Abstract
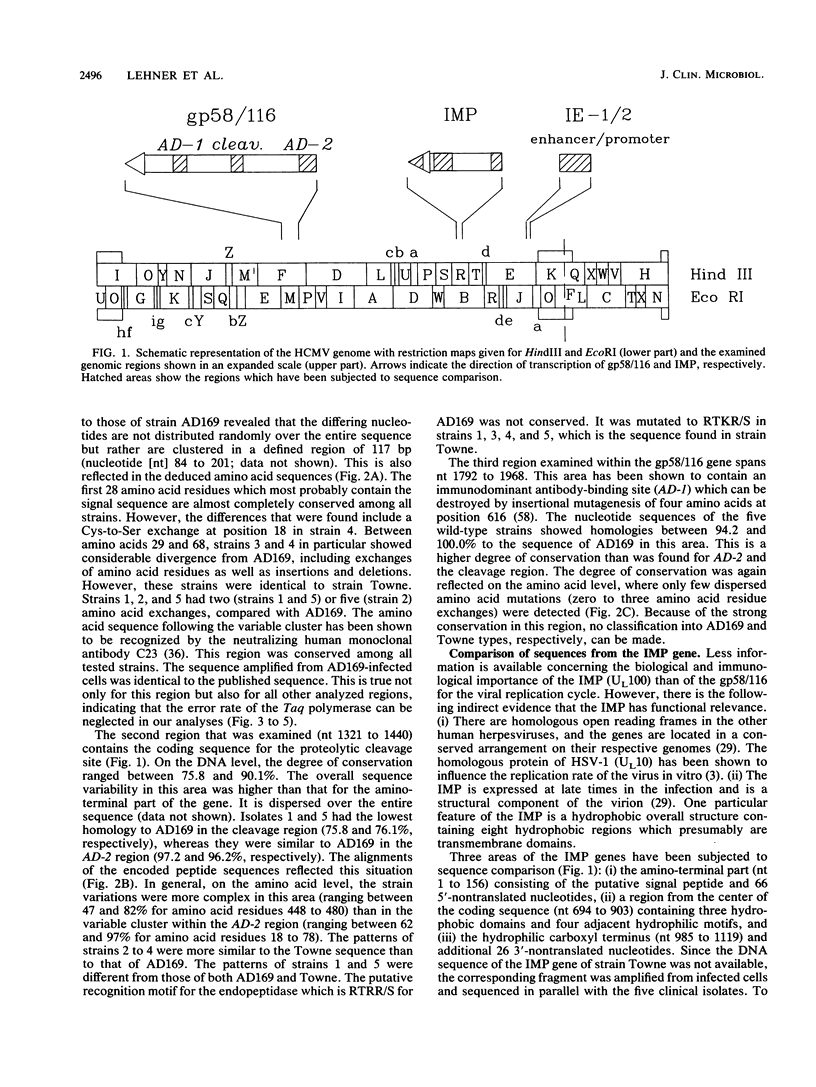
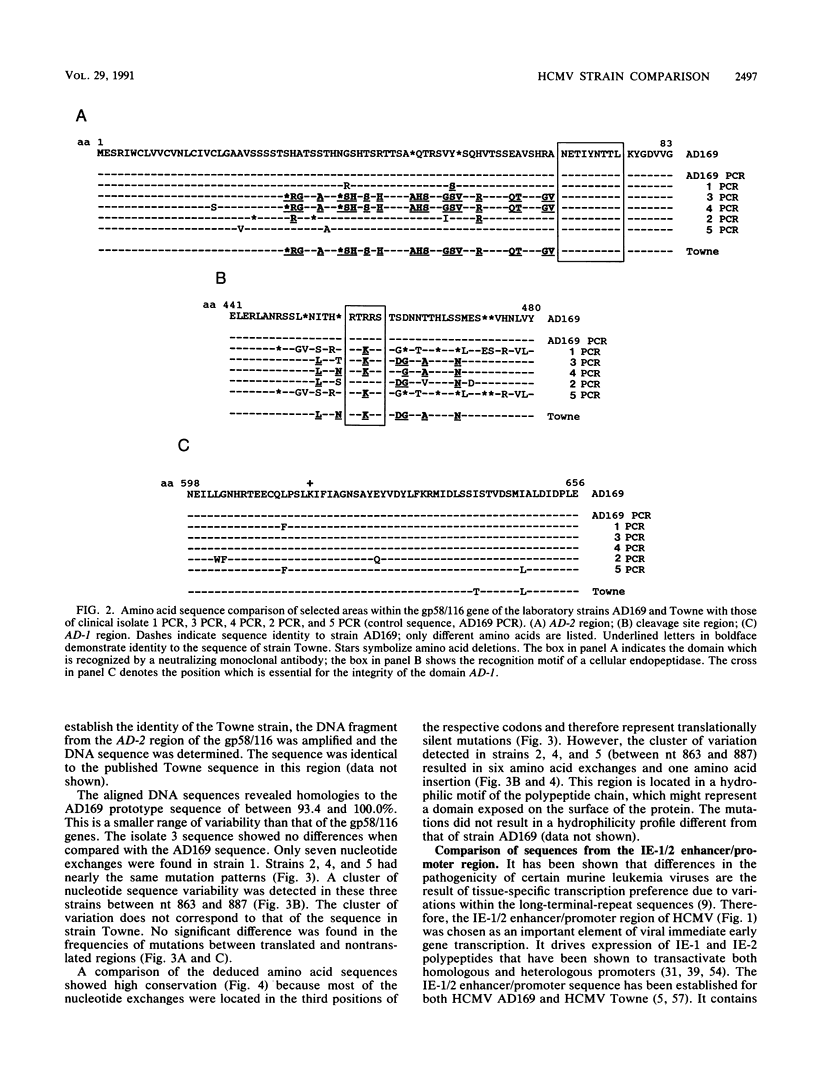
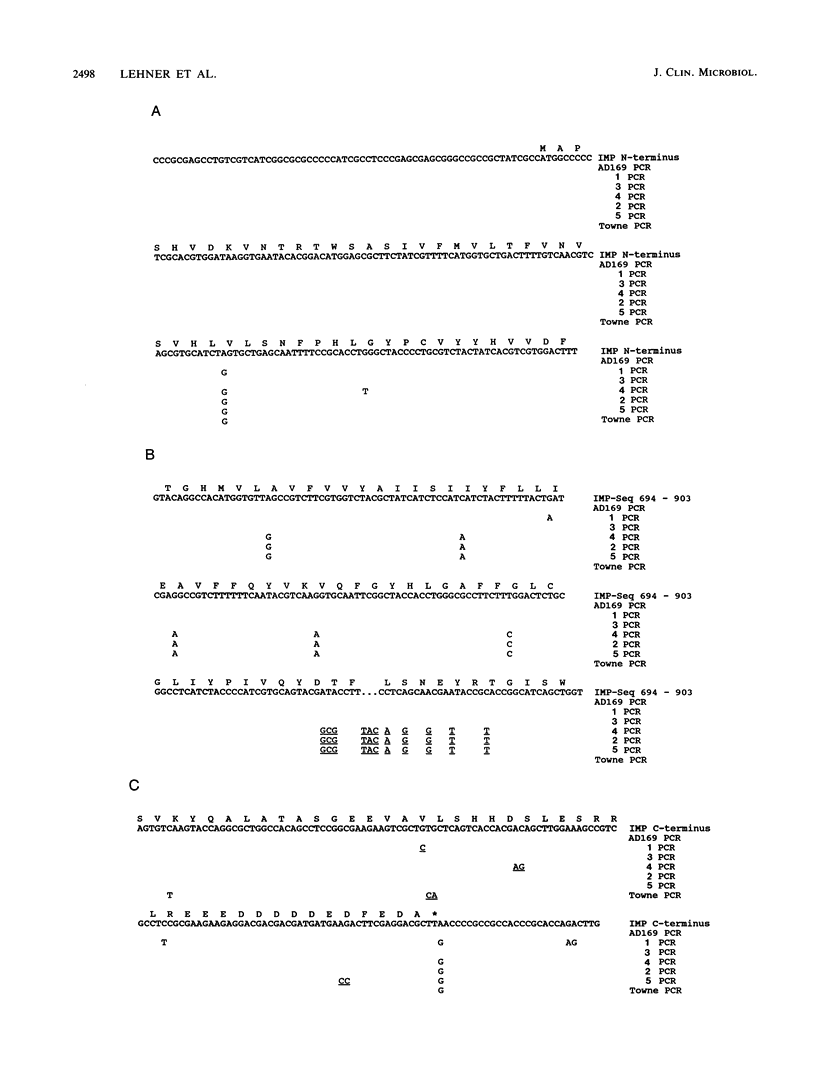
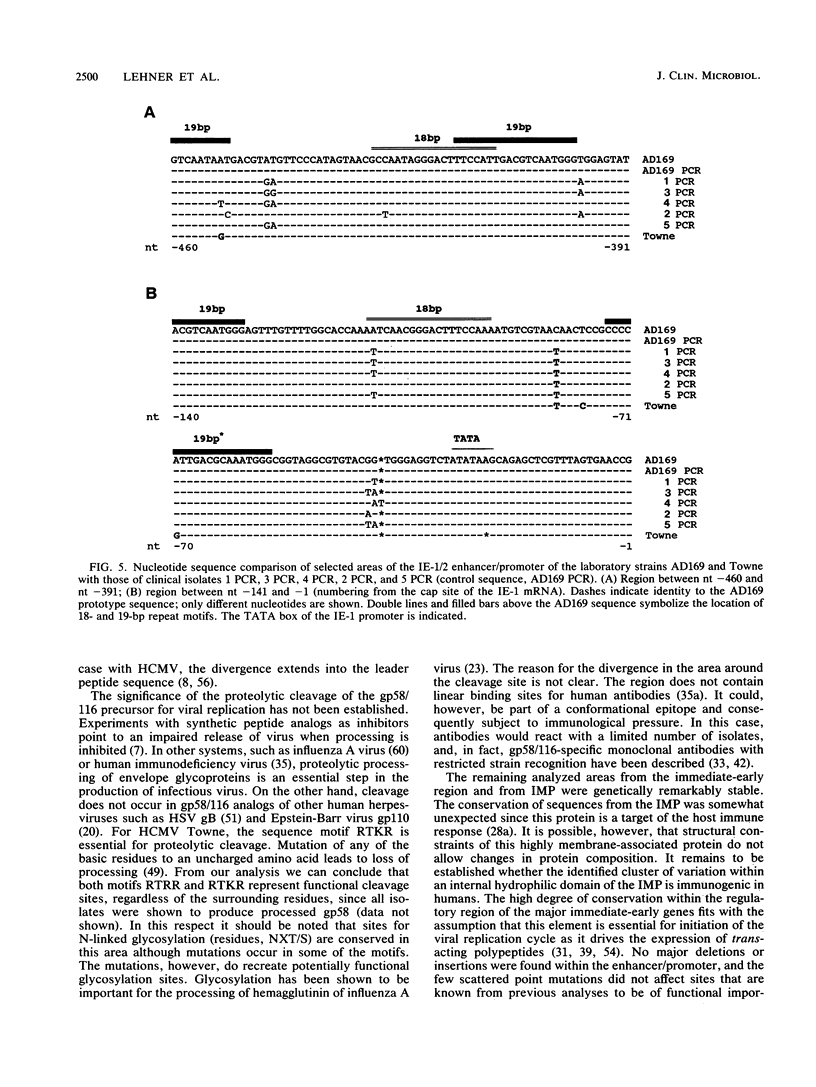
Three regions of DNA from five low-passage clinical isolates of human cytomegalovirus were amplified by polymerase chain reaction. The DNA sequences as well as the predicted amino acid sequences were compared with those of the laboratory strains AD169 and Towne. The genomic regions consisted of (i) three regions from the major glycoprotein (gp58/116, unique long [UL]55), (ii) three regions from the integral membrane protein (IMP, UL100), and (iii) a region from the major immediate-early 1 and 2 (IE-1/2) enhancer/promoter. Homologies ranged from 75.8 to 100.0% on the nucleotide level and from 47 to 100% on the amino acid level. The following two patterns were observed. (i) There are regions with a high degree of conservation with few scattered point mutations (mainly in the IE-1/2 enhancer/promoter and in the IMP gene). (ii) There are clusters of highly variable regions (parts of the gp58/116 gene and of the IMP gene). Within the areas of high variability, the strains could be classified into a limited number of subtypes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford C. A., Hayes K., Britt W. Primary cytomegalovirus infection in pregnancy: comparison of antibody responses to virus-encoded proteins between women with and without intrauterine infection. J Infect Dis. 1988 Nov;158(5):917–924. doi: 10.1093/infdis/158.5.917. [DOI] [PubMed] [Google Scholar]
- Baboonian C., Blake K., Booth J. C., Wiblin C. N. Complement-independent neutralising monoclonal antibody with differential reactivity for strains of human cytomegalovirus. J Med Virol. 1989 Oct;29(2):139–145. doi: 10.1002/jmv.1890290212. [DOI] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J Virol. 1991 Feb;65(2):938–944. doi: 10.1128/jvi.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysiewicz L. K., Hickling J. K., Graham S., Sinclair J., Cranage M. P., Smith G. L., Sissons J. G. Human cytomegalovirus-specific cytotoxic T cells. Relative frequency of stage-specific CTL recognizing the 72-kD immediate early protein and glycoprotein B expressed by recombinant vaccinia viruses. J Exp Med. 1988 Sep 1;168(3):919–931. doi: 10.1084/jem.168.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Britt W. J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984 Jun;135(2):369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Brücher K. H., Garten W., Klenk H. D., Shaw E., Radsak K. Inhibition of endoproteolytic cleavage of cytomegalovirus (HCMV) glycoprotein B by palmitoyl-peptidyl-chloromethyl ketone. Virology. 1990 Oct;178(2):617–620. doi: 10.1016/0042-6822(90)90365-x. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Debroy C., Fox B. A., Pederson N. E., Person S. The nucleotide sequence of the gB glycoprotein gene of HSV-2 and comparison with the corresponding gene of HSV-1. Virology. 1986 Dec;155(2):322–333. doi: 10.1016/0042-6822(86)90196-0. [DOI] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- Chandler S. H., McDougall J. K. Comparison of restriction site polymorphisms among clinical isolates and laboratory strains of human cytomegalovirus. J Gen Virol. 1986 Oct;67(Pt 10):2179–2192. doi: 10.1099/0022-1317-67-10-2179. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Chou S. W. Differentiation of cytomegalovirus strains by restriction analysis of DNA sequences amplified from clinical specimens. J Infect Dis. 1990 Sep;162(3):738–742. doi: 10.1093/infdis/162.3.738. [DOI] [PubMed] [Google Scholar]
- Condie R. M., O'Reilly R. J. Prevention of cytomegalovirus infection in bone marrow transplant recipients by prophylaxis with an intravenous, hyperimmune cytomegalovirus globulin. Birth Defects Orig Artic Ser. 1984;20(1):327–344. [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert K. A., Kunkel T. A. High fidelity DNA synthesis by the Thermus aquaticus DNA polymerase. Nucleic Acids Res. 1990 Jul 11;18(13):3739–3744. doi: 10.1093/nar/18.13.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickenscher H., Stamminger T., Rüger R., Fleckenstein B. The role of a repetitive palindromic sequence element in the human cytomegalovirus major immediate early enhancer. J Gen Virol. 1989 Jan;70(Pt 1):107–123. doi: 10.1099/0022-1317-70-1-107. [DOI] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Fleckenstein B., Hennighausen L. Binding of transcription factors and creation of a large nucleoprotein complex on the human cytomegalovirus enhancer. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3658–3662. doi: 10.1073/pnas.84.11.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Hennighausen L. Specific interactions between transcription factors and the promoter-regulatory region of the human cytomegalovirus major immediate-early gene. J Virol. 1988 Mar;62(3):1076–1079. doi: 10.1128/jvi.62.3.1076-1079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Ooka T., Matsuo T., Kieff E. Epstein-Barr virus glycoprotein homologous to herpes simplex virus gB. J Virol. 1987 Feb;61(2):499–508. doi: 10.1128/jvi.61.2.499-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K., Alford C., Britt W. Antibody response to virus-encoded proteins after cytomegalovirus mononucleosis. J Infect Dis. 1987 Oct;156(4):615–621. doi: 10.1093/infdis/156.4.615. [DOI] [PubMed] [Google Scholar]
- James G. S., Cloonan M. J. Demonstration of antigenic variation among isolates of human cytomegalovirus using monoclonal antibodies and indirect ELISA. J Virol Methods. 1990 Dec;30(3):301–310. doi: 10.1016/0166-0934(90)90072-n. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Webster R. G. Interplay between carbohydrate in the stalk and the length of the connecting peptide determines the cleavability of influenza virus hemagglutinin. J Virol. 1989 Aug;63(8):3296–3300. doi: 10.1128/jvi.63.8.3296-3300.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick B. A., Huang E. S., Pagano J. S. Analysis of cytomegalovirus genomes with restriction endonucleases Hin D III and EcoR-1. J Virol. 1976 Jun;18(3):1095–1105. doi: 10.1128/jvi.18.3.1095-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniess N., Mach M., Fay J., Britt W. J. Distribution of linear antigenic sites on glycoprotein gp55 of human cytomegalovirus. J Virol. 1991 Jan;65(1):138–146. doi: 10.1128/jvi.65.1.138-146.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landini M. P., Mirolo G., Coppolecchia P., Re M. C., La Placa M. Serum antibodies to individual cytomegalovirus structural polypeptides in renal transplant recipients during viral infection. Microbiol Immunol. 1986;30(7):683–695. doi: 10.1111/j.1348-0421.1986.tb02994.x. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Re M. C., Mirolo G., Baldassarri B., La Placa M. Human immune response to cytomegalovirus structural polypeptides studied by immunoblotting. J Med Virol. 1985 Dec;17(4):303–311. doi: 10.1002/jmv.1890170403. [DOI] [PubMed] [Google Scholar]
- Lehner R., Meyer H., Mach M. Identification and characterization of a human cytomegalovirus gene coding for a membrane protein that is conserved among human herpesviruses. J Virol. 1989 Sep;63(9):3792–3800. doi: 10.1128/jvi.63.9.3792-3800.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach M., Utz U., Fleckenstein B. Mapping of the major glycoprotein gene of human cytomegalovirus. J Gen Virol. 1986 Jul;67(Pt 7):1461–1467. doi: 10.1099/0022-1317-67-7-1461. [DOI] [PubMed] [Google Scholar]
- Malone C. L., Vesole D. H., Stinski M. F. Transactivation of a human cytomegalovirus early promoter by gene products from the immediate-early gene IE2 and augmentation by IE1: mutational analysis of the viral proteins. J Virol. 1990 Apr;64(4):1498–1506. doi: 10.1128/jvi.64.4.1498-1506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuho Y., Matsumoto Y., Sugano T., Fujinaga S., Minamishima Y. Human monoclonal antibodies neutralizing human cytomegalovirus. J Gen Virol. 1987 May;68(Pt 5):1457–1461. doi: 10.1099/0022-1317-68-5-1457. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Meyer H., Masuho Y., Mach M. The gp116 of the gp58/116 complex of human cytomegalovirus represents the amino-terminal part of the precursor molecule and contains a neutralizing epitope. J Gen Virol. 1990 Oct;71(Pt 10):2443–2450. doi: 10.1099/0022-1317-71-10-2443. [DOI] [PubMed] [Google Scholar]
- Onorato I. M., Morens D. M., Martone W. J., Stansfield S. K. Epidemiology of cytomegaloviral infections: recommendations for prevention and control. Rev Infect Dis. 1985 Jul-Aug;7(4):479–497. doi: 10.1093/clinids/7.4.479. [DOI] [PubMed] [Google Scholar]
- Pizzorno M. C., O'Hare P., Sha L., LaFemina R. L., Hayward G. S. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J Virol. 1988 Apr;62(4):1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin S. A., Furukawa T., Zygraich N., Huygelen C. Candidate cytomegalovirus strain for human vaccination. Infect Immun. 1975 Sep;12(3):521–527. doi: 10.1128/iai.12.3.521-527.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- Rasmussen L., Mullenax J., Nelson R., Merigan T. C. Viral polypeptides detected by a complement-dependent neutralizing murine monoclonal antibody to human cytomegalovirus. J Virol. 1985 Aug;55(2):274–280. doi: 10.1128/jvi.55.2.274-280.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sambucetti L. C., Cherrington J. M., Wilkinson G. W., Mocarski E. S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989 Dec 20;8(13):4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Snydman D. R., Werner B. G., Heinze-Lacey B., Berardi V. P., Tilney N. L., Kirkman R. L., Milford E. L., Cho S. I., Bush H. L., Jr, Levey A. S. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med. 1987 Oct 22;317(17):1049–1054. doi: 10.1056/NEJM198710223171703. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Saxena A., Scott P. I., Song G. J., Probert W. S., Britt W. J., Gibson W., Rasmussen L., Pachl C. Sequence requirements for proteolytic processing of glycoprotein B of human cytomegalovirus strain Towne. J Virol. 1990 Jun;64(6):2922–2931. doi: 10.1128/jvi.64.6.2922-2931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Thayer R. M., Probert W. S., Masiarz F. R., Chamberlain S. H., Rasmussen L., Merigan T. C., Pachl C. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology. 1988 Nov;167(1):207–225. doi: 10.1016/0042-6822(88)90071-2. [DOI] [PubMed] [Google Scholar]
- Stagno S., Pass R. F., Dworsky M. E., Britt W. J., Alford C. A. Congenital and perinatal cytomegalovirus infections: clinical characteristics and pathogenic factors. Birth Defects Orig Artic Ser. 1984;20(1):65–85. [PubMed] [Google Scholar]
- Stamminger T., Fleckenstein B. Immediate-early transcription regulation of human cytomegalovirus. Curr Top Microbiol Immunol. 1990;154:3–19. doi: 10.1007/978-3-642-74980-3_1. [DOI] [PubMed] [Google Scholar]
- Stenberg R. M., Fortney J., Barlow S. W., Magrane B. P., Nelson J. A., Ghazal P. Promoter-specific trans activation and repression by human cytomegalovirus immediate-early proteins involves common and unique protein domains. J Virol. 1990 Apr;64(4):1556–1565. doi: 10.1128/jvi.64.4.1556-1565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve L. L., Brown-Shimer S., Pachl C., Najarian R., Dina D., Burke R. L. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J Virol. 1987 Feb;61(2):326–335. doi: 10.1128/jvi.61.2.326-335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz U., Britt W., Vugler L., Mach M. Identification of a neutralizing epitope on glycoprotein gp58 of human cytomegalovirus. J Virol. 1989 May;63(5):1995–2001. doi: 10.1128/jvi.63.5.1995-2001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waner J. L., Weller T. H. Analysis of antigenic diversity among human cytomegaloviruses by kinetic neutralization tests with high-titered rabbit antisera. Infect Immun. 1978 Jul;21(1):151–157. doi: 10.1128/iai.21.1.151-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Rott R. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell. 1987 Aug 28;50(5):665–666. doi: 10.1016/0092-8674(87)90321-7. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Forman S. J., Ting Y. P., Vanderwal-Urbina E., Blume K. G. Polypeptide-specific antibody response to human cytomegalovirus after infection in bone marrow transplant recipients. J Infect Dis. 1986 Apr;153(4):780–787. doi: 10.1093/infdis/153.4.780. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Gallez-Hawkins G., Churchill M. A., Morton-Blackshere A., Pande H., Adler S. P., Schmidt G. M., Forman S. J. Comparative analysis of human cytomegalovirus a-sequence in multiple clinical isolates by using polymerase chain reaction and restriction fragment length polymorphism assays. J Clin Microbiol. 1990 Dec;28(12):2602–2607. doi: 10.1128/jcm.28.12.2602-2607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


