Abstract
Recent studies have demonstrated a clear role for pituitary adenylate cyclase-activating polypeptide (PACAP) in the regulation of gonadotropin biosynthesis and secretion, both alone and in conjunction with GnRH. First defined as a hypothalamic releasing factor, PACAP subsequently has been identified in the gonadotrope subpopulation of the anterior pituitary gland, suggesting that PACAP may act as an autocrine-paracrine factor in this tissue. In initial studies, we determined that GnRH markedly stimulated endogenous PACAP mRNA levels and promoter-reporter activity in the mature gonadotrope cell line, LβT2. GnRH-stimulated rat PACAP promoter activity was blunted with deletion from position −915 to −402 and eliminated with further truncation to position −77 relative to the transcriptional start site. Site-directed mutagenesis demonstrated a functional requirement for a cAMP response element (CRE)-like site at position −205 and an activating protein-1 (AP-1)-like site at position −275, both of which bound CRE binding protein and AP-1 family members on EMSA. Treatment with pharmacological activators or inhibitors of second messenger signaling pathways implicated the protein kinase A, protein kinase C, and MAPK pathways in the GnRH response. In support of these in vitro data, we demonstrate that JunB binds to the rat PACAP gene promoter by chromatin immunoprecipitation assay and that small interfering RNA knockdown of JunB, cFos, and CRE binding protein factors blunts PACAP expression. In summary, these results further elucidate the complex functional interactions between PACAP and GnRH in the anterior pituitary. Specifically, these studies demonstrate that GnRH-stimulated PACAP gene expression is mediated via multiple signaling pathways acting on CRE/AP-1 sites in the proximal gene promoter. Because both PACAP and GnRH regulate gonadotropin biosynthesis and secretion, these results provide important insight into the critical fine tuning of gonadotrope function and, thereby, the maintenance of normal reproductive function.
GnRH stimulates pituitary PACAP gene expression via multiple signaling pathways acting on two CRE/AP-1 sites in the proximal gene promoter.
Normal reproductive function requires the precise control of pituitary gonadotropin biosynthesis and secretion as achieved through the complex interaction of multiple hormones arising from the hypothalamus, gonads, and anterior pituitary gland itself. In addition to the well-characterized effects of hypothalamic GnRH, gonadotropin gene expression is further regulated by the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP). PACAP, like GnRH, is secreted by hypothalamic neurons into the pituitary portal vasculature, binding to specific G protein-coupled receptors (PAC1-R) on pituitary cell membranes and activating the cAMP/protein kinase A (PKA) signaling system (1,2).
A member of the secretin/glucagon/vasoactive intestinal peptide/GHRH polypeptide family, PACAP was originally isolated from the ovine hypothalamus based on its ability to stimulate cAMP formation in rat pituitary cells (3). Although first identified as a hypothalamic-releasing factor, PACAP subsequently has been determined to have widespread distribution and function, including expression in the central and peripheral nervous systems, smooth muscle of the lung and intestinal tract, and endocrine organs, including the anterior and posterior pituitary, gonads, placenta, adrenal, parathyroid, and endocrine pancreas.
In the anterior pituitary gland, PACAP modulates gonadotropin biosynthesis and secretion, acting both alone and in concert with GnRH. Although less potent than GnRH, PACAP has been demonstrated to increase secretion of LH, FSH, and free α-subunit by perifused primary pituitary cells (4,5,6). The stimulatory effects of PACAP on LH secretion have been confirmed in vivo (7). PACAP also has been shown to regulate α-, LHβ, FSHβ, and GnRH-receptor gene promoter activity and mRNA levels (8,9,10,11,12,13,14). Furthermore, PACAP stimulates gonadotrope and folliculostellate cell production of follistatin, a binding protein that blocks activin stimulation of FSH biosynthesis (15).
In 1998, Koves et al. (16,17,18) reported PACAP expression in the anterior pituitary gland. Based on colocalization with LH and FSH immunoreactivity, the majority of primary gonadotropes synthesize and secrete PACAP protein. The ability of gonadotropes to express PACAP has been confirmed in an immature gonadotrope cell line, αT3-1 (19). In a separate study, PACAP expression was not detected in non-gonadotrope hormone-secreting pituitary cells; however, PACAP mRNA was identified in the supportive folliculostellate cells (20). Thus, PACAP is both expressed by and acts on gonadotropes, forming a functional autocrine loop.
Despite its critical role in multiple physiological systems, relatively little is known about the hormones and signaling pathways that regulate PACAP gene expression. Therefore, the overall goal of our study was to define the hormonal factor(s) that regulate PACAP expression in pituitary gonadotropes. In preliminary studies, we had observed a marked increase in PACAP gene promoter activity after GnRH treatment in the LβT2 gonadotrope cell line. GnRH has been shown to bind to specific G protein-coupled receptors on the gonadotrope cell membrane, thereby activating a complex array of intracellular signaling systems including the protein kinase C (PKC), MAPK-kinase (MEK), PKA, p38 MAPK, and calcium-calmodulin systems (21,22,23,24,25). Based on this information, we hypothesized that GnRH-stimulated PACAP gene expression is mediated by a combination of these pathways acting via multiple DNA-regulatory regions. Consistent with this hypothesis, the results presented here demonstrate that GnRH increases PACAP gene expression via both the PKA and PKC intracellular signaling pathways acting on two cAMP response element (CRE)/activating protein-1 (AP-1) sites in the proximal PACAP gene promoter.
Results
Expression of PACAP mRNA in gonadotropes
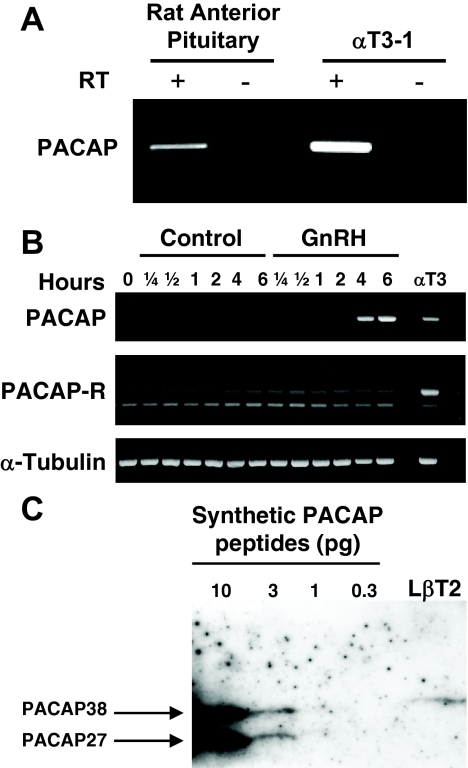
The ability of gonadotropes to biosynthesize and secrete PACAP has been reported previously in primary pituitary cells as well as in the αT3-1 cell line (19). We wished to confirm these findings as well as extend them to the more mature LβT2 cells, which express the common α-, LHβ, and FSHβ subunits, as well as the GnRH-receptor (26). As shown in Fig. 1A, PACAP transcripts were readily identified by RT-PCR in the male rat anterior pituitary gland and in the αT3-1 cell line under basal conditions. Treatment with GnRH did not appreciably change PACAP mRNA levels in this cell line (data not shown). In contrast, PACAP transcripts were not detectable in untreated LβT2 gonadotrope cells but were markedly induced by GnRH treatment, supporting a role for GnRH in the regulation of PACAP gene expression in mature gonadotropes (Fig. 1B). The LβT2 cells were also observed to express transcripts encoding the PACAP receptor, consistent with a role for PACAP in autocrine regulation of gonadotrope function. These transcripts were found to encode the short and hip or hop variants (data not shown). Western blot analysis confirmed the expression of PACAP38 peptide in these cells (Fig. 1C).
Figure 1.
Expression of PACAP and PACAP receptor in rat pituitary tissue and mouse gonadotrope cell lines. A, Total RNA from either adult male rat pituitaries or untreated mouse αT3-1 cells was reverse transcribed in the presence (+) or absence (−) of reverse transcriptase (RT) and analyzed by PCR for 35 cycles with intron-spanning PACAP-specific primers. B, Mouse LβT2 cells were treated for indicated times with 100 nm GnRH. The presence of PACAP or PACAP-R mRNA was evaluated by RT-PCR. The level of α-tubulin mRNA expression in each sample was used as a loading control. Detection of PACAP mRNA in untreated αT3-1 cells was included as a positive control. C, LβT2 membrane protein extract (20 μg/lane) was separated on a 16% tricine gel, transferred to polyvinylidine difluoride membrane, and blotted with polyclonal rabbit anti-PACAP IgG recognizing both PACAP38 and PACAP27. Serial dilutions of synthetic PACAP38 and PACAP27 peptides were included as positive controls. PACAP-R, PACAP receptor.
Dose-response experiments demonstrated maximal stimulation of PACAP mRNA expression (nearly 1000-fold) after 10 nm GnRH treatment, with a peak response achieved after 9 h of treatment (supplemental Fig. 1, A and B, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Similarly, PACAP gene promoter activity was maximally induced by 10 nm GnRH treatment using a transient transfection luciferase-reporter approach (supplemental Fig. 1C).
The GnRH response maps to CRE and AP-1 sites in the rat PACAP gene promoter
We next wished to determine the region(s) of the PACAP gene promoter that confer GnRH responsiveness using a transient transfection approach. GnRH is known to increase gene expression in gonadotrope cells via the PKC, MAPK, and calcium intracellular signaling pathways. More recently, it has been appreciated that GnRH may also activate the PKA system (21,23,24,27).
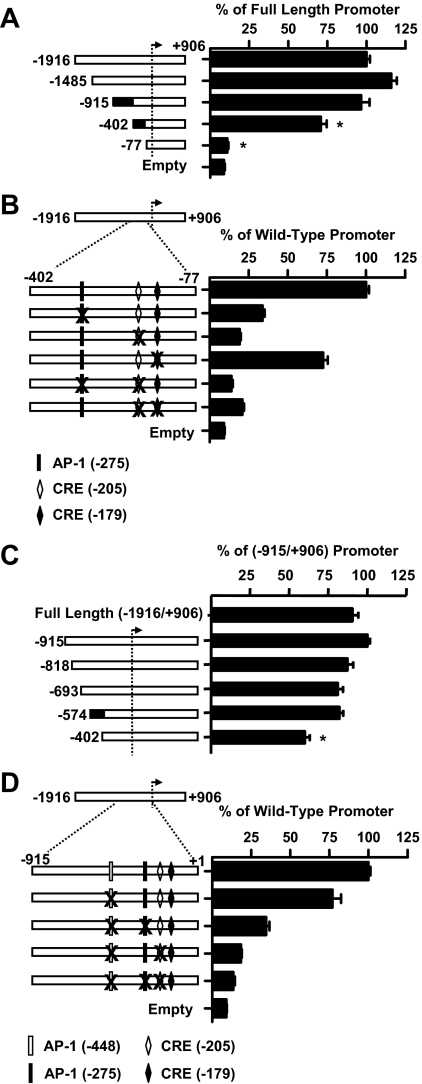
The rat PACAP gene promoter contains multiple regions with similarity to the consensus CRE or the consensus AP-1 cis-element, well-described mediators of PKA and PKC signaling, respectively (Fig. 2). Transient transfection experiments were performed in the gonadotrope LβT2 cell line to analyze the importance of the putative cis-elements. Transfection of serial 5′-deletions of the rat PACAP gene promoter fused to the pGL3 luciferase reporter demonstrated a significant loss of GnRH responsiveness between bp −915 and −402 (29%) with a further dramatic loss of promoter activity after deletion to position −77 (89%) (Fig. 3A).
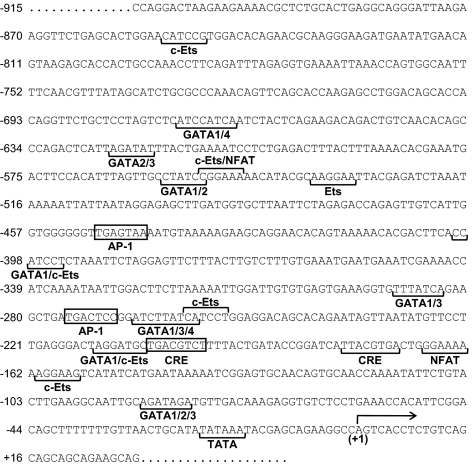
Figure 2.
Schematic of putative DNA-regulatory elements in the rat PACAP gene promoter. Putative binding sites for cEts, GATA, nuclear factor of activated T cells (NFAT), AP-1, and the CRE were identified by sequence homology to reported mouse and rat consensus sequences using TESS web-based software. Each of these factors has been shown to mediate GnRH responsiveness in other genes (54). Promoter elements shown within this study to confer GnRH responsiveness to the PACAP promoter are boxed. The AP-1-like element at position −275 and the CRE-like element at position −205 in the rat PACAP gene will be characterized in detail.
Figure 3.
Two regions of the rat PACAP gene promoter are critical for full GnRH responsiveness. Gonadotrope LβT2 cells were transiently transfected with various lengths of the rat PACAP gene promoter fused to a luciferase reporter followed by treatment with 100 nm GnRH for 6 h. A, Serial 5′-truncation analysis of the rat PACAP promoter across region −1916 to +906 relative to the transcriptional start site. *, P < 0.001 vs. the full-length construct. B, Effect of single and combinatorial site-directed mutations in the putative AP-1 and CRE identified by sequence homology in region −402 to −77 (−275 AP-1, −205 CRE, −179 CRE). All mutations resulted in a statistically significant loss of GnRH responsiveness at P < 0.001 vs. the wild-type construct. C, Detailed serial 5′-truncation analysis between positions −915 and −402. *, P < 0.001 vs. next longer construct. D, Site-directed mutation of the putative AP-1 element at position −448 and combinatorial site-directed mutagenesis of the putative −275 AP-1 and −205 CRE within region −915 to −77 identified as critical for GnRH responsiveness. All experiments were performed a minimum of three times with data expressed as the mean ± sem.
By sequence homology, two CRE-like elements, located at positions −179 and −205, and one AP-1-like element, located at position −275, were identified within region −402 to −77 (Fig. 2). These elements were mutated singly or in combination and analyzed for effects on the GnRH response (Fig. 3B). Mutation of the proximal CRE-like element at −179 showed a modest, albeit significant, blunting of the response, but the greatest losses were observed after mutation of the putative AP-1-like element at position −275 and the CRE at position −205, which blunted the GnRH response by approximately 70% and 80%, respectively, in comparison with the full-length wild-type promoter. Combined mutation of −205 CRE and −275 AP-1 led to a further (86%) reduction in promoter activity.
An additional series of 5′-truncations between region −915 and −402 were also tested to further characterize the additional GnRH-responsive region. As shown in Fig. 3C, the GnRH response was significantly decreased with deletion between positions −574 and −402 in the PACAP promoter. Site-directed mutations were introduced into the −557 GATA, −552 cEts/NFAT, −537 cEts, and −448 AP-1 sites identified by sequence analysis (Fig. 2). Stimulation of PACAP promoter activity by GnRH was blunted with mutation in the −448 AP-1 site (Fig. 3D), but not in the other putative cis-elements (data not shown). Although mutation of −448 AP-1 alone resulted in a 23% reduction in PACAP promoter reporter activity, combined mutation of −448 AP-1 with the proximal AP-1 and CRE elements did not lead to further reductions in promoter activity compared with mutation of the proximal elements alone (Fig. 3, B and D).
Taken together, these data demonstrate that the full GnRH response is dependent on an intact CRE at position −205 and AP-1 cis-elements at positions −448 and −275 in the rat PACAP gene promoter. With the exception of a single base difference in the −448 AP-1 site, these cis-elements are fully conserved in the rat, mouse, and human PACAP gene, strongly suggesting functional relevance. Also of note, mutation at these sites did not significantly alter basal expression, arguing against their importance for basal promoter activation (data not shown). For the next series of experiments, the decision was made to focus our attention on the CRE and AP-1 cis-elements within region −402/−77 because these sites were the most critical in conferring GnRH responsiveness.
The PKA and PKC signaling pathways are critical mediators of PACAP promoter activity
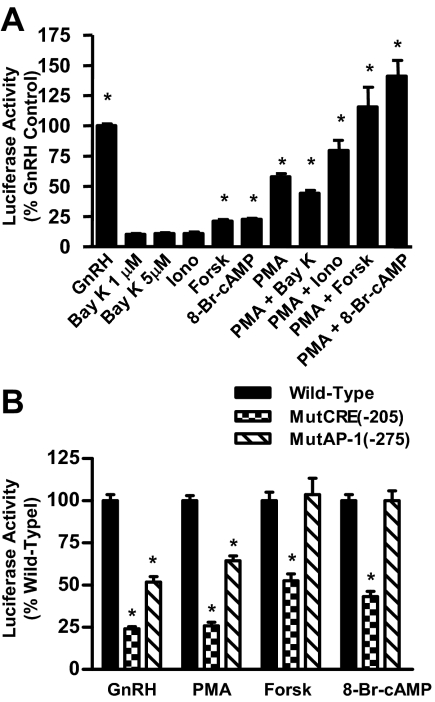
GnRH has been demonstrated to activate the PKA, PKC, and calcium-signaling systems. LβT2 cells were treated with various pharmacological activators to evaluate the effects of each of these pathways on the rat PACAP gene promoter. As shown in Fig. 4A, PACAP gene promoter activity was significantly increased by the addition of the phorbol ester, phorbol 12-myristate 13-acetate (PMA), which activates the PKC system (12-fold) and to a lesser degree by the adenylyl cyclase-activating agent, forskolin, and the stable cAMP analog, 8-bromo-cAMP (8-Br-cAMP) (4-fold and 5-fold, respectively). Furthermore, activation of these two pathways synergized to increase PACAP gene promoter activity. In contrast, neither the L-type calcium channel agonist, BayK 8644, nor the calcium ionophore, ionomycin, significantly increased PACAP gene expression when added alone, although the response to PMA was augmented with ionomycin-induced increases in intracellular calcium levels.
Figure 4.
Activation of the PKA and PKC signaling systems increases PACAP promoter activity. A, LβT2 cells were transiently transfected with the full-length PACAP reporter construct followed by treatment for 6 h with pharmacological activators of calcium (Bay K, ionomycin), PKA (forskolin, 8-Br-cAMP), and/or PKC (PMA) intracellular signaling pathways. All experiments were performed a minimum of six times with data expressed relative to the GnRH response, which was set at 100%. Data shown as the mean ± sem. *, P < 0.005 vs. vehicle control. B, Role of PACAP promoter elements in pathway-specific promoter activation. LβT2 cells were transiently transfected with wild-type or mutated PACAP reporter constructs followed by treatment for 6 h with the indicated activator. All experiments were performed a minimum of three times with the wild-type response set at 100% and data expressed as the mean ± sem. *, P < 0.005 vs. wild-type PACAP promoter response. Forsk, Forskolin; lono, lonomycin.
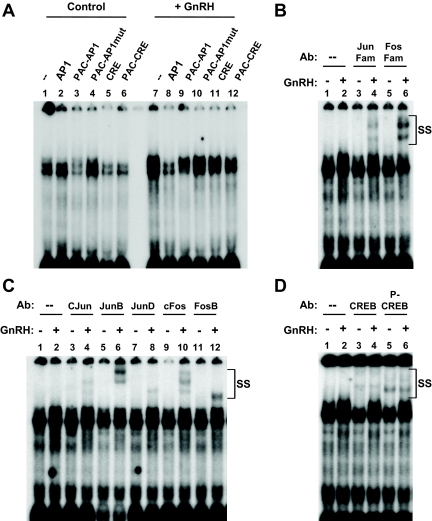
The relationship between the identified cis-elements and the PKA and PKC signaling pathways was tested in Fig. 4B. As observed previously, mutation of either −205 CRE or −275 AP-1 markedly decreased PACAP promoter activation in response to GnRH treatment. PKC-mediated promoter activation by PMA was blunted with mutation in either the −205 CRE or the −275 AP-1 cis-elements (P < 0.005 relative to the wild-type response) whereas PKA-mediated promoter activation by forskolin or 8-Br-cAMP was blunted only by mutation at −205 CRE, demonstrating that promoter activation via the PKA signaling pathway was independent of the AP-1 promoter element. These data suggest that the −205 CRE provides GnRH responsiveness via both the PKC and PKA pathways whereas promoter activity at the −275 AP-1 site is mediated primarily via the PKC pathway.
The importance of these pathways was confirmed using pharmacological inhibitors. Inhibition of the PKA or PKC pathways decreased the GnRH response by nearly 50% with an even greater loss in the presence of both inhibitors (supplemental Fig. 2A). GnRH-stimulated PACAP promoter activity was likewise blunted with inhibition of the MAPKK (MEK) and p38MAPK pathways (supplemental Fig. 2B). GnRH-dependent transcription also appears to be calcium dependent, most likely due to the release of intracellular calcium stores because blocking calcium channels had minimal effect (supplemental Fig. 2C). The importance of the cAMP/PKA system was confirmed by cotransfection of GnRH-treated LβT2 cells with expression vectors for either a phosphorylation-deficient mutant [CRE binding protein (CREB)-M2] or a DNA-binding mutant that acts as a dominant negative (K-CREB), both of which significantly blunted the ability of GnRH to stimulate −1916/+906 rat PACAP gene promoter activity (supplemental Fig. 2D).
LβT2 gonadotrope nuclear extract binds to the putative CRE and AP-1 sites in the rat PACAP gene promoter
By EMSA, LβT2 nuclear extracts were observed to bind to oligonucleotide probes containing the rat PACAP CRE region (−218/−186) and AP-1 region (−285/−259) (supplemental Fig. 3, A and B). GnRH treatment did not alter the pattern or intensity of nuclear protein binding to the CRE region. In contrast, GnRH treatment augmented DNA-protein binding on the PACAP-AP-1 region, with the observed increase continuing for 4 h.
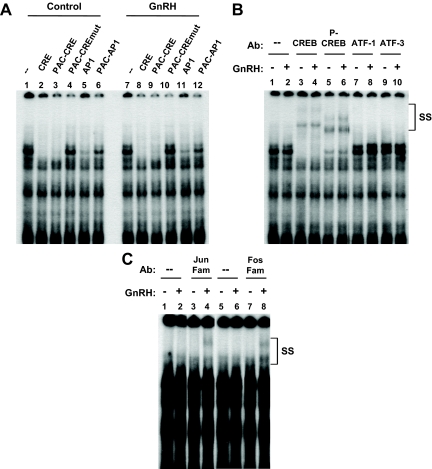
Subsequent EMSA experiments were aimed at identifying the proteins bound to these promoter regions. In analysis of the PACAP-CRE region, two major complexes were successfully competed by excess cold oligonucleotides containing a consensus CRE or the PACAP-CRE (Fig. 5A, lanes 2, 3, 8, and 9), but not by addition of an oligonucleotide containing a mutation in the PACAP-CRE sequence corresponding to the mutation used in transfection experiments (lanes 4 and 10). Of interest, complex formation also was inhibited by addition of a consensus AP-1 or the PACAP AP-1 oligonucleotide, albeit to a lesser extent than observed for the CREs (lanes 5, 6, 11, and 12). The presence of CREB in these complexes was confirmed by the addition of an antibody that recognizes both phosphorylated and nonphosphorylated members of the CREB family (Fig. 5B, lanes 3 and 4), as well as an antibody specific for the phosphorylated form of this transcription factor (lanes 5 and 6).
Figure 5.
Identification of gonadotrope nuclear proteins that bind to the putative rat PACAP gene CRE cis-element. LβT2 gonadotrope cell nuclear extracts were incubated with a 32P-labeled oligonucleotide probe spanning region −218 to −186 (PAC-CRE). A, LβT2 nuclear extracts were collected after culture with vehicle (lanes 1–6) or 100 nm GnRH for 4 h (lanes 7–12). Excess unlabeled oligonucleotide was added 20 min before the addition of labeled probe and consisted of a consensus CRE site (CRE; lanes 2 and 8), the PACAP CRE region (PAC-CRE; lanes 3 and 9), the PAC-CRE with mutation in the putative CRE site (PAC-CREmut; lanes 4 and 10), a consensus AP-1 site (AP1; lanes 5 and 11), or the PACAP AP-1 region (PAC-AP1; lanes 6 and 12). B, LβT2 nuclear extracts were collected following culture with vehicle (odd numbered lanes) or 100 nm GnRH for 4 h (even numbered lanes). Antibodies directed against CREB family members were added to the incubation mixture as indicated. P-CREB, Phosphorylated CREB. C, LβT2 nuclear extracts were collected after culture with vehicle (odd numbered lanes) or 100 nm GnRH for 4 h (even numbered lanes). Antibodies that recognize the Jun and Fos family members were evaluated for their ability to generate a supershifted complex (lanes 2 and 4 for Jun and 6 and 8 for Fos family members, respectively). SS, Supershifted complexes; Ab, antibody.
Because complex formation was partially inhibited by oligonucleotides containing AP-1 elements, we next added antibodies that recognize members of the Jun family or the Fos family (Fig. 5C). No supershift was observed with untreated nuclear extracts; however, both Jun (lane 4) and Fos (lane 8) family members were detected after GnRH treatment. Antibodies against specific AP-1 family members were also tested and demonstrated the presence of JunB and cFos within these complexes (data not shown). Taken together, these data demonstrate that the −205 CRE-like site binds both CREB and AP-1 proteins.
AP-1 sequences classically interact with members of the Jun and Fos immediate early gene families, present either as heterodimers or, less commonly, as Jun-Jun homodimers. Using the putative PACAP-AP-1 region as a probe, proteins present in LβT2 nuclear extracts were shown to bind to this region (Fig. 6A, left panel). The specificity of these complexes was confirmed by successful competition with an oligonucleotide containing either a consensus AP-1 or the PACAP-AP-1 cis-element (lanes 2 and 3), but not with a mutated PACAP-AP-1 sequence (lane 4). Surprisingly, both the consensus CRE and PAC-CRE oligonucleotides also were able to inhibit complex formation (lanes 5 and 6).
Figure 6.
Identification of gonadotrope nuclear proteins that bind the putative rat PACAP AP-1 cis-element. LβT2 gonadotrope cell nuclear extracts were incubated with a 32P-labeled oligonucleotide probe spanning region −285 to −259 (PAC-AP1). A, LβT2 nuclear extracts were collected after culture with vehicle (lanes 1–6) or 100 nm GnRH for 4 h (lanes 7–12). Excess unlabeled oligonucleotide was added 20 min before the addition of labeled probe and consisted of a consensus AP-1 site (AP1; lanes 2 and 8), the PACAP AP-1 region (PAC-AP1; lanes 3 and 9), the PAC-AP1 with mutation in the putative AP-1 site (PAC-AP1mut; lanes 4 and 10), a consensus CRE site (CRE; lanes 5 and 11), and the PACAP CRE region (PAC-CRE; lanes 6 and 12). B–D, LβT2 nuclear extracts were collected after culture with vehicle (odd numbered lanes) or 100 nm GnRH for 4 h (even numbered lanes). Antisera broadly reactive against Jun or Fos family members (B, lanes 3–6), with specificity for individual Jun or Fos isoforms (C, lanes 3–12), or CREB family members (D, lanes 3 and 4), or phosphorylated CREB (D, lanes 5 and 6) were added to the incubation mixture as indicated. SS, Supershifted complexes; Ab, antibody.
As shown in supplemental Fig. 3B, treatment of the LβT2 cells with GnRH before isolating the nuclear proteins substantially increased the intensity of the observed complexes on the AP-1 site, with particular augmentation of the larger complexes (Fig. 6A, right panel). Cold competition results were consistent with those observed from untreated cells. Addition of antibodies against Jun (Fig. 6B, lanes 3 and 4) or Fos (lanes 5 and 6) family members generated supershifts in LβT2 nuclear extracts after GnRH stimulation, but not in the basal state. Using isoform-specific antibodies, cJun, JunB, JunD, cFos, and FosB each were detected in the GnRH-induced complex (Fig. 6C). Based on the ability of CRE sequences to block complex formation, antibodies that recognize CREB family members were also tested for their effect on complex formation. As shown in Fig. 6D, CREB protein was detected by CREB antibody supershift in nuclear extracts from both basal and GnRH-treated cells. These EMSA data demonstrate the ability of both AP-1 and CREB proteins to bind to the rat PACAP −275 AP-1 region, similar to the results observed for the −205 CRE-like region.
GnRH increases JunB binding to the PACAP gene promoter in vivo
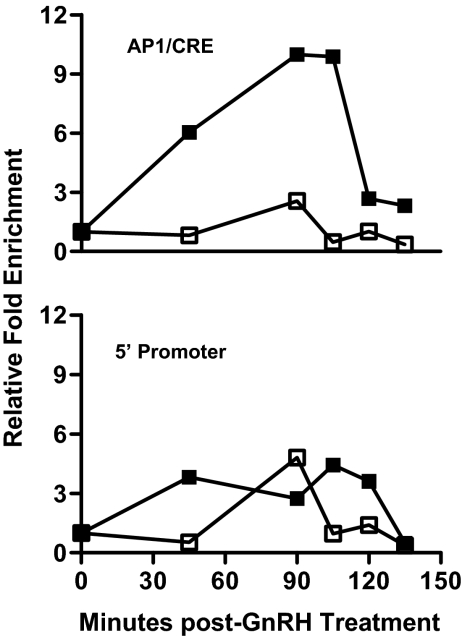
Chromatin immunoprecipitation (ChIP) analysis was performed to evaluate the ability of JunB to bind to the endogenous PACAP gene promoter in the LβT2 cell line. For these experiments, we were unable to design primers to distinguish between the −275 AP-1 and −205 CRE sites due to their proximity within the promoter. As shown in Fig. 7, GnRH increased JunB binding to the PACAP AP-1 and/or CRE sites with an approximately 10-fold enrichment over vehicle at 90 min after treatment. These data support the importance of this AP-1 protein in mediating the GnRH response.
Figure 7.
JunB binds to the PACAP promoter in the region spanning the AP-1 and CRE sites on ChIP analysis. ChIP analysis was performed using LβT2 cells treated with 100 nm GnRH and cross-linked at the times indicated after treatment. Precleared chromatin samples were immunoprecipitated with control rabbit IgG (□) or JunB specific (▪) rabbit polyclonal antibodies. Eluted DNA was analyzed by SYBR-based qPCR using primers amplifying (−308 to −168) of the mouse PACAP promoter, a region spanning the cis-elements analogous to rat AP-1 (−275) and CRE (−205) (AP1/CRE in top panel), or primers amplifying the distal mouse PACAP promoter (−2787 to −2658) (5′ Promoter in bottom panel) relative to the mouse PACAP transcriptional start site. Data shown are representative of three independent experiments with similar results.
Jun and Fos stimulate PACAP promoter activity
Based on both our in vitro and in vivo results, GnRH recruits Jun and Fos family members to the rat PACAP gene promoter within both the −275 and −205 regions. Transient transfection with expression vectors encoding these immediate early genes demonstrated minimal, if any, effect with individual overexpression; however, a subset of Jun-Fos combinations were able to stimulate basal PACAP promoter activity by up to 5-fold (supplemental Fig. 4, A and B). GnRH-induced PACAP gene promoter activity was augmented by cotransfection with combinations of Jun and Fos proteins (supplemental Fig. 4C). These experiments further support a role for AP-1 transcription factors in mediating PACAP gene expression.
Small interfering RNA (siRNA) knockdown of endogenous CREB, JunB, or cFos blunts GnRH-stimulated PACAP gene expression
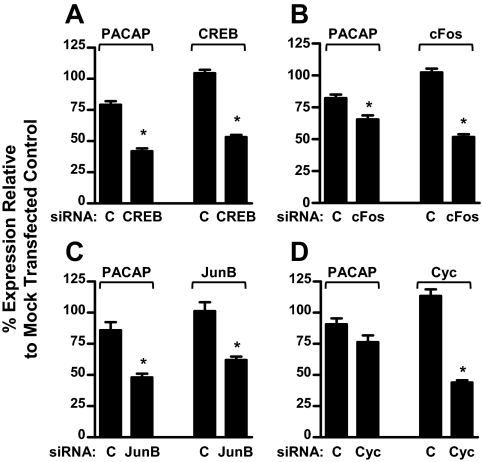
Based on EMSA analysis, CREB, Jun, and Fos proteins all bind to the PACAP gene promoter (Figs. 5 and 6). We reasoned, therefore, that decreasing the intracellular expression of these transcription factors using a siRNA approach should blunt the ability of GnRH to stimulate PACAP gene expression. GnRH-induced PACAP mRNA levels were significantly decreased in the presence of siRNAs directed against CREB, JunB, or cFos (P < 0.001) (Fig. 8, A–C). Similar results were obtained using second siRNAs directed to an alternative part of each transcript for the AP-1 proteins (data not shown). Addition of a siRNA that targets cyclophilin B, used as a negative control, did not alter PACAP expression (Fig. 8D). These data strongly support a requirement for these transcription factors in achieving maximal GnRH-stimulated PACAP gene expression.
Figure 8.
Effect of siRNA knockdown of CREB, c-Fos, and JunB on GnRH-induced PACAP mRNA expression. LβT2 cells were transfected with 30 nm siRNAs directed against CREB (panel A), cFos (panel B), JunB (panel C), control cyclophilin B (panel D), or control nontargeting siRNA (designated as “C” in each panel), and treated for 6 h with 100 nm GnRH. Mouse PACAP or siRNA target gene mRNA levels were quantified by qRT-PCR. Experiments in which the siRNA target mRNA knockdown was less than 25% were excluded from the analysis. Results are expressed as percent relative to mock-transfected controls and represent a minimum of six experiments. *, P < 0.001 vs. nontargeting control siRNA. Cyc, cyclophilin B.
Discussion
Although originally described as a hypothalamic-releasing factor, PACAP is now known to be widely expressed by the anterior pituitary gonadotropes. Substantial progress has been made in our understanding of the effects of PACAP in a wide array of physiological systems. In contrast, surprisingly little is known regarding the regulation of PACAP gene expression in any tissue and, before the current study, no information was available regarding regulation of this gene in the pituitary gland. In the studies reported here, we characterize the molecular mechanisms that mediate GnRH-induced stimulation of pituitary PACAP gene expression.
Before this report, limited evidence obtained in nongonadotrope cells had demonstrated regulation of PACAP gene expression by both peptide and steroid signals that are critical components of normal reproductive function. For example, the gonadal steroids, estradiol and progesterone, have been suggested to regulate PACAP expression in the hypothalamus and ovary (28,29). Additional studies have shown an increase in PACAP mRNA levels after treatment of granulosa cells with GnRH, LH, or FSH (30,31). In a pheochromocytoma cell line, PACAP biosynthesis was increased by nerve growth factor or after activation of the PKC or adenylate-cyclase signaling pathways (32). Their study demonstrated loss of forskolin responsiveness with mutation of a putative CRE site; however, the details of this molecular pathway were not further characterized.
Our data establish marked GnRH-induced stimulation of PACAP biosynthesis in gonadotrope cells, consistent with the observed effect of this peptide hormone on granulosa cells. Furthermore, gonadotrope expression of PACAP was increased via both the PKA and PKC pathways as reported in the neuroendocrine cell line. Our studies now demonstrate an additional role for the MAPK-signaling pathway as well as intracellular calcium in regulating PACAP gene expression. We have extended these observations and identified three DNA-regulatory elements located at positions −205, −275, and −448 in the proximal PACAP promoter that are downstream of these signaling pathways and required for full GnRH responsiveness. Based on homology to consensus sequences, the cis-element at position −205 more closely resembles a CRE, and the cis-element at position −275 more closely resembles an AP-1 binding site. Nevertheless, as demonstrated by EMSA, each of these sites binds members of both the CREB/ATF and Jun-Fos immediate-early gene families. A second AP-1 binding site located further upstream in the promoter also contributes to the GnRH response and awaits further characterization.
CREB and AP-1 are members of a large group of transcription factors, the bZIP family, that contain a highly conserved basic region involved in DNA binding and a leucine zipper, which promotes dimerization. The AP-1 proteins consist of Jun family members (c-Jun, JunB, and JunD) and Fos family members (cFos, FosB, Fra-1, and Fra-2) that dimerize to form Jun:Jun homodimers and Jun:Fos heterodimers. As observed in our studies, AP-1 proteins are generally expressed at low levels before hormonal stimulation and, therefore, the number and type of dimers present in a cell depend on cell type and activation state (33,34,35).
In contrast, CREB family members are constitutively expressed at high levels in most cell types. CREB transcriptional activity is believed to depend upon PKA-mediated phosphorylation at the Ser133 residue and subsequent recruitment of CREB-binding protein (CBP), which links CREB to the transcriptional machinery. GnRH has been shown to increase expression of phosphorylated CREB in gonadotropes (36). Nevertheless, we did not observe an increase in phospho-CREB binding to either the CRE or AP-1 cis-elements in the PACAP promoter after GnRH treatment. This result is not likely to be due to our choice of treatment duration because complex formation on the PACAP-CRE probe did not change between 0 min and 240 min. Parallel experiments further failed to demonstrate a change in the intensity of the phospho-CREB supershift after 2 h of GnRH treatment. Nevertheless, it is possible that small changes in phospho-CREB binding do occur but are below the level of detection of the EMSA technique (36). Of interest, however, we observed GnRH-mediated recruitment of Jun and Fos proteins to the CRE region. We therefore postulate that a significant proportion of the CRE-dependent GnRH response is achieved via increased Jun-Fos binding and activation at this site.
Precedent exists for DNA-regulatory sites that may be more correctly termed “compound CRE-AP-1 binding sites” as we observed in the rat PACAP gene promoter. In initial studies, it was believed that the G protein-coupled intracellular signaling systems acted as discrete, independent pathways with the cAMP/PKA system altering CREB proteins which bound to CRE sites and the PKC system inducing AP-1 protein expression with subsequent binding to AP-1 sites. More recent studies have suggested significant functional overlap between these pathways at the levels of transcription factor expression, phosphorylation, recognition of DNA-binding sites, and utilization of common cofactors. For example, expression of the aromatase gene in the ovary has been shown to be activated by cAMP and repressed by Jun via interaction with a single cis-element (37). Likewise, GnRH has been shown to increase both CREB and c-Jun interaction with a single site in the secretogranin II gene (38). Although the −205 and −275 cis-elements both bind CREB and AP-1 proteins, they are not functionally identical. Whereas the −205 site mediates GnRH, PKC, and PKA effects, the −275 site does not appear to be important for PKA responsiveness. This observed difference may be due to differential recruitment of cofactors and will require further investigation.
Our studies also addressed the role of the calcium-signaling pathway in regulating PACAP gene expression. Treatment with the ionophore, ionomycin, failed to stimulate PACAP promoter activity; however, pharmacological depletion of intracellular calcium significantly blunted the GnRH response. Inhibition of voltage-gated and L-type calcium channels had minimal effect. Taken together, these data suggest that intracellular calcium is necessary, but not sufficient, to achieve a full GnRH response in the PACAP gene and, furthermore, that this calcium is primarily derived from intracellular stores rather than calcium flux through the cell membrane. Data from studies on gonadotropin gene expression have demonstrated differential dependency on calcium and calcium flux for their expression. As for the PACAP gene, calcium signaling has been found to be necessary, but not sufficient, for expression of the ovine FSHβ-subunit gene (39). For the gonadotropin α-subunit gene, intracellular calcium, but not calcium flux, has been shown to be required for expression (40). In a third study, calcium flux was determined to be important for expression of the LHβ-subunit gene (41). Therefore, our calcium signaling results are consistent with other observations in gonadotrope cells. These results are particularly intriguing in light of a report by Fukuchi et al. (42) that calcium signaling plays a direct role in stimulating PACAP expression in cultured rat cortical neurons, implying cell-specific differences in PACAP regulation.
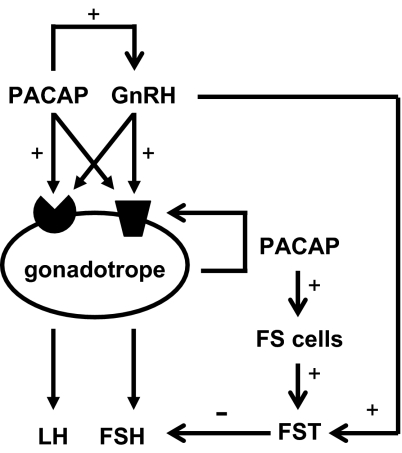
Based on our data and the results from other investigators, a picture is emerging regarding the complex interactions between PACAP and GnRH in regulation of gonadotropin gene expression. As depicted in Fig. 9, hypothalamic cells secrete both GnRH and PACAP into the pituitary portal vasculature. Within the hypothalamus, PACAP has been shown to increase GnRH mRNA expression by hypothalamic GnRH neurons (43). In the pituitary, GnRH and PACAP bind to their respective receptors on the gonadotrope cell membrane and stimulate multiple intracellular signaling pathways. These two factors further impact each other’s function at the receptor level with PACAP increasing GnRH receptor expression and GnRH modulating PACAP signaling through phosphorylation of the PACAP receptor, PAC1-R (13,44,45). As demonstrated in our current studies, GnRH stimulates PACAP gene expression by the gonadotrope. We have also observed a 2-fold increase in PACAP gene promoter activity after PACAP treatment (P < 0.001) (Grafer, C. M., and L. M. Halvorson, unpublished data). Pituitary-derived PACAP, along with hypothalamic PACAP, binds to PACAP receptors present on the gonadotropes as well as on other pituitary cell types, exerting both autocrine and paracrine effects (17,19). PACAP has been demonstrated to modulate prolactin, GH, and ACTH biosynthesis and secretion, with minimal effects on TSH expression (2). Of particular note, PACAP has been shown to stimulate follistatin production by the pituitary folliculostellate cells. Because follistatin blocks activin-mediated stimulation of FSHβ gene expression, PACAP indirectly blunts FSHβ biosynthesis (11,15). The ultimate impact of PACAP on gonadotropin biosynthesis and secretion has not been fully elucidated; however, current consensus suggests that PACAP augments GnRH action although its effects are modest when present alone.
Figure 9.
Model of interactions between PACAP, GnRH, and gonadotropins in the hypothalamic-pituitary axis. GnRH and PACAP interact within the hypothalamus and on both the gonadotropes and folliculostellate cells of the anterior pituitary to regulate gonadotropin biosynthesis and secretion (10,11,13,15,44,45). Although not depicted, PACAP receptors are also present on the other pituitary hormone-secreting cell types (43) forming a functional link between gonadotropes and gonadotrope-derived PACAP and nonreproductive endocrine systems. See Discussion section for more detail. FS, Folliculostellate cells; FST, follistatin.
In summary, the data presented here substantially expand our understanding of the signaling pathways, DNA-regulatory elements, and transcription factors that regulate expression of PACAP in the pituitary. These studies also identify an additional level of complexity in the ways in which PACAP and GnRH interact in the anterior pituitary and, as such, provide important insight into the critical fine tuning of gonadotrope function that is required for normal reproductive function.
Materials and Methods
Animals and pituitary tissue collection
Adult male Sprague Dawley rats (200–225 g) were purchased from Charles River Laboratories (Wilmington, MA). After CO2 exposure, rats were decapitated and the pituitaries were immediately processed for total RNA as described below. All procedures were performed in accordance with guidelines established by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
RNA extraction and reverse transcription
Total RNA was prepared from pooled primary pituitary cells or cultured LβT2 or αT3-1 cells using TRIzol according to manufacturer’s instructions (Invitrogen, Carlsbad, CA). Total RNA samples were DNase treated using the Turbo DNA-free kit (Ambion, Inc., Austin, TX), and 1 μg DNase-treated total RNA was reverse transcribed using Superscript II reverse transcriptase (Invitrogen) primed with random hexamer. Although primer pairs for downstream PCR applications were designed to span an intron, a parallel reaction lacking reverse transcriptase was used as an additional negative control.
PCR
Reaction conditions for each set of primer pairs were optimized to obtain results in the exponential phase of amplification for both the target and control (α-tubulin) reactions. Reactions contained 1× PCR buffer with appropriate MgCl2 concentration, 200 μm deoxynucleotide triphosphate, 400 nm of each primer, 12.5 to 100 ng cDNA, and 2.5 U Taq DNA polymerase (Promega Corp., Madison, WI). PCR conditions were 95 C ×30 sec, 50 C (PACAP) × 30 sec (55 C for α-tubulin, 60 C for PACAP-R), and 72 C × 60 sec for 35 cycles. Sequences for intron-spanning primers (Integrated DNA Technologies, Coralville, IA) are listed in supplemental Table 1. Primer sequences used for detection of PACAP receptor isoforms (PAC1 VK− and PAC1 FL+) were previously reported by Bresson-Bépoldin et al. (46).
Quantitative real-time PCR (qPCR)
cDNA (5 to 100 ng per reaction) was amplified in triplicate on either a Prism 7000 or 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) using Applied Biosystems Taqman Universal PCR Master Mix and gene-specific Taqman Gene Expression Assay primer/probe sets with universal cycling conditions. Premixed Taqman primer/probe sets amplifying 18S rRNA, PACAP, cyclophilin B, CREB1, cFos, or JunB were purchased from Applied Biosystems and are listed in supplemental Table 1. Relative proportions of target gene expression between treatment groups were calculated using the Comparative CT method as described in Applied Biosystems User Bulletin No. 2. Measured levels of target gene transcripts were normalized to levels of 18S reference transcript in each sample and expressed relative to control experimental conditions.
Western Blot analysis
Membrane fractions were prepared as previously described with minor modifications (47). A more detailed description can be found in the supplemental Materials and Methods. Briefly, the membrane fraction from LβT2 cells was isolated, electrophoresed on a 16% Novex Tricine minigel (Invitrogen), and transferred onto 0.45-μm Immobilon-P membrane (Millipore Corp., Billerica, MA). Synthetic PACAP27 and PACAP38 peptides [Phoenix Pharmaceuticals (Burlingame, CA) and Calbiochem (San Diego, CA), respectively] were used as controls. After fixation and blocking, the membrane was probed with a 1:5000 dilution of rabbit anti-PACAP27 (P/N T-4465; Peninsula Laboratories, King of Prussia, PA) followed by addition of a 1:50,000 dilution of horseradish peroxidase-conjugated antirabbit IgG secondary antibody (GE Healthcare, Piscataway, NJ). The bound secondary antibody was visualized using the SuperSignal West Femto Substrate kit (Pierce Chemical Co., Rockford, IL).
Plasmids
The rat PACAP gene promoter spanning region −1916 to +906 was subcloned into the pGL3-Basic luciferase reporter vector (Promega) (cDNA kindly provided by Drs. S. L. White and K. M. Braas, University of Vermont College of Medicine, Burlington, VT). A CRE site mutant [mutCRE(−205)] was also provided containing an AC to TG mutation present at position −203. Additional deletion constructs and site-directed mutations were generated by PCR or the QuikChange Site-Directed Mutagenesis Kit, respectively (Stratagene, La Jolla, CA). Oligonucleotide sequences can be found in supplemental Table 1. The fidelity of all constructs was verified by nucleotide sequencing. pCDNA3.1 zeo(+) expression vectors containing the full-length coding sequences for c-Fos, FosB, Fra-1, c-Jun, JunB, and JunD were generously provided by Dr. William E. Rainey (Medical College of Georgia, Augusta, GA) (48). Rous sarcoma virus expression vectors for CREB or a mutant CREB deficient in the PKA phosphorylation site (M1 CREB Ser-133→Ala) were kindly provided by Dr. Marc Montminy (49). Rc/Rous sarcoma virus expression vectors containing wild-type human CREB or a dominant-negative CREB with a single mutation in the DNA-binding domain (KCREB) were provided by Dr. Richard H. Goodman (Oregon Health Science University, Portland, OR) (50).
Transient transfection of cell lines
LβT2 and αT3-1 cells were generously provided by Dr. P.L. Mellon (University of California, San Diego, CA). Cells were maintained in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS) (vol/vol) and penicillin/streptomycin at 37 C in humidified 5% CO2. LβT2 cells (5 × 105cells/well) were grown overnight in 12-well plates and transfected with 120 ng/well luciferase reporter vector and 1.2 ng/well pRL-TK vector expressing Renilla luciferase (Promega) as an internal control using Lipofectamine (Invitrogen) according to manufacturer’s instructions. The next day, cells were treated for 6 h with the GnRH analog [des-Gly10, d-Ala6]-LH-RH ethylamide acetate hydrate (100 nm or as indicated)] (Sigma-Aldrich, St. Louis, MO), Bay K 8644 (1 μm or 5 μm), ionomycin (10 μm), forskolin (10 μm), 8-Br-cAMP (5 mm), PMA (100 ng/ml), or vehicle. The choice of dose was based on prior reports of specificity in the LβT2 cell line (23,39,51,52,53). After overnight culture in serum-free media, the pharmacological signaling inhibitors or negative controls were added 30 min before GnRH treatment: H-89 (5 μm), bisindolylmaleimide I (GF/BIM, 1 μm), calphostin (800 nm), PD 98059 (50 μm), SB203580 (10 μm), U0126 (10 μm) (Alexis Biochemicals, San Diego, CA), U0124 (10 μm), calmidazolium chloride (1 μm) (Alexis), nifedipine (1 μm), nimodipine (500 μm), BAPTA/AM (20 μm), or thapsigargin (50 nm). All modifiers were purchased from Calbiochem (San Diego, CA) unless otherwise noted. In separate experiments, expression vectors encoding CREB, mutant CREB isoforms (120 ng/well), or members of the Jun and Fos transcription factor families (30 ng/well combined total) were cotransfected with 120 ng of the full-length pGL3-PACAP reporter for 24 h, after which the cells were stimulated with 100 nm GnRH or vehicle control for 6 h. Cell extracts were prepared and luciferase activities were assessed with the Promega Dual-Luciferase Reporter Assay System using a luminometer from Berthold Detection Systems (Pforzheim, Germany).
EMSA
LβT2 cell nuclear extracts were obtained using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce) according to the manufacturer’s recommendations. Double-stranded oligonucleotides were end labeled with [γ-32P]ATP and purified over a Quick Spin G-25 Sephadex Column (Roche Applied Science; Indianapolis, IN). As appropriate, 200-fold molar excess of unlabeled oligonucleotide or 2 μl of antisera was added 20 min before the addition of labeled probe. Protein-DNA complexes were resolved on a 5% nondenaturing PAGE gel in 0.5× Tris-borate-EDTA buffer. Additional details can be found in the supplemental Materials and Methods. Oligonucleotides used for probes can be found in supplemental Table 1. Antibodies for supershift were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA): CREB-1 (sc-186), ATF-1 (sc-243), ATF-3 (sc-188), c-Jun (sc-44), c-Jun (sc-45), c-Fos (sc-253), c-Fos (sc-52), and FosB (sc-48).
siRNA interference
LβT2 cells were seeded to 12-well plates at 2 × 105/well in 10% FBS/DMEM without antibiotics. Commercially available siRNAs were transfected into the cells using DharmaFECT-1 Transfection Reagent (Dharmacon, Inc., Chicago, IL) according to manufacturer’s recommendations. Each well was transfected with 1 ml/well of 30 nm siRNA diluted in OptiMEM-1 Reduced Serum Medium (Invitrogen), complexed with 0.9 μl DharmaFECT-1 and cultured overnight. The transfection mix was replaced the next day with 1 ml per well 10% FBS/DMEM and grown an additional 36 h. After treatment for the final 6 h with 100 nm GnRH or vehicle control, the cells were harvested for total RNA extraction and gene expression analysis by qRT-PCR. The following siRNAs were obtained from Dharmacon, Inc.: mouse FOS ON-TARGETplus SMARTpool LU-041157-00, mouse JUNB ON-TARGETplus SMARTpool LU-041158-00, mouse CREB1 SMARTpool M-040959-00, and human/mouse/rat Cyclophilin B D-001136-01. Control nonsilencing siRNA (PN 1022076) was purchased from QIAGEN (Valencia, CA).
ChIP assay
Detailed methodology is described in the supplemental Materials and Methods. Briefly, LβT2 cells were cultured overnight and treated for the indicated times with 100 nm GnRH or H2O control. DNA and associated nuclear proteins were cross-linked in 1% formaldehyde, and the cells were lysed by homogenization in a Tris-Triton buffer. After sonication and centrifugation to remove the insoluble material, samples were precleared with Protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology), and 5% of each lysate was reserved at −80 C as input chromatin. Each precleared lysate was split into equal aliquots, and immunoprecipitation was performed in the presence of 2 μg anti-JunB (sc-46) or normal control rabbit IgG (Santa Cruz Biotechnology) followed by addition of Protein A/G PLUS-Agarose beads. Beads were washed sequentially in low-salt, high-salt, LiCl, and Tris-EDTA buffers, and complexes were eluted in freshly made elution buffer (Tris-EDTA, pH 8.0, plus 1% sodium dodecyl sulfate). NaCl was added to samples to a final 0.3 m, and cross-linking was reversed at 65 C overnight. Eluted chromatin was purified using the QIAquick PCR Purification Kit (QIAGEN) and analyzed in triplicate by qPCR. Relative abundances of coimmunoprecipitated PACAP promoter fragments from GnRH-treated or H2O control-treated cells were measured using by SYBR Green-based qPCR, and relative proportions of coimmunoprecipitated promoter fragments in the various treatment groups were calculated using the Comparative CT Method as previously described. Primer sets are listed in supplemental Table 1.
Statistical analysis
Statistical calculations were performed using the SigmaStat statistical software package (SPSS Science, Chicago, IL). Data were analyzed for normality followed by calculation of ANOVA or the Kruskal-Wallis ANOVA on ranks for nonparametric data. The Tukey test was used as a post hoc comparison, except for experiments with different sample sizes, in which case the Dunn’s test was employed. Statistical significance was set as indicated in each figure legend with at least a P < 0.05.
Supplementary Material
Acknowledgments
We thank Drs. Karen Braas and Victor May, who were instrumental in the development of the rat PACAP promoter constructs that launched these studies.
Footnotes
This work was supported by National Institutes of Health Grant R01 HD054782 (to L.M.H.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 2, 2009
Abbreviations: AP-1, Activating protein 1; 8-Br-cAMP, 8-bromo-cAMP; ChIP, chromatin immunoprecipitation; CRE, cAMP response element; CREB, CRE-binding protein; FBS, fetal bovine serum; PACAP, pituitary adenylate cyclase-activating polypeptide; PKA, protein kinase A; PKC, protein kinase C; PMA, phorbol 12-myristate 13-acetate; qPCR, quantitative real-time PCR; siRNA, small interfering RNA.
References
- Sherwood NM, Krueckl SL, McRory JE 2000 The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev 21:619–670 [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H 2000 Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52:269–324 [PubMed] [Google Scholar]
- Kimura C, Ohkubo S, Ogi K, Hosoya M, Itoh Y, Onda H, Miyata A, Jiang L, Dahl RR, Stibbs HH, Arimura A, Fujino M 1990 A novel peptide which stimulates adenylate cyclase: molecular cloning and characterization of the ovine and human cDNAs. Biochem Biophys Res Commun 166:81–89 [DOI] [PubMed] [Google Scholar]
- Hart GR, Gowing H, Burrin JM 1992 Effects of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, on pituitary hormone release in rats. J Endocrinol 134:33–41 [DOI] [PubMed] [Google Scholar]
- Köves K, Kántor O, Molnár J, Heinzlmann A, Szabó E, Szabó F, Nemeskéri A, Horváth J, Arimura A 2003 The role of PACAP in gonadotropic hormone secretion at hypothalamic and pituitary levels. J Mol Neurosci 20:141–152 [DOI] [PubMed] [Google Scholar]
- Tsujii T, Winters SJ 1995 Effects of pulsatile pituitary adenylate cyclase activating polypeptide (PACAP) on gonadotropin secretion and subunit mRNA levels in perifused rat pituitary cells. Life Sci 56:1103–1111 [DOI] [PubMed] [Google Scholar]
- Osuga Y, Mitsuhashi N, Mizuno M 1992 In vivo effect of pituitary adenylate cyclase activating polypeptide 38 (PACAP 38) on the secretion of luteinizing hormone (LH) in male rats. Endocrinol Jpn 39:153–156 [DOI] [PubMed] [Google Scholar]
- Burrin JM, Aylwin SJ, Holdstock JG, Sahye U 1998 Mechanism of action of pituitary adenylate cyclase-activating polypeptide on human glycoprotein hormone α-subunit transcription in αT3-1 gonadotropes. Endocrinology 139:1731–1737 [DOI] [PubMed] [Google Scholar]
- Cheng KW, Leung PC 2001 Human gonadotropin-releasing hormone receptor gene transcription: up-regulation by 3′,5′-cyclic adenosine monophosphate/protein kinase A pathway. Mol Cell Endocrinol 181:15–26 [DOI] [PubMed] [Google Scholar]
- Ferris HA, Walsh HE, Stevens J, Fallest PC, Shupnik MA 2007 Luteinizing hormone β promoter stimulation by adenylyl cyclase and cooperation with gonadotropin-releasing hormone 1 in transgenic mice and LβT2 Cells. Biol Reprod 77:1073–1080 [DOI] [PubMed] [Google Scholar]
- Katayama T, Nakashima M, Kyan H, Murakami N, Kuroda H 2000 A role of pituitary adenylate cyclase activating polypeptide (PACAP) as a regulator of paracrine interactions between folliculo-stellate cells and gonadotropes through the control of activin-follistatin interactions. J Vet Med Sci 62:731–736 [DOI] [PubMed] [Google Scholar]
- Ngan ES, Leung PC, Chow BK 2001 Interplay of pituitary adenylate cyclase-activating polypeptide with a silencer element to regulate the upstream promoter of the human gonadotropin-releasing hormone receptor gene. Mol Cell Endocrinol 176:135–144 [DOI] [PubMed] [Google Scholar]
- Pincas H, Laverrière JN, Counis R 2001 Pituitary adenylate cyclase-activating polypeptide and cyclic adenosine 3′,5′-monophosphate stimulate the promoter activity of the rat gonadotropin-releasing hormone receptor gene via a bipartite response element in gonadotrope-derived cells. J Biol Chem 276:23562–23571 [DOI] [PubMed] [Google Scholar]
- Tsujii T, Ishizaka K, Winters SJ 1994 Effects of pituitary adenylate cyclase-activating polypeptide on gonadotropin secretion and subunit messenger ribonucleic acids in perifused rat pituitary cells. Endocrinology 135:826–833 [DOI] [PubMed] [Google Scholar]
- Fujii Y, Okada Y, Moore Jr JP, Dalkin AC, Winters SJ 2002 Evidence that PACAP and GnRH down-regulate follicle-stimulating hormone-β mRNA levels by stimulating follistatin gene expression: effects on folliculostellate cells, gonadotrophs and LβT2 gonadotroph cells. Mol Cell Endocrinol 192:55–64 [DOI] [PubMed] [Google Scholar]
- Heinzlmann A, Kirilly E, Meltzer K, Szabó E, Baba A, Hashimoto H, Köves K 2008 PACAP is transiently expressed in anterior pituitary gland of rats: in situ hybridization and cell immunoblot assay studies. Peptides 29:571–577 [DOI] [PubMed] [Google Scholar]
- Köves K, Kántor O, Scammell JG, Arimura A 1998 PACAP colocalizes with luteinizing and follicle-stimulating hormone immunoreactivities in the anterior lobe of the pituitary gland. Peptides 19:1069–1072 [DOI] [PubMed] [Google Scholar]
- Szabó E, Nemeskéri A, Heinzlmann A, Suzuki N, Arimura A, Köves K 2002 Cell immunoblot assay study demonstrating the release of PACAP from individual anterior pituitary cells of rats and the effect of PACAP on LH release. Regul Pept 109:75–81 [DOI] [PubMed] [Google Scholar]
- Radleff-Schlimme A, Leonhardt S, Wuttke W, Jarry H 1998 Evidence for PACAP to be an autocrine factor on gonadotrope cells. Ann NY Acad Sci 865:486–491 [DOI] [PubMed] [Google Scholar]
- Jin L, Tsumanuma I, Ruebel KH, Bayliss JM, Lloyd RV 2001 Analysis of homogeneous populations of anterior pituitary folliculostellate cells by laser capture microdissection and reverse transcription-polymerase chain reaction. Endocrinology 142:1703–1709 [DOI] [PubMed] [Google Scholar]
- Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL 2007 p38 mitogen-activated protein kinase is critical for synergistic induction of the FSHβ gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol 21:3071–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes RC, Sidhu KK, Sosabowski JK, King P, Burrin JM 2003 Absence of pituitary adenylate cyclase-activating polypeptide-stimulated transcription of the human glycoprotein α-subunit gene in LβT2 gonadotrophs reveals disrupted cAMP-mediated gene transcription. J Mol Endocrinol 31:263–278 [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Ferris HA, Shupnik MA 2003 The calcium component of gonadotropin-releasing hormone-stimulated luteinizing hormone subunit gene transcription is mediated by calcium/calmodulin-dependent protein kinase type II. Endocrinology 144:2409–2416 [DOI] [PubMed] [Google Scholar]
- Harris D, Bonfil D, Chuderland D, Kraus S, Seger R, Naor Z 2002 Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHβ-subunit promoter. Endocrinology 143:1018–1025 [DOI] [PubMed] [Google Scholar]
- Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ 2002 Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in LβT2 cells. J Biol Chem 277:32099–32108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL 1996 Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ 2002 GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol Endocrinol 16:419–434 [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Lanz R, O'Malley BW 2004 Pituitary adenylate cyclase-activating peptide: a pivotal modulator of steroid-induced reproductive behavior in female rodents. Mol Endocrinol 18:173–183 [DOI] [PubMed] [Google Scholar]
- Ha CM, Kang JH, Choi EJ, Kim MS, Park JW, Kim Y, Choi WS, Chun SY, Kwon HB, Lee BJ 2000 Progesterone increases mRNA levels of pituitary adenylate cyclase-activating polypeptide (PACAP) and type I PACAP receptor (PAC(1)) in the rat hypothalamus. Brain Res Mol Brain Res 78:59–68 [DOI] [PubMed] [Google Scholar]
- Ko C, In YH, Park-Sarge OK 1999 Role of progesterone receptor activation in pituitary adenylate cyclase activating polypeptide gene expression in rat ovary. Endocrinology 140:5185–5194 [DOI] [PubMed] [Google Scholar]
- Park JY, Park JH, Park HJ, Lee JY, Lee YI, Lee K, Chun SY 2001 Stage-dependent regulation of ovarian pituitary adenylate cyclase-activating polypeptide mRNA levels by GnRH in cultured rat granulosa cells. Endocrinology 142:3828–3835 [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Hagihara N, Koga K, Yamamoto K, Shintani N, Tomimoto S, Mori W, Koyama Y, Matsuda T, Baba A 2000 Synergistic induction of pituitary adenylate cyclase-activating polypeptide (PACAP) gene expression by nerve growth factor and PACAP in PC12 cells. J Neurochem 74:501–507 [DOI] [PubMed] [Google Scholar]
- Abate C, Baker SJ, Lees-Miller SP, Anderson CW, Marshak DR, Curran T 1993 Dimerization and DNA binding alter phosphorylation of Fos and Jun. Proc Natl Acad Sci USA 90:6766–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenov Y, Kerppola TK 2001 Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20:2438–2452 [DOI] [PubMed] [Google Scholar]
- Rauscher 3rd FJ, Voulalas PJ, Franza Jr BR, Curran T 1988 Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev 2:1687–1699 [DOI] [PubMed] [Google Scholar]
- Duan WR, Shin JL, Jameson JL 1999 Estradiol suppresses phosphorylation of cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) in the pituitary: evidence for indirect action via gonadotropin-releasing hormone. Mol Endocrinol 13:1338–1352 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Wu Y, Li R, Hu Y 2005 Jun proteins modulate the ovary-specific promoter of aromatase gene in ovarian granulosa cells via a cAMP-responsive element. Oncogene 24:2236–2246 [DOI] [PubMed] [Google Scholar]
- Xie J, Roberson MS 2008 3′, 5′-Cyclic adenosine 5′-monophosphate response element-dependent transcriptional regulation of the secretogranin II gene promoter depends on gonadotropin-releasing hormone-induced mitogen-activated protein kinase activation and the transactivator activating transcription factor 3. Endocrinology 149:783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyev VV, Pernasetti F, Rosenberg SB, Barsoum MJ, Austin DA, Webster NJ, Mellon PL 2002 Transcriptional activation of the ovine follicle-stimulating hormone-β gene by gonadotropin-releasing hormone involves multiple signal transduction pathways. Endocrinology 143:1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BD, Sabbagh E, Chin WW, Kaiser UB 1998 Differential use of signal transduction pathways in the gonadotropin-releasing hormone-mediated regulation of gonadotropin subunit gene expression. Endocrinology 139:1835–1843 [DOI] [PubMed] [Google Scholar]
- Weck J, Fallest PC, Pitt LK, Shupnik MA 1998 Differential gonadotropin-releasing hormone stimulation of rat luteinizing hormone subunit gene transcription by calcium influx and mitogen-activated protein kinase-signaling pathways. Mol Endocrinol 12:451–457 [DOI] [PubMed] [Google Scholar]
- Fukuchi M, Tabuchi A, Tsuda M 2004 Activity-dependent transcriptional activation and mRNA stabilization for cumulative expression of pituitary adenylate cyclase-activating polypeptide mRNA controlled by calcium and cAMP signals in neurons. J Biol Chem 279:47856–47865 [DOI] [PubMed] [Google Scholar]
- Li S, Grinevich V, Fournier A, Pelletier G 1996 Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on gonadotropin-releasing hormone and somatostatin gene expression in the rat brain. Brain Res Mol Brain Res 41:157–162 [DOI] [PubMed] [Google Scholar]
- Larivière S, Garrel-Lazayres G, Simon V, Shintani N, Baba A, Counis R, Cohen-Tannoudji J 2008 Gonadotropin-releasing hormone inhibits pituitary adenylyl cyclase-activating polypeptide coupling to 3′,5′-cyclic adenosine-5′-monophosphate pathway in LβT2 gonadotrope cells through novel protein kinase C isoforms and phosphorylation of pituitary adenylyl cyclase-activating polypeptide type I receptor. Endocrinology 149:6389–6398 [DOI] [PubMed] [Google Scholar]
- Sadie H, Styger G, Hapgood J 2003 Expression of the mouse gonadotropin-releasing hormone receptor gene in α T3-1 gonadotrope cells is stimulated by cyclic 3′,5′-adenosine monophosphate and protein kinase A, and is modulated by Steroidogenic factor-1 and Nur77. Endocrinology 144:1958–1971 [DOI] [PubMed] [Google Scholar]
- Bresson-Bépoldin L, Jacquot MC, Schlegel W, Rawlings SR 1998 Multiple splice variants of the pituitary adenylate cyclase-activating polypeptide type 1 receptor detected by RT-PCR in single rat pituitary cells. J Mol Endocrinol 21:109–120 [DOI] [PubMed] [Google Scholar]
- Li M, Nakayama K, Shuto Y, Somogyvari-Vigh A, Arimura A 1998 Testis-specific prohormone convertase PC4 processes the precursor of pituitary adenylate cyclase-activating polypeptide (PACAP). Peptides 19:259–268 [DOI] [PubMed] [Google Scholar]
- Beshay VE, Havelock JC, Sirianni R, Ye P, Suzuki T, Rainey WE, Carr BR 2007 The mechanism for protein kinase C inhibition of androgen production and 17α-hydroxylase expression in a theca cell tumor model. J Clin Endocrinol Metab 92:4802–4809 [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR 1989 Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59:675–680 [DOI] [PubMed] [Google Scholar]
- Walton KM, Rehfuss RP, Chrivia JC, Lochner JE, Goodman RH 1992 A dominant repressor of cyclic adenosine 3′,5′-monophosphate (cAMP)-regulated enhancer-binding protein activity inhibits the cAMP-mediated induction of the somatostatin promoter in vivo. Mol Endocrinol 6:647–655 [DOI] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL 2004 A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Austin DA, Webster NJ 2003 Gonadotropin-releasing hormone-desensitized LβT2 gonadotrope cells are refractory to acute protein kinase C, cyclic AMP, and calcium-dependent signaling. Endocrinology 144:4354–4365 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Yamamoto H, Yonehara T, Kanasaki H, Nakanishi H, Miyamoto E, Miyazaki K 2004 Differential activation of the luteinizing hormone β-subunit promoter by activin and gonadotropin-releasing hormone: a role for the mitogen-activated protein kinase signaling pathway in LβT2 gonadotrophs. Biol Reprod 70:236–243 [DOI] [PubMed] [Google Scholar]
- Duan WR, Ito M, Park Y, Maizels ET, Hunzicker-Dunn M, Jameson JL 2002 GnRH regulates early growth response protein 1 transcription through multiple promoter elements. Mol Endocrinol 16:221–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.