Abstract
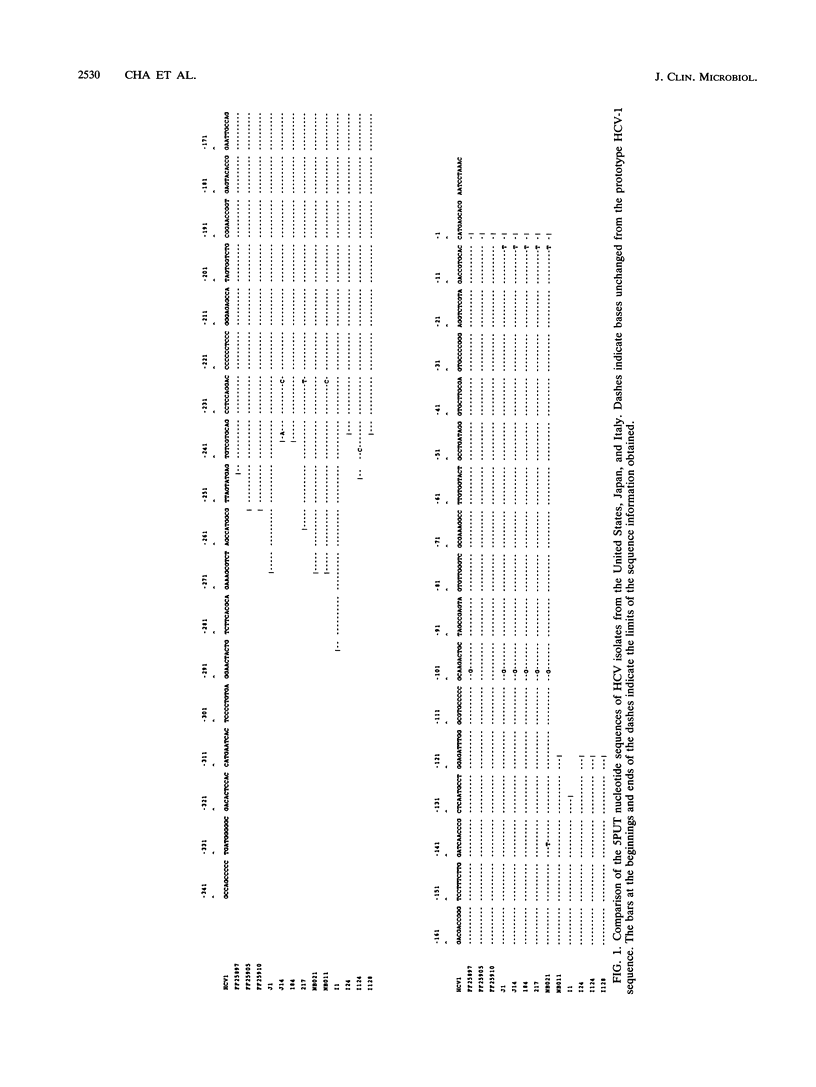
The nucleic acid sequence of the putative 5'-untranslated (5PUT) region of hepatitis C virus (HCV), determined for samples obtained from a variety of geographic origins, was found to be over 98% conserved among all isolates. On the basis of this signature sequence for HCV, a viral RNA assay was developed by using cDNA synthesis with reverse transcriptase, followed by polymerase chain reaction (PCR). The new assay was compared with the Ortho-Chiron C100-3 HCV enzyme-linked immunosorbent assay to research radioimmunoassays for antibodies to the C33c and C22 HCV antigens and to the first reported set of HCV PCR primers designed from the NS3 domain. Plasma samples from 16 Japanese patients with non-A, non-B hepatitis (NANBH) and 16 immunoassay-positive blood donors from the United States were investigated. The 5PUT PCR primers were found to be superior to the NS3 primers in sensitivity and specificity (15 of 25 versus 3 of 25 of the C100 enzyme-linked immunosorbent assay-positive samples, respectively). Samples from two C100-negative patients with acute NANBH were found to react with the 5PUT primers but not with the NS3 primers. Also, two of three patients with chronic NANBH converted from reverse transcriptase PCR positive to negative after interferon treatment. Although the clinical significance of the presence or absence of HCV RNA in samples from patients is not fully understood, the use of probes and primers from the 5PUT region (as opposed to primers from other segments) should not lead to false-negative results due to nucleic acid sequence variations in viral isolates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Alter M. J., Sampliner R. E. Hepatitis C: and miles to go before we sleep. N Engl J Med. 1989 Nov 30;321(22):1538–1540. doi: 10.1056/NEJM198911303212208. [DOI] [PubMed] [Google Scholar]
- Bradley D. W. The agents of non-A, non-B viral hepatitis. J Virol Methods. 1985 Apr;10(4):307–319. doi: 10.1016/0166-0934(85)90047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Weiner A. J., Overby L. R., Kuo G., Houghton M., Bradley D. W. Hepatitis C virus: the major causative agent of viral non-A, non-B hepatitis. Br Med Bull. 1990 Apr;46(2):423–441. doi: 10.1093/oxfordjournals.bmb.a072408. [DOI] [PubMed] [Google Scholar]
- Davis G. L., Balart L. A., Schiff E. R., Lindsay K., Bodenheimer H. C., Jr, Perrillo R. P., Carey W., Jacobson I. M., Payne J., Dienstag J. L. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989 Nov 30;321(22):1501–1506. doi: 10.1056/NEJM198911303212203. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Alter H. J. Non-A, non-B hepatitis: evolving epidemiologic and clinical perspective. Semin Liver Dis. 1986 Feb;6(1):67–81. doi: 10.1055/s-2008-1040795. [DOI] [PubMed] [Google Scholar]
- Enomoto N., Takada A., Nakao T., Date T. There are two major types of hepatitis C virus in Japan. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1021–1025. doi: 10.1016/0006-291x(90)90494-8. [DOI] [PubMed] [Google Scholar]
- Garson J. A., Preston F. E., Makris M., Tuke P., Ring C., Machin S. J., Tedder R. S. Detection by PCR of hepatitis C virus in factor VIII concentrates. Lancet. 1990 Jun 16;335(8703):1473–1473. doi: 10.1016/0140-6736(90)91510-h. [DOI] [PubMed] [Google Scholar]
- Garson J. A., Tedder R. S., Briggs M., Tuke P., Glazebrook J. A., Trute A., Parker D., Barbara J. A., Contreras M., Aloysius S. Detection of hepatitis C viral sequences in blood donations by "nested" polymerase chain reaction and prediction of infectivity. Lancet. 1990 Jun 16;335(8703):1419–1422. doi: 10.1016/0140-6736(90)91446-h. [DOI] [PubMed] [Google Scholar]
- Han J. H., Shyamala V., Richman K. H., Brauer M. J., Irvine B., Urdea M. S., Tekamp-Olson P., Kuo G., Choo Q. L., Houghton M. Characterization of the terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5' untranslated region and poly(A) tails at the 3' end. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1711–1715. doi: 10.1073/pnas.88.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., von Beroldingen C. H., Sensabaugh G. F., Erlich H. A. DNA typing from single hairs. Nature. 1988 Apr 7;332(6164):543–546. doi: 10.1038/332543a0. [DOI] [PubMed] [Google Scholar]
- Horn T., Urdea M. S. Forks and combs and DNA: the synthesis of branched oligodeoxyribonucleotides. Nucleic Acids Res. 1989 Sep 12;17(17):6959–6967. doi: 10.1093/nar/17.17.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai K., Iwata K., Nakao K., Kako M., Okamoto H. Suppression of hepatitis C virus RNA by interferon-alpha. Lancet. 1990 Jul 28;336(8709):245–245. doi: 10.1016/0140-6736(90)91768-6. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Unoura M., Kobayashi K., Kuno K., Murakami S., Hattori N. Detection of serum hepatitis C virus RNA. Lancet. 1990 Apr 21;335(8695):976–976. doi: 10.1016/0140-6736(90)91042-9. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Schmidtke J., Rösler U. Polymerase chain reaction: replication errors and reliability of gene diagnosis. Nucleic Acids Res. 1989 Mar 25;17(6):2197–2201. doi: 10.1093/nar/17.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Tanaka T., Sugai Y., Akahane Y., Machida A., Mishiro S., Yoshizawa H., Miyakawa Y. Detection of hepatitis C virus RNA by a two-stage polymerase chain reaction with two pairs of primers deduced from the 5'-noncoding region. Jpn J Exp Med. 1990 Aug;60(4):215–222. [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Yotsumoto S., Tanaka T., Yoshizawa H., Tsuda F., Miyakawa Y., Mayumi M. The 5'-terminal sequence of the hepatitis C virus genome. Jpn J Exp Med. 1990 Jun;60(3):167–177. [PubMed] [Google Scholar]
- Perrillo R. P., Schiff E. R., Davis G. L., Bodenheimer H. C., Jr, Lindsay K., Payne J., Dienstag J. L., O'Brien C., Tamburro C., Jacobson I. M. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. The Hepatitis Interventional Therapy Group. N Engl J Med. 1990 Aug 2;323(5):295–301. doi: 10.1056/NEJM199008023230503. [DOI] [PubMed] [Google Scholar]
- Rizzo T. M., Gray S. M. Cloning and sequence analysis of a cDNA encoding the capsid protein of the MAV isolate of barley yellow dwarf virus. Nucleic Acids Res. 1990 Aug 11;18(15):4625–4625. doi: 10.1093/nar/18.15.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Boonmar S., Kubo Y., Katayama T., Harada H., Ohbayashi A., Choo Q. L., Kuo G., Houghton M., Saito I. Hepatitis C viral cDNA clones isolated from a healthy carrier donor implicated in post-transfusion non-A, non-B hepatitis. Gene. 1990 Jul 16;91(2):287–291. doi: 10.1016/0378-1119(90)90102-w. [DOI] [PubMed] [Google Scholar]
- Ulrich P. P., Romeo J. M., Lane P. K., Kelly I., Daniel L. J., Vyas G. N. Detection, semiquantitation, and genetic variation in hepatitis C virus sequences amplified from the plasma of blood donors with elevated alanine aminotransferase. J Clin Invest. 1990 Nov;86(5):1609–1614. doi: 10.1172/JCI114882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdea M. S., Running J. A., Horn T., Clyne J., Ku L. L., Warner B. D. A novel method for the rapid detection of specific nucleotide sequences in crude biological samples without blotting or radioactivity; application to the analysis of hepatitis B virus in human serum. Gene. 1987;61(3):253–264. doi: 10.1016/0378-1119(87)90189-2. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. J., Kuo G., Bradley D. W., Bonino F., Saracco G., Lee C., Rosenblatt J., Choo Q. L., Houghton M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet. 1990 Jan 6;335(8680):1–3. doi: 10.1016/0140-6736(90)90134-q. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Truett M. A., Rosenblatt J., Han J., Quan S., Polito A. J., Kuo G., Choo Q. L., Houghton M., Agius C. HCV testing in low-risk population. Lancet. 1990 Sep 15;336(8716):695–695. doi: 10.1016/0140-6736(90)92194-m. [DOI] [PubMed] [Google Scholar]
- van der Poel C. L., Reesink H. W., Schaasberg W., Leentvaar-Kuypers A., Bakker E., Exel-Oehlers P. J., Lelie P. N. Infectivity of blood seropositive for hepatitis C virus antibodies. Lancet. 1990 Mar 10;335(8689):558–560. doi: 10.1016/0140-6736(90)90347-8. [DOI] [PubMed] [Google Scholar]