humans, the metabolic response to injury can sometimes have harmful effects, such as a reduced circulating volume that can occur for reasons such as fluid loss in the form of blood and fluid sequestration of plasma-like fluid in injured tissues, and can be modified by factors such as infection (Garden et al. 2007). In the initial phase of the metabolic response, energy substrate is mobilized but there is impairment of the ability to use this energy (Garden et al. 2007). If injured flies are also impaired in their ability to use energy, they may not be able to recover from injury, thus increasing their mortality in starvation conditions.
We have determined that injury affects aging patterns more strongly in males than in females, in part because of a counter-balancing reduction in female reproduction brought about by injury. We have also determined that injury reduces the starvation resistance of flies, though this effect is consistent only among males.
Given our entire set of results, it is apparent that the role of injury in aging and stress resistance is complicated by its effects on reproduction, and that this interdependence is affected by the sex of the fly. Altogether, our findings have served to undermine the simple interpretation of the interactions between injury, stress resistance, and aging that motivated this research. But they do indicate the existence of significant interactions between these characters and reproduction, interactions that should be resolved in greater mechanistic detail than we have managed here.
Acknowledgments
We thank the Summer Undergraduate Research Program (SURP) for funding this research during the summer of 2004, and UCI-MARC NIH grant GM069337 for funding this research from 2005 to the present. We are also grateful to M.K. Burke for her comments on an earlier draft of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sean Sepulveda, Department of Ecology and Evolutionary Biology, University of California, Irvine, California 92697-2525.
Parvin Shojaeian, Department of Ecology and Evolutionary Biology, University of California, Irvine, California 92697-2525.
Casandra L. Rauser, Department of Ecology and Evolutionary Biology, University of California, Irvine, California 92697-2525
Mahtab Jafari, Department of Pharmaceutical Science, University of California, Irvine, CA 92697.
Laurence D. Mueller, Department of Ecology and Evolutionary Biology, University of California, Irvine, California 92697-2525
Michael R. Rose, Department of Ecology and Evolutionary Biology, University of California, Irvine, California 92697-2525
Literature Cited
- Bishop YMM, Fienberg SE, Holland PW. Discrete multivariate analysis: theory and practice. Cambridge, Mass: MIT Press; 1975. [Google Scholar]
- Burkhard DU, Ward PI, Blanckenhor WU. Using age grading by wing injuries to estimate size-dependent adult survivorship in the field: a case study of the yellow dung fly Scathophaga stercoraria. Ecol Entomol. 2002;27:514–520. [Google Scholar]
- Carey JR. The Biology and Demography of Life Span. Princeton University Press; Princeton: 2003. Longevity. [Google Scholar]
- Carey JR, Pinter-Wollman N, Wyman M, Muller H, Molleman F, Zhang N. A search for principles of disability using experimental impairment of Drosophila melanogaster. Exp Gerontol. 2007;42:166–172. doi: 10.1016/j.exger.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. 1 Nutrition and the cost of reproduction. J Evol Biol. 1993;6:171–193. [Google Scholar]
- Djawdan M, Chippindale AK, Rose MR, Bradley TJ. Metabolic Reserves and Evolved Stress Resistance in Drosophila melanogaster. Physiol Zool. 1998;71:584–594. doi: 10.1086/515963. [DOI] [PubMed] [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. The University of Chicago Press; Chicago and London: 1990. [Google Scholar]
- Folk DG, Han C, Bradley TJ. Water acquisition and partitioning in Drosophila melanogaster: effects of selection for desiccation-resistance. J Exp Biol. 2001;204:3323–3331. doi: 10.1242/jeb.204.19.3323. [DOI] [PubMed] [Google Scholar]
- Garden J, Bradbury AW, Forsythe JLR, Parks RW. Principles and Practice of Surgery. 5. Churchill Livingstone; UK: 2007. [Google Scholar]
- Gibbs AG, Chippindale AK, Rose MR. Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J Exp Biol. 1997;200:1821–1832. doi: 10.1242/jeb.200.12.1821. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Moore KM, Sty MA, Magwire MM. Stress resistance and longevity in selected lines of Drosophila melanogaster. Neurobiol Aging. 1999;20:521–529. doi: 10.1016/s0197-4580(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Hayes EJ, Wall R. Age-grading adult insects: a review of techniques. Physiol Entomol. 1999;24:1–10. [Google Scholar]
- Hoffman AA, Parsons PA. An integrated approach to environmental stress tolerance and life history variation: desiccation tolerance in Drosophila. Biol J Linn Soc. 1989a;37:117–136. [Google Scholar]
- Hoffman AA, Parsons PA. Selection for increased desiccation resistance in Drosophila melanogaster: additive genetic control and correlated responses for other stresses. Genetics. 1989b;122:837–845. doi: 10.1093/genetics/122.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AA, Parsons PA. Evolutionary Genetics and Environmental Stress. Oxford University Press; New York: 1991. [Google Scholar]
- Hoffmann AA, Harshman LG. Desiccation and starvation resistance in Drosophila: patterns of variation at the species, population and intrapopulation levels. Heredity. 1999;83:637–643. doi: 10.1046/j.1365-2540.1999.00649.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Parsons PA. Direct and correlated responses to selection for desiccation resistance: A comparison of Drosophila melanogaster and D. simulans. J Evol Biol. 1993;6:643–657. [Google Scholar]
- Javois J, Tammaru T. Reproductive decisions are sensitive to cues of life expectancy: the case of a moth. Anim Behav. 2004;68:249–255. [Google Scholar]
- Lamb MJ, McDonald RP. Heat tolerance changes with age in normal and irradiated Drosophila melanogaster. Exp Gerontol. 1973;8:207–217. doi: 10.1016/0531-5565(73)90028-4. [DOI] [PubMed] [Google Scholar]
- Leroi AM, Chippindale AK, Rose MR. Long-term laboratory evolution of a genetic life-history trade-off in Drosophila melanogaster. 1 The role of genotype-by-environment interaction. Evol. 1994;48:1244–1257. doi: 10.1111/j.1558-5646.1994.tb05309.x. [DOI] [PubMed] [Google Scholar]
- Luckinbill LS, Arking R, Clare MJ, Cirocco WC, Buck SA. Selection for delayed senescence in Drosophila melanogaster. Evol. 1984;38:996–1003. doi: 10.1111/j.1558-5646.1984.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Mertz DB. Senescent decline in flour beetle strains selected for early adult fitness. Physiol Zool. 1975;48:1–23. [Google Scholar]
- Miller RS. Simultaneous Statistical Inference. McGraw Hill; New York: 1966. [Google Scholar]
- Mueller LD, Nasbaum TJ, Rose MR. The Gompertz equation as a predictive tool in demography. Exp Gerontol. 1995;30:553–569. doi: 10.1016/0531-5565(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Nghiem D, Gibbs AG, Rose MR, Bradley TJ. Postponed aging and desiccation resistance in Drosophila melanogaster. Exper Gerontol. 2000;35:957–969. doi: 10.1016/s0531-5565(00)00163-7. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. Springer; New York: 2000. [Google Scholar]
- Rolff J, Siva-Jothy MT. Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proc Natl Acad Sci. 2002;99:9916–9918. doi: 10.1073/pnas.152271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MR. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evol. 1984;38:1004–1010. doi: 10.1111/j.1558-5646.1984.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Rose MR, Charlesworth B. A test of evolutionary theories of senescence. Nature. 1980;287:141–142. doi: 10.1038/287141a0. [DOI] [PubMed] [Google Scholar]
- Rose MR, Passananti HB, Matos M. Methuselah Flies: A Case Study in the Evolution of Aging. World Scientific; Singapore: 2004. [Google Scholar]
- Rose MR, Vu LN, Park SU, Graves JL. Selection on stress resistance increases longevity in Drosophila melanogaster. Exp Gerontol. 1992;27:241–250. doi: 10.1016/0531-5565(92)90048-5. [DOI] [PubMed] [Google Scholar]
- Rose M. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution. 1984;38:1004–1010. doi: 10.1111/j.1558-5646.1984.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Rose MR, Charlesworth B. Genetics of life history in Drosophila melanogaster. II Exploratory selection experiments Genetics. 1981;97:187–196. doi: 10.1093/genetics/97.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service PM. The effect of mating status on lifespan, egg laying, and starvation resistance in Drosophila melanogaster in relation to selection on longevity. J Insect Physiol. 1989;35:447–452. [Google Scholar]
- Service PM. Laboratory evolution of longevity and reproductive fitness components in male fruit flies: mating ability. Evol. 1993;47:387–399. doi: 10.1111/j.1558-5646.1993.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Service PM, Fales AJ. Evolution of delayed reproductive senescence in male fruit flies: sperm competition. Genetica. 1993;91:111–125. doi: 10.1007/BF01435992. [DOI] [PubMed] [Google Scholar]
- Service PM, Vossbrink R. Genetic variation in “first” male effects on egg-laying and remating by female Drosophila melanogaster. Behav Genet. 1996;26:39–47. doi: 10.1007/BF02361157. [DOI] [PubMed] [Google Scholar]
- Service PM, Hutchinson EW, MacKinley MD, Rose MR. Resistance to environmental stress in Drosophila melanogaster selected for postponed senescence. Physiol Zool. 1985;58:380–389. [Google Scholar]
- Sokal RR. Senescence and genetic load: evidence from Tribolium. Science. 1970;167:1733–1734. doi: 10.1126/science.167.3926.1733. [DOI] [PubMed] [Google Scholar]
- Rose MR, Graves JL, Jr , Hutchinson EW. The role of selection to probe patterns of pleiotropy in fitness characters. In: Francis Gilbert., editor. Insect Life Cycles. Springer; Berlin: 1990. pp. 29–42. [Google Scholar]
- Luckinbill LS, Arking R, Clare MJ, Cirocco WC, Buck SA. Selection for delayed senescence in Drosophila melanogaster. Evolution. 1984;38:996–1003. doi: 10.1111/j.1558-5646.1984.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Wattiaux JM. Cumulative parental age effects in Drosophla subobscura. Evolution. 1968a;22:406–421. doi: 10.1111/j.1558-5646.1968.tb05908.x. [DOI] [PubMed] [Google Scholar]
- Wattiaux JM. Parental age effects in Drosophila pseudobscura. Exp Gerontol. 1968b;3:55–61. doi: 10.1016/0531-5565(68)90056-9. [DOI] [PubMed] [Google Scholar]
- Williams AE, Rose MR, Bradley TJ. CO2 release patterns in Drosophila melanogaster: the effect of selection for desiccation resistance. J Exp Biol. 1997;200:615–624. doi: 10.1242/jeb.200.3.615. [DOI] [PubMed] [Google Scholar]
- Williams AE, Bradley TJ. The effect of respiratory pattern on water loss in desiccation resistant Drosophila melanogaster. J Exper Biol. 1998;201:2953–2959. doi: 10.1242/jeb.201.21.2953. [DOI] [PubMed] [Google Scholar]