Abstract
Activating signal cointegrator-2 (ASC-2) functions as a transcriptional coactivator of many nuclear receptors and also plays important roles in the physiology of the liver and pancreas by interacting with liver X receptors (LXRs), which antagonize the development of atherosclerosis. This study was undertaken to establish the specific function of ASC-2 in macrophages and atherogenesis. Intriguingly, ASC-2 was more highly expressed in macrophages than in the liver and pancreas. To inhibit LXR-specific activity of ASC-2, we used DN2, which contains the C-terminal LXXLL motif of ASC-2 and thereby acts as an LXR-specific, dominant-negative mutant of ASC-2. In DN2-overexpressing transgenic macrophages, cellular cholesterol content was higher and cholesterol efflux lower than in control macrophages. DN2 reduced LXR ligand-dependent increases in the levels of ABCA1, ABCG1, and apolipoprotein E (apoE) transcripts as well as the activity of luciferase reporters driven by the LXR response elements (LXREs) of ABCA1, ABCG1, and apoE genes. These inhibitory effects of DN2 were reversed by overexpression of ASC-2. Chromatin immunoprecipitation analysis demonstrated that ASC-2 was recruited to the LXREs of the ABCA1, ABCG1, and apoE genes in a ligand-dependent manner and that DN2 interfered with the recruitment of ASC-2 to these LXREs. Furthermore, low-density lipoprotein receptor (LDLR)-null mice receiving bone marrow transplantation from DN2-transgenic mice showed accelerated atherogenesis when administered a high-fat diet. Taken together, these results indicate that suppression of the LXR-specific activity of ASC-2 results in both defective cholesterol metabolism in macrophages and accelerated atherogenesis, suggesting that ASC-2 is an antiatherogenic coactivator of LXRs in macrophages.
Suppression of the LXR-specific activity of ASC-2 results in accelerated atherogenesis, suggesting that ASC-2 is an anti-atherogenic coactivator of LXRs in macrophages.
Atherosclerosis, the leading cause of mortality in developed countries, is a form of chronic inflammation (1) resulting from interactions between modified lipoproteins, monocyte- derived macrophages, T lymphocytes, and normal cellular elements of the arterial wall. In particular, invasion of monocytes into the arterial wall and subsequent differentiation into cholesterol-laden macrophages, known as foam cells, is a central event in atherogenesis (2). An imbalance between uptake and disposal of cellular cholesterol is inherent to this process. Consistent with this finding, elevated serum cholesterol is sufficient to promote atherosclerosis development, even in the absence of other known risk factors. Excess cholesterol must be either stored to form foam cells or eliminated through reverse cholesterol transport (RCT). In the latter case, ATP-binding cassette (ABC) membrane transporters facilitate the transfer of excess intracellular cholesterol to extracellular acceptors, such as high-density lipoprotein (HDL) particles. The importance of RCT is underscored by the existence of Tangier disease, a rare genetic form of HDL deficiency caused by mutations in the ABCA1 gene; patients with the disease show increased risk of atherosclerosis.
The liver X receptors, LXRα and LXRβ, are members of the nuclear receptor superfamily that control cholesterol and fatty acid metabolism (3,4). LXRα is abundantly expressed in the liver, intestine, adipose tissue, and macrophages, whereas LXRβ is ubiquitously expressed at a lower level (5). The physiological ligands for LXRs are oxysterols, including 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol, and 24(S),25-epoxycholesterol. Activation of LXRs in macrophages results in increased expression of genes encoding the ABC cholesterol transporters (ABCA1 and ABCG1) and apolipoprotein E (apoE), which are involved in cholesterol efflux from macrophages to HDLs. Thus, LXRs are promising target proteins for the development of antiatherogenic drugs (3,5,6,7).
The nuclear receptor superfamily is a group of proteins that regulate, in a ligand-dependent manner, transcriptional initiation of target genes by binding to specific DNA sequences called hormone response elements (8). Genetic studies indicated that transcriptional coactivators without specific DNA-binding activity are essential for transcriptional activation. This led to the identification of many proteins interacting with the C-terminal ligand-dependent transactivation domain of nuclear receptors (9). These coactivators, including the p160 family, CBP/p300, p/CAF, TRAP/DRIP, and many others, bridge transcription factors and the basal transcription apparatus and/or remodel chromatin structures.
Activating signal cointegrator-2 (ASC-2) is a transcriptional coactivator, also known as AIB3, TRBP, RAP250, NRC, PRIP, and NCoA6 (10,11,12,13,14,15,16,17,18). The ASC-2 gene is amplified and overexpressed in human cancers, and the protein stimulates transactivation by many members of the nuclear receptor superfamily, AP-1, NFκB, SRF, and numerous other transcription factors. Interestingly, ASC-2 contains two nuclear receptor-interaction domains (NR boxes), and the function of both depends on the integrity of their core LXXLL sequences (13). The C-terminal LXXLL motif specifically interacts with LXRα and LXRβ, whereas the N-terminal motif binds to a broad range of nuclear receptors (13). Furthermore, the short fragments containing each NR box, DN1 and DN2, function as specific dominant-negative mutants of nuclear receptors interacting with each NR box. DN1 and DN2 are also specific to ASC-2 and therefore do not compete with other LXXLL-type coactivators, such as steroid receptor coactivator 1 (SRC-1) and thyroid hormone receptor-associated protein 220 (TRAP220) (19,20).
In previous studies, we demonstrated that ASC-2 acts as a crucial transcriptional coactivator in the physiological functioning of the liver and pancreas by interacting with LXRs (20,21). Because LXRs have also been identified as inhibitors of atherosclerosis (3,5,6,7), we investigated here the possible role of ASC-2 in macrophages and atherogenesis. Our results indicate that ASC-2 regulates cholesterol efflux and atherogenesis. We further show that the antiatherogenic function of ASC-2 is at least in part mediated through the LXR response elements (LXREs) in the LXR target genes ABCA1, ABCG1, and apoE. Overall, our study identifies ASC-2 as an antiatherogenic transcriptional coactivator of LXRs in macrophages.
Results
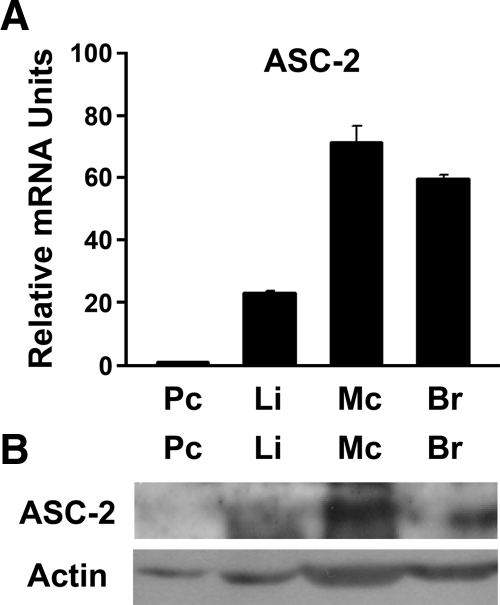
ASC-2 is highly expressed in macrophages
ASC-2 was shown to be differentially expressed in different tissues (10,22). High levels were detected in reproductive organs, such as ovary, testis, and prostate, as well as in peripheral blood leukocytes, brain, and heart. Intermediate levels were observed in pancreas and liver, and ASC-2 has been shown to play a metabolic function in these organs. However, no information is available on ASC-2 levels in macrophages, which are required for the formation of atherosclerotic lesions. Therefore, we determined the transcript level of ASC-2 in macrophages in comparison with other tissues using quantitative RT-PCR (QPCR). As shown in Fig. 1A, macrophages expressed higher levels of transcript than liver, pancreas, or brain. High expression levels of the ASC-2 protein were also observed in macrophages (Fig. 1B). Given the clear impact of ASC-2 on LXR transactivation (20) and the prominent expression of ASC-2 in macrophages, we decided to investigate the function of ASC-2 in macrophages and atherosclerosis.
Figure 1.
Abundant expression of ASC-2 in peritoneal macrophages. A, mRNA levels of ASC-2 were determined in several tissues. Total RNAs were extracted from pooled tissues (n = 3–5) and used for reverse transcription and subsequent QPCR. Results are presented as relative values to the ASC-2 mRNA level in the pancreas. B, ASC-2 protein levels were compared by immunoblot analysis. Br, Brain; Li, liver; Mc, macrophage; Pc, pancreas.
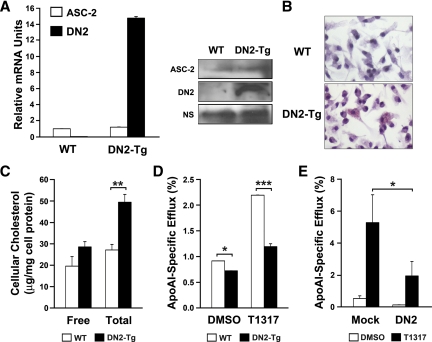
Cellular cholesterol content and cholesterol efflux in DN2-transgenic (Tg) macrophages
For this study, we isolated peritoneal macrophages from DN2-overexpressing Tg mice (20). Before using these macrophages, we examined the expression level of DN2 in DN2-Tg peritoneal macrophages using QPCR and immunoblot analysis. DN2 was overexpressed in macrophages isolated from DN2-Tg mice (Fig. 2A). In contrast, the expression level of ASC-2 was not altered in DN2-Tg macrophages (Fig. 2A). Next, to explore the cellular effects of ASC-2 on cholesterol metabolism in macrophages, we stained peritoneal macrophages isolated from DN2-Tg and wild-type mice with oil red O. Microscopic examination revealed a significant accumulation of oil red O-positive droplets in DN2-Tg macrophages, which indicates lipid accumulation (Fig. 2B). Little or no oil red O staining was observed in wild-type macrophages. Quantitative analysis of cholesterol levels showed a significantly higher level of total cholesterol in DN2-Tg macrophages than in control macrophages (Fig. 2C).
Figure 2.
Cholesterol content and efflux in DN2-Tg macrophages. A, Overexpression of DN2 in DN2-Tg macrophages. Total RNAs were extracted from pooled macrophages (n = 3) and used for reverse transcription and subsequent QPCR specifically for DN2 and ASC-2 transcripts. Protein levels of ASC-2 and DN2 were compared by immunoblot analysis. Nonspecific (NS) bands were used as a loading control. WT, Wild type. B, Oil red O-stained peritoneal macrophages from DN2-Tg mice. C, Total and free cellular cholesterol content of DN2-Tg peritoneal macrophages. D, Stimulation of cholesterol efflux by LXR ligand was lower in DN2-Tg macrophages than in control macrophages. DMSO, Dimethylsulfoxide. E, Stimulation of cholesterol efflux by LXR ligand was lower in DN2-transfected acLDL-laden foam cells than in pcDNA3-transfected foam cells. ApoAI-dependent cholesterol efflux into the medium was determined as described in Materials and Methods. Data are presented as percentages of total radioactivity in cells and medium. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
This increase in cellular cholesterol may have resulted from defective efflux. To test this possibility, apoAI-specific cholesterol efflux assays were performed. Peritoneal macrophages isolated from DN2-Tg mice were labeled for 24 h with [3H]cholesterol in the presence of an acyl-CoA: cholesterol acyltransferase (ACAT) inhibitor. This compound prevents esterification of cholesterol, resulting in a free cholesterol pool with high specific activity. As shown in Fig. 2D, under these conditions, cholesterol efflux from wild-type macrophages to apoAI was significantly enhanced when cells were treated with the synthetic LXR ligand, T0901317. However, cholesterol efflux rates were decreased in DN2-Tg macrophages. DN2 overexpression also repressed cholesterol efflux from acLDL-laden foam cells (Fig. 2E). These results clearly indicate that the LXR-specific activity of ASC-2 is necessary for the cholesterol efflux process.
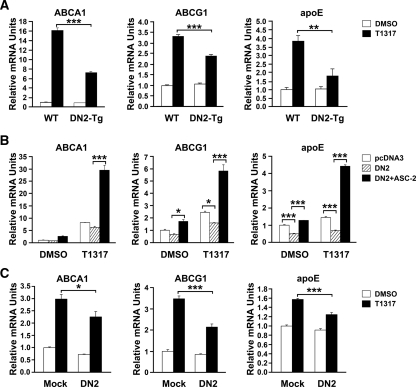
Effects of DN2 on the transcript levels of LXR target genes ABCA1, ABCG1, and apoE
To define the specific roles of ASC-2 in regulating the LXR target genes of macrophages, we first investigated the ability of the synthetic LXR ligand to modulate expression of ABCA1, ABCG1, and apoE in peritoneal macrophages obtained from DN2-Tg and wild-type mice. Treatment with T0901317 resulted in marked increases of ABCA1, ABCG1, and apoE mRNA expression in wild-type cells. In contrast, T0901317-induced expression of these genes was significantly lower in macrophages derived from DN2-Tg mice (Fig. 3A). These effects of DN2 on LXR target genes were also observed in DN2-transfected macrophages (Fig. 3B). Importantly, expression of ASC-2 restored the expression of ABCA1, ABCG1, and apoE that had been blocked by DN2 (Fig. 3B). These results demonstrate that DN2 acts as a specific dominant-negative mutant of ASC-2 to inhibit the LXR-dependent expression of ABCA1, ABCG1, and apoE in macrophages.
Figure 3.
Regulation of LXR target gene expression in DN2-overexpressing macrophages. A, Peritoneal macrophages were isolated from wild-type (WT) and DN2-Tg mice and treated for 24 h with vehicle [dimethylsulfoxide (DMSO)] or 1 μm T0901317 (T1317). mRNA levels of ABCA1, ABCG1, and apoE were measured using QPCR. B, Peritoneal macrophages were isolated from wild-type mice, transfected with DN2 and ASC-2, and treated with vehicle or T0901317. Total RNA was extracted and subjected to QPCR to assay for levels of ABCA1, ABCG1, and apoE mRNA. C, Peritoneal macrophages were isolated from mice, transfected with DN2 or pcDNA3, and incubated with 50 μg/ml acLDL and 1 μm T0901317 for 24 h. QPCR was performed to measure the levels of ABCA1, ABCG1, and apoE mRNA in acLDL-laden foam cells. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
The uptake of modified lipoproteins into macrophages is the primary reason for foam cell formation in arterial lesions. Thus, we determined the expression levels of LXR target genes in acLDL-laden foam cells, which are thought to be representative of arterial lesions in vivo. As shown in Fig. 3C, the T0901317-dependent expressions of ABCA1, ABCG1, and apoE were inhibited in DN2-transfected acLDL-laden foam cells, compared with empty vector-transfected foam cells.
DN2 decreases recruitment of ASC-2 to the promoter of ABCA1, ABCG1, and apoE
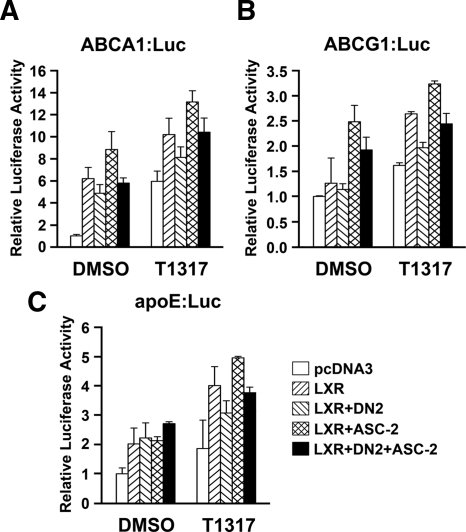
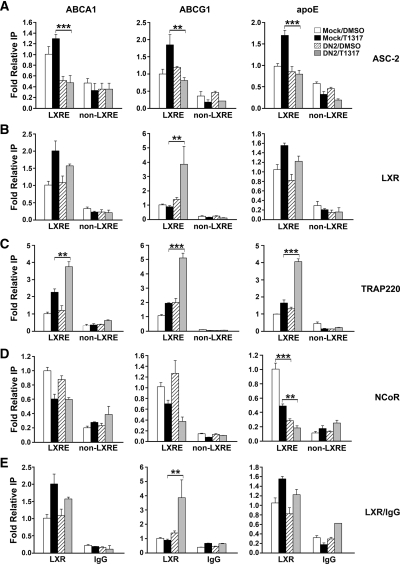
Decreased transcript levels of LXR target genes could result from decreased LXR expression. However, there was no significant change in LXR levels in DN2-overexpressing macrophages (data not shown). We therefore sought to determine the activities of luciferase reporters linked to the LXREs of ABCA1, ABCG1, and apoE upon cotransfection of each reporter with or without DN2 and/or ASC-2. As shown in Fig. 4, the reporter activities of ABCA1:Luc, ABCG1:Luc, and apoE:Luc were decreased in the presence of DN2. In addition, these repressions were relieved by cotransfecting with ASC-2. These data suggest that the reduced transcript levels of ABCA1, ABCG1, and apoE by DN2 are likely due to their reduced promoter activity. To further investigate the mechanism underlying the DN2 effects, we compared ASC-2 recruitment to the LXRE regions of ABCA1, ABCG1, and apoE in the absence and presence of DN2. A chromatin immunoprecipitation (ChIP) analysis of peritoneal macrophage chromatin using an ASC-2 antibody revealed that ASC-2 was recruited to the LXRE region of ABCA1, ABCG1, and apoE promoters in a ligand-dependent manner (Fig. 5A). However, DN2 inhibited the ligand-dependent recruitment of ASC-2 to the LXREs of ABCA1, ABCG1, and apoE (Fig. 5A). In contrast to ASC-2, DN2 overexpression did not lead to significant decreases in the LXR occupancy of these LXREs (Fig. 5B). We also evaluated the recruitment of TRAP220 and NCoR to the LXREs of the ABCA1, ABCG1, and apoE genes (Fig. 5, C and D). The data indicate that although DN2 repressed ABCA1, ABCG1, and apoE expression, DN2 inhibited neither TRAP220 recruitment to LXREs nor NCoR dissociation from the LXREs of these genes. In other words, the ligand-dependent TRAP220 recruitment and NCoR dissociation occurred even in the presence of DN2. The background immunoprecipitated LXRE-containing DNA with preimmune IgG is also shown (Fig. 5E). Taken together, these results suggest that the decreased transcript levels of ABCA1, ABCG1, and apoE by DN2 are due at least in part to the reduced recruitment of ASC-2 to the LXREs of each promoter.
Figure 4.
Regulation of promoter activities of ABCA1 (A), ABCG1 (B), and apoE (C) by ASC-2 in the RAW264.7 cell line. Luciferase reporters containing the LXRE region from ABCA1, ABCG1, or apoE were cotransfected with DN2 and/or ASC-2 into the RAW264.7 cell line. Transfected cells were treated with vehicle [dimethylsulfoxide (DMSO)] or 1 μm T0901317 (T1317). Luciferase activities were measured with a luminometer (Centro LB 960; Berthold Technologies, Oak Ridge, TN) and normalized to cotransfected β-galactosidase activity. The data shown are representative of at least two similar experiments.
Figure 5.
Effects of DN2 on the recruitment of ASC-2, LXR, TRAP220, and NCoR to the LXREs in the ABCA1, ABCG1, and apoE promoters in peritoneal macrophages. Peritoneal macrophages were isolated from thioglycolate-elicited mice and transfected with DN2. After treatment with vehicle or T0901317, ChIP analyses were performed using ASC-2 (A300-411A; Bethyl), LXR (sc-13068; Santa Cruz), TRAP220 (sc-5334; Santa Cruz), or NCoR (sc-1609X; Santa Cruz) antibodies. The background immunoprecipitation with preimmune IgG was also performed. Immunoprecipitated LXRE-containing DNA levels were quantitated using QPCR and normalized to input DNA. As a control, the non-LXRE region of each promoter was amplified using each immunoprecipitate. **, P < 0.005; ***, P < 0.001. DMSO, Dimethylsulfoxide.
ASC-2 as an antiatherogenic coactivator in macrophages
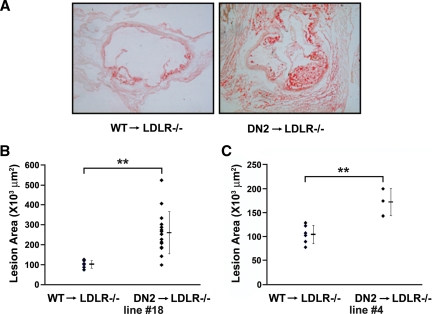
Our results indicating that ASC-2 is important for cholesterol metabolism in macrophages as a coactivator of LXRs prompted us to evaluate the functional role of ASC-2 in atherogenesis using bone marrow transplantation (BMT). Specifically, bone marrow cells from DN2-Tg mice were transplanted into lethally irradiated LDLR−/− mice, a well-characterized murine model of atherosclerosis, to assess the effect of selective inhibition of LXR-specific ASC-2 activity in macrophages. LDLR−/− mice develop atherosclerosis only after administration of a high-fat diet. The requirement for both genetic and environmental risk factors to induce atherosclerosis in LDLR−/− mice reflects an interplay between genetic and lifestyle contributions in human cardiovascular disease. One week after transplantation, mice were placed on an atherogenic diet, which was maintained for an additional 5 wk. To evaluate the extent of atherosclerosis in transplanted mice, aortic valves were sectioned, stained with oil red O, and digitally analyzed to quantify aortic valve lesion areas. As shown in Fig. 6A, oil red O-positive lesions were markedly larger in DN2-Tg BMT recipients, compared with their wild-type counterparts. In Fig. 6B, each symbol represents the mean lesion area of eight sections taken every 36 μm through the aortic valve of a single animal. The mean aortic valve lesion areas were 109,324 ± 32,287 μm2 (n = 6) and 283,363 ± 106,283 μm2 (n = 15) for recipients of wild-type BMT and DN2-Tg BMT, respectively. These values represent a remarkable increase of over 250% in the aortic valve lesion areas in DN2-Tg BMT recipient mice. Our results were independently confirmed using another DN2-Tg line (Fig. 6C) (20). These results reveal that ASC-2 is an inhibitor of atherosclerosis and a crucial determinant of macrophage lipid accumulation and foam cell formation.
Figure 6.
BMT with DN2-Tg bone marrow increases atherosclerosis in LDLR−/− mice. A, Representative oil red O-stained sections of aortic valves from each BMT group. Bright red staining by oil red O of atherosclerotic lesions was observed in cryosections of aortic valves. B and C, Quantification of the size of aortic valve lesion areas. Oil red O-stained lesions were quantified using a computer-assisted video-imaging system. Lesions in six wild-type, 15 DN2-Tg line 18 (B), and three DN2-Tg line 4 (C) mice were measured as shown. DN2, DN2-Tg; WT, wild type. **, P < 0.005.
Discussion
This study was initiated to explore the function of ASC-2 in the cholesterol metabolism of macrophages. Although ASC-2 functions as a transcriptional coactivator for numerous transcription factors, LXRs are the most plausible target transcription factors of ASC-2 in cholesterol efflux from macrophages for two reasons. First, LXRs are nuclear receptors that perform essential functions in the cholesterol efflux of macrophages and atherogenesis. Second, ASC-2 functions as a crucial coactivator of LXRs in several tissues, including liver and β-cells (20,21). The essential role of ASC-2 in LXR transactivation has also been demonstrated in mouse embryonic fibroblasts (MEFs). T0901317 enhances the expression of the LXR target genes SREBP-1c, ABCA1, and ABCG1 in wild-type MEFs but not in ASC-2−/− MEFs (23).
Here, we used DN2-Tg mice to elucidate the role of ASC-2 in macrophages and atherosclerosis. These DN2-Tg mice overexpress DN2 fragments ubiquitously due to the use of actin promoter. To address concerns about the lack of tissue specificity, two strategies were employed. First, all experiments were performed with isolated macrophages, not with DN2-Tg mice themselves. Second, most DN2-Tg effects were reevaluated in DN2-transfected peritoneal macrophages, which were isolated from wild-type mice and transfected in vitro with a DN2 expression vector. As shown in Results, the DN2-Tg effects were reproducible in DN2-transfected macrophages. Therefore, DN2 effects in DN2-Tg macrophages are likely to be attributable to the DN2 expression in the macrophages.
Although the LXXLL motif (NR box) is present in many coactivators, DN1 and DN2, which are two dominant-negative mutants of ASC-2 harboring the N- and C-terminal NR box of ASC-2, respectively, appear to be specific for ASC-2. This finding is supported by ChIP and cotransfection experiments showing that DN1/2-mediated suppression of nuclear receptor transactivation is specifically restored upon coexpression of ASC-2 but not of other LXXLL-type coactivators (SRC-1 and TRAP220) (19,20). The basis for this specificity is not entirely clear at present. However, Acevedo et al. (24) recently provided important evidence showing that information contained in the receptor-coactivator interface allows the receptor to distinguish between the two molecules even though SRCs and TRAP220 share overlapping binding sites on nuclear receptors. In support of this finding, the researchers further identified an estrogen receptor-α (ERα) AF-2 point mutant (L540Q) that selectively binds to and recruits TRAP220 but not SRCs. Their results clearly demonstrate that facilitated recruitment, rather than competition, drives the sequential recruitment of different LXXLL-type coactivator complexes to nuclear receptors.
In contrast to DN1, DN2 displays very specific affinity for LXRs (13,15,25,26). In fact, DN2 contains an unusual LXXLL motif (EAPTSLSQLLDNSGA) that does not resemble any of the motifs identified to date. Previous reports show that the C-terminal NR box does not interact with retinoic acid receptor (RAR), retinoid X receptor (RXR), thyroid hormone receptor (TR), or glucocorticoid receptor (GR) but binds strongly to LXRs (13). Conversely, LXRs do not bind to the N-terminal NR box. The phenotypes of DN2-Tg mice strongly support DN2 specificity for LXRs. DN2-Tg mice are impaired in LXR transactivation, resulting in liver phenotypes that are very similar to those previously observed in LXRα-null mice, including rapid accumulation of large amounts of cholesterol and down-regulation of the known lipid-metabolizing target genes of LXRα in the liver upon administration of a high-cholesterol diet (20). This specificity is further confirmed by recent data obtained by Li et al. (27). This group generated an NCoA6-L2m mutant mouse line in which the C-terminal LXXLL sequence of endogenous ASC-2 is mutated to AXXAL using a targeted knock-in strategy. Through characterization of the NCoA6L2m/L2m mouse model, this group showed that the C-terminal NR box is required for ASC-2 to mediate LXRα-regulated lipogenesis and cholesterol/bile acid homeostasis in the liver but not ERα function in the mammary gland, although the C-terminal NR box binds weakly to ERα.
Using DN2 as a selective inhibitor of LXR-specific activity of ASC-2, we elucidated the role of ASC-2 in the physiology of macrophages and pathophysiology of atherosclerosis. To further understand the molecular mechanism of ASC-2 in the regulation of cholesterol efflux, three genes were chosen for analysis: ABCA1, ABCG1, and apoE. ABCA1 and ABCG1, two members of the ABC transporter family, are significantly induced in lipid-laden macrophages (28,29). Loss-of-function mutations in the ABCA1 gene result in Tangier disease (30,31,32). This phenotype, along with the finding that fibroblasts from Tangier patients are impaired in their ability to donate cholesterol to apoAI, supports that ABCA1 plays a crucial role in regulating cellular cholesterol efflux. On the other hand, a recent study showed that increased expression of ABCG1 in macrophages promotes RCT, whereas knockdown or knockout of ABCG1 significantly reduces RCT in vivo (33). Additionally, apoE functions in cellular cholesterol efflux mechanisms that promote the transfer of excess intracellular cholesterol to extracellular acceptors, such as apoAI, and protects against atherosclerosis (34,35). In particular, expression of ABCA1, ABCG1, and apoE is directly regulated by LXRs (36,37,38). The analyses of these three genes revealed that ASC-2 regulates LXR-dependent transcription of ABCA1, ABCG1, and apoE and in this way promotes cholesterol efflux to the extracellular acceptor, apoAI.
Although many transcription factors are known to be involved in the initiation and progression of atherosclerosis, few studies have described transcriptional coactivators that are essential and selective for atherosclerosis-related transcription factors. Zhang et al. (39) reported that oleic acid and palmitic acid have opposing roles in regulating peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) expression in rat vascular smooth muscle cells (VSMCs), and PGC-1α inhibited oleic acid-induced VSMC proliferation and migration, which contribute significantly to the progression of atherosclerosis. PGC-1α was originally identified as a transcriptional coactivator of peroxisome proliferator-activated receptor-γ. On the other hand, the ER coactivator SRC-3 is reportedly a coactivator for the major VSMC transcription factor myocardin, which is required for VSMC differentiation into the nonproliferative, contractile state (40). Kim et al. (41) demonstrated that adenovirus-mediated overexpression of PGC-1α in human aortic smooth and endothelial cells leads to a significant reduction in intracellular and mitochondrial reactive oxygen species production as well as NAD(P)H oxidase activity. Consequently, nuclear factor-κB activity and monocyte chemoattractant protein 1 and vascular cell adhesion molecule 1 induced by TNFα are suppressed (41). Increased oxidative stress in vascular cells is implicated in the pathogenesis of atherosclerosis. These reports raise the possibility that PGC-1α and SRC-3 may play some roles in the development of atherosclerosis. However, there is no definitive or direct evidence that their activities are necessary for atherogenic or antiatherogenic processes. In contrast, ASC-2 is shown here to have antiatherogenic activity based on BMT from DN2-Tg to LDLR-null mice. Our data suggest that reduced activity of ASC-2 may accelerate the atherogenic process.
To our knowledge, this is the first study that demonstrates the involvement of a specific transcriptional coactivator in atherogenesis. In addition, this study confirms and extends our previous results on the role of ASC-2 in LXR transactivation (13,20,21). In particular, we expand the roles of ASC-2 in lipid homeostasis to include a function as an antiatherogenic coactivator of LXRs in macrophages. Thus, studies on ASC-2 and its complex, ASCOM (42), may open new pathways in the treatment of diseases of lipid homeostasis.
Materials and Methods
QPCR
Total RNA was isolated from peritoneal macrophages, liver, and pancreas using the TRIzol reagent (Invitrogen, Carlsbad, CA), and purified RNA was reverse transcribed (M-MLV reverse transcriptase; Promega, Madison, WI), according to the manufacturer’s protocol. Quantitative gene expression analysis was performed on an ABI PRISM 7000 machine (Applied Biosystems, Foster City, CA) using a SYBR Green PCR master mix. PCR primers were designed using Primer Express 3.0 software with the manufacturer’s default settings and were validated for identical efficiencies. The 18S rRNA was used as the internal control. Ratios of target gene and 18S rRNA expression levels were calculated by subtracting the cycle threshold (Ct) of the target gene from the Ct of 18S rRNA and raising 2 to the power of this difference (43). Ct values are defined as the number of PCR cycles at which the fluorescent signal reaches a fixed threshold (43). Target gene mRNA levels are thus expressed relative to 18S rRNA levels. The oligonucleotide primers used for the different genes were as follows: ASC-2, 5′-ATGGCTTCTGAGAGCGTGGA-3′ and 5′-AGGTCGAGAGGATCTTTTTCGAT-3′; ABCA1, 5′-GACCCGTACTCTCGCAGGG-3′ and 5′-CCTTGCCGGTATTTTAGCAGG-3′; ABCG1, 5′-TTCATCGTCCTGGGCATCTT-3′ and 5′-GAAATAGGCGATGAGCCGC-3′; apoE, 5′-GAGCCGGAGGTGACAGATCA-3′ and 5′-CTCCCAGGGTTGGTTGCTT-3′; and 18S rRNA, 5′-GGGAGCCTGAGAAACGGC-3′ and 5′-GGGTCGGGAGTGGGTAATTTG-3′.
Isolation of mouse peritoneal macrophages
Thioglycolate-elicited peritoneal macrophages were isolated from mice 4 d after peritoneal injection of thioglycolate broth media. Macrophages were stained with oil red O by rinsing adherent cells with 50% isopropanol for 1 min and then with 0.5% oil red O for 5 min. Free and total cholesterol levels were determined by enzymatic assay using supplier’s protocols (Biovision, Mountain View, CA).
ApoAI-dependent cholesterol efflux assay
The cholesterol efflux assay was performed as described earlier, with minor modifications (44). Briefly, cells were plated at 50% confluence in 24-well plates. On d 1, cells were transfected with pcDNA3-HA-DN2 for foam cell formation experiments. On d 2, cells were washed and incubated for 24 h in DMEM supplemented with 10% (vol/vol) lipoprotein-deficient serum (LPDS). Cells were labeled with [3H]cholesterol (1.0 μCi/ml), either in the presence of the ACAT inhibitor (58-035; 2 μg/ml) or with acLDL (50 μg/ml) in the absence of the ACAT inhibitor. T0901317 was added to cells as indicated. On d 3, cells were washed twice with PBS and incubated for 8 h in fresh medium devoid of radiolabeled cholesterol but containing the indicated concentrations of T0901317 and 0.2% (wt/vol) BSA. Cells were rewashed with PBS and incubated in 1 ml DMEM containing 0.2% (wt/vol) BSA in the absence or presence of apoAI (15 μg/ml). After 4 h, the medium was removed and centrifuged at 14,000 × g for 10 min. Radioactivity was determined by liquid scintillation counting (Tri-Carb 3100TR; PerkinElmer Life and Analytical Sciences, Shelton, CT). Cells were dissolved in isopropanol, and an aliquot was removed to determine total cell-associated radioactivity. The apoAI-dependent efflux of radioactive cholesterol from cells into the medium was determined as a percentage of total radioactivity in the cells and medium for each condition. Individual assays were performed in triplicate.
Transient transfection
Transient transfections of mouse peritoneal macrophages were performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Briefly, mouse peritoneal macrophages were seeded at 1 × 106 cells per well in six-well plates the day before transfection using DMEM supplemented with 10% fetal bovine serum (FBS). Each plasmid DNA mixture and Lipofectamine 2000 were diluted with OptiMEM I Medium (Invitrogen) and incubated for 5 min at room temperature. The DNA mixture was added to the Lipofectamine 2000 mixture and incubated for 20 min at room temperature. The medium was removed from the wells, and the DNA/Lipofectamine 2000 mixture was added to the cells, which were then incubated for 6 h at 37 C. After transfection, the cells were incubated overnight in DMEM containing 10% charcoal-stripped FBS and then treated with 1 μm T0901317 for 30 min or 16 h for subsequent experiments, ChIP assay or QPCR, respectively.
ChIP
The ChIP assays of DN2-transfected macrophages were performed as described previously, with minor modifications (45). Briefly, isolated peritoneal macrophages were incubated overnight in DMEM containing 10% FBS and transfected with pcDNA3-DN2. The transfected macrophages were incubated overnight in 10% charcoal-stripped FBS and treated with 1 μm T0901317 for 30 min. After this treatment, cells were fixed immediately with 1% formaldehyde for 15 min at room temperature and then for 20 min at 4 C. The cells were washed with cold PBS and resuspended in lysis buffer containing 50 mm Tris-HCl (pH 8.0), 1% SDS, and 10 mm EDTA. Soluble chromatin from macrophages was prepared by sonication and immunoprecipitated with ASC-2 (A300-411A; Bethyl Laboratories, Montgomery, TX), LXR (sc-13068; Santa Cruz Biotechnology, Santa Cruz, CA), TRAP220 (sc-5334; Santa Cruz Biotechnology), or NCoR (sc-1609X; Santa Cruz Biotechnology) antibodies. The final DNA extractions were analyzed by QPCR in triplicate, with reactions of input DNA performed in parallel. Ratios of immunoprecipitated DNA and input DNA levels were calculated by subtracting the Ct of the input DNA from the Ct of immunoprecipitated DNA and raising 2 to the power of this difference (43). Immunoprecipitated DNA levels are thus expressed relative to input DNA levels. The primers that encompass the LXRE region of the promoter of ABCA1, ABCG1, and apoE are as follows: ABCA1, 5′-GGGGAAAGAGGGAGAGAACAG-3′ and 5′-GAATTACTGGTTTTTGCCGC-3′; ABCG1, 5′-TGCCCTGTAGTAACCTCTGTA-3′ and 5′-TCATGTGCGACTCCTCCCA-3′; and apoE, 5′-AACCTAGAGTCTGGGCCCCTT-3′ and 5′-CATTGACCGACAGTGACCCC-3′. The primers for non-LXRE regions of ABCA1, ABCG1, and apoE promoters are as follows: ABCA1, 5′-AGGGAAAGCTCTCTGGAGCAT-3′ and 5′-CAGGAATTTCTCCATCCTTTGAGT-3′; ABCG1, 5′-TGAAATACACAGGTTCGACTAGACAA-3′ and 5′-TGTACCAACACCACCGAAGTTTA-3′; and apoE, 5′-AAGGGAAGCAAAGACTCACGAT-3′ and 5′-CCAGGCAGTAGAGAGGGTCTTAGT-3′.
BMT
Eight-week-old male LDLR-null mice were γ-irradiated (900 rad) to eliminate the majority of their bone marrow cells. The bone marrow of each γ-irradiated mouse was reconstituted via a tail vein injection with 2 × 106 bone marrow cells isolated from wild-type, LDLR−/−, or DN2-Tg mice. Mice were fed a chow diet for a week while their marrow was repopulated with donor cells. To induce atherosclerosis, transplanted mice were fed an atherogenic diet containing 15.8% (wt/wt) fat and 1.25% (wt/wt) cholesterol (no cholate;,diet 94059; Harlan Teklad, Madison, WI) for 5 wk. Atherosclerotic lesions of the aortic root were stained with oil red O and quantitated using computer-assisted image analysis (46).
Statistical analysis
Results are expressed as means ± sd. Statistical significance was assessed using the Student’s unpaired two-tailed t test or a one-way ANOVA. Differences with a P < 0.05 were considered statistically significant.
Footnotes
This work was supported by the Korea Research Foundation Grants funded by the Korean Government (MOEHRD) (KRF-2004-015-E00059, KRF-2005-041-E00062, KRF-2008-313-E00083), the grants from the Korea Science and Engineering Foundation (R01-2006-000-10806-0, M10642140001-06N4214-00110), and intramural grants (2005-352, 2006-352, 2007-352, and 2009-352) from the Asan Institute for Life Sciences, Seoul, South Korea, to S.-W.K. and National Institute of Health Grants DK064678 to J.W.L.
Disclosure Summary: All authors have nothing to declare.
First Published Online April 2, 2009
Abbreviations: ABC, ATP-binding cassette; ACAT, Acyl-CoA: cholesterol acyltransferase; acLDL, acetylated low-density lipoprotein; apoE, apolipoprotein E; BMT, bone marrow transplantation; ChIP, chromatin immunoprecipitation; Ct, cycle threshold; ERα, estrogen receptor-α; FBS, fetal bovine serum; HDL, high-density lipoprotein; LPDS, lipoprotein-deficient serum; LXR, liver X receptor; LXRE, LXR response element; MEF, mouse embryonic fibroblast; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; QPCR, quantitative PCR; RCT, reverse cholesterol transport; SRC-1, steroid receptor coactivator 1; Tg, transgenic; TRAP220, thyroid hormone receptor-associated protein 220; VSMC, vascular smooth muscle cell.
References
- Ross R 1999 Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL 2001 Atherosclerosis: the road ahead. Cell 104:503–516 [DOI] [PubMed] [Google Scholar]
- Millatt LJ, Bocher V, Fruchart JC, Staels B 2003 Liver X receptors and the control of cholesterol homeostasis: potential therapeutic targets for the treatment of atherosclerosis. Biochim Biophys Acta 1631:107–118 [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Mangelsdorf DJ 2003 Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol 17:985–993 [DOI] [PubMed] [Google Scholar]
- Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P 2002 Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci USA 99:7604–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P 2007 Ligand activation of LXRβ reverses atherosclerosis and cellular cholesterol overload in mice lacking LXRα and apoE. J Clin Invest 117:2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG 2002 Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci USA 99:11896–11901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995 The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- Caira F, Antonson P, Pelto-Huikko M, Treuter E, Gustafsson JA 2000 Cloning and characterization of RAP250, a novel nuclear receptor coactivator. J Biol Chem 275:5308–5317 [DOI] [PubMed] [Google Scholar]
- Ko L, Cardona GR, Chin WW 2000 Thyroid hormone receptor-binding protein, an LXXLL motif-containing protein, functions as a general coactivator. Proc Natl Acad Sci USA 97:6212–6217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Anzick SL, Choi JE, Bubendorf L, Guan XY, Jung YK, Kallioniemi OP, Kononen J, Trent JM, Azorsa D, Jhun BH, Cheong JH, Lee YC, Meltzer PS, Lee JW 1999 A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J Biol Chem 274:34283–34293 [DOI] [PubMed] [Google Scholar]
- Lee SK, Jung SY, Kim YS, Na SY, Lee YC, Lee JW 2001 Two distinct nuclear receptor-interaction domains and CREB-binding protein-dependent transactivation function of activating signal cointegrator-2. Mol Endocrinol 15:241–254 [DOI] [PubMed] [Google Scholar]
- Lee SK, Na SY, Jung SY, Choi JE, Jhun BH, Cheong J, Meltzer PS, Lee YC, Lee JW 2000 Activating protein-1, nuclear factor-κB, and serum response factor as novel target molecules of the cancer-amplified transcription coactivator ASC-2. Mol Endocrinol 14:915–925 [DOI] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH 2000 A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB-binding protein. Mol Cell Biol 20:5048–5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner MM, Tirkkonen M, Kallioniemi A, Isola J, Kuukasjärvi T, Collins C, Kowbel D, Guan XY, Trent J, Gray JW, Meltzer P, Kallioniemi OP 1996 Independent amplification and frequent co-amplification of three nonsyntenic regions on the long arm of chromosome 20 in human breast cancer. Cancer Res 56:3441–3445 [PubMed] [Google Scholar]
- Zhu Y, Kan L, Qi C, Kanwar YS, Yeldandi AV, Rao MS, Reddy JK 2000 Isolation and characterization of peroxisome proliferator-activated receptor (PPAR) interacting protein (PRIP) as a coactivator for PPAR. J Biol Chem 275:13510–13516 [DOI] [PubMed] [Google Scholar]
- Choi E, Lee S, Yeom SY, Kim GH, Lee JW, Kim SW 2005 Characterization of activating signal cointegrator-2 as a novel transcriptional coactivator of the xenobiotic nuclear receptor constitutive androstane receptor. Mol Endocrinol 19:1711–1719 [DOI] [PubMed] [Google Scholar]
- Kim SW, Cheong C, Sohn YC, Goo YH, Oh WJ, Park JH, Joe SY, Kang HS, Kim DK, Kee C, Lee JW, Lee HW 2002 Multiple developmental defects derived from impaired recruitment of ASC-2 to nuclear receptors in mice: implication for posterior lenticonus with cataract. Mol Cell Biol 22:8409–8414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Park K, Kwak E, Choi E, Lee S, Ham J, Kang H, Kim JM, Hwang SY, Kong YY, Lee K, Lee JW 2003 Activating signal cointegrator 2 required for liver lipid metabolism mediated by liver X receptors in mice. Mol Cell Biol 23:3583–3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeom SY, Kim GH, Kim CH, Jung HD, Kim SY, Park JY, Pak YK, Rhee DK, Kuang SQ, Xu J, Han DJ, Song DK, Lee JW, Lee KU, Kim SW 2006 Regulation of insulin secretion and β-cell mass by activating signal cointegrator 2. Mol Cell Biol 26:4553–4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liao L, Kuang SQ, Xu J 2003 Spatial distribution of the messenger ribonucleic acid and protein of the nuclear receptor coactivator, amplified in breast cancer-3, in mice. Endocrinology 144:1435–1443 [DOI] [PubMed] [Google Scholar]
- Lee S, Lee J, Lee SK, Lee JW 2008 Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol Endocrinol 22:1312–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo ML, Lee KC, Stender JD, Katzenellenbogen BS, Kraus WL 2004 Selective recognition of distinct classes of coactivators by a ligand-inducible activation domain. Mol Cell 13:725–738 [DOI] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH 2005 Nuclear hormone receptor coregulator: role in hormone action, metabolism, growth, and development. Endocr Rev 26:583–597 [DOI] [PubMed] [Google Scholar]
- Toresson G, Schuster GU, Steffensen KR, Bengtsson M, Ljunggren J, Dahlman-Wright K, Gustafsson JA 2004 Purification of functional full-length liver X receptor β produced in Escherichia coli. Protein Expr Purif 35:190–198 [DOI] [PubMed] [Google Scholar]
- Li Q, Chu MJ, Xu J 2007 Tissue- and nuclear receptor-specific function of the C-terminal LXXLL motif of coactivator NCoA6/AIB3 in mice. Mol Cell Biol 27:8073–8086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G 1999 Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun 257:29–33 [DOI] [PubMed] [Google Scholar]
- Klucken J, Büchler C, Orsó E, Kaminski WE, Porsch-Ozcürümez M, Liebisch G, Kapinsky M, Diederich W, Drobnik W, Dean M, Allikmets R, Schmitz G 2000 ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci USA 97:817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Ozcürümez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G 1999 The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet 22:347–351 [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest Jr J, Hayden MR 1999 Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 22:336–345 [DOI] [PubMed] [Google Scholar]
- Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P, Assmann G 1999 Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet 22:352–355 [DOI] [PubMed] [Google Scholar]
- Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ 2007 Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest 117:2216–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Kawamura M, Maeda T, Fujimaki Y, Fujita M, Kojima K, Teramoto T 2000 Apolipoprotein E accelerates the efflux of cholesterol from macrophages: mechanism of xanthoma formation in apolipoprotein E deficiency. J Atheroscler Thromb 6:22–27 [DOI] [PubMed] [Google Scholar]
- Linton MF, Atkinson JB, Fazio S 1995 Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science 267:1034–1037 [DOI] [PubMed] [Google Scholar]
- Kennedy MA, Venkateswaran A, Tarr PT, Xenarios I, Kudoh J, Shimizu N, Edwards PA 2001 Characterization of the human ABCG1 gene: liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J Biol Chem 276:39438–39447 [DOI] [PubMed] [Google Scholar]
- Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P 2001 LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci USA 98:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Lawn RM, Wade DP 2000 ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem Biophys Res Commun 274:794–802 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu C, Zhu L, Jiang X, Chen X, Qi X, Liang X, Jin S, Zhang P, Li Q, Wang D, Liu X, Zeng K, Zhang J, Xiang Y, Zhang CY 2007 PGC-1α inhibits oleic acid induced proliferation and migration of rat vascular smooth muscle cells. PLoS ONE 2:e1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Haque Z, Lu Q, Li L, Karas R, Mendelsohn M 2007 Steroid receptor coactivator 3 is a coactivator for myocardin, the regulator of smooth muscle transcription and differentiation. Proc Natl Acad Sci USA 104:4065–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, Kim HT, Park JY, Lee KU, Jang WG, Kim JG, Kim BW, Lee IK 2007 Effects of PGC-1α on TNF-α-induced MCP-1 and VCAM-1 expression and NF-κB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal 9:301–307 [DOI] [PubMed] [Google Scholar]
- Goo YH, Sohn YC, Kim DH, Kim SW, Kang MJ, Jung DJ, Kwak E, Barlev NA, Berger SL, Chow VT, Roeder RG, Azorsa DO, Meltzer PS, Suh PG, Song EJ, Lee KJ, Lee YC, Lee JW 2003 Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol Cell Biol 23:140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Francis GA, Knopp RH, Oram JF 1995 Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier disease. J Clin Invest 96:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando V, Strutt H, Paro R 1997 Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods 11:205–214 [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Rubin EM, Palinski W 1995 Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res 36:2320–2328 [PubMed] [Google Scholar]