Abstract
Although the ecological consequences of species invasions are well studied, the ecological impacts of genetic introgression through hybridization are less understood. This is particularly true of the impacts of hybridization on “third party” community members not genetically involved in hybridization. We also know little about how direct interactions between hybrid and parental individuals influence fitness. Here, we examined the ecological effects of hybridization between the native, threatened California Tiger Salamander (Ambystoma californiense) and the introduced Barred Tiger Salamander (Ambystoma tigrinum mavortium). Native x introduced hybrids are widespread in California, where they are top predators in seasonal ponds. We examined the impacts of early generation hybrids (first 2 generations of parental crosses) and contemporary hybrids derived from ponds where hybrids have been under selection in the wild for 20 generations. We found that most classes of hybrid tiger salamander larvae dramatically reduced survival of 2 native community members, the Pacific Chorus Frog (Pseudacris regilla) and the California Newt (Taricha torosa). We also found that native A. californiense larvae were negatively impacted by the presence of hybrid larvae: Native survival and size at metamorphosis were reduced and time to metamorphosis was extended. We also observed a large influence of Mendelian dominance on size, metamorphic timing and predation rate of hybrid tiger salamanders. These results suggest that both genetic and ecological factors are likely to influence the dynamics of admixture, and that tiger salamander hybridization might constitute a threat to additional pond-breeding species of concern in the region.
Keywords: Ambystoma, hybridization, invasion
Ever-increasing rates of global transport, structural manipulation of landscapes and other anthropogenic factors jointly increase the likelihood of hybridization between introduced and native species (1–4). A few case studies have examined the conservation implications of native-introduced species hybridization, with most focusing on the potential for genetic swamping of native genomes by introduced alleles (5–8). The ecological and conservation implications of hybridization for “third-party” community members (not genetically involved in hybridization) have only rarely been addressed, although the impacts of introduced species on native species are well documented (9–12). To our knowledge, studies of the ecological effects of hybridization among top predators do not exist.
The outcome of hybridization between lineages is a critical determinant of evolutionary processes and patterns (1, 13, 14). Hybridization can be an important source of new genetic variation (1, 15–17) or can lead to genetic homogenization and loss of diversity (2, 18–19). Hybridization can also result in geographically limited hybrid zones with low levels of gene flow across otherwise stable population boundaries (1, 20) or may lead to reinforcement of reproductive barriers due to low hybrid fitness (21). Although much attention has been paid to the role of hybridization in evolution, less has been given to its direct effects on community level ecological dynamics, with the exception of one study of Spartina cordgrass (11).
Here, we investigate the ecological effects and conservation implications of hybridization between a native and introduced predator. Hybridization between the threatened native California tiger salamander (NCTS) (Ambystoma californiense) and the introduced barred tiger salamander (IBTS) (Ambystoma tigrinum mavortium) began 60 years ago when bait dealers introduced thousands of IBTS larvae from Texas into ponds already inhabited by NCTS (22–23). Hybrid tiger salamanders now occupy ≈20% of the NCTS range, largely within the Salinas Valley (23). The transition between highly admixed and native populations is abrupt (23), and landscape genetic studies suggest that human landscape manipulations favor introduced alleles (23–24). Anecdotally, abundances of other amphibians within the hybrid zone appear low relative to NCTS sites, but these observations are confounded by the strong overlap of the hybrid zone with the highly agricultural landscape of the Salinas Valley. No studies of ecological impacts of the introduction have been performed until now. Such studies are crucial if management of biological invasions is to be based on scientific evaluation of risk and impact rather than aesthetic judgments about the desirability of nonnative species (12).
There are many reasons to expect dramatic ecological effects of introduced tiger salamanders. Decades of research have illustrated the important role of tiger salamander larvae in structuring pond-breeding amphibian communities (25–27), with effects primarily mediated through traits such as body size and gape width (28). Larval IBTS, in their natural range, attain larger average body size than NCTS (29). Introduced barred tiger salamanders also have a more plastic life history: NCTS are obligate metamorphs, transforming from the larval to the terrestrial form after a few months, whereas IBTS may extend the larval period, even becoming very large sexually mature paedomorphs (29). Finally, trophic polyphenism (presence of “typical” and “cannibal” morphs) in IBTS is well documented, whereas true cannibal morphs have not been observed in NCTS (29). All of these traits of IBTS might be expected to produce dramatically different effects on native pond communities compared with those of NCTS. However, most nonnative populations in California are hybrid rather than IBTS (24), and the inheritance of size, metamorphic timing and cannibalism in hybrids is unknown. With this in mind, we sought to answer 3 primary questions: Do NCTS and hybrids differ in phenotype? Do phenotypic differences translate into differences in ecological interactions with community members? If so, are these effects similar across community members?
“Hybrid” is not a uniform biological unit. Here, we refer to 2 genetically heterogeneous classes of hybrids: “early” generation hybrids [F1s, F2s, first generation backcrosses to NCTS (BN) and to IBTS (BI)] and contemporary generation hybrids (individuals of hybrid ancestry found currently on the landscape after up to 20 generations of selection in the wild). Early generation hybrids provide insight into the genetic basis of phenotypic variation in hybrids and a view into the dynamics between community members during the first few generations of hybridization. Contemporary hybrids lend insight into the range of phenotypic variation in hybrids after ≈20 generations of selection and the potential impacts of hybrids on extant communities. Both hybrid classes help us to understand the genetic factors underlying ecological phenotypes and to predict impacts as hybridization proceeds.
We conducted 2 experiments to answer the questions outlined above, focusing on survival and 3 components of larval tiger salamander phenotype: body size at metamorphosis, time to metamorphosis and predation on third-party community members. In both cases, our focal community consisted of NCTS and 2 additional native amphibian species commonly found within the California hybrid zone: Pseudacris regilla (Pacific Chorus Frog) and Taricha torosa (California newt). Differences in phenotype and impact were readily apparent (Fig. 1), with invasive genotypes growing larger and consuming more native frog and newt larvae.
Fig. 1.
Extreme phenotypes in contemporary hybrid and NCTS metamorphs from experiment 2 alongside mean-sized prey. The image of each animal was extracted from its original photo and size-adjusted to a common scale, but not otherwise altered. Photos were combined using Adobe Photoshop. (A) Largest NCTS. (B) Smallest NCTS. (C) Smallest hybrid. (D) Largest hybrid. (E) T. torosa larva. (F) P. regilla tadpole.
Results
Experiment 1: Early Invasion Dynamics.
Genetic differences in phenotype.
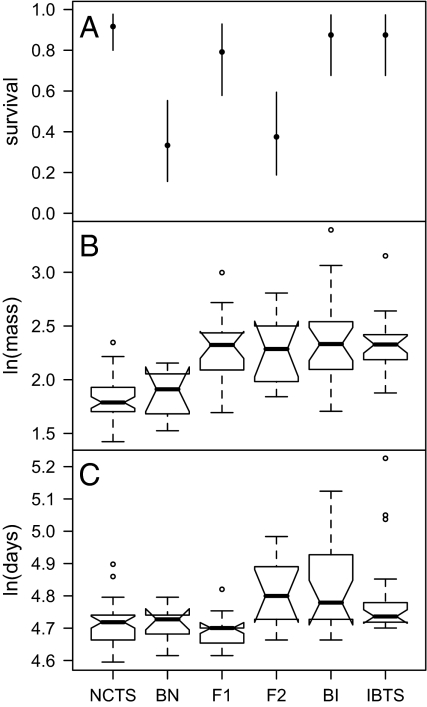
Mass at metamorphosis, time to metamorphosis, and survival were significantly different among genotypic classes (Fig. 2), with a full epistatic quantitative genetic model fitting best in all cases. Survival showed asymmetric hybrid dysfunction, with F2 and BN having low survival but BI having similar survival to parentals (Fig. 2A). F1 hybrids also had high survival, suggesting that hybrid mortality in the F2 and BN was caused by recessive epistatic interactions that were masked in the F1 (30). Introduced BTS, IBTS backcrosses (BI), and F2 hybrids remained in the larval stage longer and grew to a larger average size than NCTS and NCTS backcrosses (BN) (Figs. 2 and 3). F1 hybrids metamorphosed slightly sooner, on average, than any other group but at body masses similar to those of IBTS, implying transgressive growth rates in F1s.
Fig. 2.
Experiment 1, tiger salamander cross type responses. (A) Survival per cross type (AIC = 163.25, test vs. null likelihood ratio test: χ2 = 45.413, df = 5, P < 0.00001). Points are means; lines represent binomial 95% confidence intervals. (B) Ambystoma log mass per cross-type (AIC = 45.97, χ2 = 77.805, df = 5, P < 0.00001). Dark horizontal bands are medians; boxes show first and third quartiles; notches illustrate ≈95% confidence intervals; whisker bars show 1.5 times the interquartile range of the data (or maximum value, if smaller); open circles are outliers. Groups with nonoverlapping notches can be considered significantly different. (C) Ambystoma log time to metamorphosis per cross-type (AIC = −95.12, χ2 = 36.183, df = 5, P < 0.00001). See SI for model comparisons. NCTS data include only NCTS that emerged from control (NCTS only) ponds.
Fig. 3.
Mean size at metamorphosis for each cross type. (A) NCTS. (B) BN. (C) F2. (D) F1. (E) Contemporary hybrid. (F) BI. (G) IBTS. We selected photos of mean-sized animals for each cross type, extracted images and size-adjusted all to a common scale. Photos were not otherwise altered; images were combined using Adobe Photoshop.
Impacts on native California tiger salamanders.
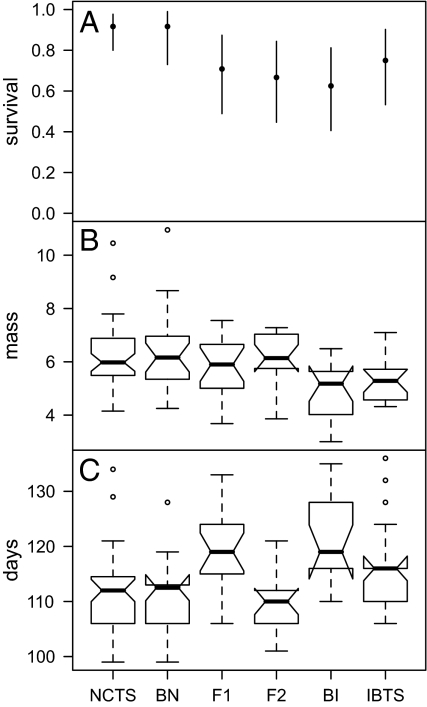
Survival of NCTS larvae was not affected by the presence of BN individuals but was lowest with BI individuals, implying an asymmetrical transgressive effect of hybrids on the survival of NCTS (Fig. 4A). Mass at metamorphosis of surviving NCTS was lowest in the presence of BI and IBTS, and not greatly affected by the other hybrid classes (Fig. 4B). Time to metamorphosis of surviving NCTS was substantially extended in the presence of F1 and BI hybrids (Fig. 4C).
Fig. 4.
Experiment 1, NCTS responses. Data illustrated as in Fig. 1. (A) NCTS survival across treatments (AIC = 169.43, test vs. null likelihood ratio test: χ2 = 15.877, df = 5, P = 0.0072). (B) NCTS mass across treatments (AIC = 422.87, χ2 = 21.560, df = 5, P = 0.0006). (C) NCTS time to metamorphosis across treatments (AIC = 913.06, χ2 = 41.985, df = 5, P < 0.00001).
Impacts on third-party species.
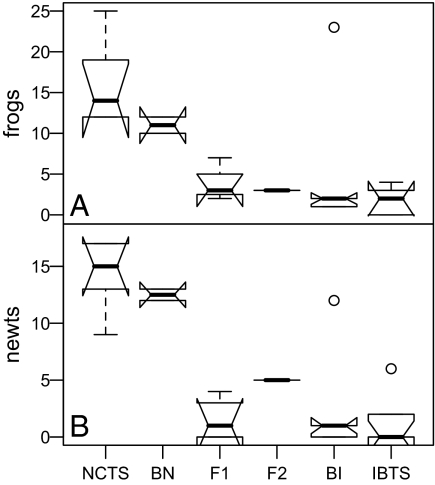
Numbers of P. regilla (Pacific chorus frogs) and T. torosa (California newts) surviving to metamorphosis were considerably lower in mesocosms with IBTS, BI, and F1 hybrids relative to NCTS, BN, and F2 hybrids. Lower impacts of BN and F2 crosses on frogs and newts might be a function of the low survival of these cross types (Fig. 2A). Including survival as a covariate in the model significantly improved the fit for both frog and newt data. In Fig. 5 we illustrate the data after removing mesocosms with >50% tiger salamander mortality and found that F1, F2, BI, and IBTS all had similarly high impacts on frogs and newts, with NCTS and BN having the lowest impact. Thus, the impact of introduced genotypes on third-party species is a function of tiger salamander phenotypes, not merely numbers of larvae.
Fig. 5.
Experiment 1, impacts on third party species. Data illustrated as in Fig. 1B. (A and B) Number of Pseudacris (A) and Taricha (B) survivors across the subset of ponds in which ≥50% of cross types survived. For the full dataset, variation among treatments was highly significant (frogs: AIC = 239.87, test vs. null likelihood ratio test: χ2 = 177.59, df = 5, P < 0.00001; newts: AIC = 101.170, χ2 = 155.38, df = 5, P < 0.00001), and adding tiger salamander survival as a random effect improved the fit for both species (frogs: AIC = 156.78, χ2 = 85.094, df = 1, P < 0.00001; newts: AIC = 98.067, χ2 = 5.1024, df = 1, P = 0.02389).
Predation rate, considered as a tiger salamander phenotype, showed a large dominance effect, with most hybrid generations resembling IBTS (Fig. 5). Comparison of quantitative genetic models [after Mather and Jinks (31)] for predation rate resulted in model 3 (additive plus dominance plus “additive × additive” epistasis) as the preferred model for frog predation (AIC = 159.81, test vs. null likelihood ratio test: χ2 = 67.2, df = 3, P < 0.00001) and the full epistatic model fit best for the newt data (AIC = 101.17, χ2 = 120.39, df = 5, P < 0.00001).
Experiment 2: Contemporary Invasion Dynamics.
We asked whether contemporary hybrid and native tiger salamander larvae differed in phenotype and whether these traits varied with absolute tiger salamander density and relative density of hybrids and NCTS in the pond (referred to as 3 levels of “genetic composition”: all-hybrid, all-NCTS or “mixed” 1:1 within ponds).
Genetic differences in phenotype.
Overall, survival was very high and did not differ significantly between NCTS and wild hybrids, or among treatments within either genetic class. Mass at metamorphosis and time to metamorphosis were significantly different among NCTS and hybrids. (Fig. 6 for NCTS; see SI for hybrid responses). A large number of tiger salamander larvae (37 individuals) did not metamorphose before the end of the experiment (when pond levels fell below 5 cm and pond temperatures exceeded 34 °C). With the exception of 2 small, sickly NCTS, these animals were all hybrid.
Fig. 6.
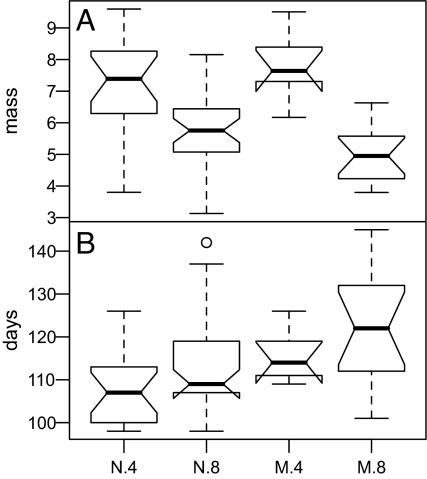
Experiment 2, NCTS responses. Labels denote genetic composition and density. N.4 refers to NCTS only at low density, N.8 to NCTS at high density. M.4 and M.8 refer to low and high density mixtures (1:1 NCTS:hybrid). Data illustrated as in Fig. 1B. (A) NCTS mass at metamorphosis was best explained by the full model (density + genetic composition + interaction) (AIC = 260.17, test vs. null likelihood ratio test: χ2 = 38.962, df = 3 P < 0.00001). (B) NCTS time to metamorphosis was affected by both density and genetic composition but not their interaction (AIC = 581.40, χ2 = 13.561, df = 2, P = 0.0011). See SI for hybrid responses.
Impacts on native California tiger salamanders.
Mass of NCTS was strongly reduced by increased larval density but not much affected by the presence of hybrids (Fig. 6). Time to metamorphosis was affected by both factors, with higher density and presence of hybrids both associated with longer times to metamorphosis (Fig. 6). Thus, wild hybrids affected growth rates of NCTS but not eventual mass at metamorphosis. Although overall NCTS survival was high, we did observe several incidences of cannibalism. Cannibalism occurred only in “mixed” treatment mesocosms, and all cases were unidirectional: hybrids eating NCTS.
Impacts on third-party species.
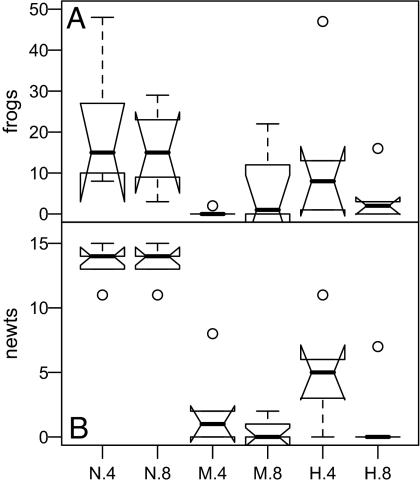
Numbers of chorus frogs and newts surviving to metamorphosis were considerably lower in mesocosms with hybrids relative to NCTS (Fig. 7). We found a nonsignificant trend toward greater third-party mortality in “mixed” treatment ponds relative to all-hybrid ponds. The effects of density were inconsistent, leading to significant statistical interactions between genetic composition and density.
Fig. 7.
Experiment 2, impacts on third party species. Labels are as in Fig. 4 plus low density and high density hybrid only (H.4 and H.8) ponds. (A) Number of P. regilla survivors was associated with both experimental factors and their interaction (full model: AIC = 282.41, test vs. null likelihood ratio test: χ2 = 180.71, df = 5, P < 0.00001); model fit was further significantly improved by adding tiger salamander survival as a random effect (AIC = 200.70, χ2 = 83.706, df = 1, P < 0.00001). (B) Number of T. torosa survivors was best described by the full model (AIC = 78.95, χ2 = 149.96, df = 5, P < 0.00001) and model fit was not improved by adding tiger salamander survival as a random effect.
Discussion
The clearest message of our results is that introduced barred tiger salamanders and introduced × native hybrids reduce survival, growth and developmental rate of native A. californiense (NCTS), Taricha torosa and Pseudacris regilla larvae when they cooccur, and these impacts are associated with biologically important, genetically based differences in hybrid phenotype compared with NCTS. This implies that the effects of barred tiger salamander introduction and hybridization in central California are likely to extend beyond direct impacts on tiger salamander populations, and probably also beyond effects on the 2 “third party” species in our experiments. The high degree of dominance in the underlying genetic basis of ecologically consequential phenotypes might contribute to both the rate and persistent ecological effects of genetic introgression in this system.
Genetic Differences in Phenotype.
NCTS were smaller at metamorphosis and faster to metamorphose when alone than nearly all classes of hybrids that we studied (exception: faster F1 time to metamorphosis). Size differences were often dramatic and readily visible (Figs. 1 and 3). Our findings also suggest that the past twenty or so generations of selection appear to have made the differences between hybrids (at the center of the hybrid zone) and NCTS even more dramatic. At metamorphosis, the vast majority of contemporary hybrid individuals from the populations we tested were bigger than the largest NCTS. This shift in trait distributions of hybrids toward larger-bodied, more persistently aquatic animals translates into a change in ecological interactions with other species and differences in ecological effects such as per capita density dependence experienced by hybrids. Although overall tiger salamander density had significant effects on both NCTS and hybrids, growth constraints were particularly exacerbated for hybrids in the presence of other hybrids compared with relaxed competitive conditions in the presence of smaller-bodied NCTS.
Nonlinearities in fitness resulting from gene interaction, density dependence, community composition and other forms of environmental variation make simple predictions of the relationship between genotype and fitness (or genotype and ecological effects) difficult. Environmental factors are likely to play an important role, given the plasticity of tiger salamander phenotypes and the potential for genetic determination of traits such as body size to vary with context (32, 33). The effects of plasticity and local adaptation on trait variation within the hybrid swarm might be fruitful subjects of further research.
Impacts on Native California Tiger Salamanders.
Tiger salamander hybrids reduced NCTS survival and impacted growth rates through cannibalism and competition. Besides outright mortality, hybrid tiger salamanders doubly impact NCTS through reduced body size at metamorphosis and increased time to metamorphosis in NCTS. Metamorphic size is known to influence adult fitness in other ambystomatid salamanders (34). In dry California upland habitat, reductions in metamorphic size may lead to differences in upland survival by increasing desiccation and predation risk or reducing competitive ability (35). More immediately, slowed growth rates may be costly during dry years (when ponds are more likely to dry before salamanders reach minimum size to metamorphose) and are likely to increase exposure to cannibalism and other forms of predation in natural habitats (36). We saw no indication of cannibalism among NCTS. Increased cannibalism by hybrids in our second experiment suggests that at minimum, cannibalism occurs, and may be under positive selection in some natural conditions.
The shift in NCTS time to metamorphosis in the presence of hybrids is particularly striking because our working assumption based on field observations outside of the hybrid zone has been that NCTS should hold an advantage in ephemeral pond environments if they need less time to reach metamorphosis. Although we did observe the expected differences between hybrids and NCTS independently, these differences were much reduced in mixed genotype treatments. Slowing of NCTS growth rates in the presence of hybrids offset natural advantages with regard to time to metamorphosis. Conversely, time to metamorphosis of contemporary hybrids sped up in mixed genotype treatments. So although some NCTS genes may confer an advantage in terms of metamorphic timing in hybrids, NCTS do not appear to benefit when competing directly with hybrids.
Impacts on Third-Party Community Members.
Taricha and Pseudacris exhibited strongly depressed recruitment in the presence of most hybrid tiger salamanders compared with NCTS. Cannibalism between tiger salamanders may exacerbate these effects, because cannibals were often the largest animals and occupied ponds from which very few or no newts or frogs emerged. Negative effects on common species bode poorly for threatened species in the region, such as Rana draytonii (California red-legged frog) and Ambystoma macrodactylum croceum (Santa Cruz long-toed salamander). Large-bodied R. draytonii tadpoles who rapidly reach a size refuge by exceeding the gape width of NCTS will likely experience higher predation in ponds with hybrids. Smaller-bodied A. m. croceum currently coexist with NCTS in some ponds, and would almost certainly be preyed upon by hybrid tiger salamanders. The small geographic range of A. m. croceum means that invasion of large, predaceous tiger salamander genotypes could represent a substantial threat to the continued persistence of the species.
Genetic Basis of Ecological Impacts.
Several authors have promoted the idea that understanding the effects of genetic variation on ecological interactions (9, 37), and biological invasions in particular (38) should yield valuable insights into evolutionary ecology and conservation biology. Our quantitative genetic analysis is among the first to go beyond establishing broad-sense heritability of ecological variation. The large influence of Mendelian dominance on size, metamorphic timing, and predation rate of hybrid tiger salamanders might have been critical in shaping the dynamics of admixture in the early generations of this invasion. Dominant advantageous alleles increase in abundance much more rapidly and reliably than recessive advantageous alleles (whose beneficial effects are largely masked when rare) (39). Because of dominance, even a small number of IBTS parents, mating randomly in a formerly NCTS population, would have a large and immediate effect on the distribution of larval phenotypes. Our previous observation of hybrid vigor (40) and the detrimental effects, shown here, of IBTS and hybrid phenotypes on growth and survival of NCTS would rapidly shift the genetic composition of invaded populations. At the landscape level, we would expect the ecological impacts of invasion to accompany introgression of introduced alleles affecting growth and feeding.
Important next steps are to investigate how selection in different pond environments influences hybrid phenotype and interaction strength between tiger salamanders and third-party species, and to better understand the relative importance of individual loci versus broad genetic background in the determination and plasticity of ecological traits. These steps will be important for issues of conservation and land management and fundamental questions about the genetic determinants of phenotypic variation and the effects of hybridization on mechanisms of coexistence within contemporary communities.
Methods
Protocols.
All research was conducted under U.S. Fish and Wildlife Service Federal Recovery Permit TE-094642, California Department of Fish and Game Collecting Permit SC-007728, and University of California Davis Institutional Animal Care and Use Committee Protocol 07-12634.
Experimental Animals.
For experiment 1, we captive-bred tiger salamanders from our salamander colony to generate all first and second generation cross types. Hybrid categories were defined entirely by the experimental crosses. We collected wild NCTS larvae from 3 vernal pools in Solano County as embryos and newly hatched larvae (<1 week of age). For experiment 2, we collected NCTS from the same ponds as above, and collected hybrid Ambystoma as embryos and newly hatched larvae from 3 cattle ponds in Salinas Valley (Monterey County) at the center of the hybrid zone, where hybrid populations that have been under selection for the maximum time. For both experiments, we collected Taricha egg masses from Ohlone Wilderness Regional Park (Alameda County), where they overlap with NCTS. We collected Pseudacris eggs and tadpoles from a variety of sites in Solano, Monterey and Alameda counties. Please see SI for details.
Experimental Design.
Experiment 1 was a single-factor design in which we varied the mixture of tiger salamanders in each pond. To each mesocosm, we added a total of 8 Ambystoma larvae, 25 Taricha larvae and 100 Pseudacris tadpoles. Because we were interested in effects of early generation crosses on native Ambystoma and third-party species, we added 4 wild NCTS larvae to each mesocosm. The remaining 4 Ambystoma larvae were captive-bred “treatment” larvae. These were: NCTS (native control), BN (backcross of F1 to NCTS), F1 (NCTS × IBTS), F2 (F1 × F1), BI (backcross of F1 to IBTS), and IBTS. To reduce the total number of treatments, we pooled reciprocal crosses (all treatments represent both cross directions). In addition to amphibians, we stocked ponds initially with zooplankton and later with California blackworms (Lumbriculus variegatus) as an additional food resource. We replicated each treatment 6 times for a total of 36 mesocosms. Mesocosms were allowed to dry naturally and the experiment ended when pond water fell below 5 cm and water temperature exceeded 34 °C. See SI for details of experimental setup and maintenance.
Experiment 2 followed a 3 × 2 factorial design, with factors Ambystoma genetic composition and Ambystoma density. The 3 levels of Ambystoma genetic composition were: (i) 100% wild NCTS larvae, (ii) 100% wild hybrid larvae, and (iii) a 1:1 ratio of hybrid:NCTS larvae. The 2 levels of Ambystoma density were: low (4 larvae total) and high (8 larvae). To each mesocosm, we also added 25 Taricha larvae and 100 Pseudacris tadpoles. We replicated this design 5 times for a total of 30 mesocosms.
Molecular Analysis.
To verify the genetic class of surviving Ambystoma, we assayed each individual for 4–6 diagnostic single nucleotide polymorphism (SNP) loci (one mtDNA and 3–5 mapped nuclear genes), which differentiate NCTS and IBTS alleles. Individuals were assayed using a fluorescence polarization platform. Markers, primer sequences and PCR conditions are described by Fitzpatrick and Shaffer (24).
Data Analyses.
All analyses were performed as linear mixed effects models fitted with restricted maximum likelihood (REML) in the lme4 package of R (41). For experiment 1, the treatment cross type was a fixed categorical effect and the block was a random effect. For experiment 2, genetic composition and density were fixed and block was random. Blocks were arbitrary rather than natural categories and we assumed no interaction between treatment and block; thus, for each response variable we test the statistical null hypothesis that there is no difference among treatments in any block (42). Results are presented as likelihood ratio tests of models with treatment and block effects vs. models including only the random block effect.
To describe genetic variation in phenotype (z) among cross types in experiment 1, we used the mixed effects framework to fit a standard quantitative genetic model (43),
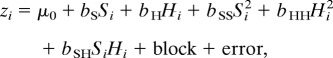 |
where Si is the ancestry index (proportional to the expected fraction of IBTS alleles in individual i based on its cross type), Hi is the heterozygosity index (proportional to the expected fraction of loci heterozygous for IBTS and NCTS), the bk are regression coefficients, and μ0 is the mean phenotype of the F2 generation (the reference generation). Nongenetic components of variation are partitioned into individual (“error”) and “block” terms. In standard quantitative genetic terms, the regression coefficients represent additive (bS), dominance (bH), and epistatic (bSS, bHH, bSH) effects of genetic differences between the parental NCTS and IBTS lineages. We fitted the typical quantitative genetic series, starting with the additive effect only and adding dominance and epistatic effects up to the full model. The best model for each dependent variable was chosen as the one with minimum AIC.
To address the question of impacts on NCTS, we modeled individual survival as a categorical variable using a logit link and binomial error term (equivalent to logistic regression). The result is a test for an association between the probability of survival of NCTS and the presence of the other genotypic categories. Masses of NCTS surviving to metamorphosis and their ages (in days) at metamorphosis were analyzed as raw or log-transformed data to test whether NCTS growth and metamorphosis patterns differed when in the presence of the other genotypic categories. All models were fitted with the NCTS-only control as the reference group such that estimated regression coefficients represent the effects of hybrids on NCTS.
Impacts on Pacific Chorus Frogs and California Newts were analyzed at the pond level. Counts of frogs and newts surviving to metamorphosis were modeled with a log link and Poisson errors in mixed models with tiger salamander cross type as a fixed treatment effect and block as a random effect.
Supplementary Material
Acknowledgments.
We thank J. Rogge, V. Gallegos, D. Dittrich-Reed, L. Gray, V. Bui, B. Wehrle, P. Trenham, M. Rogge, G. Johnson, and C. Ryan for research assistance; H.B. Shaffer for use of his laboratory, mesocosms, and salamander colony; P. Trenham for valuable input on manuscript drafts; the H.B.S. and P. Chesson laboratories for discussion; many ranchers for access to their properties; and 2 anonymous reviewers for thoughtful comments that improved this article. This work was supported by Environmental Protection Agency STAR Fellowship FP-91655501 (to M.E.R.); National Science Foundation Grant DEB-0516475 (to H.B. Shaffer, S.R. Voss, and B.M.F.); the University of California, Davis, Center for Population Biology (M.E.R.), and the University of California, Davis, John Muir Institute of Ecology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902252106/DCSupplemental.
References
- 1.Arnold ML. Natural Hybridization and Evolution. New York: Oxford Univ Press; 1997. [Google Scholar]
- 2.Allendorf FW, Leary RF, Spruell P, Wenburg JK. The problem with hybrids: Setting conservation guidelines. Trends Ecol Evol. 2001;16:613–622. [Google Scholar]
- 3.Lamont BB, He T, Endright NJ, Krauss SL, Miller BP. Anthropogenic disturbance promotes hybridization between Banksia species by altering their biology. J Evol Bio. 2003;16:551–557. doi: 10.1046/j.1420-9101.2003.00548.x. [DOI] [PubMed] [Google Scholar]
- 4.Ellstrand NC, Schierenbeck KA. Hybridization as a stimulus for the evolution of invasiveness in plants? PNAS. 2000;97:7043–7050. doi: 10.1073/pnas.97.13.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowling TE, Childs MR. Impact of hybridization on a threatened trout of the southwestern United States. Conservation Biol. 1992;6:355–364. [Google Scholar]
- 6.Rhymer JM, Williams MJ, Braun MJ. Mitochondrial analysis of gene flow between New Zealand mallards (Anas platyrhynchos) and grey ducks (A. superciliosa) Auk. 1994;111:970–980. [Google Scholar]
- 7.Utter FM, Allendorf FW. Phylogenetic relationships among species of Oncorhynchus: A consensus view. Conservat Biol. 1994;8:864–867. [Google Scholar]
- 8.Daniels MJ, Corbett L. Redefining introgressed protected mammals: When is a wildcat a wild cat and a dingo a wild dog? Wildlife Res. 2003;30:213–218. [Google Scholar]
- 9.Whitham TG, et al. Community and ecosystem genetics: A consequence of the extended phenotype. Ecology. 2003;84:559–573. [Google Scholar]
- 10.Hochwender CG, Fritz RS. Plant genetic differences influence herbivore community structure: Evidence from a hybrid willow system. Oecologia. 2004;138:547–557. doi: 10.1007/s00442-003-1472-4. [DOI] [PubMed] [Google Scholar]
- 11.Neira C, Levin LA, Grosholz ED. Benthic macrofaunal communities of three sites in San Francisco Bay invaded by hybrid Spartina, with comparison to uninvaded habitats Mar. Ecol Prog Ser. 2005;292:111–126. [Google Scholar]
- 12.Simberloff D. Non-native species do threaten the natural environment! J Agr Env Ethics. 2005;18:595–607. [Google Scholar]
- 13.Darwin C. On the Origin of Species by Means of Natural Selection. London: John Murray; 1859. [Google Scholar]
- 14.Barton NH. The role of hybridization in evolution. Mol Ecol. 2001;10:551–568. doi: 10.1046/j.1365-294x.2001.01216.x. [DOI] [PubMed] [Google Scholar]
- 15.Rieseberg LH, Archer MA, Wayne RK. Transgressive segregation, adaptation and speciation. Heredity. 1999;83:363–372. doi: 10.1038/sj.hdy.6886170. [DOI] [PubMed] [Google Scholar]
- 16.Grant PR, Grant BR. Speciation and hybridization in island birds. Phil Trans R Soc London B. 1996;351:765–772. [Google Scholar]
- 17.Dowling TE, Secor CL. The role of hybridization and introgression in the diversification of animals. Annu Rev Ecol Syst. 1997;28:593–619. [Google Scholar]
- 18.Rhymer JM, Simberloff D. Extinction by hybridization and introgression. Annu Rev Ecol Syst. 1996;27:83–109. [Google Scholar]
- 19.McDonald DB, Parchman TL, Bower MR, Hubert WA, Rahel FJ. An introduced and a native vertebrate hybridize to form a genetic bridge to a second native species. Proc Natl Acad Sci. 2008;105:10837–10842. doi: 10.1073/pnas.0712002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore WS, Price JT. In: Hybrid Zones and the Evolutionary Process. Harrison RG, editor. Oxford: Oxford Univ Press; 1993. pp. 196–225. [Google Scholar]
- 21.Servedio MR, Noor MAF. The role of reinforcement in speciation: Theory and data. Annu Rev Ecol Syst. 2003;34:339–364. [Google Scholar]
- 22.Riley SPD, Shaffer HB, Voss SR, Fitzpatrick BM. Hybridization between a rare, native tiger salamander (Ambystoma californiense) and its introduced congener. Ecol Apps. 2003;13:1263–1275. [Google Scholar]
- 23.Fitzpatrick BM, Shaffer HB. Introduction history and habitat variation explain the landscape genetics of hybrid tiger salamanders. Ecol Apps. 2007;17:598–608. doi: 10.1890/06-0369. [DOI] [PubMed] [Google Scholar]
- 24.Fitzpatrick BM, Shaffer HB. Environment-dependent admixture dynamics in a tiger salamander hybrid zone. Evolution. 2004;58:1282–1293. doi: 10.1111/j.0014-3820.2004.tb01707.x. [DOI] [PubMed] [Google Scholar]
- 25.Morin PJ, Wilbur HM, Harris RN. Salamander predation and the structure of experimental communities: Responses of Notophthalmus and microcrustacea. Ecology. 1983;64:1430–1436. [Google Scholar]
- 26.Wilbur HM, Morin PJ, Harris RN. Salamander predation and the structure of experimental communities: Anuran responses. Ecology. 1983;64:1423–1429. [Google Scholar]
- 27.Holomuzki JR, Collins JP, Brunkow PE. Trophic control of fishless ponds by tiger salamander larvae. Oikos. 1994;71:55–64. [Google Scholar]
- 28.Urban MC. Predator size and phenology shape prey survival in temporary ponds. Oecologia. 2007;154:571–580. doi: 10.1007/s00442-007-0856-2. [DOI] [PubMed] [Google Scholar]
- 29.Petranka JW. Salamanders of the US and Canada. Washington DC: Smithsonian Institute Press; 1998. [Google Scholar]
- 30.Turelli M, Orr HA. Dominance, epistasis, and the genetics of postzygotic isolation. Genetics. 2000;154:1663–1679. doi: 10.1093/genetics/154.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mather K, Jinks JL. Biometrical genetics. London: Chapman and Hall; 1971. [Google Scholar]
- 32.Maret TJ, Collins JP. Individual responses to population size structure: The role of size variation in controlling expression of a trophic polyphenism. Oecologia. 1994;100:279–285. doi: 10.1007/BF00316955. [DOI] [PubMed] [Google Scholar]
- 33.Davidowitz G, D'Amico LJ, Nijhout HF. The effects of environmental variation on a mechanism that controls insect body size. Evol Ecol Res. 2004;6:49–62. [Google Scholar]
- 34.Semlitsch RD, Scott DE, Pechmann JHK. Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology. 1988;69:184–192. [Google Scholar]
- 35.Trenham PC, Shaffer HB, Koenig WD, Stromberg MR. Life history and demographic variation in the California tiger salamander (Ambystoma californiense) Copeia. 2000;2000:365–377. [Google Scholar]
- 36.Werner EE. Amphibian metamorphosis: Growth rate, predation risk and optimal size at transformation. Am Nat. 1986;128:319–341. [Google Scholar]
- 37.Antonovics J. The input from population genetics: “The new ecological genetics.”. Syst Botany. 1976;1:233–245. [Google Scholar]
- 38.Lee CE. Evolutionary genetics of invasive species. Trends Ecol Evol. 2002;17:386–391. [Google Scholar]
- 39.Gillespie JH. Population Genetics: A Concise Guide. Baltimore: Johns Hopkins Univ Press; 1998. [Google Scholar]
- 40.Fitzpatrick BM, Shaffer HB. Hybrid vigor between native and introduced salamanders raises new challenges for conservation. Proc Natl Acad Sci. 2007;104:15793–15798. doi: 10.1073/pnas.0704791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bates D, Maechler M, Dai B. Lme4 package. 2008 Available at http://lme4.r-forge.r-project.org.
- 42.Newman JA, Bergelson J, Grafen A. Blocking factors and hypothesis tests in ecology: Is your statistics text wrong? Ecology. 1997;78:1312–1320. [Google Scholar]
- 43.Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sunderland, MA: Sinauer; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.