Abstract
The stomach-derived hormone ghrelin interacts with key CNS circuits regulating energy balance and body weight. Here we provide evidence that the central ghrelin signaling system is required for alcohol reward. Central ghrelin administration (to brain ventricles or to tegmental areas involved in reward) increased alcohol intake in a 2-bottle (alcohol/water) free choice limited access paradigm in mice. By contrast, central or peripheral administration of ghrelin receptor (GHS-R1A) antagonists suppressed alcohol intake in this model. Alcohol-induced locomotor stimulation, accumbal dopamine release and conditioned place preference were abolished in models of suppressed central ghrelin signaling: GHS-R1A knockout mice and mice treated with 2 different GHS-R1A antagonists. Thus, central ghrelin signaling, via GHS-R1A, not only stimulates the reward system, but is also required for stimulation of that system by alcohol. Our data suggest that central ghrelin signaling constitutes a potential target for treatment of alcohol-related disorders.
Keywords: appetite, ethanol, GHS-R1A, mesolimbic dopamine system, reinforcing
Ghrelin, first isolated from the rat stomach (1), has emerged as an important gut–brain signal for the control of energy balance and body weight homeostasis (2, 3). Early studies in rodents revealed an orexigenic role for ghrelin (4). Subsequently a physiological role for ghrelin in hunger, appetite, and meal initiation was proposed on the basis of the preprandial rise in plasma ghrelin levels in human subjects that correlated with hunger scores (5). In rodents, chronic exposure to ghrelin increases fat mass (2). Crucial experiments demonstrating the pro-obesity role for endogenous ghrelin include models of suppressed ghrelin signaling such as genetic deletions of ghrelin (6, 7) and/or its receptor (GHS-R1A) (8–10).
In addition to its expression in the hypothalamus, GHS-R1A is present in the hippocampus and in specific areas of importance for reward, such as the ventral tegmental area (VTA) and laterodorsal tegmental area (LDTg) (11). This distribution is in line with findings that ghrelin's central actions extend beyond energy homeostasis. Consistent with this, ghrelin injection into the VTA, an important node in the mesolimbic dopaminergic reward circuit, increases food intake in rodents (12). Recently we showed that, in addition to its well-described hypothalamic effects, ghrelin activates a key mesolimbic reward circuit involved in natural and drug-induced reinforcement, the cholinergic–dopaminergic reward link (13–15), findings that have been corroborated and extended by others (16). Specifically we showed that ghrelin increases accumbal dopamine release and also the associated locomotor stimulation, parameters that reflect an activation of the mesolimbic reward circuit (13, 14). By this route, ghrelin may increase the incentive value of signals associated with motivated behaviors of importance for survival such as food seeking (13–15, 17).
A variety of human studies suggest that common neurobiological mechanisms underlie different forms of addictive behaviors, including compulsive overeating, pathological gambling, alcoholism, nicotine dependence, and other forms of chemical addiction (18, 19). Given the hyperghrelinemia associated with certain forms of compulsive overeating (20) and also with alcohol dependence (21, 22), we hypothesize that a common mechanism, involving the central ghrelin signaling system, underlies the pathophysiology of these diseases. In the present article, we investigated whether the central ghrelin signaling system is required for alcohol reward.
Results
Ghrelin (i.c.v., VTA or LDTg) Increased and GHS-R1A Antagonists (i.c.v. or i.p.) Reduced Alcohol Intake in C57BL/6 Mice.
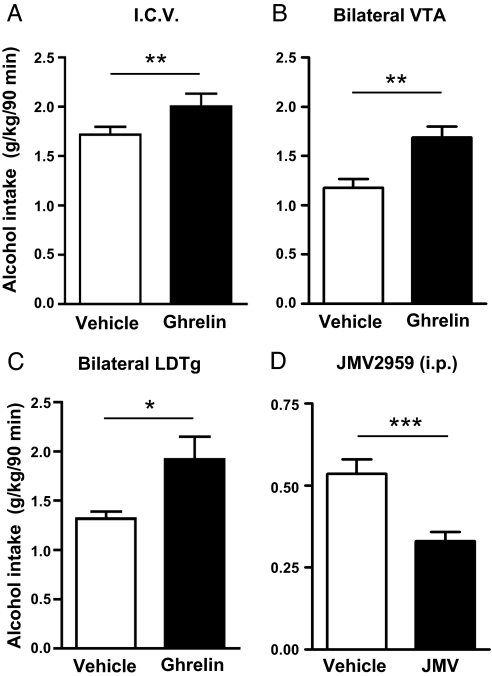
Central administration of ghrelin (2 μg i.c.v.) to C57BL/6 mice increased alcohol consumption by 16.59% relative to vehicle treatment in a 2-bottle (alcohol/water) free choice limited access paradigm (Fig. 1A). Alcohol consumption was unaffected by central injection of a lower dose (1 μg i.c.v.) of ghrelin (1.42 ± 0.13 g/kg/90 min) compared to vehicle (1.29 ± 0.12 g/kg/90 min). Given that tegmental areas involved in reward appear to be responsive to ghrelin (14, 15), we sought to determine whether ghrelin's central effects on alcohol consumption are exerted at these sites. We found that bilateral administration of ghrelin into either the VTA or the LDTg also increased alcohol consumption in comparison to vehicle controls (43.54 and 45.83%, respectively, Fig. 1 B and C, respectively). The percentage increase in alcohol consumption was significantly greater following administration to the VTA or the LDTg compared to the i.c.v. route (i.c.v. vs. VTA: P < 0.0001 and i.c.v. vs. LDTg: P < 0.001). Water intake and total fluid intake were not affected by ghrelin treatment when administered by any of these routes. As expected, food intake (normal chow) was increased by i.c.v. ghrelin administration in comparison to vehicle administration (0.57 ± 0.14 g and 0.22 ± 0.09 g, respectively, P < 0.05). Food intake was not affected by bilateral ghrelin administration into either the VTA (vehicle 0.28 ± 0.09 g; ghrelin 0.20 ± 0.003 g) or the LDTg (vehicle 0.27 ± 0.01 g; ghrelin 0.35 ± 0.11 g). The effects of i.c.v. ghrelin on both alcohol intake and food intake were absent in GHS-R1A knockout mice but not in wild-type littermates [supporting information (SI) Table S1], indicating that GHS-R1A is required for ghrelin-induced alcohol intake. Indeed, any apparent difference between the genotypes in the response to vehicle treatment did not give a statistical difference (P > 0.05). Likewise there was no difference in spontaneous alcohol intake between the genotypes in untreated mice measured during the weeks before treatment (average alcohol intake in the limited access paradigm: wild type 0.90 ± 0.07 g/kg/90 min; heterozygote 0.82 ± 0.06 g/kg/90 min; homozygote 0.99 ± 0.09 g/kg/90 min).
Fig. 1.
Ghrelin administration into brain ventricles (i.c.v.) or into specific tegmental areas increased whereas a GHS-R1A antagonist (i.p.) decreased alcohol intake in C57BL/6 mice. In a 2-bottle (alcohol/water) free choice limited access paradigm in C57BL/6 mice ghrelin increased alcohol intake (g/kg/90 min) relative to the vehicle treatment when administered into (A) the third ventricle (n = 23 in both groups; **, P < 0.01, paired t test), (B) bilaterally into the VTA (n = 5 for vehicle and n = 7 for ghrelin; **, P < 0.01, unpaired t test), or (C) bilaterally into the LDTg (n = 6 for vehicle and n = 5 for ghrelin; *, P < 0.05, unpaired t test). (D) Peripheral injection of a GHS-R1A antagonist, JMV2959, decreased alcohol consumption compared to vehicle in this paradigm in C57BL/6J mice [F (1, 28) = 15.68, P = 0.001] (n = 15 in each group; *, P < 0.001, Bonferroni post hoc test). All values represent mean ± SEM.
Consistent with the genetic model of suppressed ghrelin signaling, alcohol intake in the 2-bottle (alcohol/water) free choice limited access paradigm was suppressed in C57BL/6 mice by both of the GHS-R1A antagonists tested, BIM28163 (delivered i.c.v.; Fig. S1A) and JMV2959 (delivered i.p.; Fig. 1D). Because of new ethical permission, the limited access paradigm used to test JMV2959 used a modified protocol, which is likely to have influenced the spontaneous alcohol consumption in the control group, which was up to 3 times lower than for all other studies (Fig. 1 A–D, Fig. S1A). The reduction of alcohol intake by JMV2959 treatment was significant on days 1, 3, 4, and 5 [P = 0.002, P = 0.004, P = 0.02, P = 0.01; F (1, 28) = 15.68, P = 0.001]. BIM28163 increased food intake by 71% (P < 0.05) as reported previously (23) and increased water intake by 28% (P < 0.05), but did not affect total fluid intake. Water intake, total fluid intake, and food intake were not influenced by JMV2959 during the 90-min period of alcohol access (data not shown). However, in line with its anorexigenic properties (24), JMV2959 reduced 24-hour food intake by 12.5% on average over the 5 subsequent treatment days compared to vehicle controls (2.50 ± 0.06 g and 2.86 ± 0.06 g, respectively, P < 0.01).
The Rewarding Properties of Alcohol, as Determined by Alcohol-Induced Locomotor Stimulation and Increased Accumbal Dopamine Release, Are Reduced in Pharmacological and Genetic Models of Suppressed Ghrelin Signaling.
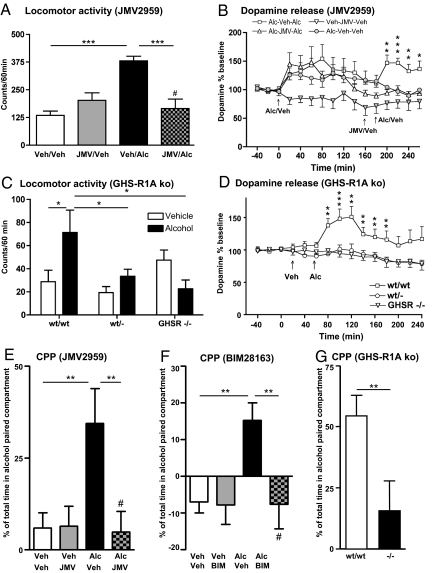
First, in Naval Medical Research Institute (NMRI) mice, we investigated the rewarding properties of alcohol by tests of alcohol-induced locomotor stimulation and, in separate studies, by measurement of alcohol-induced enhanced extracellular accumbal dopamine overflow (a measure reflecting synaptic dopamine release). We found that both parameters were consistently attenuated by both GHS-R1A antagonists: JMV2959 (Fig. 2 A and B) and BIM28163 (Fig. S1C and Fig. S1B). In line with the pharmacological models of suppressed ghrelin signaling, the locomotor stimulation and increased dopamine release induced by peripheral injection of alcohol observed in wild-type mice were abolished in both heterozygous and homozygous GHS-R1A knockout mice (Fig. 2 C and D). When making a gross comparison of all vehicle-treated groups in the pharmacological and genetic studies, there appears to be a difference in spontaneous basal locomotor activity that most likely reflects their different genetic backgrounds and different batches of mice, as is commonly observed.
Fig. 2.
Suppressed ghrelin signaling, by either ghrelin receptor (GHS-R1A) antagonist (JMV2959) or GHS-R1A knockout mice, attenuates alcohol (Alc) reward. (A) Alcohol-induced locomotor stimulation was attenuated by a single i.p. injection of JMV2959 [but not by vehicle (Veh) injection] to NMRI mice [F (3, 28) = 12.86, P = 0.001] (n = 8 in each group; ***, P < 0.001, #, P = n.s. for Veh–Veh vs. JMV–Alc, Bonferroni post hoc test). (B) The alcohol-induced increase in accumbal dopamine release was absent in GHS-R1A antagonist (JMV2959, i.p.)-mice but not in vehicle-treated NMRI mice (n = 8 in each group). In B we first demonstrated a significant effect of alcohol to increase dopamine release in comparison to vehicle treatment [treatment F (1, 14) = 0.01, P = 0.65; time F (10, 140) = 4.34, P = 0.001; treatment × time interaction F (10, 140) = 0.79, P = 0.64]. Second, we showed that pretreatment with JMV2959 (i.p.) attenuated the alcohol-induced increase in dopamine release compared to vehicle pretreatment [treatment F (1, 14) = 14.12, P = 0.002; time F (5, 70) = 2.38, P = 0.05; treatment × time interaction F (5, 70) = 1.13, P = 0.35]. This difference (P < 0.001) was evident at time intervals 200, 220, 240, and 260 min (*, P < 0.05; **, P < 0.01; ***, P < 0.001, Bonferroni post hoc test). (C) The acute locomotor stimulation of alcohol observed in wild-type mice was abolished in both heterozygous and homozygous GHS-R1A knockout mice [F (5, 56) = 4.08, P = 0.003]. No difference was found in the locomotor response between any of the genotypes treated with vehicle, indicating that deletion of GHS-R1A does not affect activity per se (wt/wt: n = 8 for Veh and n = 6 for Alc; wt/−: n = 17 for Veh and n = 16 for Alc; −/−: n = 8 for Veh and n = 7 for Alc; *, P < 0.05; Tukey/Kramer). (D) The alcohol-induced increase in extracellular accumbal dopamine release was attenuated in both heterozygous (n = 13) and homozygous GHS-R1A knockout mice (n = 11) compared to wild-type mice (n = 8) [treatment F (2, 29) = 9.55, P = 0.001; time F (14, 406) = 4.65, P = 0.001; treatment × time interaction F (28, 406) = 2.79, P = 0.001]. This difference (P < 0.001) was observed at the time intervals 80, 100, 120, 140, 160, and 180 min (**, P < 0.01; ***, P < 0.001; Bonferroni post hoc test). (E) The alcohol-induced CPP was attenuated by an acute single i.p. injection of the GHS-R1A antagonist, JMV2959, in NMRI mice [F (3, 27) = 4.96, P = 0.01]. An alcohol-induced CPP in mice pretreated with vehicle (n = 7) was obtained and pretreatment with JMV2959 (n = 8) blocked this stimulation. No significant difference was observed between vehicle–vehicle (n = 8) and JMV2959-alcohol treatment (**, P < 0.01; #, P = n.s. for Veh–Veh vs. JMV–Alc, Bonferroni post hoc test). No effect of JMV2959 per se was observed (n = 8). (F) The alcohol-induced CPP was attenuated by an acute central administration of the GHS-R1A antagonist, BIM28163 (i.c.v.), in NMRI mice [F (3, 23) = 4.98, P = 0.01]. An alcohol-induced CPP in mice pretreated with vehicle (n = 7) was obtained and pretreatment with BIM28163 (n = 6) blocked this stimulation. No significant difference was observed between vehicle–vehicle (n = 6) and BIM28163-alcohol treatment (**, P < 0.01; #, P = n.s. for Veh–Veh vs. BIM–Alc, Bonferroni post hoc test). No effect of BIM28163 per se was observed (n = 8). (G) Alcohol induces a significant CPP in wild-type mice (n = 8), but not in GHS-R1A knockout mice (n = 5) [F (1, 11) = 5.15, P = 0.04]. A significant difference between CPP in wild type compared to GHS-R1A knockout mice was obtained (**, P < 0.01, Bonferroni post hoc test). All values represent mean ± SEM.
The Rewarding Properties of Alcohol, Tested in a Conditioned Place Preference (CPP) Paradigm, Were Reduced in Genetic and Pharmacological Models of Suppressed Ghrelin Signaling.
The alcohol-induced conditioned place preference (CPP) response in models of suppressed ghrelin signaling was also used to demonstrate that the central ghrelin signaling system is required for the rewarding properties of alcohol. We found that the alcohol-induced CPP is reduced in genetic and pharmacological models of suppressed ghrelin signaling (Fig. 2 E–G). Alcohol induced a CPP response in wild-type littermate mice, but not in GHS-R1A knockout mice (Fig. 2G). Furthermore, both GHS-R1A antagonists, JMV2959 (Fig. 2E) and BIM28163 (Fig. 2F), completely abolished the alcohol-induced CPP response in NMRI mice. Similarly, pretreatment with JMV2959 before alcohol each conditioning day suppressed alcohol-induced CPP. Moreover control experiments show that JMV2959, in this model, does not condition a place preference per se. Thus, the alcohol-induced CPP was attenuated by repeated i.p. injections of the GHS-R1A antagonist, JMV2959, in NMRI mice [F (3, 42) = 4.02, P = 0.01]. An alcohol-induced CPP in mice pretreated with vehicle (11.24 ± 2.55%; n = 14) was obtained compared to vehicle–vehicle treatment (−5.80 ± 3.72%; n = 8; P < 0.01); pretreatment with JMV2959 on each conditioning day blocked this stimulation (−3.52 ± 4.11%; n = 16; P < 0.01). No significant difference was observed between vehicle–vehicle and JMV2959-alcohol treatment. No effect of JMV2959 was observed per se (−1.59 ± 7.15%; n = 8).
Control experiments showed that neither cannula insertion, i.p. injection, volume infused nor the GHS-R1A antagonist per se had any effect on locomotor activity (Fig. 2A and Fig. S1C), accumbal dopamine release (Fig. 2B and Fig. S1B) or CPP (Fig. 2 E and F). The experimental protocols used have required the use of several different mouse strains. The fact that all our data are consistent, independent of experimental protocol and strain, further strengthens our hypothesis that the central ghrelin signaling system is required for alcohol reward.
Discussion
We provide evidence that central ghrelin signaling is required for the rewarding properties of alcohol. While previous studies have identified the mesolimbic dopaminergic reward system as a target for alcohol (25) and for ghrelin (13–16) our finding that the central ghrelin signaling system is required for the rewarding properties of alcohol is entirely unique. First, we show that central administration of ghrelin into the third ventricle and into specific tegmental areas of importance for reward (the VTA or LDTg) increases alcohol intake in a limited access paradigm in C57BL/6 mice and that peripheral or central administration of GHS-R1A antagonists reduces alcohol intake in this model. In different models of reduced ghrelin signaling, GHS-R1A knockout mice and mice treated pharmacologically with 2 different GHS-R1A antagonists, we have been able to suppress well-documented effects of alcohol on the mesolimbic reward system, namely alcohol-induced locomotor stimulation and alcohol-induced accumbal dopamine release (26, 27). These effects of alcohol, considered to form part of the addiction process, are intimately associated with its reinforcing properties (28). Furthermore, our finding that CPP for alcohol is similarly suppressed by both a deletion of the GHS-R1A gene (i.e., GHS-R1A knockout) and by GHS-R1A antagonist treatment, administered to mice either during conditioning or on the test day, therefore, provides compelling evidence that disruption of ghrelin signaling blocks the rewarding properties of alcohol. Collectively our data provide a clear indication that the central ghrelin signaling system, via GHS-R1A, is required for the rewarding properties of alcohol and hence, provides a unique target for the development of drug strategies to treat alcohol-related disorders.
In contrast to the modest energy balance phenotype in GHS-R1A knockout mice (7, 9), we demonstrate a rather dramatic “nonreward” phenotype insofar as both the locomotor stimulating and dopamine releasing effects of alcohol are completely abolished in both GHS-R1A knockout and heterozygote mice. Thus, both copies of the GHS-R1A allele appear to be required for alcohol-induced reward.
Given the established role for central ghrelin signaling in the regulation of feeding (3, 4), it was important to demonstrate that ghrelin-induced alcohol intake is driven by reward and independent of the caloric value of alcohol. In the CPP procedure, mice are conditioned to associate an environment with alcohol exposure. Preference for a previously alcohol-associated compartment in the absence of alcohol itself is an established measure of drug reward (29) and is unlikely to be influenced by changes in feeding motivation or the caloric content of alcohol. Because one of the GHS-R1A antagonists, BIM28163, paradoxically increases rather than suppresses feeding, but still blocks alcohol-induced CPP, strengthens our hypothesis that suppression of ghrelin signaling interferes with the rewarding properties of alcohol and not its calorific value. Interestingly in a chronic model of alcohol exposure GHS-R1A knockout mice did not differ from wild-type mice regarding their spontaneous intake of alcohol. It seems reasonable to suggest that this reflects the recruitment of compensatory mechanisms in the GHS-R1A knockout mice, as suggested previously to explain why these mice are not lean and have normal spontaneous food intake (7) as demonstrated also in the present study. While compensatory mechanisms may be important in these chronic alcohol/food consumption protocols, they do not appear to influence the acute effects of alcohol. This is evidenced by the fact that alcohol-induced locomotor stimulation, dopamine release, and the CPP response were similarly attenuated/blocked in genetic and pharmacological models of suppressed ghrelin signaling.
Both of the GHS-R1A antagonists used in the present study blocked alcohol-induced effects on the mesolimbic dopamine system and also inhibited alcohol intake. Validation using 2 different GHS-R1A antagonists in these models was important because of their different pharmacological profile with respect to food intake. Whereas JMV2959 suppresses food intake by a GHS-R1A agonist (hexarelin) as expected (24, 30), BIM28163 paradoxically stimulates food intake (23) via an unknown mechanism that is likely to be independent of GHS-R1A and also independent of ghrelin signaling at the level of the mesolimbic dopaminergic system. Our demonstration that both GHS-R1A antagonists block alcohol intake in a free-choice limited access paradigm together with the absence of ghrelin-induced alcohol consumption in GHS-R1A knockout mice suggests that endogenous ghrelin signaling via GHS-R1A is required for the rewarding properties of alcohol. Because this receptor is constitutively active in the absence of ligand (31), it is not known to what extent alcohol reward is dependent on signaling by endogenous ghrelin. Finally, our data showing similar effects of 2 different antagonists given by central or peripheral administration indicate that neither the chemical nature of these antagonists nor the route of administration is important for their central effects on alcohol reward.
To study the impact of ghrelin on alcohol consumption, our studies involved central administration, either by the i.c.v. route or given directly into the VTA or the LDTg that express GHS-R1A (11). Previously we have shown ghrelin-induced accumbal dopamine release that is coupled with increased locomotor activity when administered via these routes (14, 15). We opted for central rather than peripheral studies of ghrelin-induced alcohol consumption to circumvent diverse peripheral effects of ghrelin and also to focus on well-defined central ghrelin-responsive circuits. It seems likely that peripherally administered ghrelin will have similar effects on alcohol reward as we have previously shown that systemic ghrelin treatment also increases accumbal dopamine release, increases locomotor activity, and induces a CPP response (32). In contrast to our data for central ghrelin administration, another study did not observe a similar increase in alcohol consumption when ghrelin was administered peripherally (33). The most likely explanation for the discrepancy relates to the protocol. Whereas we used mice that were exposed to the free choice (alcohol/water) limited access paradigm over a period of 9 weeks before the study, Lyons and colleagues (33) used mice relatively naïve to alcohol and offered only 20% alcohol to drink. It may be that the central ghrelin signaling system is important for alcohol consumption in alcohol habituated animals rather than those that are alcohol naïve.
The exact circuitry through which ghrelin modulates alcohol reward remains to be further elucidated. Appetite regulation by peripheral ghrelin is exerted, at least in part, through activation of hypothalamic receptors (8). In addition, human functional imaging studies have recently shown that peripheral ghrelin administration modulates brain responses to food images in several extrahypothalamic brain regions, including mesolimbic and tegmental areas associated with reward (34). It is unclear whether these extrahypothalamic ghrelin actions are downstream, or parallel and independent of hypothalamic GHS-R1A activation. Irrespectively, the hypothalamus is unlikely to mediate ghrelin modulation of alcohol reward, because ghrelin microinjections into discrete hypothalamic areas do not affect alcohol intake (35). Given that GHS-R1A is also expressed in several extrahypothalamic brain areas, including areas of importance for reward (the VTA and the LDTg) (11) and that local ghrelin injection into these structures increases alcohol intake it seems likely that it is ghrelin signaling within these reward nodes that is important for the rewarding properties of alcohol. Ghrelin-induced alcohol consumption was significantly lower for i.c.v. in comparison to tegmental administration, even although the dose administered was identical, indicating that the amount of ghrelin reaching these tegmental areas is likely to be lower when ghrelin is given i.c.v. The fact that we obtained a similar magnitude of alcohol-drinking response for intra-VTA and intra-LDTg administration supports the idea that activation of GHS-R1A in both tegmental areas contribute to the effect. We have previously shown that peripheral, i.c.v., and tegmental (VTA and LDTg) ghrelin administration increases dopamine release in the nucleus accumbens (13, 14, 32), a terminal area of these projections, and stimulates locomotion, an established functional marker of mesolimbic dopaminergic activation (26–28). These observations, together with the ability of nicotinic cholinergic antagonists to block ghrelin-induced activation of mesolimbic dopaminergic activity (15), suggests that ghrelin's effects to increase the incentive value of alcohol are exerted at the level of the VTA and the LDTg involving the cholinergic–dopaminergic reward link (13–15). It should be noted that GHS-R1A transcript expression is colocalized with the dopamine cell marker tyrosine hydroxylase (16). This suggests that dopamine neurons themselves express GHS-R1A and that their activity might be directly modulated by ghrelin. Consistent with this model, ghrelin has also been shown to increase the incentive value of cocaine (36, 37). Direct ghrelin actions on VTA neurons would require that peripheral ghrelin signals reach the CNS, because it is not yet clear that ghrelin is expressed centrally. Supportively, an active transport for acetylated ghrelin across the blood–brain barrier has indeed been described (38) and peripheral ghrelin injection activates the reward systems (32).
There are indications that the present findings may be of clinical relevance in alcohol-related disorders. In particular, alcohol craving in alcohol-dependent individuals, is associated with increased circulating levels of ghrelin (39, 40). Furthermore, SNPs and haplotypes in the pro-ghrelin and GHS-R1A genes have been associated with heavy alcohol consumption (41). Our data showing the importance of the central ghrelin signaling system at the level of the cholinergic–dopaminergic reward link for ghrelin-induced alcohol intake, provide an explanation for this effect and raise the possibility of a causal role for ghrelin in alcohol craving. Moreover, by increasing the incentive value of rewards such as alcohol, hyperghrelinemia may play a pathophysiological role in the disease process that leads to addiction. Reports of increased ghrelin levels during alcohol withdrawal and positive associations between ghrelin levels and alcohol dependence support the notion that the present findings are of clinical relevance (21, 22). Interestingly, it has been suggested that the high ghrelin levels in alcohol withdrawal may cause food-seeking behavior and increase the intake of high caloric food (42). Conversely, chronic food deprivation, a state associated with hyperghrelinaemia (43) is known to increase drug-seeking behavior in rats (44). These interactions between feeding and alcohol would be consistent with ghrelin's effects at the level of the mesolimbic reward system. Previously we identified the cholinergic–dopaminergic reward link as an important target for ghrelin's central effects (13–15, 32), a circuit intimately associated with the reinforcing properties of rewarding substances that include both artificial rewards like alcohol and natural ones like food.
In conclusion, using genetic and pharmacological models of suppressed ghrelin signaling, we demonstrate that the central ghrelin action, via GHS-R1A, not only stimulates the reward system but is also required for stimulation of that system by an addictive drug, alcohol. In particular, the finding that alcohol intake can be suppressed by administration of a GHS-R1A antagonist implies that orally bioavailable, brain penetrant GHS-R1A antagonists may have therapeutic potential in alcohol use disorders. Our studies also raise important questions regarding the physiological role of ghrelin, a gut–brain signal, influencing not only hunger but clearly also having a broader role in the search for rewarding substances such as alcohol.
Materials and Methods
Further details of all experimental protocols and statistic analysis are given in the SI Text.
Animals.
The results from experiments on 3 different strains of adult mice have led to similar conclusions in this study: NMRI, C57BL/6, and GHS-R1A knockout and littermate mice. GHS-R1A knockout mice on a mixed 129 Sv/Evbrd(LEX1)/C57BL/6 background and their corresponding wild-type littermates, generated through heterozygous breeding, were genoyped (backcrossed 3 times and on average 87.5% C57BL/6 mice; SI Text; Fig. S2) and used in locomotor activity, microdialysis, CPP, and alcohol consumption experiments. Outbred NMRI mice were used for studies of locomotor activity, microdialysis, and CPP testing. Alcohol consumption experiments, however, were performed on C57BL/6 mice. Details of surgical procedures and stereotaxic placements are given in the SI Text and Fig. S3.
Drugs.
Alcohol, diluted in saline (15% vol/vol), was injected i.p. at the dose of 1.75 g/kg (NMRI mice) or 1.0 g/kg (GHS-R1A mice and their littermates). Acylated ghrelin was administered i.c.v. at a dose of 1 or 2 μg/mouse or bilaterally into the VTA or LDTg at a dose of 2 μg/mouse 10 min before initiation of the experiment. The doses of the GHS-R1A antagonists, BIM28163 (5 μg i.c.v.) or JMV2959 (6 mg/kg i.p.), were determined in dose–response studies (Fig. S4 and Fig. S5, respectively). BIM28163 was administered 40 min and JMV2959 20 min before alcohol exposure.
Locomotor Activity Experiments.
GHS-R1A knockout mice and their littermate controls were i.p. injected with alcohol or an equal volume of vehicle. In separate experiments NMRI mice were pretreated with BIM28163 (i.c.v.) or JMV2959 (i.p.) before alcohol injection (i.p.). Experiments with the following treatments were also conducted: vehicle–vehicle, vehicle–alcohol, or GHSR-1A antagonist–vehicle.
In Vivo Microdialysis and Dopamine Release Measurements.
Mice were implanted unilaterally with a microdialysis probe positioned in the nucleus accumbens. For studies involving i.c.v. GHS-R1A antagonist administration, NMRI mice were also implanted with an ipsilateral guide cannula in the third ventricle. Perfusion samples were collected every 20 min and the first 4 were used as baseline. For GHS-R1A knockout mice, baseline samples were followed by an i.p. injection of vehicle and thereafter an alcohol injection. In subsequent experiments, NMRI mice were injected with an initial dose of alcohol (i.p.). Eight perfusion samples later, BIM28163 (i.c.v.) or JMV2959 (i.p.) was administered, followd by a second i.p. injection of alcohol. Experiments with the following treatments were also conducted: vehicle–GHS-R1A antagonist–vehicle, alcohol–vehicle–alcohol, or alcohol–vehicle–vehicle.
Conditioned Place Preference.
CPP tests were performed in both NMRI mice (GHS-R1A antagonist studies) and also in GHS-R1A knockout mice and their littermates. The procedure consisted of preconditioning on day 1 (in which mice were i.p. injected with vehicle and initial place preference determined during 20 min), conditioning on days 2–5 (in which the least preferred compartment was paired with alcohol injection), and postconditioning on day 6 (in which the preference for the alcohol paired compartment was assessed during 20 min). Before the test session NMRI mice were acutely injected with either of the 2 GHS-R1A antagonists (BIM28163 i.c.v. or JMV2959 i.p.) or vehicle, whereas the GHS-R1A knockout mice were untreated this day. In a separate series of experiments, the GHS-R1A antagonist (JMV2959, i.p.) or vehicle was administered before alcohol injection on each conditioning day and the NMRI mice were untreated on the test day.
Alcohol Consumption in C57BL/6 Mice and GHS-R1A Knockout Mice.
After a period of habituation to alcohol (over 9 weeks, see SI Text) a limited access paradigm was introduced for 2 weeks (i.e., 90 min of alcohol access per day for 2 weeks). Guide cannulae were positioned 4 days before treatment. For i.c.v. studies, the mice received either drug (ghrelin or BIM28163) or vehicle on day 1 and the reverse treatment on day 2, according to a balanced design. For bilateral injection studies (to VTA or LDTg) mice were injected with either ghrelin or vehicle solution and group comparisons were made, because of experimental limitations of repeated intranuclear injection via bilateral cannulae. For JMV2959, a modified alcohol consumption protocol was used in which JMV2959 or vehicle were i.p. injected daily for 5 days and, because of altered ethical permission, water was available ad libitum.
Supplementary Material
Acknowledgments.
This work was supported by the Swedish Research Council (K2006–21X-04247–33-3 and K2007–54X-20328–013); the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly; the Swedish Labour Market Insurance; the Swedish Council for Tobacco Research; the foundations of Wilhelm and Martina Lundgren, Knut and Alice Wallenberg, Adlerbert Research, Tore Nilsson, Anders Otto Swärd, Milan Valverius, Längmanska Art; the European Commission 6th Framework (EC LSHM-CT-2003–503041); Novo Nordisk (GeA/AIR); ALF Göteborg (SU7601 and SU76540); the Swedish Foundation for Strategic Research to Sahlgrenska Center for Cardiovascular and Metabolic Research (A305–188); and the Swedish Society of Medicine. We thank Gun Andersson and Kenn Johannessen for invaluable technical assistance; Michael D. Culler and Jesse Dong (Ipsen) for contributions to the discovery and characterization of BIM28163; and Gareth Leng and John-Olov Jansson for commenting on the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812809106/DCSupplemental.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Wren AM, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 5.Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–E304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- 6.Wortley KE, et al. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun YX, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun YX, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zigman JM, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfluger PT, et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol Gastrointest Liver Physiol. 2008;294:G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- 11.Guan XM, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 12.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Jerlhag E, et al. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: Implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 14.Jerlhag E, et al. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 15.Jerlhag E, Egecioglu E, Dickson SL, Svensson L, Engel JA. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors are involved in mediating the ghrelin-induced locomotor stimulation and dopamine overflow in nucleus accumbens. Eur Neuropsychopharm. 2008;18:508–518. doi: 10.1016/j.euroneuro.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Abizaid A, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keen-Rhinehart K, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 19.Holderness HC, Brooksgunn J, Warren MP. Co-moribidity of eating disorders and substance-abuse review of the literature. Int J Eat Disorder. 1994;16:1–34. doi: 10.1002/1098-108x(199407)16:1<1::aid-eat2260160102>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Cummings DE, et al. Elevated plasma ghrelin levels in Prader-Willi syndrome. Nat Med. 2002;8:643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 21.Kim DJ, et al. Increased fasting plasma ghrelin levels during alcohol abstinence. Alcohol Alcohol. 2005;40:76–79. doi: 10.1093/alcalc/agh108. [DOI] [PubMed] [Google Scholar]
- 22.Kraus T, et al. Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res. 2005;29:2154–2157. doi: 10.1097/01.alc.0000191753.82554.7e. [DOI] [PubMed] [Google Scholar]
- 23.Halem HA, et al. Novel analogs of ghrelin: Physiological and clinical implications. Eur J Endocrinol. 2004;151:S71–S75. doi: 10.1530/eje.0.151s071. [DOI] [PubMed] [Google Scholar]
- 24.Salomé N, et al. Anorexigenic and electrophysiological actions of novel ghrelin receptor (GHS-R1A) antagonists in rats. Eur J Pharmacol. 2009;612:167–173. doi: 10.1016/j.ejphar.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 25.Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev. 2004;27:713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Engel JA, et al. Biochemical and behavioral evidence for an interaction between ethanol and calcium-channel antagonists. Alcohol Alcohol. 1988;23:A13–A13. doi: 10.1007/BF01244784. [DOI] [PubMed] [Google Scholar]
- 27.Imperato A, Dichiara G. Preferential stimulation of dopamine release in the nucleus-accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- 28.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 29.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: An overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 30.Moulin A, et al. Toward potent ghrelin receptor ligands based on trisubstituted 1,2,4-triazole structure. 2. Synthesis and pharmacological in vitro and in vivo evaluations. J Med Chem. 2007;50:5790–5806. doi: 10.1021/jm0704550. [DOI] [PubMed] [Google Scholar]
- 31.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor: Identification of a potent inverse agonist. Mol Endocrinol. 2003;17:2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- 32.Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–363. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- 33.Lyons AM, Lowery EG, Sparta DR, Thiele TE. Effects of food availability and administration of orexigenic and anorectic agents on elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:1962–1968. doi: 10.1111/j.1530-0277.2008.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: Differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 36.Davis KW, Wellman PJ, Clifford PS. Augmented cocaine conditioned place preference in rats pretreated with systemic ghrelin. Regul Pept. 2007;140:148–152. doi: 10.1016/j.regpep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tessari M, et al. Correlation between serum ghrelin levels and cocaine-seeking behaviour triggered by cocaine-associated conditioned stimuli in rats. Addict Biol. 2007;12:22–29. doi: 10.1111/j.1369-1600.2007.00052.x. [DOI] [PubMed] [Google Scholar]
- 38.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 39.Addolorato G, et al. Relationship between ghrelin levels, nutritional status and craving in current alcoholics. Alcohol Clin Exp Res. 2006;30:1933–1937. doi: 10.1111/j.1530-0277.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 40.Hillemacher T, et al. Role of appetite-regulating peptides in alcohol craving: An analysis in respect to subtypes and different consumption patterns in alcoholism. Alcohol Clin Exp Res. 2007;31:950–954. doi: 10.1111/j.1530-0277.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 41.Landgren S, et al. Association of pro-ghrelin and GHS-R1A gene polymorphisms and haplotypes with heavy alcohol use and body mass. Alcohol Clin Exp Res. 2008;32:2054–2061. doi: 10.1111/j.1530-0277.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- 42.Junghanns K, et al. The consumption of cigarettes, coffee and sweets in detoxified alcoholics and its association with relapse and a family history of alcoholism. Eur Psychiat. 2005;20:451–455. doi: 10.1016/j.eurpsy.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Gualillo O, et al. Effect of food restriction on ghrelin in normal-cycling female rats and in pregnancy. Obes Res. 2002;10:682–687. doi: 10.1038/oby.2002.92. [DOI] [PubMed] [Google Scholar]
- 44.Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.