Abstract
Granulosa cells of preovulatory follicles differentiate in response to FSH, and this differentiation is augmented by estradiol. We have previously shown that FSH-mediated granulosa cell differentiation requires functional estrogen receptor-β (ERβ) by demonstrating that the granulosa cells of ERβ−/− FSH-treated mice are unable to maximally induce expression of the LH receptor (an indicator of granulosa cell differentiation) compared with ERβ+/+ controls. As a result, FSH-primed ERβ−/− granulosa cells exhibit a reduced response to a subsequent ovulatory dose of LH. In this study, we further characterized the attenuated response of ERβ−/− granulosa cells to stimulation by LH and FSH using isolated mouse granulosa cells and primary granulosa cell cultures. We observed a 50% reduction in cAMP levels in cultured ERβ−/− granulosa cells exposed to LH compared with ERβ+/+ controls. We also observed an attenuated genomic response in granulosa cells isolated from FSH-primed ERβ−/− mice compared with ERβ+/+ controls. Our data indicate that this attenuated response may result from inadequate levels of cAMP, because cAMP levels in cultured ERβ−/− granulosa cells exposed to forskolin were approximately 50% lower than in ERβ+/+ granulosa cells. Phosphorylation of cAMP regulatory element binding protein, an indicator of protein kinase A activity, was also reduced in FSH-treated ERβ−/− granulosa cells compared with ERβ+/+ controls. These are the first data to indicate that ERβ plays a role in the induction of the cAMP pathway in mouse granulosa cells and that disruption of proper ERβ signaling associated with this pathway may cause negative effects on ovulation and fertility.
Loss of ER-beta attenuates cAMP accumulation in gonadotropin- or forskolin-stimulated mouse granulosa cells
Folliculogenesis begins with recruitment of a cohort of primordial follicles and ends with the maturation of a select few to a preovulatory state capable of expelling a competent oocyte when stimulated with a sufficient bolus of LH. Maturation of preantral to preovulatory follicles is dependent on FSH to induce granulosa cell differentiation, the hallmarks of which are an increased ability to produce estradiol (via aromatization of thecal-derived androgens) and acquisition of LH-receptor (Lhcgr) expression. Although fundamental, the effects of FSH alone in the maturation of a preovulatory follicle are insufficient and must be facilitated by intrafollicular estrogens (1).
The results of early studies with estrogen receptor (ER) antagonists indicate that ERs are required for estrogen-mediated enhancement of FSH actions in granulosa cells (2). Indeed, both known nuclear forms of ER, ERα and ERβ, are present in growing follicles. ERβ is specifically localized to the granulosa cells of growing follicles, whereas ERα is predominantly expressed in the thecal and interstitial compartments (3). The importance of estrogen/ER-mediated actions in the ovary is best illustrated by the severe ovarian phenotypes observed in mice lacking ERα and/or ERβ (4). Adult ERα-null females are infertile due to a plethora of reproductive deficiencies, notably the lack of negative and positive estradiol feedback in the hypothalamic-pituitary axis, which leads to acyclic anovulation (5,6,7). In contrast, most evidence indicates that a primary role of ERβ in regard to female reproduction occurs specifically within the granulosa cells of the ovary. Adult ERβ−/− females are subfertile and possess ovaries that consistently exhibit reduced numbers of growing follicles and corpora lutea (8). ERβ−/− female mice also yield fewer offspring per pregnancy, a phenotype thought to be due to infrequent and inefficient spontaneous ovulation (8). We have previously reported (9) that ovaries and granulosa cells isolated from pregnant mare serum gonadotropin (PMSG)-treated ERβ−/− mice demonstrate an attenuated response to FSH, as indicated by reduced acquisition of Lhcgr expression and subsequent insufficient stimulation of known LH-responsive genes, namely prostaglandin-endoperoxide synthase 2 (Ptgs2) and progesterone receptor (Pgr), both of which are required for follicular rupture. Thus, ERβ is critical for FSH-mediated granulosa cell differentiation and hence maturation of the whole follicle to a preovulatory state.
FSH actions in granulosa cells are thought to be largely dependent on the cAMP-dependent protein kinase A (PKA) pathway (10). The FSH-receptor is a classic trimeric G protein-coupled receptor that when bound by FSH triggers the intracellular production of cAMP via adenylate cyclase. Increased intracellular levels of cAMP lead to release of the catalytic subunit of PKA, allowing it to phosphorylate transcription factors that then act to regulate gene expression. One such PKA-stimulated transcription factor in granulosa cells is the cAMP-regulatory element-binding protein (CREB) (10,11). Indeed, FSH increases Lhcgr expression in rat granulosa cells via the cAMP/PKA pathway, and this increase is maximized in the presence of 17β-estradiol (estradiol) (12,13,14).
In this report we provide novel data on the role of ERβ in granulosa cell differentiation and added insight toward the molecular mechanisms responsible for the facilitatory actions of estradiol on FSH-mediated granulosa cell differentiation. Microarray analysis was used to identify genes that require ERβ for maximum induction by comparing granulosa cells from ERβ+/− and ERβ−/− mice after FSH exposure. In addition we employed primary granulosa cell cultures to investigate ERβ’s role in the FSH-induced cAMP pathway in vitro. From these data we conclude that ERβ is required for optimal accumulation of cAMP in FSH-stimulated mouse granulosa cells. Therefore, the lack of ERβ results in reduced expression of several genes required for granulosa cell differentiation and ultimately ovulation of a competent oocyte.
Results
ERβ−/− granulosa cells fail to acquire LH receptor expression
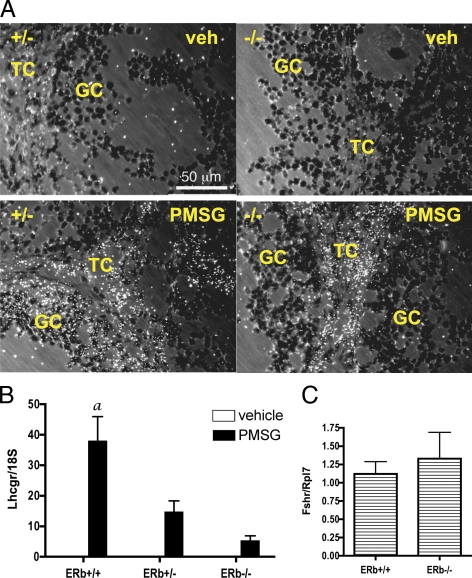
We have previously demonstrated that ERβ−/− ovaries or isolated granulosa cells exhibit less than maximal induction of Lhcgr expression when isolated from PMSG-treated mice (9). To further demonstrate that the reduced Lhcgr mRNA levels observed in ERβ−/− ovaries are specific to the granulosa cells of growing and preovulatory follicles and not reflective of cells within the ovary that constitutively express Lhcgr (i.e. thecal and interstitial), we conducted in situ hybridization analysis on ovaries from similarly treated mice (Fig. 1A). As shown in Fig. 1A, a comparison of Lhcgr mRNA hybridization in ERβ+/− and ERβ−/− ovaries after PMSG stimulation indicates clearly reduced Lhcgr expression among the granulosa cells of ERβ−/− ovaries. To further support these data, granulosa cells were isolated from mice treated with PMSG for 48 h, and Lhcgr expression was examined by quantitative RT-PCR (qRT-PCR) (Fig. 1B). We have previously shown by Northern blot analysis that the PMSG-stimulated increases in granulosa cell Lhcgr expression are reduced in ERβ−/− granulosa cells (9). We have now confirmed and extended this data by demonstrating that Lhcgr expression correlates with the loss of functional ERβ (Esr2) alleles, i.e. that ERβ+/− granulosa cell Lhcgr expression is lower than in ERβ+/+ cells but higher than in ERβ−/− cells, suggesting that ERβ is limiting for Lhcgr expression and correlates with the presence of a functional Esr2 allele. In contrast, Fshr mRNA levels in granulosa cells isolated from ERβ+/− and ERβ−/− mice are comparable (Fig. 1C).
Figure 1.
Granulosa cell expression of Lhcgr after PMSG treatment of ERβ+/+, ERβ+/−, and ERβ−/− mice. A, Immature mice were treated with saline or PMSG (3.25 IU) for 48 h, and ovaries were collected and processed for in situ hybridization to detect Lhcgr gene expression. GC, Granulosa cells; TC, thecal cells. B, Immature mice were treated with □ saline or ▪ PMSG (3.25 IU) for 48 h, granulosa cells were isolated and pooled, and total RNA was isolated for Lhcgr gene expression analysis by qRT-PCR compared with an Rpl7 control (±sem of three independent experiments). Analysis by ANOVA found a significant interaction (P = 0.002) between PMSG treatment and genotype, indicating that the response to PMSG correlates with the dosage of the ERβ allele. A Bonferroni post test was also used to compare replicate means by genotype: a, P < 0.001. C, Granulosa cells were isolated from immature mice, cultured for 2 h as for all other in vitro assays (see Materials and Methods), and total RNA isolated for Fshr gene expression analysis by qRT-PCR compared with an Rpl7 control (±sem of four independent experiments). veh, Vehicle.
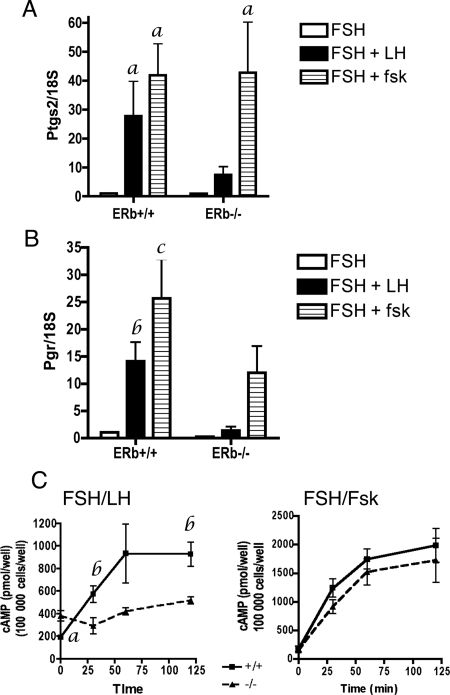
From these data we hypothesize that the failure of ERβ−/− preovulatory follicles to properly respond to an ovulatory dose of human chorionic gonadotropin (hCG) is due to their insufficient acquisition of LH-receptor (Lh-r) levels. To test this, we sought to overcome the absence of Lh-r via direct stimulation of the cAMP pathway in preovulatory granulosa cells. Granulosa cells were isolated from untreated mice, induced to differentiate in vitro with FSH treatment, and then stimulated for 4 h with LH (control) or forskolin, a direct activator of adenylate cyclase. The response was measured as stimulation of two well-known LH-responsive genes, prostaglandin-endoperoxide synthase 2 (Ptgs2) and the progesterone receptor (Pgr). As expected, Ptgs2 and Pgr mRNA levels were lower in LH-stimulated ERβ−/− vs. ERβ+/+ granulosa cells (Fig. 2, A and B). Ptgs2 mRNA levels in ERβ−/− granulosa cells were 70% lower than in ERβ+/+ granulosa cells. Similarly, Pgr mRNA levels in ERβ−/− granulosa cells were 89% lower than in ERβ+/+ granulosa cells. Therefore, LH-stimulated gene expression was reduced in primary cultures of ERβ−/− granulosa cells.
Figure 2.
Effect of LH and forskolin treatment on gene expression and cAMP levels in FSH-primed primary granulosa cells. Granulosa cells were isolated from untreated, immature mice, pooled, and then cultured in FSH (50 ng/ml) + T (5 ng/ml) for 48 h, or FSH (50 ng/ml) + T (5 ng/ml) followed by LH (100 ng/ml) or forskolin (10 μm) for 4 h. Total RNA was isolated and analyzed by qRT-PCR for (A) Ptgs2 and (B) Pgr expression. Data are expressed as the average ± sem of four independent experiments. Analysis by ANOVA found a significant interaction between LH treatment and genotype for Pgr (P = 0.002), but not for Ptgs2 (P = 0.1), indicating that the magnitude of increased Pgr expression in response to LH is dependent on the genotype, i.e. on the dosage of the ERβ allele. No significant interaction between forskolin treatment and genotype was observed for either Pgr or Ptgs2. However, the effect of both LH and forskolin treatment on individual genotypes compared with vehicle was significant for Ptgs2 (LH, P = 0.02; forskolin, P = 0.002) and for Pgr (forskolin, P = 0.001). A Bonferroni post test was also used to compare replicate means by genotype: a, P < 0.05; b, P < 0.001; c, P < 0.01. C, Granulosa cells were isolated from untreated, immature mice, pooled, and then cultured in FSH (50 ng/ml) + T (5 ng/ml) for 48 h, or FSH + T (5 ng/ml) followed by LH (100 ng/ml, left panel) or forskolin (10 μm, right panel) for 0, 0.5, 1, or 2 h. Cells were immediately frozen on dry ice at indicated times and stored at −80 C until cAMP levels were determined by ELISA. Data are expressed as average cAMP (picomoles/100,000 cells) ± sem of three independent experiments. a, P = 0.002; b, P < 0.05. No statistical significance was observed in cAMP levels between forskolin-treated ERβ+/+ and ERβ−/− granulosa cells at any time point.
Forskolin treatment of ERβ+/+ and ERβ−/− granulosa cells resulted in Ptgs2 and Pgr mRNA levels comparable to those resulting from LH treatment (Fig. 2, A and B), indicating that forskolin fully (Ptgs2) or partially (Pgr) rescues the attenuated Ptgs2 and Pgr expression in response to LH. Whereas forskolin-stimulated ERβ−/− granulosa cells exhibited Ptgs2 induction comparable to that of similarly treated ERβ+/+ granulosa cells (Fig. 2A), Pgr mRNA levels in ERβ−/− granulosa cells remained lower than in ERβ+/+ granulosa cells (Fig. 2B), suggesting that ERβ is required for the optimal expression of some genes in response to forskolin after FSH priming.
Because forskolin treatment can rescue the reduced Ptgs2 and Pgr gene expression in ERβ−/− granulosa cells, we hypothesized that LH treatment of ERβ−/− granulosa cells leads to less-than-sufficient intracellular cAMP levels. To investigate further, granulosa cells were once again isolated from untreated ERβ+/+ or ERβ−/− mice and cultured in vitro under similar conditions as above except in this case the FSH-conditioned cells were LH stimulated for 30, 60, or 120 min, and at each point cells were lysed and cAMP levels were determined. These data repeatedly indicated that LH-induced cAMP levels in ERβ−/− cells were reduced by approximately 50% at all three time points compared with control (Fig. 2C). In ERβ+/+ cells, a sharp rise in cAMP levels was observed within 1 h stimulation whereas no such increase was observed in ERβ−/− cells. In contrast, forskolin stimulated an overall increase in cAMP in cells of both genotypes (Fig. 2C), suggesting that the lack of cAMP production in ERβ−/− granulosa cells can be rescued by direct activation of adenylate cyclase.
ERβ−/− granulosa cells exhibit a compromised response to forskolin in vitro
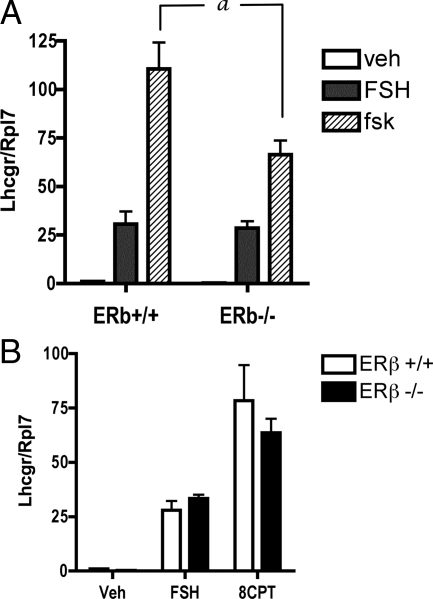
The acquisition of LH-r and LH responsiveness in preovulatory granulosa cells is well accepted to be dependent on FSH (14). The data above indicate that ERβ−/− granulosa cells fail to acquire sufficient LH-r, suggesting a poor response to the differentiating effects of FSH in the absence of ERβ. To investigate the mechanism responsible for the attenuated FSH response of ERβ−/− granulosa cells, we first assessed the induction of Lhcgr mRNA in granulosa cells treated in vitro with FSH, forskolin, or cAMP for 48 h. Surprisingly, the FSH-stimulated Lhcgr levels were similar in both ERβ+/+ and ERβ−/− granulosa cells (Fig. 3A). However, when treated with forskolin, Lhcgr mRNA levels in ERβ−/− granulosa cells were 40% lower than in ERβ+/+ granulosa cells (Fig. 3A). To determine whether ERβ may be involved in cAMP action as well as cAMP accumulation, we also treated the cells with the cAMP analog 8-(4-chlorophenylthio) adenosine 3:5-cyclic monophosphate (8CPT). However, no significant difference in Lhcgr mRNA level was observed between ERβ+/+ and ERβ−/− granulosa cells (Fig. 3B) under these conditions.
Figure 3.
Effect of FSH, forskolin treatment, and cAMP analog treatment on Lhcgr expression in primary granulosa cells isolated from ERβ+/+ and ERβ−/− mice. Granulosa cells were isolated from untreated, immature mice, pooled, and then cultured in A: FSH (50 ng/ml) + T (5 ng/ml) or forskolin (10 μm) + T (5 ng/ml) for 48 h, or B: the cAMP analog, 8CPT (1 mm) + T (5 ng/ml) for 48 h. Total RNA was prepared and Lhcgr expression analyzed by qRT-PCR. Data are expressed as average Lhcgr expression compared with an Rpl7 control ± sem of four independent experiments. a, P < 0.01 comparing forskolin-treated wild-type and ERβ−/− cells. veh, Vehicle.
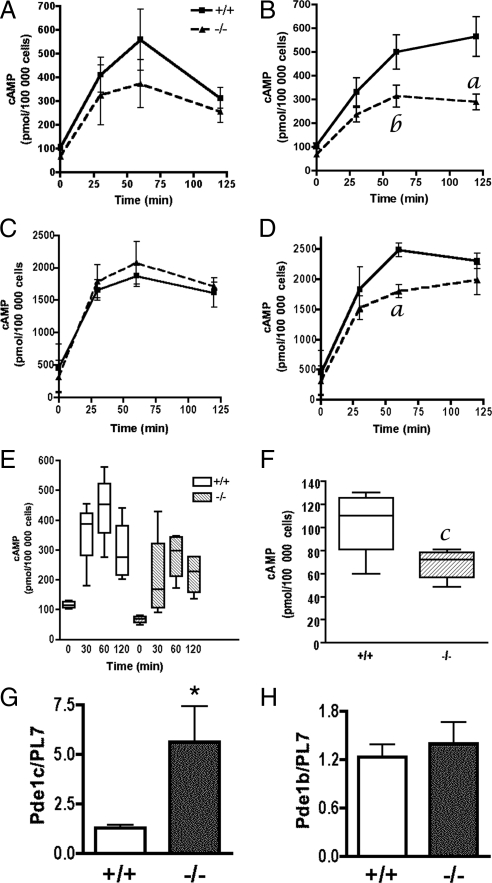
These results correlate with the levels of cAMP observed in FSH-stimulated and forksolin-stimulated ERβ+/+ and ERβ−/− granulosa cells (Fig. 4). Although there was a trend toward decreased cAMP levels in FSH-stimulated ERβ−/− granulosa cells at all time points (Fig. 4, A and E), there was not a statistically significant difference between the mean cAMP levels at any individual time point. However, median cAMP levels were lower in ERβ−/− granulosa cells than in ERβ+/+ cells after 0.5 and 1.0 h FSH (Fig. 4E), again suggesting a trend toward lower cAMP in the FSH-treated ERβ−/− granulosa cells. Consistent with previous observations (15), cAMP levels were maximal after 1 h exposure in vitro to FSH, and dropped thereafter. However, in response to forskolin, ERβ−/− granulosa cell cAMP levels were significantly lower than in ERβ+/+ granulosa cells (Fig. 4B), and this difference was maximal after 2 h of treatment, at which time cAMP levels in ERβ−/− granulosa cells were 49% lower than in ERβ+/+ granulosa cells. We also observed that freshly isolated, untreated ERβ−/− granulosa cells contained significantly less cAMP than ERβ+/+ granulosa cells (Fig. 4F). These results show that ERβ is required for the production and/or maintenance of cAMP levels in both untreated and gonadotropin-stimulated granulosa cells.
Figure 4.
Effect of FSH and forskolin on cAMP levels and phosphodiesterase expression in primary granulosa cells isolated from ERβ+/+ and ERβ−/− mice. Granulosa cells were isolated from untreated, immature mice, pooled, and then cultured in the absence or presence of the phosphodiesterase inhibitor, IBMX, for the times indicated: A and E, FSH (50 ng/ml) + T (5 ng/ml); B, forskolin (10 μm) + T (5 ng/ml); C, FSH + T (5 ng/ml) + IBMX; or D, forskolin (10 μm) + T (5 ng/ml) + IBMX for 0, 0.5, 1.0, and 2.0 h. Cells were immediately frozen on dry ice at indicated times and stored at −80 C until cAMP levels were determined by ELISA. E, Box plot of data in panel A indicating that the median cAMP levels are higher in wild-type cells than in ERβ−/− cells after 0.5 and 1.0 h treatment with FSH + T in vitro. In a box plot, the box contains the middle 50% of the data. The upper and lower edges of the box indicate the 75th and the 25th percentiles of the dataset, respectively. The line in the box indicates the median value of the data, whereas the ends of the vertical lines indicate the minimum and maximum data values. F, Box plot (see panel E for explanation of a box plot) showing cAMP levels in untreated granulosa cells. Data are expressed as cAMP levels (picomoles/100,000 cells) ± sem of five (A, B, E, and F) or three (C and D) independent experiments. G and H, Granulosa cells were isolated from untreated, immature mice, pooled, and then cultured in serum-free medium for 2 h before harvest to mimic the culture conditions used for all in vitro studies before gonadotropin or other treatment. Total RNA was prepared and analyzed by qRT-PCR for Pde1c (G) and Pde1b (H) mRNA levels. Data are expressed as average gene expression compared with an Rpl7 control ± sem of four independent experiments. a, P < 0.02; b, P < 0.07; c, P < 0.03; *, P = 0.05.
The lower cAMP levels observed in forskolin-treated ERβ−/− granulosa cells could be due to either reduced production of cAMP, or increased degradation of cAMP by phosphodiesterases, or both. To determine whether increased phosphodiesterase activity might be responsible for the lower levels of cAMP observed in forskolin-treated ERβ−/− granulosa cells, we used the nonspecific phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (IBMX), in addition to FSH or forskolin (Fig. 4, C and D). If phosphodiesterase activity is higher in ERβ−/− granulosa cells than in ERβ+/+ cells, then IBMX treatment should result in wild-type-like cAMP levels. Indeed, FSH-stimulated cAMP levels were similar in IBMX-treated ERβ+/+ and ERβ−/− granulosa cells (Fig. 4C). Interestingly, cAMP levels remained lower in forskolin-treated ERβ−/− granulosa cells after 1 h of treatment (Fig. 4D). However, after 2 h of treatment (when the difference in cAMP levels in the absence of IBMX was maximal between the genotypes) there was no significant difference between cAMP levels in ERβ+/+ and ERβ−/− granulosa cells. These results suggest that the lower cAMP levels observed in ERβ−/− granulosa cells treated with FSH or forskolin are due, in part, to increased phosphodiesterase activity in ERβ−/− granulosa cells.
To determine whether the ERβ−/− granulosa cells used in our in vitro experiments expressed higher levels of phosphodiesterases (PDEs), we investigated the mRNA levels of five PDE genes we had found to be expressed in granulosa cells (Pde1b, Pde4b, Pde7b, Pde4d, and Pde8a) (data not shown). Granulosa cells were isolated from the ovaries of untreated mice, cultured for 2 h, and analyzed for PDE gene expression. Of the five PDEs investigated, Pde1c was the only gene with mRNA levels significantly higher in ERβ−/− granulosa cells than in ERβ+/+ granulosa cells (Fig. 4G), whereas the expression of the other PDEs, such as Pde1b, was unchanged (Fig. 4H).
To determine whether the lower cAMP levels observed in forskolin-treated ER−/− granulosa cells could be due to reduced production of cAMP, adenylate cyclase mRNA levels were examined. Nine adenylate cyclase genes are expressed in mice (16), and we used qRT-PCR to identify which of these nine genes were expressed in mouse granulosa cells isolated from untreated or PMSG-treated ERβ−/− and ERβ+/+ mice. Of these, Adcy3, Adcy6, and Adcy9 were the most highly expressed under both conditions (data not shown); however, we observed no difference in Adcy3, Adcy6, and Adcy9 mRNA levels between granulosa cells isolated from vehicle or PMSG-treated ERβ−/− and ERβ+/+ mice (data not shown), suggesting that differences in adenylate cyclase mRNA levels are not responsible for the impaired cAMP levels observed in response to forskolin.
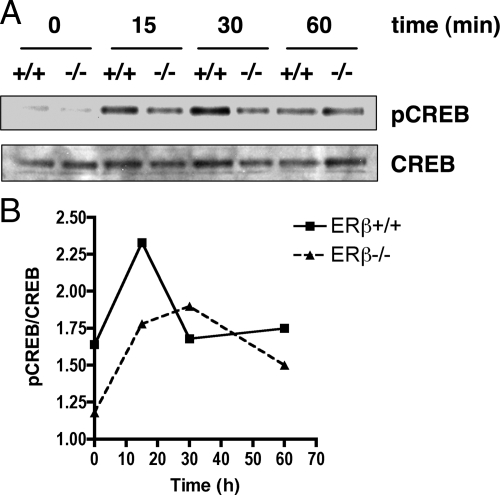
Finally, we wanted to use a complementary method to indirectly investigate the FSH-induced cAMP levels in ERβ+/+ and ERβ−/− granulosa cells by determining whether FSH-stimulated ERβ−/− granulosa cells expressed less CREB, a known phosphorylation target of PKA, than ERβ+/+ granulosa cells. Therefore, we determined the protein levels of phosphorylated CREB (pCREB) and CREB in ERβ+/+ and ERβ−/− granulosa cells treated in vitro with FSH. Western blot analysis indicated that the ratio of pCREB to CREB was lower in ERβ−/− granulosa cell than in ERβ+/+ cells at 0 and 15 min of treatment with FSH, but were approximately equal at 30 and 60 min (Fig. 5, A and B). These results suggest that the cAMP pathway is disrupted in ERβ−/− granulosa cells.
Figure 5.
pCREB levels are lower in FSH-stimulated ERβ−/− granulosa cells than in ERβ+/+ granulosa cells. A, Granulosa cells were isolated from untreated, immature mice, pooled, and then cultured in serum-free medium for 2 h before treatment with FSH (50 ng/ml) + T (5 ng/ml) for 0, 15, 30, or 60 min. Whole-cell protein extracts were prepared and analyzed for pCREB and CREB protein levels by Western blot analysis. B, Densitometric analysis of Western blot data indicating pCREB:CREB ratios at each time-point.
Granulosa cells isolated from PMSG-treated ERβ−/− mice exhibit attenuated global gene expression
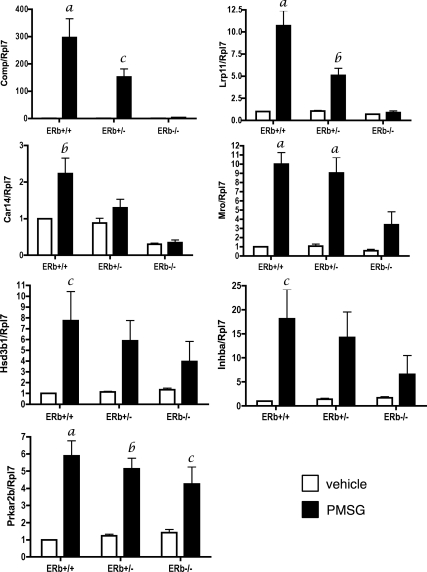
To gain insight into the mechanism by which ERβ mediates FSH-mediated differentiation of granulosa cells, we sought to identify other FSH-regulated genes that may require ERβ for maximal expression. For this we compared the pattern of global gene expression in granulosa cells isolated from ERβ+/− or ERβ−/− mice treated for 48 h with PMSG. This microarray analysis generated a ratio of gene expression of ERβ−/−:ERβ+/−. Five hundred ninety genes were differentially regulated in granulosa cells isolated from PMSG-treated ERβ−/− mice compared with cells isolated from similarly treated ERβ+/− mice and represented genes involved in metabolism, biosynthesis, regulation of the cell cycle, and blood vessel development (see Table 2). We confirmed the expression levels of seven differentially regulated genes by qRT-PCR using granulosa cells isolated from vehicle or PMSG-treated ERβ+/+, ERβ+/−, and ERβ−/−mice (Fig. 6). These genes included Cartilage oligomeric matrix protein (Comp), low-density lipoprotein receptor-related protein 11 (Lrp11), carbonic anhydrase 14 (Car14), Maestro (Mro), hydroxy-δ-5-steroid dehydrogenase, 3β- and steroid δ-isomerase 1 (Hsd3b1), inhibin β-A (Inhba), and protein kinase, cAMP-dependent regulatory, type II β (Prkar2b). With the exception of Comp, the expression of these genes is regulated, at least in part, by the cAMP/PKA pathway (17,18,19,20). Statistically significant differences between genotypes in response to PMSG were observed for Comp, Lrp11, Car14, and Mro, with levels highest in ERβ+/+ granulosa cells, lowest in ERβ−/− granulosa cells. Levels in ERβ+/− granulosa cells were lower than in ERβ+/+ granulosa cells, but higher than in ERβ−/− granulosa cells. Although a similar trend was observed for Hsd3b1 and Inhba, the differences between genotypes were not statistically significant. No effect of genotype on PMSG response was observed for Prkar2b. These results show that granulosa cells lacking ERβ demonstrate an attenuated genomic response to FSH, and that, for some genes, the number of functional Esr2 alleles correlates directly with the level of attenuated gene expression. The data also suggest that loss of ERβ does not affect the response of all cAMP or PKA-regulated genes to PMSG.
Table 2.
GO biological process categories of genes regulated by PMSG in ERβ+/− but not ERβ−/− granulosa cells
| Term | % | P value |
|---|---|---|
| Protein metabolism | 17.3 | 0.003 |
| Cellular macromolecule metabolism | 16.0 | 0.012 |
| Negative regulation of cellular physiological process | 5.00 | 0.001 |
| Generation of precursor metabolites and energy | 4.83 | 0.008 |
| Organic acid metabolism | 4.00 | 0.002 |
| Cellular lipid metabolism | 3.83 | 0.005 |
| Regulation of cell cycle | 3.33 | 0.003 |
| Alcohol metabolism | 2.67 | 0.002 |
| Lipid biosynthesis | 2.33 | 0.003 |
| Blood vessel development | 2.00 | 0.004 |
| Negative regulation of transferase activity | 0.83 | 0.012 |
Granulosa cells were isolated from mice treated with PMSG for 48 h. Genes (n = 590) were differentially regulated in ERβ−/− compared with ERβ+/− granulosa cells. Of these, 64% were associated with a GO Biological Process 3 category (P < 0.01).
Figure 6.
ERβ is required for maximal expression of many FSH-regulated genes identified by microarray analysis. Immature mice were treated with saline (□) or PMSG (▪) (3.25 IU) for 48 h, and granulosa cells were isolated and pooled, and total RNA was isolated for gene expression analysis by quantitative RT-PCR. Data are expressed as the average ± sem of three independent experiments. Analysis by ANOVA found a significant interaction between PMSG treatment and genotype for Comp (P = 0.002), Lrp11 (P = 0.0002), Car14 (P = 0.04), and Mro (P = 0.03), indicating that the magnitude of increased Comp, Lrp11, Car14, and Mro expression in response to PMSG is dependent on the genotype, i.e. on the presence of the ERβ allele. For Hsd3b1, Inhba, and Prkarb, no significant interaction between PMSG treatment and genotype was observed. However, the effect of PMSG treatment on individual genotypes compared with vehicle was considered significant for Hsd3b1 (P = 0.003), Inhba (P = 0.002), and Prkarb (P < 0.0001). A Bonferroni post test was also used to compare replicate means by genotype: a, P < 0.001; b, P < 0.01; c, P < 0.05.
The extensive number of cAMP-responsive genes that were differentially regulated by PMSG in ERβ−/− granulosa cells suggests that the levels of cAMP in PMSG-stimulated ERβ−/− granulosa cells are less than sufficient and that this insufficiency explains, at least in part, the global reduction in gene expression in the absence of ERβ.
Discussion
We have previously shown from in vivo ovarian studies (9) and in vitro follicle culture analysis (21) that granulosa cells of ERβ−/− preovulatory follicles exhibit an attenuated response to FSH-induced differentiation, as evidenced by reduced Lhcgr expression and a correspondingly attenuated response to LH stimulation (9). In this study, we now demonstrate that this lack of sufficient Lhcgr expression specifically occurs in ovarian granulosa cells and results in markedly lower cAMP levels in FSH-primed, LH-stimulated ERβ−/− granulosa cells. We also show that the attenuated FSH-mediated differentiation observed in ERβ−/− granulosa cells may be due, at least in part, to dysregulation of the cAMP pathway, indicating a role for ERβ in FSH-stimulated cAMP signaling.
ERβ−/− granulosa cells exhibit reduced cAMP levels in response to LH
Preovulatory follicles are characterized by Lh-r expression in granulosa cells, a response that requires the actions of both FSH and estradiol (13,22,23,24), the former of which is known to act via adenylate cyclase/cAMP signaling (25,26,27,28). Our in situ hybridization experiments show that PMSG treatment robustly increases Lhcgr mRNA levels in granulosa cells of ERβ+/− mice but not in the granulosa cells of similarly treated ERβ−/− mice (Fig. 1). This lack of Lhcgr mRNA results in a dramatically attenuated response to LH in vitro as evidenced by the impaired expression of Ptgs2 and Pgr in response to LH after FSH priming of primary granulosa cells. Induction of both Ptgs2 (29,30,31,32,33) and Pgr (34,35,36,37) occurs via the cAMP/PKA pathway. The lack of Lh-r appears to be the primary reason for the attenuated Ptgs2 and Pgr expression because direct activation of adenylate cyclase by forskolin (effectively bypassing the need for Lh-r) induced similar wild type-like levels of Ptgs2 and Pgr mRNA levels in ERβ−/− granulosa cells. As predicted from these results, cAMP levels in LH-stimulated ERβ−/− granulosa cells were approximately half those of ERβ+/+ granulosa cells. In response to forskolin, ERβ+/+ and ERβ−/− granulosa cell cAMP levels were not significantly different. These results indicate that it is the loss of Lhcgr expression and the concomitant reduction in LH-generated cAMP that is the primary cause of the attenuated response of genes such as Ptgs2 and Pgr to hCG treatment in ERβ−/− mice.
However, mechanisms other than loss of Lhcgr expression are also likely to play a role in the reduced genomic response of ERβ−/− granulosa cells to LH because forskolin-induced Pgr levels remained markedly lower in ERβ−/− granulosa cells (Fig. 2B). Given the large number of genes differentially regulated in PMSG-treated ERβ−/− mice compared with ERβ+/− mice, it is likely that the differential expression of genes other than Lhcgr may contribute to the reduced cAMP levels observed in LH-treated ERβ−/− granulosa cells. However, neither adenylate cyclase nor PDE genes were identified by microarray analysis or qRT-PCR as differentially regulated in granulosa cells of PMSG-treated ERβ−/− mice compared with ERβ+/− mice. These results suggest that neither differences in adenylate cyclase nor PDE mRNA levels after FSH exposure are responsible for the impaired cAMP levels observed in response to LH. We also investigated whether disrupted coupling of Lh-r to the G protein, Gsα, might be responsible for reduced LH-induced cAMP levels in ERβ−/− granulosa cells; however, qRT-PCR again indicated no difference in Gsα mRNA levels in granulosa cells isolated from PMSG-treated ERβ−/− and ERβ+/+ mice (data not shown). Finally, we investigated whether PMSG-stimulated ERβ−/− granulosa cells might express higher mRNA levels of Rgs2, which inhibits G protein signaling and is expressed in mouse and rat granulosa cells (38,39). Again, qRT-PCR indicated no difference in expression between Rgs2 mRNA levels between granulosa cells isolated from PMSG-treated ERβ−/− and ERβ+/+ mice. Therefore, more studies are required to determine mechanisms other than loss of Lh-r, which may be responsible for the reduced cAMP levels observed in LH-stimulated ERβ−/− granulosa cells.
Identification of genes requiring FSH and ERβ for maximal expression in preovulatory granulosa cells
Several hundred genes were identified with expression levels that were significantly different in granulosa cells isolated from PMSG-treated ERβ−/− mice compared with ERβ+/− mice. We selected seven genes to confirm these genotypic differences in PMSG response; for six of these there is evidence for regulation, at least in part, by the cAMP/PKA pathway (17,18,19). We found that loss of the Esr2 allele significantly decreased the PMSG response for some, but not all, cAMP-responsive genes (Fig. 6). One reason for this differential response to the loss of ERβ is that despite attenuated levels of cAMP in ERβ−/− cells, sufficient cAMP remained to maintain the response to PMSG (i.e. a minimum threshold of cAMP was met). This differential response between cAMP-regulated genes may also indicate that in our model system, other signaling mechanisms contribute to the regulation of these PMSG-responsive genes in addition to the cAMP/PKA pathway. Finally, microarray analyses indicated that mRNA levels of certain genes, such as Car14, were lower in granulosa cells from ERβ−/− compared with ERβ+/− mice even before being treated with PMSG (Fig. 6). This may be due to the lower levels of circulating FSH in prepubertal mice or may indicate a role for ERβ before gonadotropin exposure or formation of antral follicles, as has been suggested in some reports (40). We did observe significantly lower cAMP levels in untreated granulosa cells isolated from ERβ−/− mice than in ERβ+/+ mice (Fig. 4F), suggesting that the two granulosa cell populations are not identical biochemically after isolation. However, we have previously shown that the number of primordial, primary, and preantral follicles is comparable in prepubertal ERβ−/− and ERβ+/+ mice (21), indicating that ERβ is unlikely to play a major role in regulating preantral follicle development.
We previously observed that FSH-stimulated granulosa cell differentiation was attenuated in granulosa cells isolated from ERβ−/− mice treated with PMSG as evidenced by reduced expression of LH receptor (Lhcgr) mRNA and Cyp19a1 mRNA (17). During that study, ERβ+/− mice were frequently used as controls because they do not demonstrate ovulatory or fertility defects as do the ERβ−/− mice. However, confirmation of our microarray data using PMSG-treated ERβ+/+, ERβ+/−, and ERβ−/− mice (Figs. 1 and 6) now indicate that gene expression correlates with the dosage of functional Esr2 (ERβ) alleles, indicating that ERβ is a limiting factor in the FSH-mediated regulation of these genes. These data also implicate that women with one allelic mutation in ERβ may present with reduced fertility and that complete loss of ERβ expression would not be necessary to impact fertility. This hypothesis is consistent with a report in which single nucleotide polymorphisms of ERβ were associated with severe idiopathic ovulatory defects in women (41). Interestingly, recent studies indicate that ablation of full-length ERβ and all ERβ transcriptional variants in mice renders both sexes sterile (42), suggesting that expression of ERβ transcriptional variants, as well as gene dosage, may impact human fertility.
cAMP levels are significantly reduced in forskolin-stimulated ERβ−/− granulosa cells
There is previous evidence for a role for estradiol in enhancing both the level and action of cAMP after FSH stimulation of granulosa cells (12,13,14). Knecht et al. (13) demonstrated that cotreatment of granulosa cells with FSH and estradiol resulted in 50% higher production of cAMP than in cells treated with FSH alone. They also observed that estradiol enhanced the production of Lh-r in response to FSH, PGE2, forskolin, or 8-bromo-cAMP, indicating that estradiol enhances the actions of cAMP, regardless of its means of production. In our study, Lhcgr expression was 40% lower in forskolin-stimulated ERβ−/− primary granulosa cells than in similarly-treated ERβ+/+ cells (Fig. 3A), indicating that ERβ is required for maximal Lhcgr expression even when adenylate cyclase is activated directly, consistent with the reduced cAMP levels observed under the same conditions (Fig. 4B) and previous publications indicating a role for estradiol in the production of cAMP in response to FSH. These results suggested that cAMP levels in forskolin-stimulated ERβ−/− granulosa cells would be lower than in similarly treated ERβ+/+ cells. Although a trend toward lower cAMP levels in FSH-stimulated ERβ−/− granulosa cells compared with wild-type controls was observed, the difference at individual time points was not statistically significant (Fig. 4A).
Surprisingly, Lhcgr mRNA levels were similar in ERβ+/+ and ERβ−/− granulosa cells treated in vitro with FSH, in contrast to the dramatic loss of expression in granulosa cells when exposed to PMSG in vivo (Fig. 1B). It is unclear why Lhcgr expression is not lower in FSH-treated ERβ−/− granulosa cells than in ERβ+/+ cells; however, several explanations are possible. First, because isolated granulosa cells lack the environment provided by the intact follicle, signaling or other factors may be absent that would normally contribute to Lhcgr mRNA levels in vivo. This could result in suboptimal Lhcgr induction in ERβ+/+ cells, resulting in Lhcgr levels comparable to those in ERβ−/− cells. On the other hand, an ERβ-dependent inhibitory factor that would normally suppress Lhcgr expression in ERβ+/+ cells may be absent, resulting in overproduction of Lhcgr in ERβ−/− cells. Further experiments will be required to understand why FSH-stimulated Lhcgr levels are comparable in both ERβ+/+ and ERβ−/− cells.
ERβ−/− granulosa cells stimulated directly with a cAMP analog did not express significantly lower levels of Lhcgr than ERβ+/+ cells. These results are in contrast to previous observations by others (12,13,14) who found that estradiol plays a role in cAMP action as well as its production. However, differences between our study and theirs may be explained by differences in species and estrogen priming in the model systems. In our study, we used immature, non-estrogen-primed mice. However, the studies in which estradiol was found to augment cAMP activity used either hypophysectomized immature estradiol-primed rats (12,14) or immature diethylstilbestrol-primed rats (13). In addition, as our endpoint we measured Lhcgr mRNA levels, whereas in the previously described studies, hCG binding was measured. This difference may also contribute to our observed results. Thus, our studies suggest that in our model system, ERβ plays a greater role in augmenting cAMP accumulation than in cAMP action.
Pde1c expression is increased in ERβ−/− granulosa cells
Our experiments using the PDE inhibitor, IBMX, indicate that elevated PDE activity in cultured ERβ−/− granulosa cells may be responsible, in part, for the lower cAMP levels observed in response to in vitro FSH or forskolin stimulation (Fig. 4, C and D). This hypothesis is supported by the observation that Pde1c mRNA levels are 5-fold higher in untreated primary ERβ−/− granulosa cells than in ERβ+/+ granulosa cells (Fig. 4G). Pde1c is a member of the Ca2+/calmodulin-dependent family of PDEs. CaM-PDE activity has been previously detected in granulosa cells (15). Ca2+/calmodulin-dependent phosphodiesterase activity is present in granulosa cells isolated from hypophysectomized estrogen-primed immature female rats, and FSH treatment stimulates cAMP degradation by this CaM-PDE (15). Our data suggest that Pde1c activity is higher in ERβ−/− granulosa cells than in ERβ+/+ granulosa cells and may be one explanation for the reduced cAMP levels observed in vitro in FSH or forskolin-stimulated ERβ−/− granulosa cells.
There is significant evidence to suggest that one mechanism by which estradiol augments FSH-mediated granulosa cell differentiation is by enhancing cAMP production and/or accumulation (12,13,14). In summary, we have now shown that it is ERβ that mediates this estradiol effect, and that in the absence of ERβ, the cAMP pathway is disrupted in response to FSH, leading to the dysregulation of many FSH-regulated genes, an impaired response to LH, and impaired ovulation in ERβ−/− mice.
Materials and Methods
Animals and treatment
All animals were handled according to NIH guidelines and in compliance with the National Institute of Environmental Health Sciences Institutional Animal Care and Use Committee. The generation of ERβ−/− mice has been described previously (8). All animals were maintained under a 12-h light, 12-h dark schedule and fed NIH-31 mouse chow. Prepubertal ERβ-null (ERβ−/−) female mice were generated via breeding homozygous (ERβ−/−) males with heterozygous (ERβ+/−) females. All females were weaned at 21 d of age and genotyped as previously described (8). For in vivo studies, mice between 23 d and 25 d of age were treated with 3.25 IU PMSG (Sigma Chemical Co., St. Louis, MO) between 1300 and 1500 h. For microarray analysis, this treatment was followed 48 h later by treatment with 2.2 IU hCG (Sigma). Both PMSG and hCG were dissolved in 0.85% saline solution and injected sc in a total volume of 0.1 ml.
Granulosa cell isolation
For in vivo studies, mice were treated with PMSG as described above. Ovaries were removed and immediately transferred to a 100-mm cell culture dish containing 15 ml ice-cold M199 medium supplemented with 1 mg/ml BSA, 2.5 μg/ml Fungizone, and 50 μg/ml gentamicin (all reagents from Invitrogen, Carlsbad, CA). Ovaries were pooled according to genotype and treatment, after which the granulosa cells from each were expressed by manual puncture with 25-gauge needles followed by pressure applied with a sterile spatula. Follicular debris was removed manually, and the granulosa cell suspension was filtered through a 150-μm Nitex nylon membrane (Sefar America, Inc., Depew, NY) mounted in Swinnex filters (Millipore Corp., Billerica, MA). The granulosa cells were then pelleted by centrifugation at 250 × g for 5 min at 4 C, followed by two washes in DMEM/F-12 medium. The final cell pellet was frozen at −80 C until RNA extraction or cultured in vitro as described below.
For analysis of Lhcgr mRNA levels after in vitro FSH, forskolin, or 8-(4-chlorophenylthio) adenosine 3:5-cyclic monophosphate (8CPT) treatment, granulosa cells were plated in DMEM/F12 in 60-mm plastic dishes, 2 million cells per dish, then allowed to adhere for 2 h before treatment (37 C, 5% CO2). The cells were then treated with either recombinant hFSH (50 ng/ml; National Hormone & Peptide Program, the National Institute of Diabetes and Digestive and Kidney Diseases, and Dr. Parlow) or forskolin (10 μm; Sigma-Aldrich), or 8CPT (1 mm) for 48 h. Testosterone (T)(5 ng/ml, Steraloids, Inc., Newport, RI) was included in the medium with FSH, forskolin, and 8CPT to serve as a substrate for estradiol production. After incubation, the cells were frozen immediately or treated for an additional 4 h with 100 ng/ml LH (human, National Hormone & Peptide Program), and then frozen. RNA was later isolated from frozen cells.
RNA isolation and quantitative PCR gene expression assays
Frozen pellets of granulosa cells were solubilized in Trizol (Invitrogen, Carlsbad, CA) and RNA was isolated according to the manufacturer’s protocol. RNA was further treated with deoxyribonuclease I, and then reverse transcribed using Superscript II (Invitrogen). cDNA levels were detected using quantitative PCR with the ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) and SYBR Green I dye, and primers were generated using Applied Biosystems Primer Express Software version 2.0 (Table 1). Taqman Gene Expression Assay (Applied Biosystems) probe/primer sets were used to analyze Lhcgr, Ptgs2, and Pgr expression. Fold changes in gene expression were determined by quantitation of cDNA from target (treated) samples relative to a calibrator sample (vehicle). For all SYBR green assays (see Table 1 for primer sequences), the gene for ribosomal protein L7 (Rpl7) was used as the endogenous control for normalization of initial RNA levels. For all Taqman assays, the gene for 18S (Rn18s) was used as the endogenous control for normalization of initial RNA levels. Rn18s Taqman probe sequence: 5′-ATGGTTCCTTTGGTCGCTCGCTCC-3′. See Table 1 for Rn18s primer sequences. Expression ratios were calculated according to the mathematical model described by Pfaffl (45), in which ratio = (EtargetΔCt(target)/(Econtrol)ΔCt(control) and E = efficiency of the primer set, calculated from the slope of a standard curve of log (nanograms of cDNA) vs. Ct value for a sample that contains the target according to the formula E =10−(1/slope) and ΔCt = Ct(vehicle) − Ct(treated sample).
Table 1.
Primer sequences for quantitative PCR (SYBR green)
| Gene | Accession | Forward primer | Reverse primer |
|---|---|---|---|
| Car14 | NM_011797 | 5′-GCTGTCCTAGGCATCCTCATTG-3′ | 5′-TGTATCTTATTTCATGTAGACGACTCAGAAT-3′ |
| Comp | NM_016685 | 5′-ACGGACCTGGACGGTTTTC-3′ | 5′-CCTCCTGCCCCGAATTG-3′ |
| Ddah1 | NM_026993 | 5′-CGCAAAGGTCTATGAGAAACTCAAG-3′ | 5′-AGCAAGCCGTCCACCTTTT-3′ |
| Lrp11 | NM_172784 | 5′-GGCTGGGAAGGATGTGGTTT-3′ | 5′-AGGCACCTTCATGTCCACTGA-3′ |
| Mro | NM_027741 | 5′-CGGAGGGAGGCTCTGAGAA-3′ | 5′-TGGCCAAATGTGGTGAATCA-3′ |
| Pde1c | NM_011054 | 5′-AACTCTCTGAAACACCTGCAACCT-3′ | 5′-CCGTAACCTCTGGGACGTTTT-3′ |
| Pde1b | NM_008800 | 5′-GACTGGTTGGCCTCCACCTT-3′ | 5′-CGGAACTTGGGTTTCTCTTCTG-3′ |
| Pde8a | NM_008803 | 5′-GACCACAGATATCCCCGACAGA-3′ | 5′-CGTACTACGCCAACAATAACTGTGT-3′ |
| Pde4b | NM_019840 | 5′-GCGAGATGGCTTCAAACAAGT-3′ | 5′-TCCCTGATCTGCTCATCTCTGA-3′ |
| Pde7b | NM_013875 | 5′-CACGGCTCCTGGCTCACTT-3′ | 5′-TTGATATCCGTGGCCAAGATG-3′ |
| Rn18s | NR_003278 | 5′-GAAACTGCGAATGGCTCATTAA-3′ | 5′-GAATCACCACAGTTATCCAAGTAGGA-3′ |
| Rpl7 | NM_011291 | 5′-AGCTGGCCTTTGTCATCAGAA-3′ | 5′-GACGAAGGAGCTGCAGAACCT-3′ |
| Thbs4 | NM_011582 | 5′-AGGTGGCCAGCCTCCAA-3′ | 5′-GTCGATTAAAGTCTCCTGTGCTTGT-3′ |
Western blot analysis
For Western blot analyses, granulosa cells were isolated and pooled from untreated mice as described above, and then were plated in 60-mm plastic dishes in M199 medium at 5 million cells per dish and allowed to adhere for 2 h (37 C, ambient) before treatment. The cells were then treated with 100 ng/ml FSH + T (5 ng/ml) for 0, 15, 30, and 60 min and then frozen immediately. Whole-cell extracts were prepared using the M-Per kit (Pierce Biotechnology, Rockford, IL). Granulosa cell extract (3 μg) was separated on a 10% NuPage gel run in 3[N-morpholino]propanesulfonic acid buffer (Invitrogen), transferred to a nitrocellulose membrane, blocked for 1 h in 5% milk/Tris-buffered saline with Tween-20, and then incubated in phospho (Ser133)-CREB antibody (no. 9198) diluted 1:2000 or CREB (no. 9197) antibody (Cell Signaling Technology, Inc., Danvers, MA) diluted 1:2000.
In situ hybridization
Mice were treated with vehicle or PMSG for 48 h, after which the ovaries were removed and snap frozen in 2-methylbutane (isopentane) and then stored at −70 C. In situ hybridization analysis for the detection of Lhcgr in whole ovaries was then carried out by Phylogeny, Inc. (Columbus, OH). The antisense probe hybridized to bp 738–1190 of the Lhcgr coding region.
Microarray analysis of granulosa cells
Granulosa cell isolation
Immature mice were treated with PMSG (3.25 IU) for 48 h. Granulosa cells from each of both treatment groups (ERβ+/− PMSG, ERβ−/− PMSG) were isolated, total RNA was extracted, and fluorescently labeled cRNA was generated for microarray analysis as described below. Gene expression values representing the (ERβ−/− PMSG:ERβ+/− PMSG) ratio were calculated for each genotype.
Gene expression analyses were conducted using Agilent Mouse Oligo arrays (pattern no. 011978) (Agilent Technologies, Palo Alto, CA). Total RNA was amplified using the Agilent Low RNA Input Fluorescent Linear Amplification Kit protocol. Cy3- or Cy5-labeled cRNA was produced according to the manufacturer’s protocol from 500 ng of total RNA per sample. For each two-color comparison, 750 ng of each of Cy3- and Cy5-labeled cRNAs were mixed and fragmented using the Agilent In Situ Hybridization Kit protocol. Hybridizations were performed for 17 h in a rotating hybridization oven using the Agilent 60-mer oligo microarray processing protocol. Two slides were hybridized for each sample pairing to allow for dye reversals (technical replicates). Microarray analysis of PMSG samples was conducted from one biological replicate (Fig. 6). Slides were washed as indicated in this protocol and then scanned with an Agilent Scanner. Data were obtained using the Agilent Feature Extraction software (version 7.5), using defaults for all parameters.
The Agilent Feature Extraction Software performed error modeling, adjusting for additive and multiplicative noise. The resulting data were processed using the Rosetta Resolver system (version 7.1) (Rosetta Biosoftware, Kirkland, WA). The ratio intensity value for each gene feature on the array was averaged across technical replicates using the error-weighted approach described by Weng et al. (43). A P value for each gene probe is computed based upon the reproducibility of the expression measurements across the technical replicates. Gene features with a P value <0.001 (reflecting the technical variability of the array chip) were considered differentially expressed. The resulting lists were combined and clustered hierarchically. Note that the microarray data between genotypes could not be analyzed for statistical significance because of limited sample size.
The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (44) and are accessible through Gene Expression Omnibus Series accession no. GSE11585. They are also published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org.
Functional analysis
The Database for Annotation, Visualization and Integrated Discovery 2.1 (DAVID 2.1) Functional Annotation tool (45) was used to determine Gene Ontology functions (Ref. 46 and Table 2). All analyses were conducted with Maximum EASE Score/P value set to 0.05. Only groups satisfying a P value ≤ 0.01 have been listed.
Determination of cAMP levels
Freshly isolated granulosa cells from untreated mice were resuspended in DMEM/F12 medium and transferred into 96-well cell culture plates at 1 × 105 cells per well. The cells were incubated for 2 h (37 C, 5% CO2) and then treated with recombinant FSH (50 ng/ml) + T (5 ng/ml) and incubated for an additional 30, 60, or 120 min under the same conditions. For analysis of LH-stimulated cAMP levels, cells were treated with FSH (50 ng/ml) + T (5 ng/ml) for 48 h, and then stimulated with LH (100 ng/ml) for 30, 60, or 120 min. After treatment, the plates were covered in foil and frozen on dry ice and then stored at −80 C until cAMP analysis was conducted. cAMP levels were determined using the cAMP enzyme immunoassay Biotrak kit (no. RPN 225) (Amersham Biosciences, Piscataway, NJ) according to the manufacturer’s instructions for Protocol no. 4 (total cellular cAMP measurement using the nonacetylation enzyme immunoassay procedure with the novel lysis reagents).
Statistical analysis
Lhcgr (Fig. 1), Comp, Lrp11, Car14, Mro, Hsd3b1, Inhba, Prkar2b (Fig. 6) mRNA levels in response to PMSG for ERβ+/+, ERβ+/−, and ERβ−/−
Three independent experiments were conducted. The data were analyzed by ANOVA, followed by a Bonferroni post test to compare replicate means by genotype (see figure legends for significance values).
Ptgs2 and Pgr mRNA levels in response to FSH treatment followed by LH or forskolin (Fig. 2)
Four independent experiments were conducted. The data were analyzed by ANOVA, followed by a Bonferroni post test to compare replicate means by genotype (see figure legends for significance values).
cAMP levels in response to LH, FSH, and forskolin (Fig. 2C and Fig. 5, A–F) and Pde1c mRNA levels (untreated cells) (Fig. 5, G and H)
Data from three (Fig. 2C) or five (Fig. 5) independent experiments were analyzed by two-sample Student’s t-tests (Fig. 5, A–F). For Pde1 mRNA levels (Fig. 5, G and H), data from four independent experiments were analyzed by an unpaired two-tailed Student’s t test.
Lhcgr mRNA levels in response to FSH or 8CPT (Fig. 3B)
Data from two independent experiments (one conducted in duplicate, the other in singlicate) were analyzed using a two-sided Mann-Whitney test. No significant difference was observed between Lhcgr levels induced by CPT in wild-type and ERβ-null granulosa cells (P = 0.7).
Lhcgr mRNA levels in response to FSH or forskolin (Fig. 3A)
Four independent experiments were conducted. Significance of the three main effects (genotype and FSH/forskolin treatment) and genotype-by-treatment interaction effects were determined through ANOVA. All effects were significant at or below a P < 0.002 threshold except for the genotype-by-FSH interaction. Thus all genotype/treatment groups significantly differed from each other except for FSH-treated animals. Under FSH treatment, genotype did not significantly change the expression level.
Acknowledgments
We thank Grace Kissling (National Institute of Environmental Health Sciences) and Andrew D. Fernandes (The University of Western Ontario) for their assistance with statistical analysis, and Adrian Vincent Buensuceso (The University of Western Ontario) for carrying out a subset of the qRT-PCR experiments in Fig. 6.
Footnotes
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (all authors), as well as The University of Western Ontario, The Children’s Health Research Institute, and the London Regional Cancer Program (to B.D.).
Current address for B.J.D.: The University of Western Ontario, London, Ontario, Canada N6A 3K7.
Current address for J.F.C.: Taconic Biotech, 5 University Place, Rensselaer, New York 12144.
Current address for S.F.G.: Illumina, 9885 Town Centre Drive, San Diego, California 92121.
Disclosure Summary: All authors have nothing to declare.
First Published Online March 26, 2009
Abbreviations: 8CPT, 8-(4-Chlorophenylthio) adenosine 3:5-cyclic monophosphate; CREB, cAMP-regulatory element-binding protein; ER, estrogen receptor; hCG, human gonadotropin; IBMX, 3-isobutyl-1-methylxanthine; Lh-r, LH receptor; pCREB, phosphorylated CREB; PDE, phosphodiesterase; PKA, protein kinase A; PMSG, pregnant mare serum gonadotropin; qRT-PCR, quantitative RT-PCR; T, testosterone.
References
- Richards JS 1994 Hormonal control of gene expression in the ovary. Endocr Rev 15:725–751 [DOI] [PubMed] [Google Scholar]
- Nakano R, Nakayama T, Iwao M 1982 Inhibition of ovarian follicle growth by a chemical antiestrogen. Horm Res 16:230–236 [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR 2000 Expression of estrogen receptor β is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod 62:310–317 [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Harrell JC, Korach KS 2005 Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol 67:285–308 [DOI] [PubMed] [Google Scholar]
- Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS 1999 Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-α knockout mouse. Endocrinology 140:5855–5865 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Rodriguez KF, Johnson JA, Poirier D, Korach KS 2006 The intraovarian actions of estrogen receptor-α are necessary to repress the formation of morphological and functional Leydig-like cells in the female gonad. Endocrinology 147:3666–3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi F, Couse JF, Rodriguez KF, Emmen JM, Poirier D, Korach KS 2007 Estrogen receptor-α mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17α-hydroxylase/17,20 lyase) expression. FASEB J 21:586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS 2005 Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 146:3247–3262 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Maizels ET 2006 FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 18:1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador LM, Park Y, Cottom J, Maizels ET, Jones JC, Schillace RV, Carr DW, Cheung P, Allis CD, Jameson JL, Hunzicker-Dunn M 2001 Follicle-stimulating hormone stimulates protein kinase A-mediated histone H3 phosphorylation and acetylation leading to select gene activation in ovarian granulosa cells. J Biol Chem 276:40146–40155 [DOI] [PubMed] [Google Scholar]
- Jonassen JA, Bose K, Richards JS 1982 Enhancement and desensitization of hormone-responsive adenylate cyclase in granulosa cells of preantral and antral ovarian follicles: effects of estradiol and follicle-stimulating hormone. Endocrinology 111:74–79 [DOI] [PubMed] [Google Scholar]
- Knecht M, Darbon JM, Ranta T, Baukal AJ, Catt KJ 1984 Estrogens enhance the adenosine 3′,5′-monophosphate-mediated induction of follicle-stimulating hormone and luteinizing hormone receptors in rat granulosa cells. Endocrinology 115:41–49 [DOI] [PubMed] [Google Scholar]
- Richards JS, Jonassen JA, Rolfes AI, Kersey K, Reichert Jr LE 1979 Adenosine 3′,5′-monophosphate, luteinizing hormone receptor, and progesterone during granulosa cell differentiation: effects of estradiol and follicle-stimulating hormone. Endocrinology 104:765–773 [DOI] [PubMed] [Google Scholar]
- Conti M, Kasson BG, Hsueh AJ 1984 Hormonal regulation of 3′,5′-adenosine monophosphate phosphodiesterases in cultured rat granulosa cells. Endocrinology 114:2361–2368 [DOI] [PubMed] [Google Scholar]
- Hanoune J, Defer N 2001 Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41:145–174 [DOI] [PubMed] [Google Scholar]
- Escamilla-Hernandez R, Little-Ihrig L, Orwig KE, Yue J, Chandran U, Zeleznik AJ 2008 Constitutively active protein kinase A qualitatively mimics the effects of follicle-stimulating hormone on granulosa cell differentiation. Mol Endocrinol 22:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurten RC, Levy LO, Shey J, Durica JM, Richards JS 1992 Identification and characterization of the GC-rich and cyclic adenosine 3′,5′-monophosphate (cAMP)-inducible promoter of the type II β cAMP-dependent protein kinase regulatory subunit gene. Mol Endocrinol 6:536–550 [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguère V 1997 Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol Endocrinol 11:353–365 [DOI] [PubMed] [Google Scholar]
- Tuuri T, Erämaa M, Van Schaik RH, Ritvos O 1996 Differential regulation of inhibin/activin α- and β A-subunit and follistatin mRNAs by cyclic AMP and phorbol ester in cultured human granulosa-luteal cells. Mol Cell Endocrinol 121:1–10 [DOI] [PubMed] [Google Scholar]
- Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS 2005 In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)α and ERβ null mice indicate a role for ERβ in follicular maturation. Endocrinology 146:2817–2826 [DOI] [PubMed] [Google Scholar]
- Farookhi R, Desjardins J 1986 Luteinizing hormone receptor induction in dispersed granulosa cells requires estrogen. Mol Cell Endocrinol 47:13–24 [DOI] [PubMed] [Google Scholar]
- Kessel B, Liu YX, Jia XC, Hsueh AJ 1985 Autocrine role of estrogens in the augmentation of luteinizing hormone receptor formation in cultured rat granulosa cells. Biol Reprod 32:1038–1050 [DOI] [PubMed] [Google Scholar]
- Segaloff DL, Wang HY, Richards JS 1990 Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol 4:1856–1865 [DOI] [PubMed] [Google Scholar]
- Knecht M, Catt KJ 1982 Induction of luteinizing hormone receptors by adenosine 3′,5′-monophosphate in cultured granulosa cells. Endocrinology 111:1192–1200 [DOI] [PubMed] [Google Scholar]
- Nimrod A 1981 The induction of ovarian LH-receptors by FSH is mediated by cyclic AMP. FEBS Lett 131:31–33 [DOI] [PubMed] [Google Scholar]
- Segaloff DL, Limbird LE 1983 Luteinizing hormone receptor appearance in cultured porcine granulosa cells requires continual presence of follicle-stimulating hormone. Proc Natl Acad Sci USA 80:5631–5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Liu X, Segaloff DL 2001 Identification of an SAS (Sp1c adjacent site)-like element in the distal 5′-flanking region of the rat lutropin receptor gene essential for cyclic adenosine 3′,5′-monophosphate responsiveness. Endocrinology 142:2013–2021 [DOI] [PubMed] [Google Scholar]
- Sirois J, Levy LO, Simmons DL, Richards JS 1993 Characterization and hormonal regulation of the promoter of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Identification of functional and protein-binding regions. J Biol Chem 268:12199–12206 [PubMed] [Google Scholar]
- Sirois J, Simmons DL, Richards JS 1992 Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. Induction in vivo and in vitro. J Biol Chem 267:11586–11592 [PubMed] [Google Scholar]
- Sirois J, Richards JS 1993 Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for the role of a cis-acting C/EBP β promoter element. J Biol Chem 268:21931–21938 [PubMed] [Google Scholar]
- Morris JK, Richards JS 1993 Hormone induction of luteinization and prostaglandin endoperoxide synthase-2 involves multiple cellular signaling pathways. Endocrinology 133:770–779 [DOI] [PubMed] [Google Scholar]
- Morris JK, Richards JS 1995 Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology 136:1549–1558 [DOI] [PubMed] [Google Scholar]
- Park OK, Mayo KE 1991 Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol 5:967–978 [DOI] [PubMed] [Google Scholar]
- Natraj U, Richards JS 1993 Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology 133:761–769 [DOI] [PubMed] [Google Scholar]
- Park-Sarge OK, Mayo KE 1994 Regulation of the progesterone receptor gene by gonadotropins and cyclic adenosine 3′,5′-monophosphate in rat granulosa cells. Endocrinology 134:709–718 [DOI] [PubMed] [Google Scholar]
- Clemens JW, Robker RL, Kraus WL, Katzenellenbogen BS, Richards JS 1998 Hormone induction of progesterone receptor (PR) messenger ribonucleic acid and activation of PR promoter regions in ovarian granulosa cells: evidence for a role of cyclic adenosine 3′,5′-monophosphate but not estradiol. Mol Endocrinol 12:1201–1214 [DOI] [PubMed] [Google Scholar]
- Ujioka T, Russell DL, Okamura H, Richards JS, Espey LL 2000 Expression of regulator of G-protein signaling protein-2 gene in the rat ovary at the time of Ovulation. Biol Reprod 63:1513–1517 [DOI] [PubMed] [Google Scholar]
- Wu YL, Chuang HH, Kou YR, Lee TS, Lu SH, Huang YC, Nishi Y, Yanase T 2008 Regulation of LH receptor and PGF2α receptor signaling by the regulator of G protein signaling 2 (RGS2) in human and mouse granulosa cells. Chin J Physiol 51:282–291 [PubMed] [Google Scholar]
- Hegele-Hartung C, Siebel P, Peters O, Kosemund D, Müller G, Hillisch A, Walter A, Kraetzschmar J, Fritzemeier KH 2004 Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci USA 101:5129–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundarrajan C, Liao WX, Roy AC, Ng SC 2001 Association between estrogen receptor-β gene polymorphisms and ovulatory dysfunctions in patients with menstrual disorders. J Clin Endocrinol Metab 86:135–139 [DOI] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M 2008 Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERβ-null mutant. Proc Natl Acad Sci USA 107:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L, Dai H, Zhan Y, He Y, Stepaniants SB, Bassett DE 2006 Rosetta error model for gene expression analysis. Bioinformatics 22:1111–1121 [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE 2002 Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis Jr G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA 2003 DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4:P3 [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G 2000 Gene Ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25:25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]