Abstract
The Src homology 2 (SH2) domain-containing adapter protein SH2B1β plays a role in severe obesity, leptin and insulin resistance, and infertility. SH2B1β was initially identified as a Janus tyrosine kinase 2 (JAK2) substrate, and it has been implicated in cell motility and regulation of the actin rearrangement in response to GH and platelet-derived growth factor. SH2B1β is also required for maximal actin-based motility of Listeria. Here we have used a low-speed pelleting assay and electron microscopy to demonstrate that SH2B1β has two actin-binding sites and that it cross-links actin filaments in vitro. Wild-type SH2B1β localized to cell ruffles and along filopodia, but deletion of amino acids 150–200 (the first actin-binding site) led to mislocalization of the protein to filopodia tip complexes where it colocalized with vasodilator-stimulated phosphoprotein (VASP). Based on studies performed in VASP-deficient MVD7−/− cells, with or without green fluorescent protein-VASP reconstitution, we concluded that the proper intracellular localization of native SH2B1β required the presence of the first SH2B1β actin-binding site and VASP. Finally, we found that both SH2B1β actin-binding domains were required for maximal GH- and prolactin-induced cell ruffling. Together, these results suggest that SH2B1β functions as an adapter protein that cross-links actin filaments, leading to modulation of cellular responses in response to JAK2 activation.
Adapter protein SH2B1β directly binds to and cross-links actin filaments that lead to modulation of actin cytoskleton in response to JAK2 activation.
Cytokine receptors lack intrinsic tyrosine kinase activity, but they constitutively associate with Janus tyrosine kinases (JAKs), facilitating activation of signaling cascades. When a cytokine binds to its receptor, it induces conformational changes within the receptor that lead to dimerization of the receptor-associated JAKs (1,2). Dimerization of the JAKs allows for auto- and/or transphosphorylation of tyrosines within the kinase’s activation loop, causing a conformational change that fully activates the JAK (3). JAK activation leads to the autophosphorylation of multiple tyrosines as well as the phosphorylation of the associated receptor. Phosphorylated tyrosines on JAK and their associated cytokine receptors provide binding sites for downstream signaling molecules, which bind via phosphotyrosine binding (4) and Src homology 2 (SH2) domains. Such downstream signaling molecules include the family of transcription factors known as signal transducers and activators of transcription (STATs) (5), protein phosphatases, and other adapter proteins such as Shc, Grb2, Cbl, and p85 phosphatidylinositol 3-kinase. These interactions lead to the stimulation of classical Ras/Raf/ERK and phosphatidylinositol 3-kinase/Akt pathways, culminating in activation of transcription factors, including Fos and Jun (for review see Ref. 6).
The widely expressed SH2 domain-containing protein SH2B1β was initially identified as a binding partner and substrate of JAK2 (7) (for review see Ref. 8). SH2-B is a member of the SH2B family (SH2-B, APS, and Lnk), which contains conserved N-terminal dimerization, central pleckstrin homology (PH), and C-terminal SH2 domains. SH2-B, APS, and Lnk were renamed recently by the HUGO Gene Nomenclature Committee as SH2B1, SH2B2, and SH2B3, respectively. The SH2B1 (SH2-B) gene encodes four isoforms (α, β, γ, and δ) by alternative mRNA splicing; these four forms have identical dimerization, PH, and SH2 domains but differ at their C termini (9,10). SH2B1β was originally identified as a JAK2-binding protein in a yeast two-hybrid screen (7). In cultured cells, SH2B1β was tyrosyl phosphorylated by JAK2 and potentiated JAK2 activation in response to GH (7,11,12) and leptin (13). One or more SH2B1β isoforms was tyrosyl phosphorylated by activated forms of the platelet-derived growth factor (PDGF) (9,14,15,16), IGF-I (9,15,16), nerve growth factor (17,18), insulin (10,15,19), and fibroblast growth factor (20) receptors, suggesting that SH2B1β may play a fundamental role in a variety of cell functions. Deletion of the SH2B1 gene resulted in severe obesity and both leptin and insulin resistance as well as infertility, which might be a consequence of resistance to IGF-I (21,22,23). Thus, knockout mice support a role for SH2B1 as a positive regulator of JAK2 signaling pathways initiated by leptin, insulin, and potentially, by IGF-I.
Several lines of evidence show that SH2B1β is involved in signaling to the actin cytoskeleton. First, SH2B1β increases membrane ruffling and pinocytosis induced by GH and PDGF (24). Second, SH2B1β is required for optimal actin-based cell motility and binds Rac (25). Third, SH2B1β is also required for maximal actin-based motility of Listeria monocytogenes, an intracellular bacterial pathogen that can invade a broad range of cell types and cause a variety of syndromes in human and animals. SH2B1β increases the rate of bacterial propulsion in infected cells and in cell extracts in a vasodilator-stimulated phosphoprotein (VASP)-dependent fashion (26). Finally, both SH2B2 and SH2B3 have been implicated as regulators of the actin cytoskeleton. SH2B3 (Lnk) was identified in a yeast two-hybrid screen as a binding partner for the actin-binding protein filamin (27), and SH2B2 (APS) colocalizes with filamentous actin (F-actin) during the capping of B-cell receptor in SH2B2 transgenic B cells (28). Mast cells from SH2B2−/− mice showed reduced F-actin assembly while degranulation was augmented, suggesting that SH2B2 plays a role in the negative regulation of mast cell degranulation by controlling actin dynamics (29). A role for SH2B family members in the regulation of the actin cytoskeleton is further supported by the finding that SH2B2 binds to Vav3, a guanine nucleotide exchange factor for Rac (30) and to Enigma, the protein participating in insulin-induced actin remodeling (31,32). However, the mechanisms by which the effects of SH2B family proteins on the actin cytoskeleton are mediated remain largely unknown.
In this work, we extend initial findings implicating SH2B1β in the regulation of the actin cytoskeleton by providing strong evidence that SH2B1β has two actin-binding sites: amino acids 150–200 that strongly bind to F-actin and amino acids 615–670 that bind F-actin less strongly. The first actin-binding site is responsible for the proper intracellular localization of SH2B1β to cell ruffles and along the filopodia. The localization of SH2B1β also depends on the protein VASP because in MVD7−/− cells (generated from VASP/Mena double-knockout mice), SH2B1β is mis-colocalized to filopodia tip complexes. This mis-colocalization of SH2B1β is rescued by reexpression of VASP in MVD7−/− cells. Using a low-speed pelleting assay and electron microscopy, we demonstrate that SH2B1β cross-links F-actin in vitro. Finally, we show that both actin-binding domains are required for maximal GH- and prolactin (PRL)-induced cell ruffling.
Results
SH2B1β is an actin-binding protein
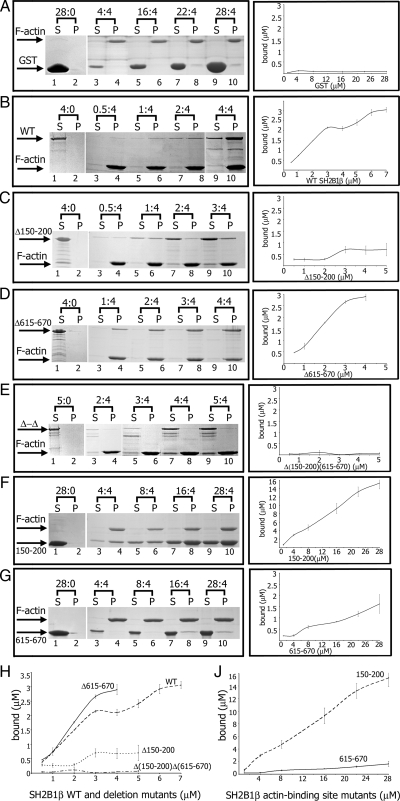
To identify the region of SH2B1β to which actin binds, we coexpressed green fluorescent protein (GFP)-actin with various myc-tagged truncated forms of SH2B1β and showed that amino acids 150–200 and 615–670 contain binding sites for actin (data not shown). Direct binding of SH2B1β to pure F-actin was tested in a cosedimentation assay. A series of glutathione S-transferase (GST)-tagged truncated forms of SH2B1β were used in this assay (Fig. 1). Before performing the assay, GST-tagged wild-type (WT) SH2B1β or SH2B1β mutants were centrifuged at 150,000 × g for 2 h to remove any insoluble protein aggregates. Control samples without actin showed that GST alone, WT SH2B1β, or any SH2B1β mutants did not self-aggregate to form pellets (Fig. 2, A–G, lanes 1 and 2). Next, a constant concentration of F-actin (4 μm) was mixed with increasing concentrations of each of the different forms of SH2B1β or with GST alone (Fig. 2). These soluble proteins will sediment in the pellet fraction only if they bind to F-actin. WT SH2B1β was detected in the pellet fractions (Fig. 2B, lanes 4, 6, 8, and 10), demonstrating an association of the WT protein with F-actin. However, some amount of SH2B1β remained in the supernatants, in agreement with our previous observation (26). In contrast, GST alone was not detected in the pellet fractions (Fig. 2A), suggesting that the GST tag does not affect the binding of GST-tagged proteins to actin. When we deleted amino acids 150–200, the SH2B1β (Δ150–200) mutant still retained F-actin-binding activity, although SH2B1β (Δ150–200) bound less F-actin than WT SH2B1β (Fig. 2, C and H). Indeed, a SH2B1β (150–200) mutant that contained only this actin-binding site bound to F-actin much better than WT protein (Fig. 2, F and J). In addition, a SH2B1β (Δ150–200) mutant also lacking the C-terminal 615–670 amino acids (Δ-Δ mutant) failed to bind to F-actin (Fig. 2, E and H), suggesting that the amino acids 615–670 include a second actin-binding site. Indeed, a mutant containing residues 615–670 bound to F-actin (Fig. 2, G and J) although substantially less strongly than WT protein or the 150–200 mutant. Interestingly, SH2B1β (Δ615–670), which lacks the second actin-binding site, exhibited increased F-actin binding when compared with WT SH2B1β (Fig. 2, D and H), suggesting that the C-terminal second actin-binding site may inhibit the interaction of WT protein with F-actin, for example, by masking the first actin-binding site. When we performed similar experiments in the EGTA-F buffer (Ca2+ was replaced by EGTA), we did not see a significant difference in the amount of WT SH2B1β bound to F-actin (data not shown), suggesting that this binding is Ca2+ independent.
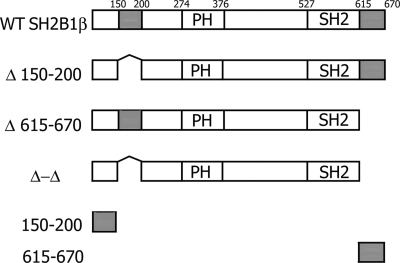
Figure 1.
Schematic representation of WT and mutant forms of rat SH2B1β used in the study. Actin-binding domains (amino acids 150–200 and 615–670) are shown in gray. PH is the PH domain (amino acids 274–376), and SH2 is the SH2 domain (amino acids 527–620). Proline-rich regions (amino acids 13–24, 89–103, and 469–496), and dimerization domain (amino acids 24–85) are not shown.
Figure 2.
SH2B1β binds F-actin. Before each experiment, WT SH2B1β and mutants were centrifuged in the absence of F-actin to remove any insoluble protein aggregates. A defined concentration of F-actin (4 μm) was mixed with increasing concentrations of the forms of SH2B1β or GST alone. Ratios above gel images indicate concentrations of recombinant proteins (in micromolar) to the constant concentration of F-actin (4 μm). After ultracentrifugation at 150,000 × g for 2 h, equivalent amounts of pellet (P) and supernatant (S) fraction were subjected to SDS-PAGE followed by Coomassie blue staining (A–G, left column). Protein bands were quantified using Bio-Rad Quantity One software. The amount of WT GST-SH2B1β or GST- SH2B1β mutants bound to F-actin was calculated from the known concentrations of proteins in the assay and the ratios of each protein in the supernatant and pellet. Plots of concentration (x-axis) vs. amount bound (y-axis) for WT SH2B1β and all mutants are shown in the right column. Plots of concentration (x-axis) vs. amount bound (y-axis) for WT SH2B1β and deletion mutants (H) and two actin-binding site mutants (J) are shown together. Bars represent mean ± se; n = 3.
Overall, these observations demonstrate that SH2B1β contains two F-actin binding sites and that one site, including amino acids 150–200, binds F-actin much better than the other, amino acids 615–670.
SH2B1β cross-links actin filaments in vitro
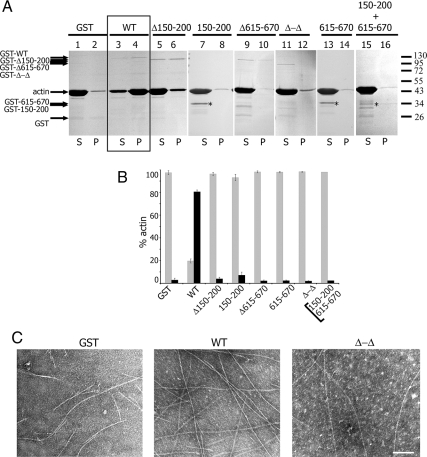
Because SH2B1β has two actin-binding sites, we hypothesized that SH2B1β might organize filaments into higher-order structures. Therefore, we examined filament aggregation using a low-speed pelleting assay, in which filament aggregates (cross-linked networks or bundles) pellet, whereas single actin filaments remain in the supernatant. GST-tagged WT SH2B1β caused actin to pellet at low speed (Fig. 3A, lane 3 vs. 4, and B). Conversely, GST alone and GST-tagged mutants Δ150–200, 150–200, Δ615–670, Δ-Δ, and 615–670 did not pellet actin filaments at low speed (Fig. 3A, lanes 1, 5, 7, 9, 11, and 13 vs. 2, 6, 8, 10, 12, and 14, and 3B). These data suggest that both actin-binding domains of SH2B1β are required for organizing the actin filaments into higher-order structures. When both actin-binding domain mutants 150–200 and 615–670 were added together, they did not pellet actin filaments (Fig. 3A, lanes 15 and 16, and 3B). This suggests that to aggregate actin filaments, the intact SH2B1β molecule with both actin-binding sites is required.
Figure 3.
SH2B1β cross-links actin filaments. For the low-speed centrifugation assay (A and B), 0.1 μm GST-SH2B1β WT or GST-SH2B1β mutants were incubated with 8 μm F-actin for 30 min in actin polymerizing buffer (F-buffer) and then sedimented at 16,000 × g for 1 h at 24 C. A, Equivalent amounts of pellet (P) and supernatant (S) fraction were subjected to SDS-PAGE, followed by Coomassie blue staining. Asterisks indicate the bands corresponding to the 150–200 and 615–670 mutants. B, Actin bands were quantified and plotted to indicate the percentage of actin bundled/cross-linked (pellet fraction, black bar) or not bundled/cross-linked (supernatant fraction, gray bar) for each GST-SH2B1β or GST-SH2B1β mutant. Bars represent mean ± se; n = 3. C, Electron micrographic images of negatively stained F-actin in the presence of GST (left), SH2B1β WT (middle), and Δ-Δ mutant (right). Scale bar, 100 nm.
The low-speed pelleting assay shows that WT SH2B1β causes actin aggregation but does not address the morphology of these aggregates. For example, based on criteria established by Harris et al. (33), bundles are defined as ordered aggregates in which filaments are oriented along their long axes and a cross-linked network as aggregates in which filaments are not parallel to each other. Therefore, the morphology of SH2B1β-bound actin aggregates was assessed by electron microscopy. The small amount of actin that polymerized in the presence of GST alone showed individual filaments (Fig. 3C, left). However, the addition of GST-SH2B1β WT induced formation of a cross-linked actin network (Fig. 3C, middle). As predicted, the deletion of both actin-binding sites dramatically decreased the appearance of cross-linking (Fig. 3C, right), suggesting that SH2B1β is an F-actin cross-linker. These observations are consistent with the results obtained by the low-speed centrifugation assay.
SH2B1β localizes to membrane ruffles and filopodia
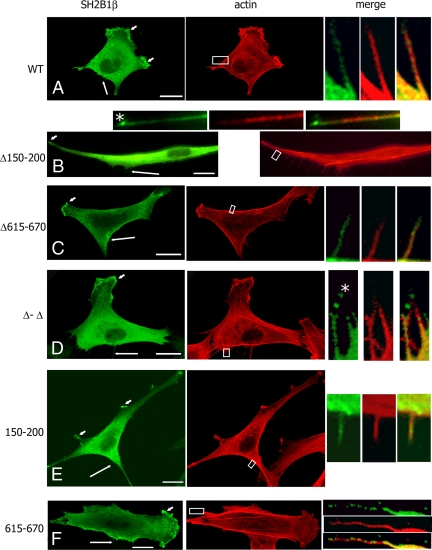
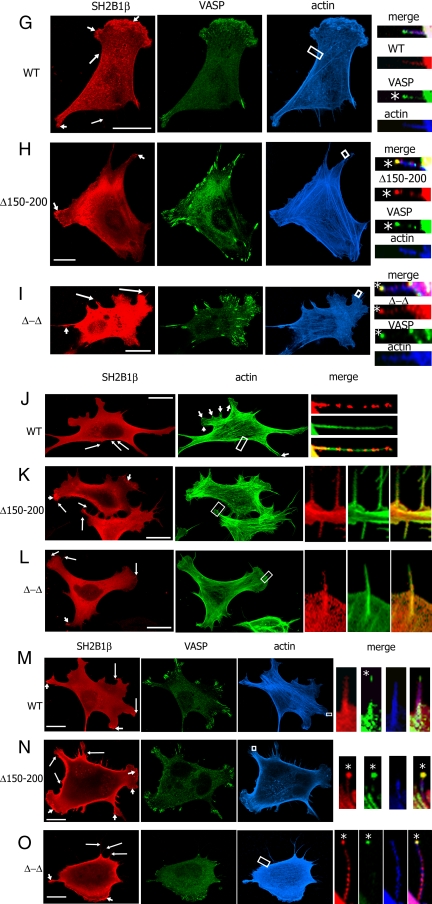
Previous experiments in 3T3 F442A cells showed that SH2B1β localizes to membrane ruffles (24). To examine the role of the two SH2B1β actin-binding sites in this localization, the localization of myc-tagged SH2B1β mutants was examined. WT SH2B1β and SH2B1β Δ150–200, Δ615–670, Δ-Δ, 150–200, and 615–670 mutants colocalized with actin in cell ruffles (Fig. 4, short arrows) and filopodia (Fig. 4, long arrows and small boxes). Interestingly, in addition to cell ruffles and filopodia length, the Δ150–200 and Δ-Δ mutants also concentrated at the tips of filopodia (Fig. 4, B and D, asterisks). In contrast, WT, Δ615–670, 150–200, and 615–670 mutants were distributed along the filopodia and were not concentrated at the tip (Fig. 4, A, C, E, and F). These data suggest that the first actin-binding site (amino acids 150–200) is responsible for the proper intracellular localization of SH2B1β.
Figure 4.
Intracellular localization of SH2B1β depends on both the first actin-binding domain of SH2B1β and VASP protein. A–F, 3T3 F442A cells overexpressing the indicated forms of myc-SH2B1β were stained with α-myc (green) and phalloidin-rhodamine (red); G–I, 3T3 F442A cells overexpressing GFP-VASP (green) and the indicated forms of myc-SH2B1β were stained with α-myc (red) and phalloidin-647 (blue); J–L, MVD7−/− cells overexpressing the indicated forms of myc-SH21Bβ were stained with α-myc (red), and phalloidin-488 (green). (M-O) MVD7−/− stably overexpressing GFP-VASP (green) were transfected with cDNA encoding the indicated forms of myc-SH2B1β, stained with α-myc (red) and phalloidin-647 (blue). Long arrows indicate filopodia, and short arrows indicate ruffles. The boxed regions were enlarged and merged. Asterisks denote the tips of filopodia. Scale bars, 20 μm.
Similar aggregates at the tip of filopodia were previously revealed by electron microscopy, and the existence of a filopodium tip complex has been suggested (34,35,36,37). The exact molecular composition of this complex is still largely unknown, although some proteins that are enriched at filopodial tips have been identified, including Ena/VASP proteins (38,39). GFP-VASP appears as a bright dot at filopodia tips (38,39,40) and therefore may serve as a marker of the tip complex. To confirm that Δ150–200 and Δ-Δ mutants concentrated at the tip complexes, we overexpressed GFP-VASP together with WT, Δ150–200, or Δ-Δ mutants of SH2B1β in 3T3 F442A cells and performed immunofluorescence analysis. As predicted from the results outlined above, WT SH2B1β was not colocalized with GFP-VASP at the tips of filopodia (Fig. 4G). In contrast, the SH2B1β Δ150–200 (Fig. 4H) and Δ-Δ mutants (Fig. 4I) colocalized with GFP-VASP at the tips of filopodia (asterisks in Fig. 4, H and I) confirming that amino acids 150–200 of SH2B1β are essential for the correct localization of SH2B1β along the filopodia, whereas deletion of these amino acids leads to mislocalization to the tip complexes.
Intracellular localization of SH2B1β depends on VASP
The localization of WT SH2B1β in Listeria actin tails depends on the presence of VASP in the tails (26). To test whether the intracellular localization of SH2B1β depends on VASP, the localization of SH2B1β mutants was examined in the MVD7−/− mouse fibroblastic cell line generated from VASP/Mena double-knockout mice (41,42,43). The localization of WT SH2B1β to the ruffles of MVD7−/− cells was impaired when compared with its localization in 3T3 F442A cells (Fig. 4J, short arrows indicating the actin ruffles in the phalloidin-labeled image vs. Fig. 4A, short arrows indicating the actin ruffles labeled with α-SH2B1β). Localization of WT SH2B1β in filopodia of MVD7−/− cells differs from that seen in 3T3 F442A cells; instead of localizing along the length of filopodia as in F442A cells (Fig. 4A), SH2B1β localized in patches along the filopodia (Fig. 4J). Interestingly, we did not see this patch-like localization when we overexpressed the Δ150–200 or Δ-Δ mutants. Both first actin-binding site-deficient mutants localized in the cell ruffles in MVD7−/− cells (Fig. 4, K and L, short arrows), and although both of these mutants localized in filopodia, they failed to localize at filopodia tips (Fig. 4, K and L, small boxes) in contrast to their localization in 3T3F 44A cells. These data suggest that VASP is sufficient for the proper localization of SH2B1β. To confirm this, GFP-VASP was reexpressed in MVD7−/− cells. As predicted, GFP-VASP localized at the tip complexes, but WT SH2B1β did not (asterisk in Fig. 4M). In contrast, both SH2B1β mutants lacking the first actin-binding site (Δ150–200 and Δ-Δ) colocalized with VASP at the filopodia tips (asterisks in Fig. 4, N and O). Additionally, WT and mutated forms of SH2B1β localized in cell ruffles (small arrows) and along the filopodia (long arrows). These data confirm that the proper intracellular localization of SH2B1β depends on both the first SH2B1β actin-binding site (amino acids 150–200) and VASP.
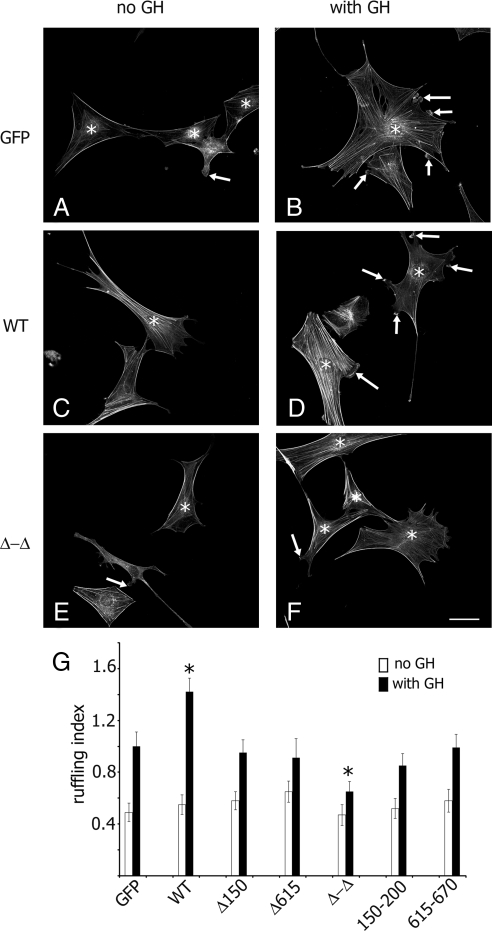
Actin-binding domains of SH2B1β are required for maximal GH- and PRL-dependent ruffling
Both the N terminus and C terminus of SH2B1β participate in GH-dependent cell ruffling (24). To determine whether the actin-binding sites of SH2B1β are required for cytokine-stimulated actin reorganization, GH-dependent ruffling was assessed in 3T3F F442A cells. GFP (control) and SH2B1β WT or mutants were expressed in 3T3 F442A cells, and actin ruffling was assessed (the number of phalloidin-rhodamine-stained ruffles per cell) after treatment with GH. Before stimulation, cells possess typical flattened fibroblast morphology with numerous actin stress fibers (Fig. 5, A, C, and E). GH induces the formation of cell ruffles rich in polymerized actin. As expected, cells overexpressing myc- SH2B1β WT exhibited more pronounced ruffling in response to GH compared with cells expressing GFP alone (Fig. 5, B, D, and G). To examine whether the actin-binding sites of SH2B1β are required for GH-induced membrane ruffling, we tested whether mutant forms of SH2B1β might act as dominant negatives. We showed that the SH2B1β Δ150–200, Δ615–670, 150–200, and 615–670 mutants failed to enhance the GH-dependent ruffling in contrast to WT protein (Fig. 5G). Expression of the Δ-Δ mutant inhibited cell ruffling (Fig. 5, F and G). Hence, this mutant is a putative dominant-negative protein and supports the hypothesis that both actin-binding domains of SH2B1β are required for the actin cross-linking activity of SH2B1β and maximal GH-induced actin ruffling.
Figure 5.
Both actin-binding sites of SH2B1β are required for maximal GH-induced membrane ruffling. A–F, 3T3 F44A cells expressing GFP or the indicated forms of myc-tagged forms of SH2B1β were serum deprived (A, C, and E) and treated with 500 ng/ml GH for 10 min (B, D, and F). F-actin was visualized by staining with phalloidin-rhodamine (shown), and the transfected cells were visualized by staining with α-myc. Arrows indicate ruffles, and asterisks denote transfected cells. Scale bar, 50 μm. G, Ruffling index as the number of ruffles per cell was counted. Bars represent mean ± se. *, P < 0.05 compared with cells expressing GFP and treated with GH. Δ150–200 and Δ615–670 are designated as Δ150 and Δ615, respectively. Each experiment was repeated three times; 100 cells were assessed for ruffling in each experiment for each type of transfection.
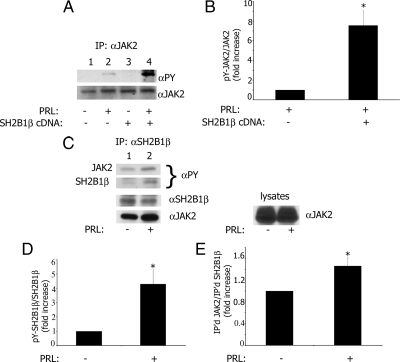
Next, we sought to determine whether the actin-binding sites of SH2B1β participate in prolactin-dependent actin reorganization. PRL rapidly induced ruffling in T47D cells (44) and is a potent activator of JAK2 (45,46,47). SH2B1β is tyrosyl phosphorylated by JAK2 and potentiates JAK2 activation in response to GH (7,10,11,12) and leptin (13,48). However, a potential role for SH2B1β in PRL-dependent signaling remains to be determined. To address whether SH2B1β is activated by PRL, and whether SH2B1β can potentiate PRL-induced activation of JAK2, SH2B1β was overexpressed in PRL receptor-containing T47D cells and tyrosyl phosphorylation of JAK2 was determined in immunoprecipitated endogenous JAK2 by immunoblotting with α-phosphotyrosine (α-PY). Prolactin induced tyrosyl phosphorylation of JAK2 as expected (Fig. 6A, lane 2 vs. lane 1). The quantification of tyrosyl-phosphorylated JAK2 (pY-JAK2) and JAK2 bands from lane 2 vs. lane 4 showed that SH2B1β increased the tyrosyl phosphorylation of JAK2 about 7.5-fold in response to PRL (Fig. 6B). These data demonstrate that overexpression of SH2B1β significantly enhanced PRL-dependent tyrosyl phosphorylation of JAK2. To demonstrate PRL-dependent tyrosyl phosphorylation of SH2B1β, T47D cells were treated with PRL and endogenous SH2B1β was immunoprecipitated with α-SH2B1β and immunoblotted with α-PY. PRL induced tyrosyl phosphorylation of both JAK2 and SH2B1β (Fig. 6C, lane 2 vs. 1, α-PY blot) and promoted association of SH2B1β with JAK2 (lane 2 vs. 1 in α-JAK2 blot). The quantification of pY-SH2B1β and SH2B1β bands from lane 1 vs. lane 2 showed that PRL increased the tyrosyl phosphorylation of SH2B1β about 4-fold (Fig. 6D). The quantification of immunoprecipitated SH2B1β and co-immunoprecipitated JAK2 bands (lane 2 vs. lane 1 in α-SH2B1β and α-JAK2 blots) showed that PRL increased association of SH2B1β with JAK2 about 1.5-fold.
Figure 6.
SH2B1β enhances tyrosyl phosphorylation of JAK2 in response to prolactin. A, SH2B1β was either overexpressed (lanes 3 and 4) or was not (lanes 1 and 2) in T47D cells. The cells were deprived of serum and treated without (lanes 1 and 3) or with (lanes 2 and 4) 150 ng/ml PRL for 20 min. JAK2 was immunoprecipitated with α-JAK2, immunoblotted with α-PY, and reblotted with α-JAK2. B, The graph represents densitometric analysis of the bands obtained for tyrosyl-phosphorylated JAK2 normalized with total JAK2. Bar represents mean ± se. *, P < 0.05 compared with cells not expressing SH2B1β; n = 3. C, PRL promotes association of SH2B1β with JAK2 and stimulates tyrosyl phosphorylation of SH2B1β. T47D cells were treated with (lane 2) or without (lane 1) PRL as above, and endogenous SH2B1β was immunoprecipitated with α-SH2B1β, immunoblotted with α-PY, and reblotted with α-JAK2 and α-SH2B1β. D, The graph represents densitometric analysis of the bands obtained for tyrosyl-phosphorylated SH2B1β normalized with total SH2B1β. Bar represents mean ± se. *, P < 0.05 compared with cells not treated with PRL; n = 3. E, The graph represents densitometric analysis of the bands obtained for co-immunoprecipitated JAK2 normalized with amount of immunoprecipitated SH2B1β. Bar represents mean ± se. *, P < 0.05 compared with cells not treated with PRL; n = 3.
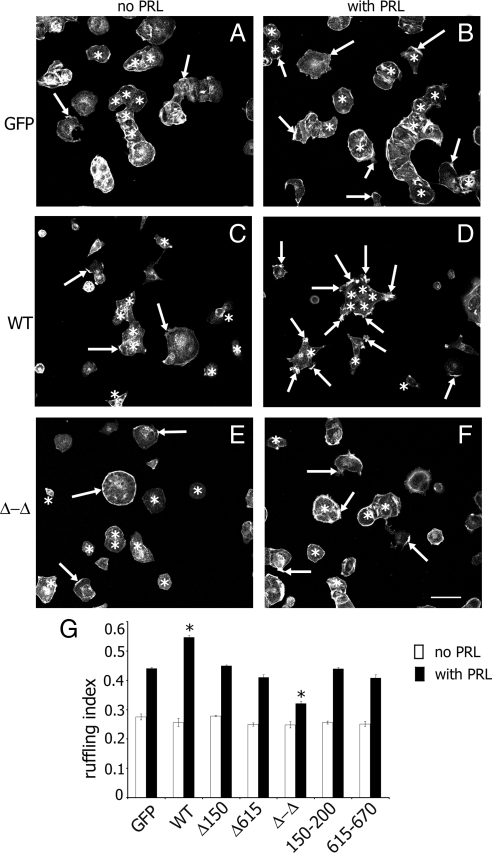
Next, we tested the effect of the two actin-binding sites of SH2B1β on PRL-dependent ruffling. GFP (control), WT, or mutants of SH2B1β were expressed in T47D cells, and actin ruffling was assessed as before. Overexpression of WT SH2B1β enhanced the ability of PRL to induce cell ruffling. In contrast, overexpression of the Δ-Δ mutant inhibited PRL-induced cell ruffling (Fig. 7), suggesting that this mutant is a dominant negative for PRL-dependent ruffling. The cells expressing SH2B1β Δ150–200, Δ615–670, 150–200, and 615–670 mutants exhibited ruffling similar to that observed in GFP-expressing cells, suggesting that both actin-binding sites in the context of the full-length molecule are required for cell ruffling.
Figure 7.
Actin-binding sites of SH2B1β are involved in PRL-induced membrane ruffling. A–F, T47D cells expressing GFP or the indicated forms of myc-tagged forms of SH2B1β were serum deprived (A, C, and E) and treated with 150 ng/ml PRL for 15 min (B, D, and F). F-actin was visualized by staining with phalloidin-rhodamine (shown) and the transfected cells were visualized by staining with α-myc. Arrows indicate ruffles, and asterisks denote transfected cells. Scale bar, 50 μm. G, Ruffling index as a number of ruffles per cell was counted. Bar represents mean ± se. *, P < 0.05 compared with cells expressing GFP and treated with PRL. Δ150–200 and Δ615–670 are designated as Δ150 and Δ615, respectively. Each experiment was repeated four times; 100 cells were assessed for ruffling in each experiment for each type of transfection.
Our data strongly suggest that both actin-binding domains of SH2B1β are required for maximal cytokine (GH and PRL)-induced actin ruffling.
SH2B1β participates in cell motility
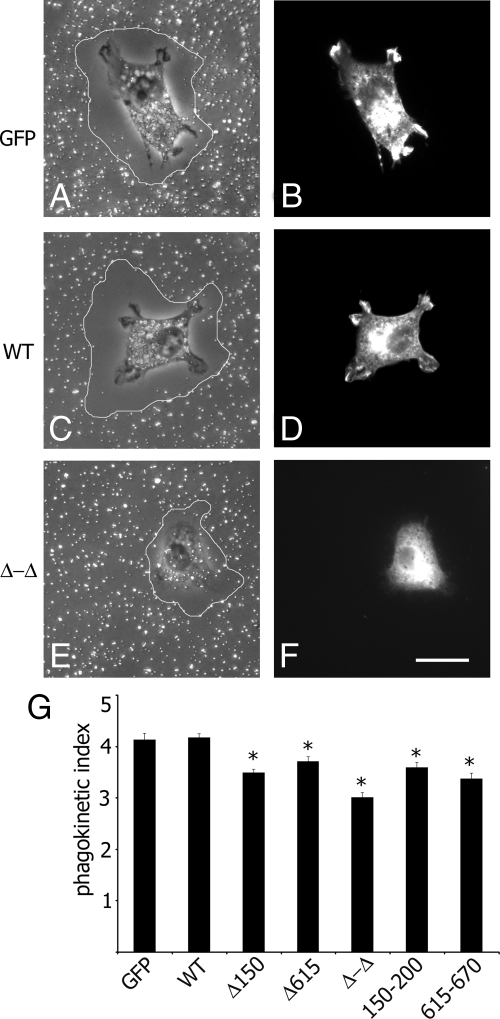
The effects of the SH2B1β actin-binding sites on cell motility were examined using a gold particle motility (phagokinetic) assay. This assay measures the disappearance of colloid gold particles from a coverslip, a process requiring both phagocytosis and locomotion. Transfected cells were plated on coverslips coated with colloid gold particles (Fig. 8, A–F). Cells remove particles while they move, thereby producing areas that are free of colloid gold. The phagokinetic index was calculated and plotted in Fig. 8G. The cells expressing Δ150–200, Δ615–670, Δ-Δ or 150–200, and 615–670 mutants of SH2B1β exhibited reduced cell motility as compared with the control or the cells overexpressing WT SH2B1β. These results suggest that SH2B1β participates in cell motility and that the mutant variants disrupt normal SH2B1β function.
Figure 8.
Maximal phagokinesis of 3T3 F442A cells requires SH2B1β. A–F, 3T3 F442A cells expressing GFP or the indicated forms of myc-tagged SH2B1β were plated on colloid gold-covered coverslips, and the areas that became free of colloid gold in 7 h were visualized (outlined). The cells overexpressing GFP or the indicated forms of myc-SH2B1β were stained with α-myc and phalloidin-rhodamine (not shown). Individual transfected cells were located with a FITC filter set (B, D, and F) and then observed by differential interference contrast (A, C, and E). Scale bar, 20 μm. G, A phagokinetic index was calculated as the ratio of the particle-free area to the cross-sectional area of the cells. Bars represent mean ± se. *, P < 0.05 compared with cells expressing GFP. Δ150–200 and Δ615–670 are designated as Δ150 and Δ615, respectively. n = 111, 247, 153, 159, 95, 85, and 63 for cells expressing GFP, WT, Δ150–200, Δ615–670, Δ-Δ, 150–200, and 615–670 of SH2B1β, respectively.
Discussion
Many cytokines, including GH and PRL, have been shown to promote actin reorganization (for example, Refs. 24,44, and 49,50,51). Actin is one of the most abundant cellular proteins and plays an important role in many dynamic processes such as cell motility, membrane ruffling, and cytokinesis. In addition, actin plays a role in signal transduction. Thus, various components involved in cytokine- and growth factor-induced signal transduction appeared to be associated with the actin cytoskeleton. One of the links between the cytoskeleton and signaling molecules are adapter proteins. Adapter proteins are important molecules that usually lack intrinsic enzymatic activity but, instead, serve to connect proteins together. One group of adapter proteins that has a role in a variety of signaling pathways is the SH2B family of adapters (for review see Ref. 8). One of them, SH2B1β, has been shown to link JAK2-dependent signaling to the actin cytoskeleton. This protein is required for GH-induced membrane ruffling and pinocytosis (24) and maximal cell motility (25). Additionally, it regulates the intracellular motility of the bacteria L. monocytogenes. Previous studies showed that SH2B1β cosedimented with purified F-actin (26) and coprecipitated with the small GTPase Rac1 (25). Here we extended these findings and showed that SH2B1β has two actin-binding sites: amino acids 150–200 on the N terminus that binds actin in vitro much stronger than the second C-terminal site (amino acids 615–670) that binds actin weakly. Using a low-speed pelleting assay and electron microscopy, we showed that SH2B1β cross-links actin filaments in vitro. In support of SH2B1β binding to and cross-linking F-actin, SH2B1β colocalized with actin in Listeria tails (26), membrane ruffles (24) (Fig. 4, A, G, and M), and lamellipodia and filopodia (Fig. 4, A, G, and M) in vivo. The actin structure of Listeria tails is thought to be similar to that found in lamellipodia but not stress fibers. For example, actin filaments in both Listeria tails and lamellipodia have dendritic organization generated by the Arp2/3 complex (52). Our previous finding that SH2B1β localizes along Listeria actin tails is consistent with our current finding that SH2B1β is excluded from stable stress fibers and enriched in highly dynamic filopodia and lamellipodia. Filopodia are actin-based structures composed of actin filaments and various actin-associated proteins. They play important roles in cell-cell signaling, guidance toward chemoattractants, embryonic development, wound healing, metastasis, and adhesion to the extracellular matrix (for review see Refs. 53 and 54). Two mechanisms for the formation of filopodia have been suggested, each using different sets of actin-regulating proteins, creating some controversy in the field (35,55). In this context, our data on the localization of the actin cross-linker SH2B1β in filopodia are of particular interest. Of additional interest is the observation that SH2B1β localization in filopodia depends on the presence of VASP. This was demonstrated by the fact that localization of SH2B1β in filopodia was dramatically changed in MVD7−/− cells generated from VASP/Mena double-knockout mice. This effect was reversible; after the reexpression of VASP in MVD7−/− cells, SH2B1β again localized along the length of filopodia. Interestingly, when the first actin-binding site was deleted (Δ150–200 and Δ-Δ mutants), SH2B1β mutants mislocalized to the top of filopodia into a filopodium tip complex where they colocalized with VASP. The exact molecular composition of this complex is still largely unknown, although some proteins are enriched at filopodial tips including Ena/VASP proteins (38,39), myosin X (56), Dia2 (57,58), and some others (for review see Ref. 53,54, and 59). How can we explain that SH2B1β mutants deficient in amino acids 150–200 translocate into the tip complex where they colocalize with VASP? The N terminus of SH2B1β contains several proline-rich regions. The first of these contains a FPSPP sequence (amino acids 12–16) that is very similar to the known VASP-binding consensus motif FPPPP (60). For example, Listeria protein ActA has three to four copies of this motif that binds to proteins of the Ena/VASP family (60,61,62). In some cases, individual prolines in the central core can be exchanged for other amino acids (60), but the first proline is absolutely essential for binding to VASP (63). Although SH2B1β does not interact directly with VASP, it may bind indirectly via some proteins, like ActA in the Listeria system (26). We hypothesize here that in WT SH2B1β, amino acids 150–200 mask the first proline-rich FPSPP motif, preventing it from binding to VASP. However, when amino acids 150–200 are deleted, these mutants (Δ150–200 and Δ-Δ) can now bind to VASP, leading to mislocalization of these mutants into the tip complexes and colocalization with VASP there. Whether VASP binds to the FPSPP sequence of SH2B1β directly in the absence of amino acids 150–200 remains to be determined. However, an attractive possibility is that amino acids 150–200 of SH2B1β play a role not only in binding to actin but also in regulating the proper cellular localization of SH2B1β.
A role for SH2B1β in actin-dependent cell ruffling and cell motility has been proposed previously (24,25). We have extended those findings and mapped two actin-binding sites within SH2B1β that are required for such important cell functions as maximal cytokine-dependent cell ruffling and cell motility. Membrane ruffling has been observed in many cell types in response to various extracellular factors and on motile cells where they are believed to be required for directed cell migration. Thus, the formation of membrane ruffles may be a sign of increased response to external stimuli and of elevated cell migration (for review see Refs. 64 and 65). Our findings that deletion of both identified actin-binding sites conferred dominant-negative activity upon SH2B1β suggest a role for the WT protein in actin cross-linking in GH- and PRL-dependent cell ruffling. Based upon our findings, we propose that upon ligation of GH and/or PRL receptors, SH2B1β translocates to activated membrane receptor-JAK2 complexes where it cross-links actin filaments and facilitates cytokine-dependent cell ruffling. Upon tyrosyl phosphorylation by JAK2, SH2B1β may also create additional docking sites to recruit actin-regulating SH2 domain-containing proteins to the plasma membrane to facilitate membrane ruffling. Indeed, JAK2-phosphorylated tyrosines 439 and 494 of SH2B1β participate in GH-dependent ruffling (66). Thus, the dominant-negative Δ-Δ mutant might still be able to bind and therefore sequester JAK2 and/or other effector molecules from binding to endogenous SH2B1β but is unable to bind and cross-link actin filaments for cell ruffling. We are currently investigating which actin-regulating proteins associate with tyrosyl-phosphorylated SH2B1β.
We have shown here that full-length SH2B1β, Δ150–200, Δ615–670, Δ-Δ, 150–200, and 615–670 mutants localize in actin ruffles. This localization may be enhanced by one or both actin-binding domains. However, we also suspect that additional regions of SH2B1β contribute to the localization via interaction with proteins that colocalize to actin ruffles. One candidate is VASP because VASP localizes in cell ruffles (67), and localization of the Δ-Δ mutant is dependent on the presence of VASP as demonstrated in Fig. 4. Another protein that may recruit SH2B1β and its mutants to ruffles is Rac1, because SH2B1β co-immunoprecipitates with Rac1 and amino acids 85–106 of SH2B1β (which are different from the actin-binding sites) are required for this binding (25). Membrane ruffles are active centers of signal transduction containing several signaling molecules (64). The signaling protein Grb2 localizes to actin ruffles (68) and constitutively binds to a proline-rich region of SH2B1β through its SH3 domain (18). Thus, Grb2 and/or other SH3 domain-containing proteins (such actin-binding proteins as cortactin, spectrin, and myosin I) (69,70,71,72) that localize to membrane ruffles, could bring the Δ-Δ mutant of SH2B1β to ruffles by one of its proline-rich regions that binds to the SH3 domains. Association with these other proteins may explain why the Δ-Δ mutant localizes to actin ruffles in the absence of direct binding to actin. We are currently investigating which actin-regulating proteins associate with the actin-binding site-deficient mutants of SH2B1β.
We have demonstrated here that the cells expressing Δ150–200, Δ615–670, Δ-Δ or 150–200, and 615–670 mutants of SH2B1β impaired cell motility as compared with the control or the cells overexpressing WT SH2B1β. Interestingly, these data confirmed the previous observation that overexpression of amino acids 504–670 of SH2B1β also reduced the phagokinetic index in 3T3 F442A and 293T cells (25). Cell motility is a more complex process than actin ruffling that involves the coordinated extension of lamellipodia, subsequent formation of new focal adhesion complexes at the leading edge, generation of traction and tension within the cell cortex, and release of focal adhesion complexes at the rear of the cell (73). The phagokinetic assay, which we used in our study, measures a combination of two processes that are dependent upon changes in the actin cytoskeleton: cellular movement and phagocytosis. Thus, the simplest explanation for why mutated forms of SH2B1β inhibit cell motility is that the mutants are able to sequester actin and/or actin-binding proteins (in the cases of 150–200 and 615–670 mutants) and/or other effector molecules (in the cases of Δ150, Δ615 and Δ-Δ mutants) away from endogenous SH2B1β, thereby preventing the assembly of active signaling complexes at sites of actin rearrangement needed for cell migration.
PRL was previously shown to act as a chemoattractant for human breast carcinoma, but the mechanisms by which PRL impacts the actin cytoskeleton and cell motility are largely unknown (44). Our current data may provide insight into one mechanism of PRL-stimulated metastatic dissemination of breast cancer. Activated PRL receptors couple activated JAK2 tyrosine kinase with adapter protein SH2B1β, which in turn directly binds to F-actin and may cross-link the actin filaments via its two actin-binding sites. This cross-linking activity is required for maximal cell ruffling and cell migration. GH also increases migration of certain cell types (49), and our current findings may contribute to an understanding of this process as well. Additionally, studies in Dictyostelium, Drosophila, and vertebrates have shown that the JAK pathways are required for an unusually broad set of developmental decisions including cell migration (for review see Ref. 74). Whether the actin cross-linking activity of adapter protein SH2B1β contributes to this process is still to be determined.
Materials and Methods
Plasmids, antibodies, and cells
cDNAs encoding myc-tagged and GST-tagged SH2B1β were described previously (11,75). To generate a series of truncated SH2B1β mutants, restriction sites were inserted at appropriate positions in myc-tagged or GST- tagged SH2B1β by using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Mutations were verified by DNA sequencing. cDNA encoding GFP-VASP was described previously (42). Porcine GH and human PRL were purchased from the National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases (Dr. Parlow). Polyclonal antibodies (AB) raised against SH2B1 (7) were provided by Dr. Carter-Su and used for immunoprecipitation and immunoblotting. α-JAK2 serum was provided by Dr. Carter-Su (76) and used for immunoprecipitation. Monoclonal α-JAK2 AB (AHO1352, clone 691R5; Biosource, Camarillo, CA) and α-PY (clone 4G10; Upstate Biotechnology, Inc., Lake Placid, NY) were used for immunoblotting. α-myc AB (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and phalloidin-rhodamine, phalloidin-AlexaFluor488, and phalloidin-AlexaFluor 647 (Invitrogen, Carlsbad, CA) were used for immunocytochemistry. The stocks of mouse 3T3 F442A fibroblasts were provided by Dr. Green (Harvard University, Cambridge, MA). The MVD7−/− cells, derived from embryonic fibroblasts taken from VASP/Mena double-knockout mice, and MVD7−/− reexpressing GFP-VASP (41) were provided by Dr. Gertler (Massachusetts Institute of Technology, Boston, MA). T47D cells were provided by Dr. Ethier (Karmanos Cancer Institute, Detroit, MI).
Immunocytochemistry
For localization studies, 3T3 F442A, MVD7−/−, or MVD7−/− cells stably expressing GFP-VASP were transfected with cDNA encoding myc-tagged versions of SH2B1β and GFP-VASP if indicated using the Amaxa method (Lonza Cologne AG, Cologne, Germany) according to the manufacturer’s protocol. The cells were replated on coverslips and processed for immunocytochemistry. The coverslips were fixed (25) and incubated with α-myc followed by goat-α-mouse-AlexaFluor 594 or goat-α-mouse-AlexaFluor 488 (Invitrogen). Staining by secondary antibody reagent alone was negligible (not shown). The actin was stained by phalloidin-rhodamine, phalloidin-AlexaFluor 488, or phalloidin-AlexaFluor 647. Confocal imaging was performed with an Olympus 1X70 laser scanning confocal microscope.
Actin preparation and cosedimentation assay
Rabbit skeletal muscle actin (Cytoskeleton Inc., Denver, CO) was subjected to an actin polymerization-depolymerization cycle before use in experiments. G-actin was polymerized by addition of 50 mm KCl, 2 mm MgCl2, and 1 mm ATP. After polymerization, F-actin was centrifuged at 150,000 × g for 90 min, the pellet was resuspended in G-buffer [2 mm Tris-HCl (pH 8.5), 0.2 mm CaCl2, 0.2 mm ATP, and 0.5 mm dithiothreitol], and dialyzed overnight against G-buffer at 4 C. Next, G-actin was spin-clarified at 150,000× g for 1 h to remove degraded actin. G-actin was polymerized as above. After polymerization, the F-actin was centrifuged at 150,000 × g for 90 min, and the pellet was resuspended in F-buffer [10 mm Tris-HCl (pH 8.0), 50 mm KCl, 2 mm MgCl2, 1 mm ATP, and 0.2 mm CaCl2], containing 0.05% sodium azide, and stored at a concentration of 140 μm at 4 C. For experiments with EGTA, F-actin after polymerization was resuspended and dialyzed against an EGTA-G-buffer [2 mm Tris-HCl (pH 8.0), 0.1 mm ATP, 0.2 mm EGTA, 0.05 mm MgCl2, and 0.1 mm dithiothreitol], polymerized as described above, pelleted, and finally resuspended in EGTA-F-buffer [10 mm Tris-HCl (pH 8.0), 50 mm KCl, 2 mm MgCl2, 1 mm ATP, and 0.2 mm EGTA].
GST or GST-tagged WT SH2B1β or SH2B1β mutants were purified using a glutathione-agarose affinity column (Sigma-Aldrich, St. Louis, MO). The purity of the eluted proteins was monitored by SDS-PAGE. Before performing cosedimentation assays, GST-tagged WT SH2B1β or SH2B1β mutants were centrifuged at 150,000 × g for 2 h to remove any insoluble protein aggregates. For cosedimentation assays, increasing amounts (0.5–28 μm) of GST-tagged WT SH2B1β or mutants were incubated with 4 μm F-actin for 30 min at 24 C in F-buffer and then sedimented at 150,000 × g for 2 h at 24 C. Supernatants were separated from pellets, and pellets were resuspended in F-buffer. Equivalent amounts of supernatant and pellet fractions were subjected to SDS-PAGE. Gels were stained with Coomassie blue, and protein bands were quantified using Bio-Rad (Hercules, CA) Quantity One software. The amount of WT GST-SH2B1β or GST- SH2B1β mutants bound to F-actin was calculated from the known concentrations of proteins in the assay and the ratios of each protein in the supernatant and pellet. The results were plotted to indicate the concentration of WT GST-SH2B1β or GST- SH2B1β mutants vs. amount bound to 4 μm F-actin.
Low-speed centrifugation
For low-speed pelleting assay, 0.1 μm GST-SH2B1β WT or GST- SH2B1β mutants were incubated with 8 μm F-actin for 30 min at 24 C in F-buffer and then sedimented at 16,000 × g for 1 h at 24 C. Supernatants were separated from pellets, and pellets were resuspended in F-buffer. Both supernatant and pellet fractions were subjected to SDS-PAGE. Gels were stained with Coomassie blue, and actin bands were quantified as described above. The results were plotted to indicate the percentage of actin bundled/cross-linked (pellet fraction) or not bundled/cross-linked (supernatant fraction) for each GST, GST-SH2B1β WT, or GST- SH2B1β mutant.
Electron microscopic analysis
To test actin bundling/cross-linking activity, 15 μm F-actin was incubated with either 1 μm GST-SH2B1β, 1 μm GST-SH2B1β (Δ-Δ), or 1 μm GST in F-buffer for 30 min at 24 C. After centrifugation at 16,000 × g for 1 h at 24 C, pellets, if present, were dissolved in F- buffer and immediately applied to poly-lysine-coated glow-discharge carbon grids. The actin on grids was fixed with 2.5% glutaraldehyde and stained with 1% phosphotungstic acid, each for 1 min. Electron microscopy was performed with a Zeiss EM10 electron microscope at 60 kV.
Coimmunoprecipitation and immunoblotting
T47D cells were serum deprived for 72 h and JAK2 or SH2B1β were immunoprecipitated from cell lysates using α-JAK2 or α-SH2B1β, respectively, and protein A-agarose. Proteins were resolved by SDS-PAGE followed by immunoblotting with the indicated antibodies. For some experiments, T47D cells were transiently transfected with cDNA encoding SH2B1β using the Amaxa method and processed as above. Nitrocellulose patterns were scanned, and the amount of protein was quantified using Multi-Analyst (Bio-Rad) software. Amount of phosphorylated protein was then normalized with the total amount of immunoprecipitated protein for each lane.
Assessment of membrane ruffling
To measure the effect of SH2B1β mutants on membrane ruffling, cells expressing the indicated proteins were deprived of serum and treated as indicated in the figure legends. Cells were rapidly rinsed three times with PBS [10 mm sodium phosphate (pH 7.4), 140 mm NaCl] and fixed for 30 min at room temperature in 4% formaldehyde in PBS. Cell were permeabilized with 0.1% Triton X-100 in PBS for 15 min, rinsed three times with PBS, and incubated with α-myc followed by goat-α-mouse-AlexaFluor 488. F-actin was stained with phalloidin-rhodamine. Transfected cells expressing myc-tagged forms of SH2B1β were located with a fluorescein isothiocyanate (FITC) filter set using a Zeiss Axiovert 200 microscope. The number of ruffles per transfected cell was determined. Only cells with similar levels of GFP or myc-tagged indicated forms of SH2B1β expression were scored. Each transfection was repeated at least three times with similar results.
Phagokinetic assay
For the phagokinetic assay, 3T3 F442A cells were transfected with cDNA encoding the indicated proteins using the Amaxa method and plated on colloid gold-covered coverslips 24 h after transfection (25,77). Cells remove particles while they move, thereby producing areas that are free of colloid gold. The incubation time (7 h) was experimentally determined to avoid the overlapping of the particle-free areas produced by neighbor cells. After 7 h, the coverslips were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100 for 15 min, and incubated with α-myc followed by goat-α-mouse-AlexaFluor 488. F-actin was stained with phalloidin-rhodamine. Individual transfected cells were located with a FITC filter set using a Zeiss Axiovert 200 microscope. Only cells with similar levels of GFP or myc-tagged indicated forms of SH2B1β expression were scored. Differential interference contrast images were collected, and particle-free areas were quantified using Image Tool software. The particle-free area was measured in three independent experiments. The phagokinetic index was calculated as a ratio of particle-free to cross-sectional area of the cell. The images presented in the figure are representative of three independent experiments.
Acknowledgments
We thank Drs. Carter-Su and Argetsinger (University of Michigan, Ann Arbor, MI) for providing the cDNAs encoding WT SH2B1β, α-JAK2, and α-SH2B serums and for their helpful advice. We are grateful to Dr. Gertler (Massachusetts Institute of Technology, Boston, MA) for sending MVD7−/− and MVD7−/− stably overexpressing GFP-VASP cells and to Dr. Ethier (Karmanos Cancer Institute, Detroit, MI) for providing T47D cells. We thank Drs. Heckman and Cayer (Bowling Green State University, Bowling Green, OH) for help with electron microscopy. We are grateful to Dr. Way (Cancer Research UK, London Research Institute, London, UK) and Dr. Svitkina (University of Pennsylvania, Philadelphia, PA) for very helpful discussion and Dr. Svitkina for sending actin. We thank Drs. Leaman, Vestal, and Komuniecki (University of Toledo, OH) for helpful discussion and for comments on the manuscript.
Footnotes
This work was supported by grants from the National Institutes of Health (R21 DK074689 and R15 CA135378 (both for M.D.).
Current address for J.L.: Department of Biomedical Engineering, Johns Hopkins University, Baltimore, Maryland 21218.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 2, 2009
Abbreviations: AB, Antibody; F-actin, filamentous actin; FITC, fluorescein isothiocyanate; GFP, green fluorescent protein; GST, glutathione S-transferase; JAK, Janus tyrosine kinase; PDGF, platelet-derived growth factor; PH, pleckstrin homology; PRL, prolactin; α-PY, α-phosphotyrosine; SH2, Src homology 2; STAT, signal transducer and activator of transcription; VASP, vasodilator-stimulated phosphoprotein; WT, wild type.
References
- Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ 2005 Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol 12:814–821 [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Saharinen P, Pesu M, Holt 3rd VE, Silvennoinen O, O'Shea JJ 2004 The Janus kinases (Jaks). Genome Biol 5:253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ, O'Shea JJ 1998 Jaks and STATs: biological implications. Annu Rev Immunol 16:293–322 [DOI] [PubMed] [Google Scholar]
- Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL 2005 Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol 345:1–20 [DOI] [PubMed] [Google Scholar]
- Darnell Jr JE, Kerr IM, Stark GR 1994 Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415–1421 [DOI] [PubMed] [Google Scholar]
- Ingley E, Klinken SP 2006 Cross-regulation of JAK and Src kinases. Growth Factors 24:89–95 [DOI] [PubMed] [Google Scholar]
- Rui L, Mathews LS, Hotta K, Gustafson TA, Carter-Su C 1997 Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol 17:6633–6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maures TJ, Kurzer JH, Carter-Su C 2007 SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab 18:38–45 [DOI] [PubMed] [Google Scholar]
- Yousaf N, Deng Y, Kang Y, Riedel H 2001 Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J Biol Chem 276:40940–40948 [DOI] [PubMed] [Google Scholar]
- Nelms K, O'Neill TJ, Li S, Hubbard SR, Gustafson TA, Paul WE 1999 Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm Genome 10:1160–1167 [DOI] [PubMed] [Google Scholar]
- Rui L, Carter-Su C 1999 Identification of SH2-Bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA 96:7172–7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Werner ED, Oh BC, Frantz JD, Dhe-Paganon S, Hansen L, Lee J, Shoelson SE 2005 Kinase activation through dimerization by human SH2-B. Mol Cell Biol 25:2607–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhou Y, Carter-Su C, Myers Jr MG, Rui L 2007 SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol 21:2270–2281 [DOI] [PubMed] [Google Scholar]
- Rui L, Carter-Su C 1998 Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bβ with PDGF receptor and phosphorylation of SH2-Bβ. J Biol Chem 273:21239–21245 [DOI] [PubMed] [Google Scholar]
- Riedel H, Yousaf N, Zhao Y, Dai H, Deng Y, Wang J 2000 PSM, a mediator of PDGF-BB-, IGF-I-, and insulin-stimulated mitogenesis. Oncogene 19:39–50 [DOI] [PubMed] [Google Scholar]
- Wang J, Riedel H 1998 Insulin-like growth factor-I receptor and insulin receptor association with a Src homology-2 domain-containing putative adapter. J Biol Chem 273:3136–3139 [DOI] [PubMed] [Google Scholar]
- Rui L, Herrington J, Carter-Su C 1999 SH2-B is required for nerve growth factor-induced neuronal differentiation. J Biol Chem 274:10590–10594 [DOI] [PubMed] [Google Scholar]
- Qian X, Riccio A, Zhang Y, Ginty DD 1998 Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21:1017–1029 [DOI] [PubMed] [Google Scholar]
- Kotani K, Wilden P, Pillay TS 1998 SH2-Bα is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem J 335(Pt 1):103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M, Wang CS, Donoghue DJ 2002 Interaction of fibroblast growth factor receptor 3 and the adapter protein SH2-B. A role in STAT5 activation. J Biol Chem 277:15962–15970 [DOI] [PubMed] [Google Scholar]
- Ohtsuka S, Takaki S, Iseki M, Miyoshi K, Nakagata N, Kataoka Y, Yoshida N, Takatsu K, Yoshimura A 2002 SH2-B is required for both male and female reproduction. Mol Cell Biol 22:3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Yang H, White MF, Rui L 2004 Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol 24:7435–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Li M, Duan C, Rui L 2005 Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab 2:95–104 [DOI] [PubMed] [Google Scholar]
- Herrington J, Diakonova M, Rui L, Gunter DR, Carter-Su C 2000 SH2-B is required for growth hormone-induced actin reorganization. J Biol Chem 275:13126–13133 [DOI] [PubMed] [Google Scholar]
- Diakonova M, Gunter DR, Herrington J, Carter-Su C 2002 SH2-Bβ is a Rac-binding protein that regulates cell motility. J Biol Chem 277:10669–10677 [DOI] [PubMed] [Google Scholar]
- Diakonova M, Helfer E, Seveau S, Swanson JA, Kocks C, Rui L, Carlier MF, Carter-Su C 2007 Adapter protein SH2-Bβ stimulates actin-based motility of Listeria monocytogenes in a vasodilator-stimulated phosphoprotein (VASP)-dependent fashion. Infect Immun 75:3581–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Li Y, Schembri-King J, Jakes S, Hayashi J 2000 Identification of actin binding protein, ABP-280, as a binding partner of human Lnk adaptor protein. Mol Immunol 37:603–612 [DOI] [PubMed] [Google Scholar]
- Iseki M, Kubo C, Kwon SM, Yamaguchi A, Kataoka Y, Yoshida N, Takatsu K, Takaki S 2004 Increased numbers of B-1 cells and enhanced responses against TI-2 antigen in mice lacking APS, an adaptor molecule containing PH and SH2 domains. Mol Cell Biol 24:2243–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo-Akashi C, Iseki M, Kwon SM, Takizawa H, Takatsu K, Takaki S 2004 Roles of a conserved family of adaptor proteins, Lnk, SH2-B, and APS, for mast cell development, growth, and functions: APS-deficiency causes augmented degranulation and reduced actin assembly. Biochem Biophys Res Commun 315:356–362 [DOI] [PubMed] [Google Scholar]
- Yabana N, Shibuya M 2002 Adaptor protein APS binds the NH2-terminal autoinhibitory domain of guanine nucleotide exchange factor Vav3 and augments its activity. Oncogene 21:7720–7729 [DOI] [PubMed] [Google Scholar]
- Barrès R, Gonzalez T, Le Marchand-Brustel Y, Tanti JF 2005 The interaction between the adaptor protein APS and Enigma is involved in actin organisation. Exp Cell Res 308:334–344 [DOI] [PubMed] [Google Scholar]
- Barrès R, Grémeaux T, Gual P, Gonzalez T, Gugenheim J, Tran A, Le Marchand-Brustel Y, Tanti JF 2006 Enigma interacts with adaptor protein with PH and SH2 domains to control insulin-induced actin cytoskeleton remodeling and glucose transporter 4 translocation. Mol Endocrinol 20:2864–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ES, Rouiller I, Hanein D, Higgs HN 2006 Mechanistic differences in actin bundling activity of two mammalian formins, FRL1 and mDia2. J Biol Chem 281:14383–14392 [DOI] [PubMed] [Google Scholar]
- Steketee MB, Tosney KW 2002 Three functionally distinct adhesions in filopodia: shaft adhesions control lamellar extension. J Neurosci 22:8071–8083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG 2003 Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol 160:409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G, Leopardi S, Printup S, Madden BC 2002 Filopodia are conduits for melanosome transfer to keratinocytes. J Cell Sci 115:1441–1451 [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM 1998 Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci 18:8900–8911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LM, Gates MA, Witke W, Menzies AS, Wehman AM, Macklis JD, Kwiatkowski D, Soriano P, Gertler FB 1999 Mena is required for neurulation and commissure formation. Neuron 22:313–325 [DOI] [PubMed] [Google Scholar]
- Rottner K, Behrendt B, Small JV, Wehland J 1999 VASP dynamics during lamellipodia protrusion. Nat Cell Biol 1:321–322 [DOI] [PubMed] [Google Scholar]
- Applewhite DA, Barzik M, Kojima S, Svitkina TM, Gertler FB, Borisy GG 2007 Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol Biol Cell 18:2579–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Loureiro JJ, Libova I, FässlerR, Wehland J, Gertler FB 2000 Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101:717–728 [DOI] [PubMed] [Google Scholar]
- Geese M, Loureiro JJ, Bear JE, Wehland J, Gertler FB, Sechi AS 2002 Contribution of Ena/VASP proteins to intracellular motility of listeria requires phosphorylation and proline-rich core but not F-actin binding or multimerization. Mol Biol Cell 13:2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro JJ, Rubinson DA, Bear JE, Baltus GA, Kwiatkowski AV, Gertler FB 2002 Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/vasodilator-stimulated phosphoprotein (VASP) function during cell migration. Mol Biol Cell 13:2533–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus MV, Reilly SC, Clevenger CV 1999 Prolactin as a chemoattractant for human breast carcinoma. Endocrinology 140:5447–5450 [DOI] [PubMed] [Google Scholar]
- Campbell GS, Argetsinger LS, Ihle JN, Kelly PA, Rillema JA, Carter-Su C 1994 Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci USA 91:5232–5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun JJ, Ali S, Sofer L, Ullrich A, Kelly PA 1994 Prolactin-induced proliferation of Nb2 cells involves tyrosine phosphorylation of the prolactin receptor and its associated tyrosine kinase JAK2. J Biol Chem 269:14021–14026 [PubMed] [Google Scholar]
- Rui H, Lebrun JJ, Kirken RA, Kelly PA, Farrar WL 1994 JAK2 activation and cell proliferation induced by antibody-mediated prolactin receptor dimerization. Endocrinology 135:1299–1306 [DOI] [PubMed] [Google Scholar]
- Duan C, Li M, Rui L 2004 SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem 279:43684–43691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedermann CJ, Reinisch N, Braunsteiner H 1993 Stimulation of monocyte chemotaxis by human growth hormone and its deactivation by somatostatin. Blood 82:954–960 [PubMed] [Google Scholar]
- Goh EL, Pircher TJ, Wood TJ, Norstedt G, Graichen R, Lobie PE 1997 Growth hormone-induced reorganization of the actin cytoskeleton is not required for STAT5 (signal transducer and activator of transcription-5)-mediated transcription. Endocrinology 138:3207–3215 [DOI] [PubMed] [Google Scholar]
- Reddy GR, Pushpanathan MJ, Ransom RF, Holzman LB, Brosius 3rd FC, Diakonova M, Mathieson P, Saleem MA, List EO, Kopchick JJ, Frank SJ, Menon RK 2007 Identification of the glomerular podocyte as a target for growth hormone action. Endocrinology 148:2045–2055 [DOI] [PubMed] [Google Scholar]
- Cameron LA, Svitkina TM, Vignjevic D, Theriot JA, Borisy GG 2001 Dendritic organization of actin comet tails. Curr Biol 11:130–135 [DOI] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P 2008 Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 9:446–454 [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB 2007 Filopodia: the fingers that do the walking. Sci STKE 2007:re5 [DOI] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H 2005 The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol 15:129–133 [DOI] [PubMed] [Google Scholar]
- Berg JS, Cheney RE 2002 Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol 4:246–250 [DOI] [PubMed] [Google Scholar]
- Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J 2005 The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol 7:619–625 [DOI] [PubMed] [Google Scholar]
- Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T 2007 Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol 5:e317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Stradal T, Vignal E, Rottner K 2002 The lamellipodium: where motility begins. Trends Cell Biol 12:112–120 [DOI] [PubMed] [Google Scholar]
- Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl UD, Walter U, Gertler FB, Wehland J, Chakraborty T 1997 A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J 16:5433–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Theriot JA, Portnoy DA 1996 The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J Cell Biol 135:647–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner MP, Urbanke C, Barzik M, Otten S, Sechi AS, Wehland J, Heinz DW 2001 ActA from Listeria monocytogenes can interact with up to four Ena/VASP homology 1 domains simultaneously. J Biol Chem 276:40096–40103 [DOI] [PubMed] [Google Scholar]
- Ball LJ, Kühne R, Hoffmann B, Häfner A, Schmieder P, Volkmer-Engert R, Hof M, Wahl M, Schneider-Mergener J, Walter U, Oschkinat H, Jarchau T 2000 Dual epitope recognition by the VASP EVH1 domain modulates polyproline ligand specificity and binding affinity. EMBO J 19:4903–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ 1994 Membrane ruffling and signal transduction. Bioessays 16:321–327 [DOI] [PubMed] [Google Scholar]
- Borm B, Requardt RP, Herzog V, Kirfel G 2005 Membrane ruffles in cell migration: indicators of inefficient lamellipodia adhesion and compartments of actin filament reorganization. Exp Cell Res 302:83–95 [DOI] [PubMed] [Google Scholar]
- O'Brien KB, Argetsinger LS, Diakonova M, Carter-Su C 2003 YXXL motifs in SH2-Bβ are phosphorylated by JAK2, JAK1, and platelet-derived growth factor receptor and are required for membrane ruffling. J Biol Chem 278:11970–11978 [DOI] [PubMed] [Google Scholar]
- Reinhard M, HalbrüggeM, Scheer U, Wiegand C, Jockusch BM, Walter U 1992 The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J 11:2063–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D, Rotin D, Batzer A, Mandiyan V, Schlessinger J 1993 SH3 domains direct cellular localization of signaling molecules. Cell 74:83–91 [DOI] [PubMed] [Google Scholar]
- Bretscher A 1989 Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol 108:921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad PA, Giuliano KA, Fisher G, Collins K, Matsudaira PT, Taylor DL 1993 Relative distribution of actin, myosin I, and myosin II during the wound healing response of fibroblasts. J Cell Biol 120:1381–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilainen J, Palovuori R, Sormunen R, Wasenius VM, Lehto VP 1993 Binding of the α-fodrin SH3 domain to the leading lamellae of locomoting chicken fibroblasts. J Cell Sci 105(Pt 3):647–654 [DOI] [PubMed] [Google Scholar]
- Wu H, Parsons JT 1993 Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol 120:1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF 1996 Cell migration: a physically integrated molecular process. Cell 84:359–369 [DOI] [PubMed] [Google Scholar]
- Hou SX, Zheng Z, Chen X, Perrimon N 2002 The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev Cell 3:765–778 [DOI] [PubMed] [Google Scholar]
- Rui L, Gunter DR, Herrington J, Carter-Su C 2000 Differential binding to and regulation of JAK2 by the SH2 domain and N-terminal region of SH2-Bβ. Mol Cell Biol 20:3168–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C 1993 Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler G 1977 The phagokinetic tracks of 3T3 cells. Cell 11:395–404 [DOI] [PubMed] [Google Scholar]