Abstract
Mechanisms underlying the initiation of parturition remain unclear. Throughout most of pregnancy, uterine quiescence is maintained by elevated progesterone acting through progesterone receptor (PR). Although in most mammals, parturition is associated with a marked decline in maternal progesterone, in humans, circulating progesterone and uterine PR remain elevated throughout pregnancy, suggesting a critical role for functional PR inactivation in the initiation of labor. Both term and preterm labor in humans and rodents are associated with an inflammatory response. In preterm labor, intraamniotic infection likely provides the stimulus for increased amniotic fluid interleukins and migration of inflammatory cells into the uterus and cervix. However, at term, the stimulus for this inflammatory response is unknown. Increasing evidence suggests that the developing fetus may produce physical and hormonal signals that stimulate macrophage migration to the uterus, with release of cytokines and activation of inflammatory transcription factors, such as nuclear factor κB (NF-κB) and activator protein 1 (AP-1), which also is activated by myometrial stretch. We postulate that the increased inflammatory response and NF-κB activation promote uterine contractility via 1) direct activation of contractile genes (e.g. COX-2, oxytocin receptor, and connexin 43) and 2) impairment of the capacity of PR to mediate uterine quiescence. PR function near term may be compromised by direct interaction with NF-κB, altered expression of PR coregulators, increased metabolism of progesterone within the cervix and myometrium, and increased expression of inhibitory PR isoforms. Alternatively, we propose that uterine quiescence during pregnancy is regulated, in part, by PR antagonism of the inflammatory response.
Uterine quiescence throughout pregnancy and its increased contractility at term are regulated by a complex interplay of pro- and anti-inflammatory signals between fetus and mother.
Preterm labor is the major cause of neonatal morbidity and mortality in developed countries. In the United States, the incidence of preterm birth has increased steadily over the past two decades and now approaches approximately 13% of all live births (www.marchofdimes.com/peristats). Preterm birth is even more prevalent among certain racial and ethnic groups. For example, about 18.0% of Black infants are born prematurely, whereas the prematurity rate in White infants is about 11% (www.marchofdimes.com/peristats). This racial disparity remains, even after adjustments are made for socioeconomic status, geographic location, and access to healthcare. Of the approximately 500,000 babies born prematurely in the United States each year, about 25,000 develop respiratory distress syndrome, which is caused by the immaturity of the neonatal lungs and their inability to produce adequate amounts of pulmonary surfactant, a developmentally regulated, glycerophospholipid-rich lipoprotein that reduces alveolar surface tension and is essential for air breathing (1). The relatively high incidence of preterm birth is due, in part, to our incomplete understanding of the pathways that maintain uterine quiescence throughout pregnancy as well as those that promote increased uterine contractility and cervical ripening leading to labor. There is increasing evidence to suggest that both term and preterm labor are associated with an inflammatory response within the maternal uterus and cervix and that uterine quiescence during most of pregnancy is maintained by the antiinflammatory actions of progesterone acting via its nuclear receptor [progesterone receptor (PR)]. In this mini-review, we will consider the roles of fetal hormones and factors in the induction of the inflammatory response leading to parturition. We also will review findings to support the concept that maintenance of uterine quiescence vs. induction of contractility is precisely controlled by the relative impact of the antiinflammatory actions of progesterone/PR vs. those of transcription factors, such as nuclear factor-κB (NF-κB) and activating protein-1 (AP-1). These transcription factors serve to up-regulate inflammatory response pathways and impair PR function. Mechanisms that result in the attenuation of PR function leading to labor also will be considered.
Inflammatory Pathways and the Initiation of Labor
As mentioned above, both term and preterm labor are associated with an inflammatory response. This is exemplified by increased concentrations of proinflammatory cytokines (i.e. IL-1β) in amniotic fluid (2) and infiltration of the myometrium, cervix, and fetal membranes by neutrophils and macrophages (3,4,5). The invading immune cells secrete cytokines and chemokines (6), resulting in activation of NF-κB and other proinflammatory transcription factors in the myometrium (4,7) and in cervical epithelial and amnion cells (8,9,10). Activated NF-κB, in turn, increases expression of genes promoting myometrial contractility, including the prostaglandin F2α receptor (11), the gap junction protein connexin 43 (12), the oxytocin receptor (13), and cyclooxygenase-2 (COX-2) (14). In preterm labor, intraamniotic infection associated with chorioamnionitis may provide the stimulus for increased amniotic fluid interleukins and inflammatory cell migration (15). At term, mechanical stretch (16,17) caused by the growing fetus, as well as hormonal signals produced by the developing fetus near term (4,18,19,20), promote production of chemokines leading to macrophage migration and up-regulation of inflammatory response pathways.
Fetal Signals and the Initiation of Labor
Whereas infection with associated chorioamnionitis has been suggested to serve as a stimulus for enhanced leukocyte activation and proinflammatory cytokine production leading to preterm labor (6), the signals for the increased inflammatory response associated with labor at term has remained less clear. There is increasing evidence to suggest that the fetus may generate signals that contribute to the initiation of labor. Described below are the fetal tissues and signaling molecules that have been proposed to serve a role in the initiation of parturition at term in different animal models.
Fetal adrenal gland: cortisol
In sheep, maturation of the hypothalamic-pituitary-adrenal axis during the last 2–3 wk of gestation with increased cortisol production by the fetal adrenal gland has been suggested to serve a role in the initiation of labor (21). The elevated fetal cortisol production has been proposed to enhance COX-2 expression in the placenta, leading to increased production of prostaglandins, which stimulate expression of 17α-hydroxylase/17,20-lyase (product of the CYP17 gene). This, in turn, is proposed to enhance placental production of C19-steroids, which are metabolized to estrogens by placental aromatase P450 (product of the CYP19 gene). The increased estrogens are suggested to augment uterine contractility by antagonizing PR function (19). Importantly, the surge of fetal cortisol also increases maturation of the fetal lung and its capacity to synthesize surfactant (22), which as discussed below, may also serve as a fetal signal for initiation of labor.
Placenta: CRH
The primate placenta is unique in its ability to produce CRH (23), whereas it lacks the capacity to express CYP17. Thus, the human placenta cannot synthesize C19-steroids, which instead are produced in large quantities by the fetal adrenal glands (24). In the human, it has been suggested that CRH, which is secreted in increasing amounts by the placenta near term, provides a fetal signal for the initiation of labor (25,26). The increased fetal CRH is proposed to up-regulate secretion by the fetal pituitary of ACTH, which enhances production of cortisol and dehydroepiandrosterone (DHEA) sulfate by the fetal adrenal. DHEA sulfate subsequently is metabolized to DHEA and aromatized within the placental syncytiotrophoblast to estrogens, which as mentioned above, have been proposed to oppose the action of progesterone/PR to maintain uterine quiescence. Interestingly, progesterone has a pronounced inhibitory effect on CRH expression by cultured human trophoblast cells (27,28). CRH mRNA also is developmentally induced to relatively high levels in bronchiolar epithelium of fetal mouse lung between 13.5 and 17.5 d postcoitum (dpc) and is then extinguished at 18.5 dpc (29). This rise in CRH expression precedes the developmental induction of synthesis of the major surfactant protein, surfactant protein A (SP-A), by the fetal lung. Developmental induction of SP-A expression was found to be delayed together with lung maturation in CRH-null mouse fetuses of CRH-deficient mothers (30). Therefore, CRH may contribute to the initiation of labor by acting indirectly to enhance fetal ACTH and adrenal cortisol production and/or directly to stimulate fetal lung maturation and the production of surfactant components. In this regard, we and others have suggested that augmented surfactant production by the maturing fetal lung may serve as a fetal signal for the initiation of labor.
Fetal lung: surfactant lipids and proteins
The synthesis and secretion of pulmonary surfactant by the fetal lung is initiated during the third trimester of gestation. Surfactant, a glycerophospholipid-rich lipoprotein, is produced by alveolar type II pneumocytes and is secreted into amniotic fluid with fetal breathing movements. Surfactant contains four essentially lung-specific proteins, SP-A, SP-B, SP-C, and SP-D (31,32). Whereas SP-B and SP-C are lipophilic proteins that work together with surfactant lipids to reduce alveolar surface tension (31,33), SP-A and SP-D are C-type lectins that function as part of the innate immune system. As such, they bind to a variety of microbial pathogens and enhance their phagocytosis by immune cells (e.g. macrophages) within the lung alveolus (32,34,35).
Lopez-Bernal et al. (36) observed that pulmonary surfactant isolated from human amniotic fluid stimulated the synthesis of prostaglandin E in discs of human amnion. They postulated that surfactant phospholipids secreted by the fetal lung into amniotic fluid provide a source of arachidonic acid as precursor for prostaglandin synthesis by the amnion. Mitchell and colleagues (20) reported that a substance in amniotic fluid caused a marked increase in prostaglandin E2 production by cultured human amnion cells. Although they postulated that this might arise from the fetal kidney, the nature and source of this substance were never identified. Johnston and colleagues (37) proposed that platelet-activating factor, a highly bioactive phospholipid component of fetal lung surfactant that is secreted into amniotic fluid near term, may play an important role in the activation of myometrial contractility.
Our findings suggest that SP-A, secreted by the fetal lung into amniotic fluid in large amounts near term, may provide an important signal for the initiation of labor (4). Postnatally, SP-A enhances immune function within the lung alveolus by activating alveolar macrophages, increasing cytokine production, and activating NF-κB (34,35,38,39). SP-A synthesis by the fetal lung is initiated only after about 80% of gestation is complete, in concert with augmented synthesis of surfactant glycerophospholipids, and reaches maximal levels before term (40). In studies using human fetal lung type II cells in culture, we observed that SP-A gene expression is itself increased by proinflammatory stimuli; SP-A expression is up-regulated by IL-1 via activation of NF-κB (41) and by hormones and factors that increase cAMP (41,42). Furthermore, others have demonstrated that intraamniotic administration of IL-1 in pregnant rabbits increased SP-A expression in fetal lung (43) and caused preterm birth (44). By contrast, cAMP and IL-1 induction of SP-A expression in human fetal lung type II cells is blocked by glucocorticoids, which exert antiinflammatory actions through glucocorticoid receptor (GR) antagonism of NF-κB activation and binding to the hSP-A promoter (45,46). Glucocorticoids also cause histone modifications indicative of repressed chromatin (45).
In the mouse, SP-A mRNA, which is barely detectable in fetal lung at 16 dpc, is up-regulated at 17 dpc and increases markedly toward term (19 dpc) (47,48). SP-A protein, which is absent in amniotic fluid at 16 dpc, is readily detectable at 17 dpc and increases to extremely high levels at 19 dpc (4). This gestational increase in SP-A secretion by the fetal lung was associated with a parallel increase in IL-1β protein expression in macrophages isolated from amniotic fluid, migration of activated macrophages to the pregnant uterus, and activation of uterine NF-κB (4). Accordingly, SP-A treatment of mouse amniotic fluid macrophages at 15, 17, and 19 dpc enhanced IL-1β expression. Using a transgenic mouse model in which fetal macrophages stained positively for β-galactosidase, we obtained evidence that a proportion of the macrophages present within the maternal uterus near term are derived from the fetus (4).
To directly assess the capacity of SP-A to initiate labor in vivo, parallel groups of mice were injected with purified SP-A or with a control, SP-A-depleted preparation (4). The majority of mice injected with SP-A on 15 dpc delivered prematurely on d 16–17 of gestation. Fetuses in the uninjected horn were not delivered and were ultimately resorbed. Importantly, fetal macrophage migration and NF-κB activation were detected within the SP-A-injected uterine horn by 4.5 h but not in the uninjected horn, suggesting a local inflammatory response. To further evaluate the role of endogenous SP-A in the initiation of labor, another series of 15-dpc pregnant mice were intraamniotically injected with an antibody raised against SP-A to deplete endogenous levels of the surfactant protein in amniotic fluid. Interestingly, all of the mice in this group delivered viable pups 24 h late (20 dpc) (4).
Based on these collective findings, we suggest that augmented production of SP-A by the maturing fetal lung near term provides a hormonal stimulus for activation of a cascade of inflammatory signals within the maternal uterus that culminate in the enhanced myometrial contractility leading to parturition (49). This hormonal signal, which is transmitted to the uterus by fetal macrophages, reveals that the fetal lungs are sufficiently developed to withstand the critical transition from an aqueous to an aerobic environment. Our findings further suggest that NF-κB serves as a key transcriptional mediator of the uterine inflammatory response leading to labor. We propose that activated NF-κB promotes increased uterine contractility by direct and indirect mechanisms. For example, NF-κB can bind directly to the promoters of genes that mediate increased uterine contractility, including the prostaglandin F2α receptor (11), the gap junction protein connexin 43 (12), the oxytocin receptor (13), and COX-2 (14). Alternatively, NF-κB can block the capacity of the PR to activate genes that control uterine quiescence.
Progesterone/PR Maintains Uterine Quiescence via Antiinflammatory Actions
Uterine quiescence is maintained throughout most of pregnancy by elevated levels of circulating progesterone acting through PR. However, to date, the PR target genes that prevent uterine contractility remain to be identified. Recent findings suggest that progesterone/PR action to maintain uterine quiescence may be indirect; by inhibiting activation of inflammatory response pathways and expression of contractile genes within the uterus and cervix and blocking the production of chemokines that promote chemotaxis of immune cells. Interestingly, even during the estrous cycle in rodents, migration of macrophages and neutrophils to the uterus is stimulated by estrogen and inhibited by progesterone (50,51,52). This antagonistic effect of progesterone on estrogen-induced macrophage/neutrophil migration fails to occur in mice with a deletion of the PR gene (PRKO mice) and is, therefore, PR dependent (52). Furthermore, PRKO mice manifest a massive uterine inflammatory response upon estrogen (52) or estrogen plus progesterone treatment (53).
Expression of the β-chemokine, monocyte chemoattractant protein-1 (MCP-1/CCL-2), which attracts and activates macrophages, was found to be stimulated by NF-κB and inhibited by progesterone/PR in choriodecidual and breast cancer cells (54). Notably, MCP-1 was reported to be up-regulated in myometrium of women in labor, as compared with myometrium from term pregnant women not in labor (55) and of pregnant rats before and during parturition (16). Furthermore, MCP-1 expression and macrophage infiltration were greatly increased in the pregnant rat uterus after PR blockade by RU486 treatment, which caused preterm parturition, and were inhibited by progestin treatment, which delayed parturition (16). In unilaterally pregnant rats, increased MCP-1 mRNA was evident only in the gravid horn, implying a possible role of fetal-derived factors and/or of uterine stretch (16).
Using telomerase-immortalized human myometrial cells, we observed that progesterone/PR plays a major antiinflammatory role via antagonism of both NF-κB activation and induction of COX-2 (56,57). A similar phenomenon was noted in studies using human fetal lung type II cells (58), amnion epithelial cells, lower uterine segment fibroblasts (59), and human breast cancer cells (60). By use of chromatin immunoprecipitation analysis, we found that progesterone treatment of the human myometrial cells blocked IL-1 induction of in vivo binding of NF-κB p65 to both proximal and distal NF-κB response elements in the COX-2 promoter (61). The progesterone-mediated decrease in p65 binding to the COX-2 promoter might be caused, in part, by a direct physical interaction of PR with p65 as was previously observed in vitro (62), resulting in a repression of NF-κB DNA-binding and transcriptional activity. On the other hand, the antiinflammatory effect of progesterone within the myometrium also may be mediated by increased expression of IκBα, a crucial inhibitor of NF-κB transactivation. Progesterone caused a rapid induction of IκBα mRNA and protein expression in the immortalized myometrial cells, which preceded its effect to inhibit IL-1β-induced COX-2 expression (61). Moreover, coincubation with IL-1β and progesterone prevented the IL-1β-induced decline in IκBα protein levels, suggesting an effect of progesterone/PR to block IκBα degradation via the proteasome pathway (63). As a consequence of the increased IκBα expression, more NF-κB is likely sequestered in an inactive state in the cytoplasm. Progesterone inhibition of NF-κB activation by induction of IκBα has also been observed in macrophage cell lines (64) and in T47D cells (60,65). The identification of IκBα as a progesterone target gene that mediates myometrial quiescence is of great interest considering that relatively few PR target genes have been identified.
The molecular mechanisms that mediate progesterone induction of IκBα gene expression have not been defined. Glucocorticoids acting through the GR are known to induce IκBα expression in a cell type- and promoter-specific manner (66,67). It is conceivable that glucocorticoids and progestins may induce IκBα expression through similar mechanisms, because the GR and PR are structurally homologous and bind to a common response element in DNA [GR/PR response element (GRE/PRE)]. Although no consensus GRE/PRE has been identified in the 5′-flanking region of the IκBα gene (68), a reporter construct containing 623 bp of IκBα 5′-flanking sequence was sufficient for glucocorticoid induction of IκBα promoter activity in transfected cells (69). This region contains a GRE half-site that was suggested to mediate glucocorticoid/GR effects to enhance binding of other activating transcription factors, including NF-κB, Ets-1, and Sp1 (68).
Importantly, it appears that PR also inhibits NF-κB activation and COX-2 induction via ligand-independent mechanisms. In studies using human breast cancer cells in which PR-A and PR-B protein levels were completely suppressed using small interfering RNA-mediated knockdown, COX-2 expression was up-regulated more than 30-fold (61). This phenomenon was observed in the absence of progesterone treatment. Conversely, up-regulation of PR protein expression in breast cancer cells transfected with an RNA duplex complementary to a PR promoter sequence, markedly inhibited IL-1β induction of COX-2 expression in a progesterone-independent manner (70).
Decline in PR Function at Term Is Multifactorial and Induced by the Inflammatory Response
As discussed above, it has long been appreciated that progesterone acting through PR plays a critical role in maintaining uterine quiescence throughout most of pregnancy. The finding in rodents that circulating maternal progesterone levels decline precipitously near term (71) has led to the concept that labor is associated with progesterone withdrawal. Although in humans and guinea pigs, circulating progesterone levels remain elevated throughout pregnancy and into labor, as do myometrial levels of PR (19), treatment with PR antagonists mifepristone (RU486) or onapristone can cause increased cervical ripening and spontaneous labor or increased sensitivity to labor induction by oxytocin or prostaglandins (72,73,74,75). It should be noted that even in mice, maternal progesterone levels at term remain well above the Kd for binding to PR. These collective findings have led to the concept that parturition in all species is initiated by a complex and concerted series of biochemical events that antagonize the ability of the PR to regulate target genes in the uterus that maintain myometrial quiescence. Recent studies have elucidated potential mechanisms involved in the reduction in PR function in target tissues associated with the onset of labor. These include 1) increased expression of progesterone-metabolizing enzymes, 2) direct interaction of PR with NF-κB, 3) increased expression of inhibitory PR isoforms, and 4) altered expression of PR coactivators and corepressors.
Increased expression of progesterone-metabolizing enzymes
The initiation of labor in mice is accompanied by an increase in expression of the progesterone-metabolizing enzymes 20α-hydroxysteroid dehydrogenase (20α-HSD) and 5α-reductase type I in the uterus (76) and cervix (77,78), respectively. Consequently, mice with a targeted deletion in the gene encoding 5α-reductase type I, which metabolizes progesterone to inactive products, fail to deliver due to defects in cervical ripening (77,78). This occurred despite the precipitous fall in maternal circulating progesterone levels near term, suggesting that local metabolism of progesterone within the cervix also is required for progesterone withdrawal and the initiation of spontaneous labor in the mouse. 20α-HSD−/− mice manifest delayed parturition and increased fetal demise at birth (79,80). Whereas in one study, progesterone levels were found to remain elevated in 20α-HSD−/− mice at term (80), in the other, progesterone levels declined significantly (79), suggesting that its local metabolism in PR target tissues, such as the uterus, may be critical.
Antagonistic interaction of PR and NF-κB
A mutual antagonism between the PR and the p65 subunit of NF-κB has been reported in COS-1 and HeLa cells; activation of NF-κB by TNF-α inhibited PR transcriptional activity, whereas PR also repressed p65-mediated transcription (62). Also, in amnion epithelial cells, PR overexpression repressed NF-κB transcriptional activity, whereas IL-1β activated NF-κB and inhibited PR transcriptional activity (8). Based on findings that PR and p65 interact in vitro (62), it was suggested that mutual repression resulted from the formation of an inactive complex of these proteins on the DNA that was incapable of interacting with essential coregulators.
Altered expression of PR isoforms
Human PR exists as two major isoforms, hPR-A (94 kDa), hPR-B (114 kDa). These arise by alternative transcription initiation from different promoters (81) and by alternative translation initiation from different AUG sites (82). Both PR-A and PR-B isoforms bind to PREs in DNA; however, hPR-A lacks one of three transcriptional activation domains that are present in hPR-B and has been reported to repress PR-B transcriptional activity in certain cell and gene contexts (83,84). The ratio of PR-A to PR-B mRNA was reported to be increased about 10-fold in lower uterine segment myometrium from women in labor, as compared with tissues from women not in labor at term (85). PR-A also was found to inhibit PR-B transcriptional activity in cultured human myometrial cells (86), suggesting a potential antagonistic role of PR-A on PR-B function in the uterus. In this regard, mice deficient in Krüppel-like factor 9 (KLF9) manifested delayed parturition in association with aberrant uterine PR-A expression, as compared with wild type. However, the KLF9-deficient mice also exhibited altered expression of NF-κB p65 (87). The finding that mice with a selective knockout of PR-B are fertile and deliver normally (88) suggests that PR-A can mediate actions of progesterone to maintain myometrial quiescence.
A third N-terminally truncated PR isoform, PR-C, has been characterized (89). PR-C lacks part of the DNA-binding domain and is restricted primarily to the cytoplasm (90). Because PR-C cannot bind DNA but can bind progesterone (91), it may inhibit PR function by sequestering available hormone away from PR-B. PR-C also can bind to PR-B and reduce its capacity to bind to DNA (89). In term fundal myometrium from women before and after the initiation of labor, we observed a marked labor-associated increase in protein and mRNA expression of a truncated (∼60 kDa) PR isoform; this was spatially and temporally associated with increased activation of NF-κB (7). A temporal increase in expression of a truncated PR isoform also was observed in mouse uterus during late gestation. It should be noted that the identity of truncated PR isoforms (92,93) has recently been queried, requiring additional studies in this area.
Membrane G protein-coupled PR isoforms (mPRα and mPRβ) linked to Giα, adenylyl cyclase inhibition, p38 MAPK activation, and increased myosin light-chain phosphorylation also have been characterized in human myometrial cells (94). Increased expression of mPR in myometrium from women in labor, as compared with not in labor was suggested to contribute to increased myometrial contractility (94). Collectively, these findings suggest that increased expression of alternative PR binding proteins and isoforms may contribute to a decline in PR function near term.
Altered expression of PR coregulators
Previously, we observed that expression of cAMP response element-binding protein (CREB)-binding protein (CBP), steroid receptor coactivator (SRC)-2 and SRC-3 were decreased in fundal myometrial tissues of women in labor, as compared with those from women not in labor. We also found marked decreases in SRC and CBP coactivator levels in uterine tissues of pregnant mice at term (95). This decline in coactivators in uterine tissues of women in labor and mice at term was associated with decreased levels of acetylated histone H3. Interestingly, administration of trichostatin A (TSA), a potent histone deacetylase inhibitor, to pregnant mice late in gestation increased histone acetylation and delayed parturition by 24–48 h (95). This suggests that the decline in PR coactivator expression and in histone acetylation in the uterus near term may impair PR regulation of genes that maintain uterine quiescence and increase sensitivity of the uterus to contractile stimuli. In cultured human myometrial cells, TNFα antagonism of progesterone/PR-mediated transcription was associated with decreased expression of SRC-1 and SRC-2 (96). This suggests that the decline in coactivator expression in the myometrium near term may be induced by an increased inflammatory response. Moreover, a putative corepressor, termed polypyrimidine tract-binding protein-associated splicing factor (PSF), which promotes PR degradation via the proteasome pathway, inhibits its binding to the PRE and its transcriptional activity, was reported to increase in pregnant rat myometrium at term (97). Thus, PR transcriptional activity near term may be compromised via altered expression of coregulators in response to enhanced inflammatory signals.
Conclusions
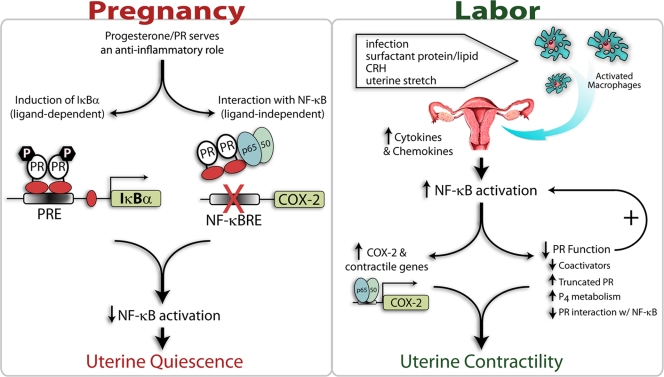
The findings presented herein suggest that the suppression of uterine contractility during pregnancy and its increase near term leading to labor are regulated by a complex interplay of signals between fetus and mother. Maintenance of the uterus in a state of near quiescence throughout most of gestation is regulated, in part, by the actions of the PR to block activation of the inflammatory transcription factor NF-κB (Fig. 1). These actions of PR are mediated by ligand-dependent and -independent mechanisms. On the one hand, PR acts in a ligand-dependent manner to markedly up-regulate expression of the NF-κB inhibitor IκBα in myometrial cells. Increased cellular levels of IκBα serve to sequester NF-κB proteins p50 and p65 as an inactive complex in the cytoplasm. PR also acts (possibly via direct protein-protein interaction) in a dominant ligand-independent manner to block NF-κB activation and DNA binding to and transactivation of contractile genes (e.g. COX-2). Although it has been suggested that progesterone/PR action within the pregnant myometrium involves activation of genes that maintain quiescence/block contractility, a direct PR target gene within the myometrium that mediates these effects has not been identified. We suggest that IκBα, which is markedly up-regulated by progesterone/PR in myometrial cells and serves to block NF-κB transcriptional activation by cytokines, bacterial lipopolysaccharide, and other stimulatory factors, may serve as a key progesterone/PR target gene in the maintenance of the quiescent state.
Figure 1.
Mechanisms for progesterone/PR regulation of uterine quiescence during pregnancy and induction of uterine contractility in preterm and term labor. During most of pregnancy, the uterus is maintained in a quiescent state by the PR, which acts in a ligand-dependent and -independent manner to block activation of the inflammatory transcription factors (e.g. NF-κB). PR acts in a ligand-dependent manner to up-regulate expression of the NF-κB inhibitor IκBα in myometrial cells. Alternatively, PR acts in a dominant ligand-independent manner (likely via direct protein-protein interaction) to block NF-κB activation, DNA binding, and transactivation of contractile genes within the uterus. Labor can be initiated preterm as a result of bacterial infection, resulting in enhanced migration of macrophages to the maternal uterus with release of cytokines/chemokines and activation of NF-κB. However, at term, enhanced macrophage activation and migration and increased uterine NF-κB activity are likely induced by signals produced by the maturing fetus. These include increased secretion of surfactant proteins and lipids by the fetal lung into amniotic fluid, augmented production of CRH by the placenta, and enhanced uterine stretch caused by the growing conceptus. Within the uterus, the activated NF-κB directly acts to increase expression of contractile genes and causes an impairment of PR function by effecting 1) down-regulation of PR coactivators, 2) increased expression of inhibitory PR isoforms, 3) increased metabolism of progesterone to inactive products, and possibly 4) direct inhibitory interaction with PR. These concerted events culminate in a further increase in NF-κB activation and expression of contractile genes, leading to labor.
On the other hand, the initiation of term and preterm labor by signals from the fetus (fetal growth and enhanced uterine stretch, increased SP-A secretion by the fetal lung, and/or increased CRH secretion by the placenta) or infection, respectively, leads to macrophage activation and migration to the pregnant uterus with the release of proinflammatory cytokines and activation of NF-κB and other inflammatory transcription factors (Fig. 1). The activated NF-κB, in turn, binds to enhancers in the regulatory regions of contractile genes, such as COX-2, resulting in transcriptional activation and the production of prostaglandins that promote uterine contractility. The inflammatory response also causes an impairment of PR function by inhibiting the expression of critical PR coactivators (95,96), enhancing expression of inhibitory PR isoforms and increasing local metabolism of progesterone within the uterus (76) and cervix (77,78). This, in turn, may result in the decreased capacity of progesterone/PR to activate IκBα expression and of PR to inhibit NF-κB activation by direct protein-protein interaction. This down-regulation of PR function causes the further activation of contractile genes, which culminates in the initiation of labor.
Footnotes
Our research was supported by National Institutes of Health Grants 5 P01 HD011149 and R37 HL050022 and the March of Dimes Birth Defects Foundation (Research Grant No. 21-FY07-601).
Disclosure Summary: The author has nothing to declare regarding potential conflicts of interest.
First Published Online March 12, 2009
Abbreviations: AP-1, Activator protein 1; COX-2, cyclooxygenase-2; DHEA, dehydroepiandrosterone; dpc, days postcoitum; GR, glucocorticoid receptor; GRE/PRE, GR/PR response element; 20αHSD, 20α-hydroxysteroid dehydrogenase; MCP-1, monocyte chemoattractant protein-1; NF-κB, nuclear factor κB; PR, progesterone receptor; SP-A, surfactant protein A; SRC, steroid receptor coactivator.
References
- Avery ME, Mead J 1959 Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child 97:517–523 [DOI] [PubMed] [Google Scholar]
- Cox SM, Casey ML, MacDonald PC 1997 Accumulation of interleukin-1β and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Hum Reprod Update 3:517–527 [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE 1999 Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 14:229–236 [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Mendelson CR 2004 Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA 101:4978–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE 2003 Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 9:41–45 [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S 2007 The role of inflammation and infection in preterm birth. Semin Reprod Med 25:21–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR 2006 Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of NF-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 20:764–775 [DOI] [PubMed] [Google Scholar]
- Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR 2001 Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod 7:581–586 [DOI] [PubMed] [Google Scholar]
- Lee YS, Terzidou V, Lindstrom T, Johnson M, Bennett PR 2005 The role of CCAAT/enhancer-binding protein β in the transcriptional regulation of COX-2 in human amnion. Mol Hum Reprod 11:853–858 [DOI] [PubMed] [Google Scholar]
- Elliott CL, Allport VC, Loudon JA, Wu GD, Bennett PR 2001 Nuclear factor-κB is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol Hum Reprod 7:787–790 [DOI] [PubMed] [Google Scholar]
- Olson DM 2003 The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol 17:717–730 [DOI] [PubMed] [Google Scholar]
- Chow L, Lye SJ 1994 Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol 170:788–795 [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Husslein P, Soloff MS 1984 Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol 150:734–741 [DOI] [PubMed] [Google Scholar]
- Soloff MS, Cook Jr DL, Jeng YJ, Anderson GD 2004 In situ analysis of interleukin-1-induced transcription of COX-2 and IL-8 in cultured human myometrial cells. Endocrinology 145:1248–1254 [DOI] [PubMed] [Google Scholar]
- Rauk PN, Chiao JP 2000 Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol 43:152–159 [DOI] [PubMed] [Google Scholar]
- Shynlova O, Tsui P, Dorogin A, Lye SJ 2008 Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol 181:1470–1479 [DOI] [PubMed] [Google Scholar]
- Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR 2004 Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod 10:109–113 [DOI] [PubMed] [Google Scholar]
- Shaw G, Renfree MB 2001 Fetal control of parturition in marsupials. Reprod Fertil Dev 13:653–659 [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ 2000 Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 2000 21:514–550 [DOI] [PubMed] [Google Scholar]
- Mitchell MD, MacDonald PC, Casey ML 1984 Stimulation of prostaglandin E2 synthesis in human amnion cells maintained in monolayer culture by a substance(s) in amniotic fluid. Prostaglandins Leukot Med 15:399–407 [DOI] [PubMed] [Google Scholar]
- Liggins GC, Fairclough RJ, Grieves SA, Kendall JZ, Knox BS 1973 The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res 29:111–159 [DOI] [PubMed] [Google Scholar]
- Liggins GC 1969 Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol 45:515–523 [DOI] [PubMed] [Google Scholar]
- Robinson BG, Arbiser JL, Emanuel RL, Majzoub JA 1989 Species-specific placental corticotropin releasing hormone messenger RNA and peptide expression. Mol Cell Endocrinol 62:337–341 [DOI] [PubMed] [Google Scholar]
- Rainey WE, Rehman KS, Carr BR 2004 The human fetal adrenal: making adrenal androgens for placental estrogens. Semin Reprod Med 22:327–336 [DOI] [PubMed] [Google Scholar]
- Florio P, Cobellis L, Woodman J, Severi FM, Linton EA, Petraglia F 2002 Levels of maternal plasma corticotropin-releasing factor and urocortin during labor. J Soc Gynecol Invest 9:233–237 [PubMed] [Google Scholar]
- Torricelli M, Giovannelli A, Leucci E, De Falco G, Reis FM, Imperatore A, Florio P, Petraglia F 2007 Labor (term and preterm) is associated with changes in the placental mRNA expression of corticotrophin-releasing factor. Reprod Sci 14:241–245 [DOI] [PubMed] [Google Scholar]
- Jones SA, Brooks AN, Challis JR 1989 Steroids modulate corticotropin-releasing hormone production in human fetal membranes and placenta. J Clin Endocrinol Metab 68:825–830 [DOI] [PubMed] [Google Scholar]
- Karalis K, Goodwin G, Majzoub JA 1996 Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med 2:556–560 [DOI] [PubMed] [Google Scholar]
- Keegan CE, Herman JP, Karolyi IJ, O'Shea KS, Camper SA, Seasholtz AF 1994 Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology 134:2547–2555 [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Bae DS, Brown TT, Vogt SK, Alvarez JG, Sunday ME, Majzoub JA 1999 Proliferation and differentiation defects during lung development in corticotropin-releasing hormone-deficient mice. Am J Respir Cell Mol Biol 20:181–188 [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Weaver TE 2002 Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 347:2141–2148 [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Takahashi M, Nishitani C 2007 Pulmonary collectins in innate immunity of the lung. Cell Microbiol 9:1871–1879 [DOI] [PubMed] [Google Scholar]
- Hawgood S, Shiffer K 1991 Structures and properties of the surfactant-associated proteins. Annu Rev Physiol 53:375–394 [DOI] [PubMed] [Google Scholar]
- Crouch E, Wright JR 2001 Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol 63:521–554 [DOI] [PubMed] [Google Scholar]
- Wright JR 2005 Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68 [DOI] [PubMed] [Google Scholar]
- López-Bernal A, Newman GE, Phizackerley PJ, Turnbull AC 1988 Surfactant stimulates prostaglandin E production in human amnion. Br J Obstet Gynaecol 95:1013–1017 [DOI] [PubMed] [Google Scholar]
- Toyoshima K, Narahara H, Furukawa M, Frenkel RA, Johnston JM 1995 Platelet-activating factor. Role in fetal lung development and relationship to normal and premature labor. Clin Perinatol 22:263–280 [PubMed] [Google Scholar]
- Kremlev SG, Phelps DS 1994 Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. Am J Physiol 267:L712–L719 [DOI] [PubMed] [Google Scholar]
- Phelps DS 2001 Surfactant regulation of host defense function in the lung: a question of balance. Pediatr Pathol Mol Med 20:269–292 [PubMed] [Google Scholar]
- Mendelson CR, Boggaram V 1990 Hormonal and developmental regulation of pulmonary surfactant synthesis in fetal lung. Baillieres Clin Endocrinol Metab 4:351–378 [DOI] [PubMed] [Google Scholar]
- Islam KN, Mendelson CR 2002 Potential role of nuclear factor κB and reactive oxygen species in cAMP and cytokine regulation of surfactant protein-A gene expression in lung type II cells. Mol Endocrinol 16:1428–1440 [DOI] [PubMed] [Google Scholar]
- Odom MJ, Snyder JM, Mendelson CR 1987 Adenosine 3′,5′-monophosphate analogs and β-adrenergic agonists induce the synthesis of the major surfactant apoprotein in human fetal lung in vitro. Endocrinology 121:1155–1163 [DOI] [PubMed] [Google Scholar]
- Bry K, Lappalainen U, Hallman M 1997 Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest 99:2992–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bry K, Hallman M 1993 Transforming growth factor-β2 prevents preterm delivery induced by interleukin-1α and tumor necrosis factor-α in the rabbit. Am J Obstet Gynecol 168:1318–1322 [DOI] [PubMed] [Google Scholar]
- Islam KN, Mendelson CR 2008 Glucocorticoid/glucocorticoid receptor inhibition of surfactant protein-A (SP-A) gene expression in lung type II cells is mediated by repressive changes in histone modification at the SP-A promoter. Mol Endocrinol 22:585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn JL, Islam KN, Young PP, Mendelson CR 2004 Glucocorticoid inhibition of SP-A gene expression in lung type II cells is mediated via the TTF-1-binding element. Am J Physiol Lung Cell Mol Physiol 286:L767–L776 [DOI] [PubMed] [Google Scholar]
- Korfhagen TR, Bruno MD, Glasser SW, Ciraolo PJ, Whitsett JA, Lattier DL, Wikenheiser KA, Clark JC 1992 Murine pulmonary surfactant SP-A gene: cloning, sequence, and transcriptional activity. Am J Physiol Lung Cell Mol Physiol 263:L546–L554 [DOI] [PubMed] [Google Scholar]
- Alcorn JL, Hammer RE, Graves KR, Smith ME, Maika SD, Michael LF, Gao E, Wang Y, Mendelson CR 1999 Analysis of genomic regions involved in regulation of the rabbit surfactant protein A gene in transgenic mice. Am J Physiol Lung Cell Mol Physiol 277:L349–L361 [DOI] [PubMed] [Google Scholar]
- Mendelson CR, Condon JC 2005 New insights into the molecular endocrinology of parturition. J Steroid Biochem Mol Biol 93:113–119 [DOI] [PubMed] [Google Scholar]
- Hunt JS, Miller L, Platt JS 1998 Hormonal regulation of uterine macrophages. Dev Immunol 6:105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, Wira CR 1998 Influence of the estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol 39:209–216 [DOI] [PubMed] [Google Scholar]
- Tibbetts TA, Conneely OM, O'Malley BW 1999 Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol Reprod 60:1158–1165 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Kelly RW, Carr GG, Riley SC 1997 The inhibition of synthesis of a β-chemokine, monocyte chemotactic protein-1 (MCP-1) by progesterone. Biochem Biophys Res Commun 239:557–561 [DOI] [PubMed] [Google Scholar]
- Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, Branch DW, Silver RM, Adashi EY 2005 Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta 26:661–671 [DOI] [PubMed] [Google Scholar]
- Havelock JC, Keller P, Muleba N, Mayhew BA, Casey BM, Rainey WE, Word RA 2005 Human myometrial gene expression before and during parturition. Biol Reprod 72:707–719 [DOI] [PubMed] [Google Scholar]
- Hardy DB, Mendelson CR 2006 Progesterone receptor (PR) antagonism of the inflammatory signals leading to labor. Fetal Maternal Med Rev 17:281–289 [Google Scholar]
- Hardy DB, MacDonald E, Smith M, Mendelson CR 2006 Developmental regulation of the eicosanoid pathway mediates the induction of surfactant protein-A (SP-A) expression in the fetal lung. J Soc Gynecol Invest 13:242A [Google Scholar]
- Loudon JA, Elliott CL, Hills F, Bennett PR 2003 Progesterone represses interleukin-8 and cyclo-oxygenase-2 in human lower segment fibroblast cells and amnion epithelial cells. Biol Reprod 69:331–337 [DOI] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Chen CC, Mendelson CR 2008 Progesterone receptor inhibits aromatase and inflammatory response pathways in breast cancer cells via ligand-dependent and ligand-independent mechanisms. Mol Endocrinol 22:1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR 2006 Progesterone receptor (PR) plays a major antiinflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Wissink S, van der Saag PT, van der Burg B 1996 Negative interaction between the RelA(p65) subunit of NF-κB and the progesterone receptor. J Biol Chem 271:6217–6224 [DOI] [PubMed] [Google Scholar]
- Baldwin Jr AS 1996 The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol 14:649–683 [DOI] [PubMed] [Google Scholar]
- Miller L, Hunt JS 1998 Regulation of TNF-α production in activated mouse macrophages by progesterone. J Immunol 160:5098–5104 [PubMed] [Google Scholar]
- Deroo BJ, Archer TK 2002 Differential activation of the IκBα and mouse mammary tumor virus promoters by progesterone and glucocorticoid receptors. J Steroid Biochem Mol Biol 81:309–317 [DOI] [PubMed] [Google Scholar]
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M 1995 Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science 270:286–290 [DOI] [PubMed] [Google Scholar]
- Scheinman RI, Cogswell PC, Lofquist AK, Baldwin Jr AS 1995 Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science 270:283–286 [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Archer TK 2001 Glucocorticoid receptor activation of the IκBα promoter within chromatin. Mol Biol Cell 12:3365–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck S, Bender K, Kullmann M, Göttlicher M, Herrlich P, Cato AC 1997 IκBα-independent downregulation of NF-κB activity by glucocorticoid receptor. EMBO J 16:4698–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR 2007 Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat Chem Biol 3:166–173 [DOI] [PubMed] [Google Scholar]
- Virgo BB, Bellward GD 1974 Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology 95:1486–1490 [DOI] [PubMed] [Google Scholar]
- Frydman R, Lelaidier C, Baton-Saint-Mleux C, Fernandez H, Vial M, Bourget P 1992 Labor induction in women at term with mifepristone (RU 486): a double-blind, randomized, placebo-controlled study. Obstet Gynecol 80:972–975 [PubMed] [Google Scholar]
- Elliott CL, Brennand JE, Calder AA 1998 The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstet Gynecol 92:804–809 [DOI] [PubMed] [Google Scholar]
- Stenlund PM, Ekman G, Aedo AR, Bygdeman M 1999 Induction of labor with mifepristone: a randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand 78:793–798 [PubMed] [Google Scholar]
- Chwalisz K 1994 The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod 9(Suppl 1):131–161 [DOI] [PubMed] [Google Scholar]
- Condon JC, Mendelson CR 2004 Fetal macrophages, which contain elevated 20α-hydroxysteroid dehydrogenase (20α-HSD) activity and invade the pregnant uterus near term, may contribute to the onset of labor by causing local progesterone withdrawal. J Soc Gynecol Invest 11:224A [Google Scholar]
- Mahendroo MS, Cala KM, Russell DW 1996 5α-Reduced androgens play a key role in murine parturition. Mol Endocrinol 10:380–392 [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Porter A, Russell DW, Word RA 1999 The parturition defect in steroid 5α-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 13:981–992 [DOI] [PubMed] [Google Scholar]
- Ishida M, Choi JH, Hirabayashi K, Matsuwaki T, Suzuki M, Yamanouchi K, Horai R, Sudo K, Iwakura Y, Nishihara M 2007 Reproductive phenotypes in mice with targeted disruption of the 20α-hydroxysteroid dehydrogenase gene. J Reprod Dev 53:499–508 [DOI] [PubMed] [Google Scholar]
- Piekorz RP, Gingras S, Hoffmeyer A, Ihle JN, Weinstein Y 2005 Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20aldo-keto reductase family-hydroxysteroid dehydrogenase. Mol Endocrinol 19:431–440 [DOI] [PubMed] [Google Scholar]
- Kastner P, Bocquel MT, Turcotte B, Garnier JM, Horwitz KB, Chambon P, Gronemeyer H 1990 Transient expression of human and chicken progesterone receptors does not support alternative translational initiation from a single mRNA as the mechanism generating two receptor isoforms. J Biol Chem 265:12163–12167 [PubMed] [Google Scholar]
- Conneely OM, Maxwell BL, Toft DO, Schrader WT, O'Malley BW 1987 The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun 149:493–501 [DOI] [PubMed] [Google Scholar]
- Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP 2000 The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol 20:3102–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP 1993 Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 7:1244–1255 [DOI] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R 2002 Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 87:2924–2930 [DOI] [PubMed] [Google Scholar]
- Pieber D, Allport VC, Hills F, Johnson M, Bennett PR 2001 Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod 7:875–879 [DOI] [PubMed] [Google Scholar]
- Zeng Z, Velarde MC, Simmen FA, Simmen RC 2008 Delayed parturition and altered myometrial progesterone receptor isoform A expression in mice null for Kruppel-like factor 9. Biol Reprod 78:1029–1037 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LL, Norris BM, Baker CJ 1997 An N-terminally truncated third progesterone receptor protein, PRC, forms heterodimers with PRB but interferes in PRB-DNA binding. J Steroid Biochem Mol Biol 62:287–297 [DOI] [PubMed] [Google Scholar]
- Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB 1990 5′-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol Endocrinol 4:1833–1840 [DOI] [PubMed] [Google Scholar]
- Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG 1996 An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol 10:1379–1387 [DOI] [PubMed] [Google Scholar]
- Samalecos A, Gellersen B 2008 Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinology 149:5872–5887 [DOI] [PubMed] [Google Scholar]
- Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S 2007 Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab 92:1927–1933 [DOI] [PubMed] [Google Scholar]
- Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P 2006 Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol 20:1519–1534 [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR 2003 A decline in progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of labor. Proc Natl Acad Sci USA 100:9518–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite RS, Brown AG, Strauss 3rd JF 2004 Tumor necrosis factor-α suppresses the expression of steroid receptor coactivator-1 and -2: a possible mechanism contributing to changes in steroid hormone responsiveness. FASEB J 18:1418–1420 [DOI] [PubMed] [Google Scholar]
- Dong X, Shylnova O, Challis JR, Lye SJ 2005 Identification and characterization of the protein-associated splicing factor as a negative coregulator of the progesterone receptor. J Biol Chem 280:13329–13340 [DOI] [PubMed] [Google Scholar]