Abstract
The calcium sensing receptor (CaSR) is a Family C/3 G protein-coupled receptor that translates changes in extracellular Ca2+ into diverse intracellular signals. Loss-of-function mutations in human CaSR cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. CaSR must navigate a number of endoplasmic reticulum quality control checkpoints during biosynthesis, including a conformational/functional checkpoint. Here we examine the biosynthesis of 25 CaSR mutations causing familial hypocalciuric hypercalcemia /neonatal severe hyperparathyroidism using immunoprecipitation, biotinylation, and functional assays. We define classes of CaSR mutants based on their biosynthetic profile. Class I CaSR mutants are not rescued to the plasma membrane. To dissect the organellar compartments that class I mutants can access, we engineered a cleavage site for the proprotein convertase furin into the extracellular domain of wild-type CaSR and class I mutants. Based on absence or presence of cleavage fragments, we find most mutants are degraded from the endoplasmic reticulum (no furin-mediated cleavage), whereas others access the Golgi (furin-mediated cleavage) before degradation. Class II CaSR mutants show increased expression and/or enhanced plasma membrane localization upon treatment with MG132 or the pharmacochaperone NPS R-568, permitting assay of functional activity. Of the 10 CaSR mutants that exhibit plasma membrane localization, only two did not show enhanced functional activity after rescue with NPS R-568. The established approaches can be used with current and newly identified CaSR mutations to identify the location of biosynthetic block and to determine the likelihood of rescue by allosteric agonists.
This study establishes approaches to characterize the trafficking defects of loss-of-function CaSR mutants, and the likelihood of ”rescue” by the pharmacochaperone NPS R-568.
Serum-ionized calcium levels are maintained over a very narrow range of 1.1–1.3 mm by calcium sensing receptor (CaSR)-mediated regulation of parathyroid chief cells (1). CaSR exhibits high cooperativity for activation by extracellular Ca2+ and acutely regulates PTH secretion and synthesis (2,3). CaSR activity also controls parathyroid cell proliferation. Regulation of parathyroid cell function by CaSR is inversely related to the extracellular Ca2+ concentration, i.e. as serum Ca2+ decreases, PTH secretion is increased (2).
The central role of CaSR in regulating plasma Ca2+ concentrations has been confirmed by the identification of mutations that cause human diseases. More than 200 mutations have been identified in the human CaSR gene (www.casrdb.mcgill.ca/), the majority being loss-of-function missense mutations in the extracellular domain (ECD) and transmembrane domains of CaSR. Familial hypocalciuric hypercalcemia, FHH (OMIM 14598), is an autosomal dominant disorder resulting from a loss-of-function mutation in a single allele of CaSR, which causes a mild to moderate hypercalcemia (depending on the mutation) without hypercalciuria. In fact, the combination of hypercalcemia in the face of a reduced rate of urinary calcium excretion is diagnostic of CaSR loss-of-function mutations (4). Serum levels of PTH are generally normal despite hypercalcemia. Individuals with homozygous (or compound heterozygous) loss-of-function mutations of CaSR have a severe clinical phenotype, neonatal severe primary hyperparathyroidism, NSHPT (OMIM 239200), which manifests with a constellation of symptoms typical of hyperparathyroidism in the early neonatal period, including multiple fractures, respiratory difficulties, dehydration, and hypotonia (reviewed in Ref. 5). Parathyroidectomy is generally required. Activating mutations of CaSR have also been identified in humans (5,6), causing autosomal dominant hypoparathyroidism (OMIM 601298) or Bartter’s syndrome type V (OMIM 601199.0035), characterized by hypocalcemia, low serum PTH levels, and hypercalciuria that may lead to nephrolithiasis (reviewed in Ref. 7).
Loss-of-function mutations reduce the efficacy of extracellular Ca2+ for activation of CaSR assayed by measuring various signaling pathway outputs, generally inositol phosphate production, increases in intracellular Ca2+ and/or activation of the MAPK pathway monitored by ERK1/2 phosphorylation (reviewed in Ref. 8). Mutations within the venus flytrap (VFT) module of the ECD may either alter the affinity of CaSR for Ca2+ or allosteric agonists like amino acids (9,10) or alter the structure of the VFT to make the active conformation less likely or less stable (11). Mutations within the cysteine-rich domain (CysRD) or heptahelical transmembrane domain (TMD) most likely affect the ability of CaSR to achieve the active conformation capable of interacting with heterotrimeric G proteins or other proteins required to initiate signaling (12,13,14,15). The current lack of high-affinity agonists or antagonists for CaSR makes direct measurement of receptor affinity for Ca2+ or allosteric agonists impossible. Most critical for understanding the contribution of CaSR loss-of-function mutations to human disease severity, however, is the documented reduction in signaling capacity. An alternative source of reduced signaling of CaSR loss-of-function mutations is defective trafficking to or stability at the plasma membrane. We have recently shown that a number of CaSR loss-of-function mutations cause folding/biosynthetic trafficking defects that severely limit or eliminate plasma membrane targeting of CaSR (16). Treatment of the mutants, R66C, R185Q, R680C, R795W, and V817I, with either MG132 (which prevents degradation by the proteasome) or NPS R-568 (a membrane permeant allosteric agonist of CaSR) increased the abundance of the receptors in human embryonic kidney (HEK)293 cells. In addition, R185Q, R680C, R795W, and V817I were rescued to the plasma membrane, increasing CaSR-mediated signaling. Some CaSR mutants causing FHH/NSHPT therefore represent biosynthetic trafficking mutants akin to the ΔF508 mutation of the cystic fibrosis transmembrane conductance regulator (17,18). We have shown that NPS R-568 represents a pharmacochaperone for CaSR, rescuing functional receptors to the plasma membrane (16). Not all of the CaSR loss-of-function mutants were rescued, however. Whereas the abundance of the R66C mutant was increased by treatment with either MG132 or NPS R-568, there was no significant rescue to the plasma membrane or CaSR-mediated signaling.
The current report examines additional mutations identified in patients with FHH or NSHPT that are localized to the ECD or TMD of human CaSR and establishes methods that can be used to categorize the type of defect in maturation and/or function caused by the mutation. We determined the degree of rescue of mutant expression and plasma membrane targeting and sorted the mutants into class I, which are trapped within biosynthetic compartment(s) in the cells, and class II, which can be rescued to the plasma membrane. We used a furin-mediated cleavage strategy to identify CaSR mutants that access the Golgi complex before degradation. These studies establish that the majority of CaSR loss-of-function mutations cause a defect in biosynthetic trafficking, but that a significant number can be rescued, with full or partial function, to the plasma membrane with membrane-permeant allosteric agonists of CaSR, typified by NPS R-568. The established approaches can be used with current and newly identified CaSR mutations to identify the location of biosynthetic block and to determine the likelihood of rescue by allosteric agonists.
Results
CaSR loss-of-function mutants differ in their ability to reach the plasma membrane
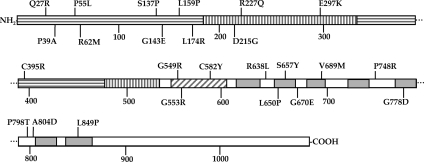
More than 235 DNA mutations in CaSR have been identified in patients (tabulated in the CaSR database, www.casrdb. mcgill.ca/), with the majority resulting in missense mutations in the ECD and TMD of CaSR. We have previously shown that five representative loss-of-function mutations in the CaSR ECD (R66C, R185Q) and TMD (R680C, R795W, V817I) are defective in trafficking to the plasma membrane, and their expression can be rescued by blocking proteasomal degradation with MG132 (16). All mutants except R66C were also rescued to the plasma membrane and exhibited increased function after overnight treatment with NPS R-568, an allosteric agonist of CaSR (16). To test the generality of these findings, we generated 25 additional documented CaSR loss-of-function mutations in the background of human CaSR containing an external FLAG epitope inserted immediately after the cleaved signal sequence and characterized the biosynthetic trafficking and maturation of these mutants. Figure 1 illustrates the locations of the loss-of-function mutations within the domain structure of CaSR. The ECD of CaSR comprises two subdomains, a venus flytrap domain (VFTD) and a CysRD having structural homology to metabotropic glutamate receptors (19,20). Mutations Q27R, P39A, P55L, R62M, S137P, G143E, L159P, L174R, and C395R are located in lobe 1 of the VFTD, and mutations D215G, R227Q, and E297K are located in lobe 2 of the VFTD. Mutations G549R, G553R, and C582Y are located in the CysRD, and the remaining mutations are located in the transmembrane domain helices (L650P, S657Y, V689M, G778D, L849P) or extracellular (G670E, P748R) or intracellular loops (R638L, P798T, A804D). Mutations in the carboxyl-terminal domain of CaSR are relatively rare, and no mutations from this region were studied.
Figure 1.
Schematic illustration of location of CaSR loss-of-function mutations. CaSR contains 1078 amino acids. The amino-terminal, ECD is comprised of a VFTD, divided into lobes I (horizontal bars) and II (vertical bars), and a CysRD (diagonal bars). The TMD contains seven helices (gray boxes). Extra- and intracellular loops and carboxyl terminus are white.
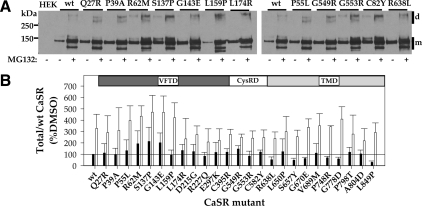
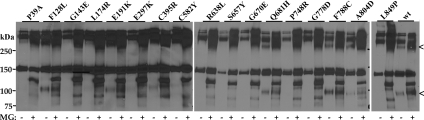
Wild-type (wt) CaSR is expressed in HEK293 cells as several forms representing distinct steps along the biosynthetic pathway. The unglycosylated CaSR protein, a form generally not seen in the absence of MG132, is approximately 120 kDa (21). The core-glycosylated, high-mannose form of CaSR, which is predominantly present in the endoplasmic reticulum (ER), is approximately 140 kDa (21,22,23), and the maturely glycosylated form of CaSR, approximately 160 kDa, is present in the Golgi complex and beyond (plasma membrane, endosomes) (22,23). Also observed on Western blots of CaSR, despite cell lysis in the presence of iodoacetamide and sample buffers containing high concentrations of mercaptoethanol or dithiothreitol, are dimers and higher order oligomers of CaSR (>280 kDa). We have previously shown that treatment with the proteasomal inhibitor MG132 or the membrane-permeant CaSR allosteric agonist, NPS R-568, increases abundance, and for some mutants, maturation of receptors via distinct mechanisms (16). For the current studies, we transiently transfected HEK293 cells with wt CaSR or loss-of-function mutants and treated cells with dimethylsulfoxide (DMSO) (vehicle) or 10 μm MG132 for 12–14 h before cell lysis for immunoprecipitation with anti-FLAG antibody. Figure 2A illustrates Western blots for some of the CaSR loss-of-function mutants studied, chosen to illustrate representative receptors from each domain (VFTD, CysRD, and TMD) and the range of observed phenotypes. Each blot contains control [DMSO, (−)] and MG132-treated (+) samples. For wt CaSR and all mutants examined, MG132 treatment leads to the appearance of a band at approximately 120 kDa (core protein without glycosylation) and a general increase in receptor abundance. All of the CaSR mutants (three to five independent transfections) were subjected to overnight DMSO/MG132 treatment followed by immunoprecipitation with anti-FLAG antibody and Western blotting with anti-CaSR antibody. Blots were scanned, and the total CaSR immunoreactivity in each lane was quantified [sum of monomeric (m) and dimer (d) forms; indicated by bars at right of blots] and normalized to the abundance of CaSR treated with DMSO from the same blot. Figure 2B illustrates the results for all CaSR loss-of-function mutants studied. The solid line indicates the abundance of wt CaSR under control conditions (DMSO treatment). It is clear that many CaSR loss-of-function mutants are expressed at levels equal to or greater than wt CaSR even in DMSO (black bars) and that, for most mutants, treatment with MG132 further increases receptor abundance (open bars). MG132 also enhances maturation for wt CaSR and for many of the mutants (Fig. 2A), although this is difficult to quantify on Western blots because of the large differences in relative abundance of mature vs. immature forms of CaSR.
Figure 2.
Expression of wt CaSR and loss-of-function mutants assessed by immunoprecipitation. A, Western blots of immunoprecipitates of wt CaSR and representative loss-of-function mutants treated for 14 h before cell lysis with DMSO or 10 μm MG132. Both monomer (m) and dimer (d) immunoreactivity is illustrated. Receptors were immunoprecipitated with anti-FLAG antibody, and blots were probed with LRG anti-CaSR antibody, as described in Materials and Methods. B, Plot of normalized expression of wt CaSR and mutants with domain localization of the mutants indicated. For wt CaSR and each mutant, the sum of monomer and dimer immunoreactivity was normalized to that obtained for wt CaSR (% DMSO) on the same blot. Bars indicate average ± sd for three to six Western blots. Black bars, DMSO treatment; open bars, 10 μm MG132; solid line indicates total expression of wt CaSR after DMSO treatment (=100%).
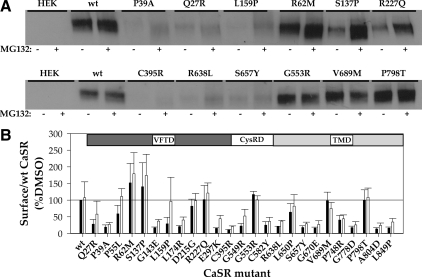
To more directly explore the effect of MG132 treatment on maturation of CaSR, we used surface biotinylation of HEK293 cells expressing wt or mutant CaSRs, treated before biotinylation and cell lysis with DMSO or MG132 for 12–14 h. Biotinylated surface proteins were isolated with streptavidin-conjugated agarose and solubilized in sample buffer without sulfhydryl-reducing agents to facilitate quantitation, because under these conditions CaSR resides solely in dimer or oligomer forms (>250 kDa). Western blots were probed with anti-CaSR antibody (Fig. 3A illustrates representative mutants), and the abundance of wt CaSR or mutants was determined. Each blot contained untransfected HEK293 cells and wt CaSR-expressing cells (treated with DMSO or MG132) to permit normalization of abundance to that of wt CaSR in DMSO from the same blot. Figure 3B illustrates the results of three to five experiments for each loss-of-function CaSR mutant compared with wt CaSR. It is clear that some CaSR loss-of-function mutants are not significantly localized to the plasma membrane in the absence or presence of MG132 despite high levels of expression relative to wt CaSR, and we define these as class I mutants: Q27R, P39A, G143E, L174R, E297K, C395R, G549R, C582Y, R638L, S657Y, G670E, P748R, G778D, A804D, and L849P. Also in class I is R66C (16). More interesting are the class II mutants, which are localized to the plasma membrane without or with MG132, although MG132 can increase surface localization. We define the following mutants as class II based on this criterion: P55L, R62M, S137P, L159P, D215G, R227Q, G553R, L650P, V689M, and P798T. Also in class II are the mutants R185Q, R680C, R795W, and V817I, which we characterized in a previous study (16).
Figure 3.
Surface localization of wt CaSR and loss-of-function mutants assessed by biotinylation. A, Western blots of untransfected HEK293 cells, wt CaSR, or representative loss-of-function mutants treated overnight with DMSO or 10 μm MG132 and biotinylated before cell lysis. Sample buffer did not contain reducing agents so that all CaSR immunoreactivity was present in the dimer/oligomer form. B, Plot of surface expression of wt CaSR and mutants with domain localization of the mutants indicated. For wt CaSR and each mutant, immunoreactivity was normalized to that obtained for wt CaSR (% DMSO) on the same blot. Bars indicated average ± sd for three to six Western blots. Solid line indicates surface expression of wt CaSR after DMSO treatment (=100%).
Class I CaSR mutants: dissection of the site of biosynthetic block
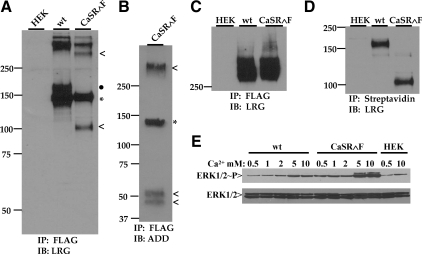
Class I mutants traffic to the plasma membrane poorly or not at all, even in the presence of MG132. These mutants are trapped in the biosynthetic pathway but may be blocked at distinct quality control checkpoints, depending upon the mutation. In general, membrane proteins undergo cotranslational core glycosylation at the endoplasmic reticulum (ER), followed by multiple rounds of chaperone-assisted folding, and are released from the ER to the ER-Golgi intermediate compartment (ERGIC) (reviewed in Ref. 24). Trafficking to the ERGIC requires packaging of receptor cargo into coat protein complex (COP) II vesicles under the control of Sar1 guanosine triphosphatase (25,26). Membrane proteins then undergo further maturation, remodeling of N-linked glycosylation, and possible addition of O-linked glycosylation, as they progress through the Golgi (27). Retrieval of misfolded proteins that escape to the ERGIC occurs via COPI vesicles. In yeast, trafficking through the ERGIC and retrieval by COPI vesicles are required for proteasomal degradation, perhaps because the COPI vesicles target retrieved proteins to quality control sites at the ER (28). To establish a biochemical approach for determining the biosynthetic compartment(s) that CaSR loss-of-function mutants can access, we took advantage of the proprotein convertase furin, a trans-Golgi network (TGN)-resident type I membrane protease with a catalytic site facing the Golgi lumen (29). Furin has broad specificity for cleavage of arginine-rich sequences and activates prohormones, receptors, and ion channels during their transit through the Golgi (29). We incorporated the furin cleavage site between residues D371 and T372 of the ECD of wt CaSR and loss-of-function mutants (termed CaSR∧F). The engineered cleavage site is located between two epitopes for anti-CaSR antibodies (30), ADD (against residues 214–235) and LRG (residues 374–391), which can be used to identify the cleavage products of CaSR∧F on Western blots. Figure 4A shows a representative Western blot from HEK293 cells transiently expressing wt CaSR or wt CaSR∧F, immunoprecipitated with anti-FLAG antibody (both constructs contain a FLAG epitope immediately C terminal of the signal sequence). Samples were run under reducing conditions, and the blot was probed with anti-CaSR antibody LRG, which recognizes both CaSR and the larger, C-terminal fragment of CaSR∧F. The furin cleavage site (ARRRKKR↓GLDV; arrow indicates site of cleavage) adds approximately 1.2 kDa to the mass of wt CaSR, and this is reflected in location of the uncleaved form on the western blot (*). The mature form of CaSR (≈160 kDa, dark asterisk) is absent from the CaSR∧F lane, but a band is present at approximately 108 kDa (<), representing the maturely glycosylated but cleaved fragment of CaSR∧F that contains the LRG epitope. The smaller cleavage fragment, which appears as a doublet of 48/54 kDa, can be visualized on Western blots under reducing conditions by probing with anti-CaSR antibody ADD (Fig. 4B). The sum of the two fragments (LRG 108 kDa plus ADD 48/54 kDa) yields the molecular mass of mature, wt CaSR. For all subsequent experiments, we used the anti-CaSR LRG antibody to visualize the uncleaved ER form and the larger fragment of the cleaved form of CaSR∧F.
Figure 4.
Comparison of wt CaSR and CaSR∧F structure, trafficking, and function. A, Cells expressing CaSR or CaSR∧F were immunoprecipitated with anti-FLAG antibody and Western blotted with anti-CaSR LRG antibody (against residues 374-391 of human CaSR) under reducing conditions (0.1 m DTT) as described in Materials and Methods. Monomeric CaSR forms resolve at 140 kDa (immature, light asterisk) and 160 kDa (mature, filled circle). The immature uncleaved CaSR∧F is observed at 140 kDa (light asterisk), and the larger fragment of the mature, cleaved form (containing LRG epitope) is resolved at approximately 108 kDa (indicated with <). Reduction of receptors is not complete even in 0.1 m DTT, and the cleaved form of CaSR∧F is also apparent in the dimer band region of the blot (indicated with <). B, Western blot of CaSR∧F processed as described in panel A. The blot was probed with anti-CaSR ADD antibody (against residues 214-235 of human CaSR). The immature, uncleaved form (light asterisk) is observed at 140 kDa, and the cleaved fragments containing the ADD epitope are resolved as a doublet at 48/54 kDa (indicated with <). C, Cells transfected with either CaSR or CaSR∧F immunoprecipitated with anti-FLAG antibody in the absence of reducing agents were run on Western blots and probed with anti-CaSR LRG antibody. Both wt and CaSR∧F are resolved in dimeric form above 250 kDa. D, Cells expressing wt or CaSR∧F were biotinylated as described in Materials and Methods and proteins isolated with streptavidin-agarose. Samples were eluted under reducing conditions and Western blots probed with anti-CaSR LRG antibody. E, Transiently transfected cells were starved in 0.5 mm Ca2+/0.2% BSA in DMEM for 12 h before stimulation with varying extracellular Ca2+ for 10 min 37 C. Lysates were processed for Western blotting with anti-phospho-ERK1/2 antibody, stripped, and reprobed with anti-ERK1/2 antibody. Untransfected HEK293 cells stimulated with either 0.5 or 10 mm Ca2+ were used as controls. IB, Immunoblotting; IP, immunoprecipitation.
The ability to isolate the 108-kDa fragment of cleaved CaSR∧F implies that the cleavage products do not dissociate in vivo, because the FLAG epitope used for immunoprecipitation resides in the smaller cleaved fragment. To confirm this idea, we isolated CaSR∧F with anti-FLAG antibody and performed immunoblotting in the absence of reducing agents. Figure 4C illustrates that, under these conditions, both wt CaSR and CaSR∧F have comparable masses. To identify the form(s) of CaSR∧F present at the plasma membrane, we treated transiently transfected cells with a membrane-impermeant biotinylation reagent, isolated biotinylated proteins with streptavidin-conjugated agarose, and ran the samples under reducing conditions. Figure 4D illustrates that the mature form of wt CaSR (160 kDa) and the cleaved form of CaSR∧F (represented here by the fragment of 108 kDa) were biotinylated and thus present at the plasma membrane. Finally, we determined whether CaSR∧F was functional by assessing the ability to activate ERK1/2 phosphorylation as extracellular Ca2+ was increased. Figure 4E illustrates that CaSR∧F at 5 and 10 mm extracellular Ca2+ was able to active ERK1/2 phosphorylation more robustly than wt CaSR. Overall, Fig. 4 illustrates that CaSR∧F is cleaved during maturation but does not dissociate and traffics to the plasma membrane and functions as well or better than wt CaSR. CaSR∧F cleavage can therefore serve as a marker for maturation of the receptor within the secretory pathway.
We generated all of the class I mutants in the CaSR∧F background, expressed them in HEK293 cells, and treated cells overnight with DMSO or MG132 before cell lysis to resolve the forms of CaSR∧F present. For controls, we used wt CaSR∧F as well as four gain-of-function CaSR mutants, F128L, E191K, Q681H, and F788C (also generated in the CaSR∧F background), which we have previously shown to be resistant to ERAD and robustly trafficked to the plasma membrane (1). Two distinct patterns of furin-mediated cleavage products were observed, allowing further division of class I mutants into class Ia, cleaved by furin in a manner analogous to wt CaSR, and class Ib, not cleaved, as illustrated by representative mutants in Fig. 5. Blots were overexposed to permit visualization of minor bands. We confirm that wt CaSR and the gain-of-function mutants (F128L, E191K, Q681H, F788C) are cleaved by furin, indicating transit through the TGN. Some mutants, class Ia, including E297K, C395R, and A804D, were processed in a manner comparable to wt CaSR∧F, i.e. they were maturely glycosylated and cleaved by furin, generating a fragment at approximately 108 kDa. The furin-cleaved receptor was observed under control conditions (DMSO), but its abundance was increased by MG132 treatment, indicating that a significant fraction of the cleaved form was degraded by the proteasome (Fig. 5). Class Ib mutants (Fig. 5), including Q27R, P39A, G143E, L174R, G549R, C582Y, R638L, S657Y, G670E, P748R, G778D, and L849P, showed no cleaved forms of the receptor in the absence of MG132. CaSR immunoreactivity was apparent in the presence of MG132, although not at the mature mass (note multiple bands <108 kDa). These results suggest that CaSR loss-of-function mutants are targeted for degradation by the proteasome via distinct routes, from the ER as well as the Golgi.
Figure 5.
Biochemical analysis of trafficking of CaSR and loss-of-function mutants. Representative class I mutants were treated overnight with DMSO or 10 μm MG132 before cell lysis and resolved under reducing conditions on Western blots. Monomer and dimer forms are illustrated, and blots are overexposed to assess presence of minor amounts of cleaved forms below the 150 kDa marker. In the absence of MG132, cleavage of wt CaSR and F128L, E191K, C395Y, Q681H, F788C, and A804D was observed at both monomer and dimer zones of blot (indicated with <).
Class II CaSR mutants: plasma membrane abundance and function
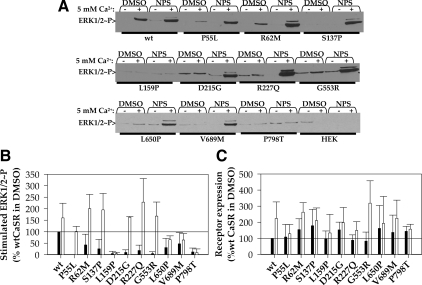
Because class II CaSR mutants reach the plasma membrane, they may exhibit some functional activity as calcium sensors that can be enhanced by rescue, i.e. increased plasma membrane targeting. We determined the ability of class II mutants to activate the MAPK pathway, monitoring ERK1/2 phosphorylation in HEK293 cells expressing wt CaSR or mutants. Figure 6A illustrates representative Western blots of lysates from cells treated with either 0.5 or 5 mm extracellular Ca2+ for 10 min at 37 C. Figure 6B illustrates the tabulated results of three to seven such experiments, normalized to the stimulated increase in ERK1/2 phosphorylation observed for wt CaSR. The loss-of-function mutants P55L, L159P, D215G, R227, G553R, and P798T had little or no ability to induce ERK1/2 phosphorylation in the presence of 5 mm Ca2+ under control (DMSO) conditions. We next determined whether enhanced plasma membrane expression induced by 12–14 h treatment with NPS R-568 could increase functional activity of the mutants, particularly of those that exhibited little activity under control conditions. Representative blots are illustrated in Fig. 6A and tabulated data are shown in Fig. 6B. Exposure to NPS R-568 rescued the activity of all mutants except L159P and P798T. Mutants L650P and V689M showed modest increases, to 70% of wt CaSR activity, whereas mutants P55L, R62M, S137P, D215G, R227Q, and G553R showed levels of stimulated ERK1/2 phosphorylation equal to or greater than wt CaSR. Finally, mutants P55L, D215G, and G553R were rescued from no functional activity to levels greater than wt CaSR (DMSO), an effect we have previously shown for mutant R680C (16). Class II mutants can therefore be sorted into class IIa, with function rescued by NPS R-568, and class IIb, which does not exhibit functional rescue. Overnight treatment with NPS R-568 increases the abundance of wt CaSR and some loss-of-function mutants (16). Figure 6C confirms that the NPS R-568 treatment enhances stimulated ERK1/2 phosphorylation, at least in part, by increasing the abundance of wt CaSR and mutants.
Figure 6.
Class II mutant activation of ERK1/2 phosphorylation. A, Western blots illustrating ERK1/2 phosphorylation in the presence of 0.5 (−) or 5 mm (+) extracellular Ca2+ (10 min, 37 C) for HEK293 cells expressing wt CaSR or class II loss-of-function mutants after overnight treatment with DMSO or 10 μm NPS R-568 in starvation medium (DMEM plus 0.5 mm Ca2+ plus 0.2% BSA). B, Functional rescue of class II mutants by overnight treatment with NPS R-568. Averaged results of three to seven experiments such as in panel A for cells treated overnight in DMSO (black bars) or NPS R-568 (open bars). Bars indicate average ± sd, and solid line indicates stimulated ERK1/2 phosphorylation of wt CaSR upon DMSO treatment. C, Increased expression of class II mutants after overnight treatment with NPS R-568. Cells treated as in panel A were immunoprecipitated with anti-FLAG M2 antibody and subjected to Western blotting under reducing conditions. Monomer + dimer bands for each lane were quantified, and all results were normalized to the abundance of wt CaSR in DMSO (indicated by solid line in blot). Averaged results of three to five independent transfections are plotted. Bars indicate average ± S.D.; black bars, DMSO treatment; open bars, 10 μm NPS R-568 treatment.
Discussion
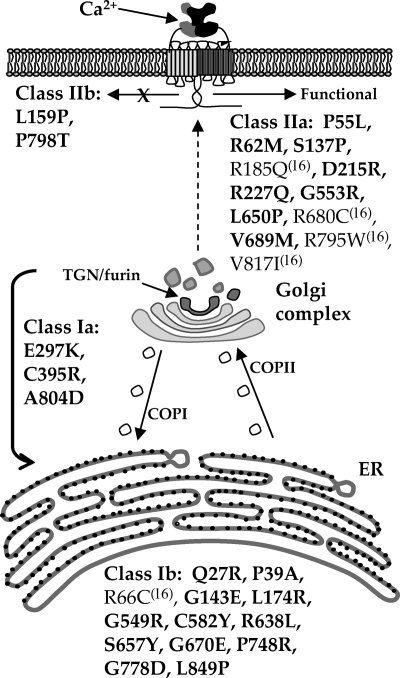
In this report, we have characterized the defects in 25 CaSR loss-of-function mutants. When combined with our previous study (16), we have sampled 30 representative CaSR loss-of-function mutants covering the ECD and TMD of human CaSR. We have used a multifaceted approach to characterize the cell biological effects of the loss-of-function point mutations, and our results strongly implicate folding and/or trafficking defects for the majority of these mutants. Figure 7 illustrates the distribution of mutants studied and their classification based on the organellar site of block. Of particular interest is that roughly 50% of the mutants are blocked from trafficking to the plasma membrane. We used a furin-mediated cleavage strategy to aid in determining whether any of the mutants were able to access the Golgi compartment. Only three of 16 mutants (class Ia) were able to traverse from the ER to the Golgi (TGN), where cleavage by furin would occur (29). These results suggest that class Ib mutants are targeted for degradation from the ER or ERGIC. Our results provide a mechanistic explanation for the lack of functional activity of some CaSR mutants studied by Ca2+ imaging or stimulated inositol phosphate production (34,35) and corroborate studies that demonstrate a lack of plasma membrane targeting for other mutants, e.g. R66C (36). Class Ia mutants (E297K, C395R, A804D) are interesting because they undergo significant furin-mediated cleavage but are not present in significant amounts at the plasma membrane as assayed by surface biotinylation. These mutants are therefore either retrieved to the ER via COPI vesicles for proteasome-mediated degradation or transiently trafficked to the plasma membrane before targeting to the lysosome.
Figure 7.
Schematic illustration of CaSR maturation through the secretory pathway. Results from this report (bold) and a previous study (16) allow sorting of CaSR loss-of-function mutants into class Ia, which traffic to the Golgi and class Ib, which are not able to exit the endoplasmic reticulum. Class II mutants reach the plasma membrane and can also be divided into two categories: class IIa mutants exhibit some activity that can be enhanced by rescue with NPS R-568, whereas class IIb mutants are not able to activate signaling despite enhanced plasma membrane localization.
Fifty percent of the loss-of-function CaSR mutants examined could be rescued to the plasma membrane with either MG132 or the membrane-permeant allosteric agonist NPS R-568. After rescue with NPS R-568, mutants P55L, R62M, S137P, D215G, R227Q, and G553R displayed activities equal to or greater than wt CaSR, and all but two mutants (L159P and P798T) were able to stimulate some level of ERK1/2 phosphorylation. The loss-of-function mutations studied were chosen because they have been documented to cause FHH or NSHPT and have been characterized in HEK293 cells to demonstrate a reduced apparent affinity for extracellular Ca2+ (reviewed in Ref. 37; www.casrdb. mcgill.ca/). Our current results suggest that increasing the amount of mutant receptor at the plasma membrane, i.e. increasing Vmax of signaling output, can rescue mutant receptor function to levels equivalent to wt CaSR. Rescue of the activity of a number of CaSR loss-of-function mutants by acute application of l-phenylalanine [an allosteric modulator that binds to the CaSR ECD (38)] and NPS R-467 (39) or NPS R-568 (40) has also been demonstrated. Treatment of patients harboring CaSR loss-of-function mutants with calcimimetics will likely invoke both mechanisms, i.e. calcimimetics will increase the biosynthetic success rate resulting in enhanced receptor targeting to the plasma membrane (Vmax) and will also act at plasma membrane-localized receptors to increase the efficacy of plasma Ca2+ in receptor activation (EC50). Indeed, a number of pathological conditions result in reduced expression of wt CaSR, e.g. parathyroid adenomas (41), colon cancers (42), breast cancer (43), and treatment with calcimimetics to enhance biosynthetic success, may serve to modulate disease progression.
With this survey of CaSR loss-of-function mutants we define CaSR as a member of the growing family of conformationally defective proteins (44) that cause human disease. A number of G protein-coupled receptors have been characterized in this regard, including V2 vasopressin receptors, in which trafficking defects cause nephrogenic diabetes insipidus and rescue can be achieved by V2R antagonists (45,46). GnRH receptor mutations that restrict trafficking cause hypogonadotropic hypogonadism, which can be rescued with membrane-permeant peptidomimetics (reviewed in Ref. 44). As the biosynthesis of other G protein-coupled receptors is examined, it seems likely that loss-of-function mutants that limit trafficking to the plasma membrane will be identified. The development of pharmacochaperones that rescue such trafficking defects will likely be a novel and important therapeutic strategy (reviewed in Refs. 44,47 and 48).
In conclusion, we have examined 30 representative loss-of-function point mutations localized throughout the ECD and TMD of human CaSR. We have defined two distinct classes of mutants that are either trapped within intracellular compartments (class I) or that localize to the plasma membrane without or with the aid of the pharmacochaperone NPS R-568 (class II). The approaches delineated here can be used to dissect the location of biosynthetic block and the sensitivity to pharmacochaperone rescue for novel CaSR mutations. Of considerable clinical interest is the finding that more than 50% of the studied loss-of-function CaSR mutants can be rescued and stabilized in functional form at the plasma membrane by the calcimimetic NPS R-568. Such biosynthetic rescue may provide a novel treatment approach for both FHH/NSHPT patients and for pathological conditions resulting from reduced wt CaSR expression.
Materials and Methods
Materials
Human CaSR and NPS R-568 were obtained from Dr. K. Seuwen (Novartis Pharmaceuticals, AG, Basel, Switzerland).
Plasmid construction
Human CaSR with an amino-terminal Flag epitope (31) was used as the template. The human CaSR clone contained the human polymorphism R990G. The furin cleavage site (ARRRKKRGLDV) was inserted between human CaSR amino acids D371 and T372 using inverse PCR mutagenesis (32) with Pfu UltraHighFidelity DNA polymerase (Stratagene, La Jolla, CA). All constructs were verified by sequencing the entire coding region (GeneWiz, Inc., Trenton, NJ).
Cell culture and transfection
HEK293 cells (American Type Culture Collection (ATCC); Manassas, VA) were cultured as per ATCC recommendations in MEM plus 10% fetal bovine serum, penicillin/streptomycin at 37 C, 5% CO2. Cells were transiently transfected with NovaFECTOR (Venn Nova, Inc., Pompano Beach, FL) according to manufacturer’s protocol and cultured at 37 C, 5% CO2, for 72 h before experiments. For cell treatments, stock solutions of MG132 or NPS R-568 in DMSO were added to DMEM plus 0.2% BSA. Cells were incubated at the indicated concentrations for 12–14 h before harvest. Control solutions contained equivalent volumes of DMSO.
Biotinylation
HEK293 cells were transiently transfected in six-well plates (0.5 μg cDNA/well). After 72 h, cells were treated with 50 μg/ml EZ-Link Sulfo-NHS-LC-Biotin (Pierce Chemical Co., Rockford, IL) in PBS for 15 min at 4 C, washed twice with cold PBS-EDTA, and lysed in PBS containing 5 mm EDTA, 0.5% Triton X-100, 10 mm iodoacetamide, and Cømplete EDTA-free protease inhibitor tablet (Roche, Indianapolis, IN). Biotinylated proteins were isolated by incubation for 30 min at 4 C with Streptavidin agarose (Invitrogen, Carlsbad, CA) and processed as for Western blotting.
Immunoprecipitation, SDS-PAGE, and Western blotting
Transfected HEK293 cells were lysed (PBS plus 5 mm EDTA, 0.5% Triton X-100, Complete protease inhibitor tablet (Boeringher Mannheim, Mannheim, Germany), 10 mm iodoacetamide), and proteins immunoprecipitated with M2 anti-Flag antibody (Sigma Chemical Co., St. Louis, MO) and resolved by SDS-PAGE on either 4–15% or 7.5% gels (Criterion; Bio-Rad Laboratories, Hercules, CA). Reducing sodium dodecyl sulfate sample buffer contained 0.1 m dithiothreitol (DTT). After transfer to nitrocellulose membranes, the presence of CaSR was detected with anti-CaSR polyclonal antibody (LRG, 1:2000, or ADD, 1:1000; custom-generated by Genemed Synthesis, Inc., San Antonio, TX) and visualized by enhanced chemiluminescence (SuperWest Pico Chemiluminescent substrate; Pierce) and quantitated using AlphaEaseFC software version 4.0.0 (Alpha Innotech Corp., San Leandro, CA).
CaSR functional assays
CaSR-mediated changes in ERK1/2 phosphorylation were measured as previously described (33) using cells transfected in 12- or 24-well plates stimulated with 0.5 or 5 mm Ca2+ for 10 min at 37 C after overnight starvation in 0.5 mm Ca2+-containing DMEM plus 0.2% BSA (Cohn fraction V; Sigma). For some experiments, the starvation solution also contained vehicle (DMSO) or 10 μm NPS R-568. Lysates were run on 4–15% gradient gels (Criterion; Bio-Rad), and Western blots were probed with anti-ERK1/2 phospho-specific antibodies (Cell Signaling Technologies, Inc., Danvers, MA). Blots were stripped and reprobed with anti-ERK1/2 antibody (Cell Signaling Technologies, Inc.). Parallel experiments on wt or mutant CaSR-transfected cells were treated with DMSO or NPS R-568 overnight and then processed for immunoprecipitation with anti-FLAG antibody.
Acknowledgments
We thank Ying (April) Huang, Ph.D. and Heather Walter, Weis Center for Research, for preliminary experiments, and members of the Breitwieser laboratory for helpful discussions.
Footnotes
This work was supported by National Institutes of Health Grant GM077563 and funds from the Geisinger Clinic (to G.E.B.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 23, 2009
Abbreviations: CaSR, Calcium-sensing receptor; COP, coat protein complex; CysRD, cysteine-rich domain; DMSO, dimethylsulfoxide; DTT, dithiothreitol; ECD, extracellular domain; ER, endoplasmic reticulum; ERGIC, ER-Golgi intermediate compartment; FHH, familial hypocalciuric hypercalcemia; HEK, human embryonic kidney; NSHPT, neonatal severe primary hyperparathyroidism; OMIM, Online Mendelian Inheritance in Man; TGN, trans-Golgi network; TMD, transmembrane domain; VFT, venus flytrap; VFTD, VFT domain; wt, wild type.
References
- Tfelt-Hansen J, Brown EM 2005 The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci 42:35–70 [DOI] [PubMed] [Google Scholar]
- Brown EM 1983 Four parameter model of the sigmoidal relations between parathyroid hormone release and extracellular calcium concentration in normal and abnormal parathyroid tissue. J Clin Endocrinol Metab 56:572–581 [DOI] [PubMed] [Google Scholar]
- Breitwieser GE, Gama L 2001 Calcium-sensing receptor activation induces intracellular calcium oscillations. Am J Physiol Cell Physiol 280:C1412–C1421 [DOI] [PubMed] [Google Scholar]
- Christensen SE, Nissen PH, Vestergaard P, Heickendorff L, Brixen K, Mosekilde L 2008 Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: a follow-up study on methods. Clin Endocrinol (Oxf) 69:713–720 [DOI] [PubMed] [Google Scholar]
- Egbuna OI, Brown EM 2008 Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations. Best Pract Res Clin Rheumatol 22:129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhardt A, Bai M, Lagarde JP, Rigaud M, Zhang Z, Jiang Y, Kottler ML, Brown EM, Garabédian M 2001 Activating mutations of the calcium-sensing receptor: management of hypocalcemia. J Clin Endocrinol Metab 86:5313–5323 [DOI] [PubMed] [Google Scholar]
- Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaître X, Paillard M, Planelles G, Déchaux M, Miller RT, Antignac C 2002 Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13:2259–2266 [DOI] [PubMed] [Google Scholar]
- Ward DT 2004 Calcium receptor-mediated intracellular signalling. Cell Calcium 35:217–228 [DOI] [PubMed] [Google Scholar]
- Hu J, Spiegel AM 2007 Structure and function of the human calcium-sensing receptor: insights from natural and engineered mutations and allosteric modulators. J Cell Mol Med 11:908–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhou Y, Yang W, Butters R, Lee HW, Li S, Castiblanco A, Brown EM, Yang JJ 2007 Identification and dissection of Ca2+-binding sites in the extracellular domain of Ca2+-sensing receptor. J Biol Chem 282:19000–19010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Reyes-Cruz G, Goldsmith PK, Gantt NM, Miller JL, Spiegel AM 2007 Functional effects of monoclonal antibodies to the purified amino-terminal extracellular domain of the human Ca2+ receptor. J Bone Miner Res 22:601–608 [DOI] [PubMed] [Google Scholar]
- Hu J, Hauache O, Spiegel AM 2000 Human Ca2+ receptor cysteine-rich domain: Analysis of function of mutant and chimeric receptors. J Biol Chem 275:16382–163889 [DOI] [PubMed] [Google Scholar]
- Ray K, Adipietro KA, Chen C, Northup JK 2007 Elucidation of the role of peptide linker in calcium-sensing receptor activation process. J Biol Chem 282:5310–5317 [DOI] [PubMed] [Google Scholar]
- Hu J, Reyes-Cruz G, Chen W, Jacobson KA, Spiegel AM 2002 Identification of acidic residues in the extracellular loops of the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+ and a positive allosteric modulator. J Biol Chem 277:46622–46631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedlich SU, Gama L, Seuwen K, Wolf RM, Breitwieser GE 2004 Homology modeling of the transmembrane domain of the human calcium sensing receptor and localization of an allosteric binding site. J Biol Chem 279:7254–7263 [DOI] [PubMed] [Google Scholar]
- Huang Y, Breitwieser GE 2007 Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J Biol Chem 282:9517–9525 [DOI] [PubMed] [Google Scholar]
- Amaral MD 2005 Processing of CFTR: traversing the cellular maze—how much CFTR needs to go through to avoid cystic fibrosis? Pediatr Pulmonol 39:479–491 [DOI] [PubMed] [Google Scholar]
- Wang X, Koulov AV, Kellner WA, Riordan JR, Balch WE 2008 Chemical and biological folding contribute to temperature-sensitive ΔF508 CFTR trafficking. Traffic 9:1878–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K 2000 Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407:971–977 [DOI] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H 2007 Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci USA 104:3759–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Niwa J, Sobue G, Breitwieser GE 2006 Calcium-sensing receptor ubiquitination and degradation mediated by the E3 ubiquitin ligase dorfin. J Biol Chem 281:11610–11617 [DOI] [PubMed] [Google Scholar]
- Fan G, Goldsmith PK, Collins R, Dunn CK, Krapcho KJ, Rogers KV, Spiegel AM 1997 N-linked glycosylation of the human Ca2+ receptor is essential for its expression at the cell surface. Endocrinology 138:1916–1922 [DOI] [PubMed] [Google Scholar]
- Ray K, Clapp P, Goldsmith PK, Spiegel AM 1998 Identification of the sites of N-linked glycosylation on the human calcium receptor and assessment of their role in cell surface expression and signal transduction. J Biol Chem 273:34558–34567 [DOI] [PubMed] [Google Scholar]
- Hebert DN, Molinari M 2007 In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev 87:1377–1408 [DOI] [PubMed] [Google Scholar]
- Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman JE, Balch WE 1994 Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol 125:51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J 2001 Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol 155:557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markkanen PM, Petaja-Repo UE 2008 N-glycan-mediated quality control in the endoplasmic reticulum is required for the expression of correctly folded δ-opioid receptors at the cell surface. J Biol Chem 283:29083–29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxis C, Vogel F, Wolf DH 2002 ER-Golgi traffic is a prerequisite for efficient ER degradation. Mol Biol Cell 13:1806–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G 2002 Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol 3:753–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith PK, Fan G, Miller JL, Rogers KV, Spiegel AM 1997 Monoclonal antibodies against synthetic peptides corresponding to the extracellular domain of the human Ca2+ receptor: characterization and use in studying concanavalin A inhibition. J Bone Miner Res 12:1780–1788 [DOI] [PubMed] [Google Scholar]
- Gama L, Wilt SG, Breitwieser GE 2001 Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem 276:39053–39059 [DOI] [PubMed] [Google Scholar]
- Gama L, Breitwieser GE 1999 Generation of epitope-tagged proteins by inverse PCR mutagenesis. Biotechniques 26:814–816 [DOI] [PubMed] [Google Scholar]
- Zhang M, Breitwieser GE 2005 High affinity interaction with filamin A protects against calcium-sensing receptor degradation. J Biol Chem 280:11140–11146 [DOI] [PubMed] [Google Scholar]
- Pearce SH, Bai M, Quinn SJ, Kifor O, Brown EM, Thakker RV 1996 Functional characterization of calcium-sensing receptor mutations expressed in human embryonic kidney cells. J Clin Invest 98:1860–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath H Odelberg S, Jackson CE, Teh BT, Hayward N, Larsson C, Buist, NRM, Krapcho KJ, Hung, BC, Capuano IV, Garrett JE, Leppert MF 1996 Clustered inactivating mutations and benign polymorphisms of the calcium receptor gene in familial benign hypocalciuric hypercalcemia suggest receptor functional domains. J Clin Endocrinol Metab 81:1312–1317 [DOI] [PubMed] [Google Scholar]
- Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN 2006 Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum Mol Genet 15:2200–2209 [DOI] [PubMed] [Google Scholar]
- Hu J, Spiegel AM 2003 Naturally occurring mutations of the extracellular Ca2+-sensing receptor: implications for its structure and function. Trends Endocrinol Metab 14:282–288 [DOI] [PubMed] [Google Scholar]
- Mun HC, Culverston EL, Franks AH, Collyer CA, Clifton-Bligh RJ, Conigrave AD 2005 A double mutation in the extracellular Ca2+-sensing receptor’s venus flytrap domain that selectively disables L-amino acid sensing. J Biol Chem 280:29067–29072 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jiang Y, Quinn SJ, Krapcho K, Nemeth EF, Bai M 2002 l-Phenylalanine and NPS R-467 potentiate the function of the extracellular calcium-sensing receptor through distinct sites. J Biol Chem 277:33736–33741 [DOI] [PubMed] [Google Scholar]
- Rus R, Haag C, Bumke-Vogt C, Bahr V, Mayr B, Mohlig M, Schulze E, Frank-Raue K, Raue F, Schofl C 2008 Novel inactivating mutations of the calcium-sensing receptor: the calcimimetic NPS R-568 improves signal transduction of mutant receptors. J Clin Endocrinol Metab 93:2797–2803 [DOI] [PubMed] [Google Scholar]
- Yano S, Sugimoto T, Tsukamoto T, Chihara K, Kobayashi A, Kitazawa S, Maeda S, Kitazawa R 2003 Decrease in vitamin D receptor and calcium-sensing receptor in highly proliferative parathyroid adenomas. Eur J Endocrinol 148:403–411 [DOI] [PubMed] [Google Scholar]
- Kállay E, Bonner E, Wrba F, Thakker RV, Peterlik M, Cross HS 2003 Molecular and functional characterization of the extracellular calcium-sensing receptor in human colon cancer cells. Oncol Res 13:551–559 [DOI] [PubMed] [Google Scholar]
- Liu G, Hu X, Chakrabarty S 2009 Calcium sensing receptor down-regulates malignant cell behavior and promotes chemosensitivity in human breast cancer cells. Cell Calcium 45:216–225 [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM 2004 Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 5:821–837 [DOI] [PubMed] [Google Scholar]
- Wüller S, Wiesner B, Löffler A, Furkert J, Krause G, Hermosilla R, Schaefer M, Schülein R, Rosenthal, W, Oksche A 2004 Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biol Chem 279:47254–47263 [DOI] [PubMed] [Google Scholar]
- Robben JH, Sze M, Knoers NVAM, Deen PMT 2007 Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: relevance to therapy of nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 272:253–260 [DOI] [PubMed] [Google Scholar]
- Bernier V, Bichet DG, Bouvier M 2004 Pharmacological chaperone action on G protein-coupled-receptors. Curr Opin Pharmacol 4:528–533 [DOI] [PubMed] [Google Scholar]
- Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA 2007 G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev 59:225–250 [DOI] [PubMed] [Google Scholar]