Abstract
Human regulatory T cells (Treg) have been variously defined as CD4+CD25+, CD4+CD25high or CD4+CD25highFOXP3+ cells which are responsible for maintaining peripheral tolerance. Their isolation from human peripheral blood or tissues depends on the expression level of CD25(IL-2Rα) - a surface marker which is also expressed on activated effector helper T cells. CD39, a cell surface associated ectonucleotidase, can be used to purify Treg with strong suppressor functions. The CD4+CD39+ T cells catalyze cleavage of adenosine triphosphate (ATP) to adenosine monophosphate (AMP), which is then further cleaved to adenosine. CD4+CD39+ T cells largely overlap with CD4+CD25highFOXP3+ but not CD4+CD25+ T-cell subset, and mediate equally potent immune suppression. Thus, CD39 surface marker can be successfully used for routine isolation of functionally-active human Treg from the peripheral blood of healthy donors or patients with cancer for studies of their role in health and disease.
Keywords: CD39, ectonucleotidase, regulatory T cells, FOXP3, CD4+CD25high
INTRODUCTION
Human thymus-derived natural regulatory T cells (nTreg) play a key role in maintaining tolerance (Strauss et al., 2007a). nTreg represent only a small subset of CD4+ T lymphocytes in the peripheral circulation and are responsible for the balance of immune responses, which is essential for health (Sakaguchi et al., 2005). nTreg excess or loss is associated with disease. Their accumulations in patients with cancer suppress anti-tumor immunity and promote tumor progression, while autoimmune diseases are associated with a decrease in the number and/or function of nTreg (Sakaguchi et al., 1985; Asano et al., 1996; Woo et al., 2001; Liyanage et al., 2002; Strauss et al., 2007a, b). The role of nTreg in transplant tolerance and graft-versus-host disease (GVHD) is being extensively investigated (Coghill et al., 2008).
Studies of human nTreg have been limited, largely because of their considerable diversity and a lack of a unique surface marker(s) that would allow for their isolation and purification. Consequently, much of the current knowledge about nTreg is derived from murine models, which offer an ample opportunity for nTreg isolation in numbers sufficient for functional studies, nTreg adoptive transfers or their depletion (Sakaguchi et al., 2007; Nagahama et al., 2007; Curiel, 2008). However, it appears that extrapolation from murine to human nTreg is not always accurate, and that human nTreg might be more heterogenous or utilize somewhat different mechanisms of suppression than their murine counterparts (Ziegler, 2006). Hence, there is a need for nTreg isolation and characterization from human body fluids and tissues to be able to evaluate their contribution to the control of beneficial as well as disease-associated immune responses.
Human nTreg have been phenotypically defined as CD4+CD25+, CD4+CD25high or CD4+CD25highFOXP3+ cells endowed with a capacity to suppress immune responses of activated T cells (Jonuleit et al., 2001; Levings et al., 2001; Dieckmann et al., 2001). More recently, with the realization that suppression mediated by Treg is regulated by the forkhead transcription factor (FOXP3), this protein has been widely considered as an identity marker for Treg. However, it has been reported that FOXP3 expression is not restricted to Treg, as activated T cells as well as tumor cells may be FOXP3+ (Morgan et al., 2005; Ziegler, 2006; Karanikas et al., 2008). For the purpose of Treg isolation, FOXP3 is not useful, because it is an intracytoplasmic protein (Zhang and Zhao, 2007). We and others have largely depended on the isolation of CD4+CD25+ T cells as Treg, using immunobead-based separation methods (Jeal, 2008). However, CD4+CD25+ populations might contain both activated CD4+ effector and suppressor T cells, which leads to confounding results. This could be particularly confusing in patients whose T cells are chronically activated such as in cancer or chronic infections. The alternative approach of sorting for CD4+CD25high T cells is labor-intensive and biased by an arbitrary definition of CD25 expression as “high.” Furthermore, it cannot be excluded that some CD4+CD25high T cells are effector T cells.
Recently, the presence on nTreg of CD39, a rate-limiting ectonucleotidase responsible for enzymatic cleavage of adenosine triphosphate (ATP) to adenosine monophosphate (AMP), has been reported on the surface of murine and human nTreg (Deaglio et al., 2007; Borsellino et al., 2007). In this communication, we show that CD39 is a surface marker that can be successfully and reliably used for isolation from the peripheral blood of functionally-active human nTreg. The capability to obtain purified nTreg is essential for establishing their role in human health and disease.
MATERIALS AND METHODS
Healthy volunteers and cancer patients
Venous peripheral blood samples were obtained from 15 healthy donors (NC) as well as 15 patients with head and neck squamous cell carcinoma (HNSCC). All subjects signed an informed consent approved by the Institutional Review Board of the University of Pittsburgh. The NC group included 4 males and 11 females with a mean age of 39 years (range, 24 to 58 years). The patients with HNSCC were age-matched with NC and included 10 males and 5 females. All had active disease and were not previously treated with oncologic therapy.
Collection of peripheral blood mononuclear cells (PBMC)
Blood samples (20–30mL) were drawn into heparinized tubes and centrifuged on Ficoll-Hypaque gradients (GE Healthcare Bioscience). PBMC were recovered, washed in AIM-V medium (Invitrogen), counted in a trypan blue dye and immediately used for experiments.
Separation of Treg
CD4+CD25neg as well as CD4+CD25+ T cells were freshly isolated from buffy coats using the Regulatory T cell Separation Kit and AutoMACS (Miltenyi Biotech). Briefly, cells were washed twice and first, CD4+ T cells were negatively selected from the total PBMC, followed by positive selection on anti-CD25 magnetic beads, separating CD4+CD25neg cells and CD4+CD25+ cells. CD4+CD25high cells (i.e., those with the MFI <120) and CD4+CD39+ cells were single-cell sorted following staining with anti-CD4 and anti-CD25 or anti-CD39 antibodies and using a Beckman Coulter cell sorter.
Antibodies
The following anti-human monoclonal antibodies were used for staining or cell sorting: anti-CD3-ECD, anti-CD4-ECD, anti-CD4-PC5, anti-CD8-PC5, anti-CD25-PC5, anti-GITR-FITC, anti-FOXP3-FITC, anti CD39-FITC, antiCD39-PE, antiCD26-PE and anti-CTLA4-PE. Antibodies and appropriate isotypes, which served as negative controls for surface as well as intracellular staining, were purchased from Beckman Coulter. Anti-FOXP3, anti-CD39-FITC and anti-CD39-PE were purchased from eBioscience. All antibodies were pre-titered on human T cells to determine optimal staining dilutions.
Surface and intracellular staining
Freshly isolated or activated cells were stained for flow cytometry as previously described (Strauss et al., 2007a, b). Briefly, for staining of surface markers, cells were incubated with the relevant pre-titered antibodies for 30 min at 4°C in the dark and then fixed with 2% (w/v) paraformaldehyde for 15 min. For intracellular staining, cells were additionally permeabilized with 0.1% (w/v) saponin and stained with antibodies specific for intracellular markers for 30 min at 4°C in the dark. Cells were washed twice with 0.1% saponin in PBS, resuspended in a flow solution and immediately analyzed by flow cytometry. Appropriate isotype controls were included for each sample.
Flow cytometry
Flow cytometry was performed using a EPICS® XL-MCL flow cytometer equipped with Expo32 software (Beckman Coulter). Lymphocyte were identified based on characteristic properties of the cells in the forward (FSC) and side scatter (SSC). FSC and SSC were set in a linear scale, and 105 cells were acquired for analysis, which was performed using the Coulter EXPO 32vl.2 analysis program. For additional analyses, gates were restricted to the CD3+CD4+, CD4+CD25+, CD4+CD39+ and CD4+CD25high cells.
Suppression Assays
Single cell-sorted CD4+CD39+ or CD4+CD25high or MACS sorted CD4+CD25+ cells were tested for suppressor activity in co-culture assays with autologous CD4+CD25neg responder cells (RC). To evaluate their proliferation, RC separated by MACS-sorting, were stained with 1.5μM CFSE (Molecular Probes/Invitrogen) and incubated at 37°C for 15 min. The unbound CFSE was quenched by using an equal amount of FCS (Invitrogen), and, subsequently, cells were washed twice with PBS. CFSE-labeled autologous CD4+CD25− 105cells/well were incubated in wells of flat-bottom 96-well plates at RC/suppressor (S) ratios of 1:1, 2:1, 5:1 and 10:1. RC were stimulated with plate-bound OKT-3 (2μg/mL) and soluble anti-CD28 mAb (2μg/mL) (Miltenyi) in the presence of 150 IU/mL IL-2 for 5 days. S cells were considered to mediate suppression, when they significantly inhibited proliferation of RC in co-culture assays. All CFSE data were analyzed using the ModFit software provided by Verity Software (Topsham). The percentage of suppression was calculated by using the mean proliferation index (PI) of RC alone compared to the PI of cultures containing RC + S cells. The program determines the percentage of cells within each detected peak, whereas the sum of all peaks in the control culture is set to be 100% proliferation or 0% suppression.
Statistical analysis
All data were presented as the means of at least three experiments ± 1 standard deviation (SD). The data were analyzed using the paired Student’s t test. Spearman correlations were performed to estimate relationships between percentages of T cells positive for a given marker or expression levels (MFI) of these markers. The p values < 0.05 were considered to be significant.
RESULTS
The frequency of CD4+CD39+ T cells in the peripheral blood of NC and cancer patients
Figure 1 shows that the frequency of CD4+CD25+, CD4+CD25high and CD4+CD39+ cells was higher in the peripheral blood lymphocytes of a representative HNSCC patient than in a NC. Within the CD4+ T cell subset, 8% of the cells were CD25+, 0.5% were CD25high (Table I) and CD39+ cells represented 7% of CD4+ cells in NC. In HNSCC patients 14% of CD4+ cells expressed CD25, 3% were CD25high (Table I) and 13% of the cells were CD39+ (Figure 2A, B). In this NC, 5% of CD4+ cells expressed FOXP3, while the proportion of FOXP3+ T cells was greater in a HNSCC patient, accounting for 12% of the total CD4+ cells (Figure 2C). At the population level, CD3+CD4+CD39+ T cells represented 7±4% (mean ± SD) of total CD4+ T cells, while HNSCC patients had an increased frequency (12±3%; p< 0.002) of these cells in the peripheral blood. The numbers and quality of the three Treg cell subsets isolated from NC and cancer patients are presented in Table I.
Figure 1. Percentages of circulating CD4+CD39+, CD4+CD25+ and CD4+CD25high cells in PBMC of NC and HNSCC.
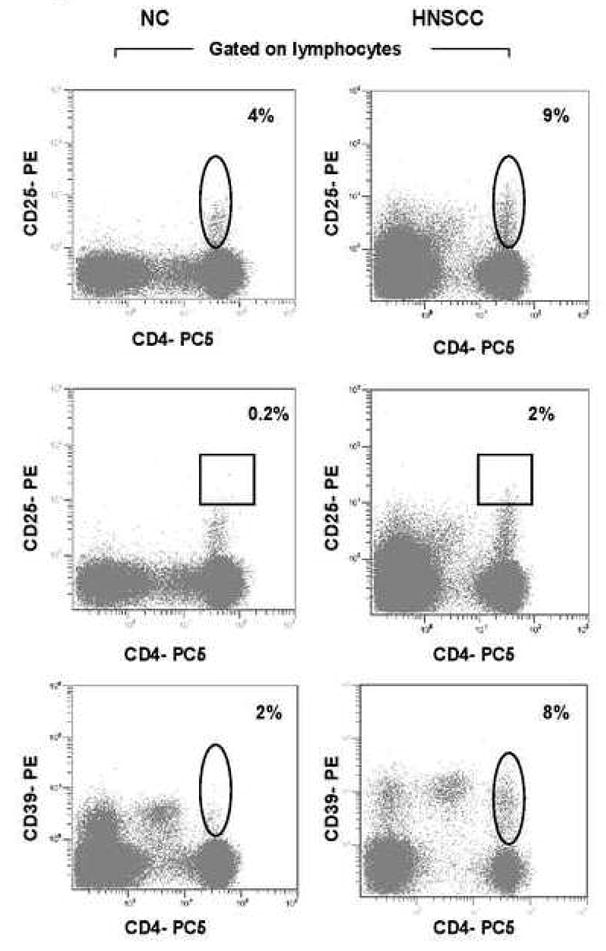
(A) PBMC obtained from one representative NC and one HNSCC were stained and examined for expression of CD25+ cells and CD25high cells in lymphocytes. (B) PBMC obtained from one representative NC and one HNSCC were stained and examined for expression of CD39+ cells in lymphocytes. The outlined regions show CD39+ cells in the CD3+CD4+ T-cell subset.
Table I.
Parameters defined for the isolation of the CD4+CD25+, CD4+CD25high and CD4+CD39+ subsets from peripheral blood mononuclear cells in normal controls and patients with HNSCC1.
| CD4+CD25+ | CD4+CD25high | CD4+CD39+ | |
|---|---|---|---|
| Frequency within the CD4+ T cells fraction | |||
| Normal donors (cell #) | 12±4 (2.4×106±0.3) | 0.5±0.2 (1×105±0.2) | 7±3 (1.4×106±0.4) |
| Patients (cell #) | 21±5 (4.2×106±0.3)* | 1.2±0.3 (2.4×105±0.4)* | 13±5 (2.6×106±0.4)* |
| Purity of cells | |||
| Normal donors | 96±0.6 | 98±0.6 | 98±0.6 |
| Patients | 96±0.6 | 98±0.6 | 98±0.6 |
| % Recovery after isolation | |||
| Normal donors | 64±10 | 65±11 | 70±13 |
| Patients | 62±8 | 60±8 | 71±12 |
| % Suppression (1S:1RC ratio) | |||
| Normal donors | 16±5 | 33±7 | 36±1 |
| Patients | 49±5* | 72±3* | 81±6* |
The data are mean percentages ± SD (mean cell numbers ± SD are in parentheses) from three representative individuals in each group. The cell numbers were calculated based on 50 × 106 PBMC used for each experiment. The asterisks indicate significant differences (p ≤ 0.05) between NC and patients with cancer.
Figure 2. Percentages of CD25+, CD39+ and FOXP3 expression in PBMC of NC and HNSCC.
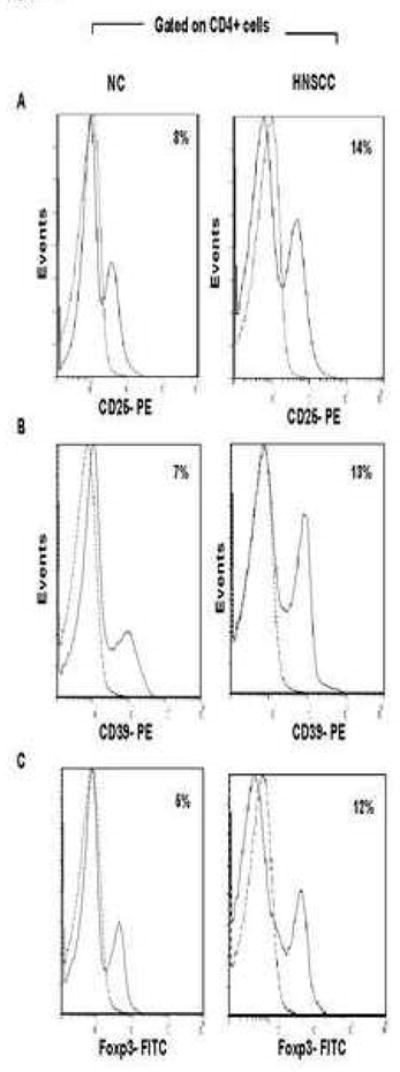
PBMC of 15 NC and 15 HNSCC were stained and analyzed on flow cytometry. Gates were set on CD3+CD4+. (A) Histoplots for CD25 expression on CD4+ cells in one representative individual of each group is shown. (B) Histoplots show CD39 expression in CD4+ cells of one representative individual of each group. (C) Histoplots show FOXP3 expression in CD4+ cells of one representative individual of each group.
Phenotypic characteristics of CD4+CD25+, CD4+CD25high and CD4+CD39+ Treg in NC and cancer patients
To examine phenotypic profiles of these Treg subsets following their purification from peripheral blood, multiparameter flow cytometry was performed. Figures 3A, D show that CD4+CD25+ population in the patients and NC contains relatively low percentages of FOXP3+, CTLA4+, GITR+, CD39+ or CD25high T cells compared to the CD4+CD25high or CD4+CD39+ subsets. This suggests that the majority of T cells in this subset are CD4+CD25low/intermediate conventional T cells. No correlation was observed between the percentages of FOXP3+ and CD39+ T cells or FOXP3+ within the CD4+CD25+ subset in NC or patients with HNSCC (Figure 3A). Because FOXP3 (a transcription factor regulating suppressor activity) and CD25 (an IL-2Rα) are functional markers for Treg, we also examined their expression levels (i.e., MFI) in CD4+CD25+ T cells. In NC and especially in patients, FOXP3 and CD25high expression was low, and no positive correlations were evident between expression levels of CD39 and FOXP3 or CD39 and CD25high for the CD4+CD25+ subset (data not shown). These results are consistent with the conclusion that the CD4+CD25+ population contains a mix of CD4+ effector T cells and nTreg.
Figure 3. Multiparameter flow cytometry analyses of CD4+CD25+, CD4+CD25high and CD4+CD39+ Treg subsets.
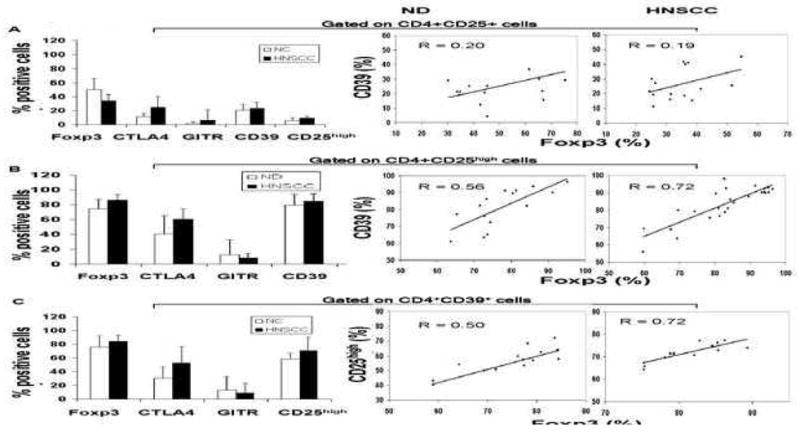
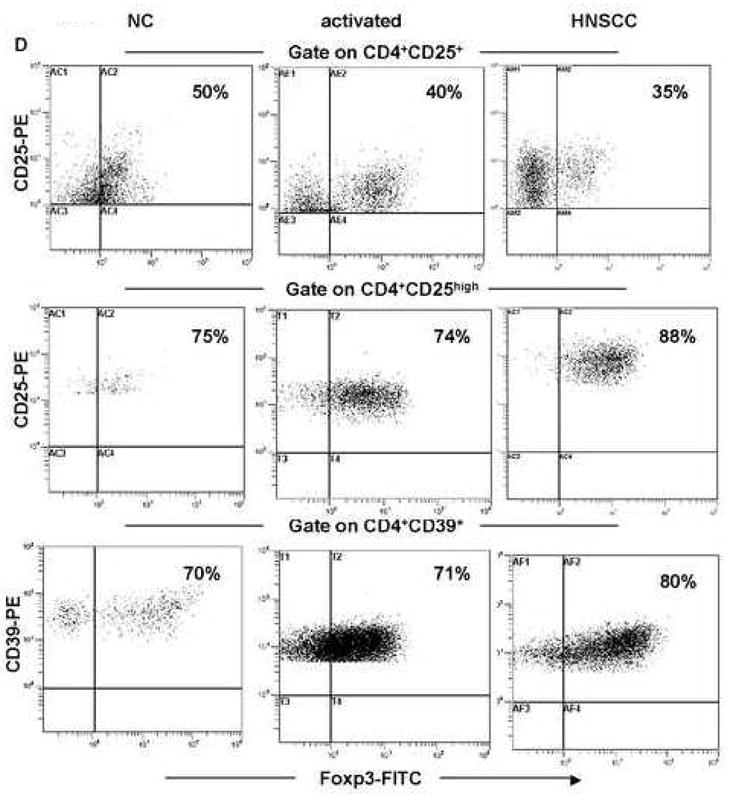
PBMC of 15 NC and 15 HNSCC were stained for flow cytometry and analyzed as described in Material and Methods. Gates were set on CD4+CD25+ (in A), CD4+CD25high (in B) or CD4+CD39+ (in C) T cells and expression of the commonly used Treg markers was determined. The mean % ± SD of T cells positive for these markers within each subset is shown. Spearman correlations for the percentages of CD39+ and FOXP3+ T cells in the CD4+CD25+ T cell subset (A) and in the CD4+CD25high subset (B) as well as for percentages of CD25high and FOXP3+ T cells in the CD4+CD39+ T cell subset (C) was determined in NC and HNSCC patients. The dot blots (in D) of FOXP3+ T cells in the CD4+CD25+, CD4+CD25high and CD4+CD39+ T cell subsets obtained from a representative NC and a representative HNSCC patient. Cells obtained from a NC were examined before or after 24h activation with OKT3/anti-CD28A and IL-2. The percentages of positive cells are shown in the upper right quadrants.
On the other hand, the frequency of FOXP3+ and CD39+ cells within the CD4+CD25high Treg subset exceeded 90% in both NC and cancer patients (Figures 3B, D), while the percentages of CTLA-4+ cells ranged from 50 to 60%. The percentages of CD4+CD39+ and CD4+FOXP3+ T cells in this subset correlated at R=0.56 in NC and R=0.72 in HNSCC patients (p<0.05). Expression levels of these proteins in the CD4+CD25high subset also showed a positive correlation, with R=0.90 for NC and R=0.61 for HNSCC patients (p<0.01) (data not shown).
The CD4+CD39+ cell subset contained up to 90% of FOXP3+ cells (Figures 3C, D), although only 50% to 70% of these cells in NC and 50–90% in HNSCC patients were CD25high. GITR+ cells represented less than 10% of the CD4+CD39+ subset in the circulation of both cohorts. CTLA-4+ cells had the frequency similar to that seen in the CD4+CD25high subset. The absence/low expression of CD127 in Treg cells has been recently described and used for Treg isolation (Liu et al., 2006; Hartigan-O’Connor et al., 2007; Peters et al., 2008). Also, CD26, a widely distributed cell membrane-associated glycoprotein with intrinsic dipeptidyl peptidase IV activity, which is associated with adenosine deaminase in humans has been recently reported by us to be low/absent on Treg1. CD4+CD39+ cells express low levels of CD127 and CD26, whereas CD4+CD39neg cells have abundant expression of CD127 and CD26 (Table II). A strong positive correlation was observed between the frequency of CD25high and FOXP3+ cells within the CD4+CD39+ subset in HNSCC patients (R=0.72) p<0.05, which was weak in NC (R=0.50) (Figure 3C). The results indicate that not all CD4+CD39+ T cells co-express FOXP3 or are CD25high, although the majority (up to 80%) do, especially in patients with cancer (Figure 3D). Expression levels of CD25high and FOXP3 in CD4+CD39+ cells correlated weakly at R=0.43, and a higher expression of IL-2Rα than FOXP3 was evident in these cells (data not shown). Because of the possibility that more activated Treg than “naïve” Treg express CD25+, CD25high and FOXP3+ T cells, we also evaluated these markers on ex vivo activated Treg by flow cytometry (Figure 3D). The representative dot blots show that OKT3-activated Treg contained similar percentages of CD4+CD25high and CD4+CD39+ T cells that were also FOXP3+. In contrast, activated CD4+CD25+ T cells contained fewer FOXP3+ T cells, probably because of the presence of activated effector T cells rather than Treg in this subset.
Table II.
Phenotypic characteristic of single-cell sorted CD4+CD39+ cells and CD4+CD39− cells from normal donors1.
| Phenotype | CD4+CD39+ | CD4+CD39neg |
|---|---|---|
| FOXP3 | 76 ± 16 | 12 ± 6* |
| CD25high | 58 ± 8 | 10 ± 4* |
| CTLA-4 | 30 ± 17 | 5 ± 4* |
| GITR | 18 ± 11 | 7 ± 2 |
| CD1272 | 10 ± 4 | 38 ± 7* |
| CD62L | 91 ± 5 | 82 ± 8 |
| CD26 | 17 ± 8 | 83 ± 10* |
| CD45RO | 55 ± 15 | 12 ± 1* |
| CD95 | 75 ± 10 | 22 ± 11* |
The data are mean percentages ± SD of experiments done with cells from 15 individuals in each group. PBMC were stained using antibodies for surface or intracellular markers. Cells were analyzed on flow cytometry. The asterisks indicate significant differences (p ≤ 0.05) between both groups.
Data represents mean percentage ± SD of experiments performed with cells of three individuals.
In aggregate, the phenotypic analysis of the three CD4+ T cell subsets before and after in vitro activation, showed that the peripheral blood-derived, CD4+CD39+ T cell subset and the CD4+CD25high T cell subset contained a comparably high frequency of FOXP3+ T cells.
Suppressor function of circulating CD4+CD39+, CD4+CD25high T cells and CD4+CD25+ cells
To analyze suppressor activity of CD4+CD39+, CD4+CD25high and CD4+CD25+ cells, these cell subsets were single-cell sorted and co-incubated with autologous CD4+CD25neg responder cells (RC) at different suppressor (S)/RC ratios. The purity, recovery and suppressor function of each sorted subset is shown in Table I. The representative suppression data for one NC and one HNSCC patient are shown in Figures 4A, B. While all three Treg subsets mediated suppression of RC proliferation, CD4+CD25+ T cells obtained from NC were only weakly suppressive. In patients with cancer, suppression levels mediated by all three subsets were much higher (Figure 4B). In NC and patients with cancer, suppression activity linearly decreased upon S cell dilution (Figures 4C, D). In all tested NC and patients, CD4+CD39+ Treg mediated the highest suppression, followed by CD4+CD25high subset and then by weakly suppressive CD4+CD25+ cells. The percent suppression for all three Treg subsets in cancer patients was significantly higher (p<0.001) than that for Treg of NC (Figure 4). Single-cell sorted CD4+CD39neg cells did not mediate suppression of RC proliferation (data not shown).
Figure 4. Suppression of proliferation of CFSE-labeled CD4+CD25neg responder cells mediated by CD4+CD25+, CD4+CD25high or CD4+CD39+ Treg.
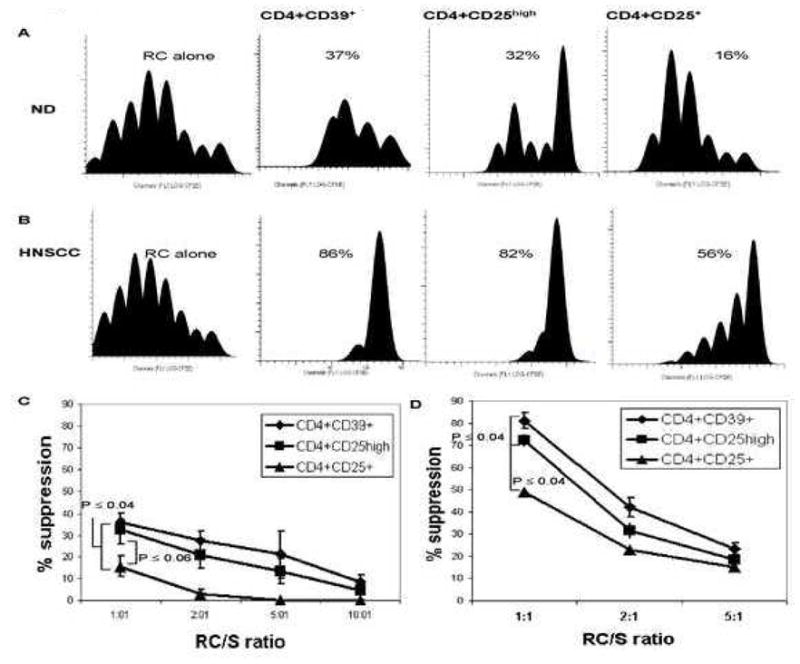
Autologous responder cells (RC) were CFSE-labeled, stimulated with plate-bound OKT-3 and soluble anti-CD28 and co-cultured with either CD4+CD25+, CD4+CD25high or CD4+CD39+ cells (S) in the presence of 150 IU/mL of IL-2 for 5 days. The Treg subsets were isolated as described in Material and Methods. Cells were analyzed by flow cytometry, gating on CD3+CD4+ and CD4+CFSE+ T-cell subsets. CFSE plots show suppression of RC proliferation mediated by S cells in one representative NC (A) and one representative HNSCC patient (B) at the 1RC:1S ratio. Data are from one experiment of three performed with cells of different NC or HNSCC patients. The percent suppression of RC proliferation is indicated in each panel. (C) Suppression of RC proliferation mediated by CD4+CD25+, CD4+CD25high or CD4+CD39+ cells (S) obtained from NC (n=3) at various RC: S ratios (p≤0.001) is shown. (D) Suppression of autologous RC proliferation mediated by the same S cell subsets obtained from HNSCC patients (n=3) at various RC/S is shown. The p values are for differences in suppression mediated by CD4+CD25+ cells to either CD4+CD25high or CD4+CD39+ cells in the 1RC:1S ratio.
DISCUSSION
To date, most investigators have utilized FOXP3 as a “benchmark” for human Treg despite the newly available information that transient expression of this transcription factor occurs in non-regulatory activated T cells (Ziegler, 2006; Mottet and Goishayan, 2007; Roncarolo and Gregori, 2008; Ahmadzdeh et al, 2008). Also, FOXP3 is not expressed in all Treg and, more importantly, is not a cell surface molecule. Therefore, it cannot be used for isolation of human Treg. Another surface marker, CD25 (a high affinity IL-2Rα) has been adopted for this purpose, based on the rationale that all Treg are dependent on IL-2 for their function and expansion (Fontenot et al., 2005; Bayer et al., 2007). Consequently, CD4+CD25+ and CD4+CD25high T-cell subsets have been designated as Treg subsets, which has led to a considerable confusion regarding phenotypic and functional features of Treg in humans. Our results clearly show that the CD4+CD25+ subset contains CD4+ effector cells in addition to Treg, and that Treg represent a minority of CD4+CD25+ T cells. This finding is consistent with weak suppression mediated by this T-cell subset in the presence of exogenous IL-2. On the other hand, the definition of CD25high T cells by quantitative flow cytometry has been largely arbitrary and inconsistent, as no definite expression criteria have been so far defined for the “high” CD25 expression level on CD4+ T cells. In view of this unsatisfactory situation, which is exacerbated by the differences that seem to exist between murine and human Treg, there is an urgent need for a more stringent and effective strategy of Treg isolation in humans.
The recent identification of a new surface marker, CD39 ectonucleotidase, for the isolation and characterization of human Treg is a highly desirable development. The advantage of this marker is that it recognizes Treg with suppressor activity mediated via pericellular adenosine, which is the end product of enzymatic degradation of ATP (Robson et al., 2006). Thus, CD39 defines Treg based not only on the phenotypic but also functional characteristics. Also, CD39 expression on Treg is associated with that of another enzyme, ecto-5″neuleotidase or CD73, which mediates cleavage of AMP to adenosine. Adenosine has been considered to be a strong suppressor of T cell functions (Zarek & Powell, 2007). However, before this new surface membrane-associated enzyme can be accepted as the Treg marker, it is necessary to consider properties of the T-cell subset it defines relative to those of widely used CD4+CD25+ and CD4+CD25highFOXP3+ T cell subsets.
The CD4+CD39+ T-cell subset represents 7±4% of CD3+CD4+ T cells in the circulation of NC and 12±3% in the blood of patients with cancer. The frequency and expression levels of CD39 correlate with those of FOXP3 in CD4+ T cells, especially in the peripheral blood of cancer patients, where nTreg are both more numerous and more functionally active (Strauss et al., 2007a). They also correlate with the frequency and expression levels of CD25high receptor. Thus, it appears that CD4+CD39+ T cell subset largely but nor completely overlaps with the CD4+CD25high subset, and that single-sorted populations of these cells have similar but not identical phenotypic and functional attributes. Using antibodies specific for CD39 instead of IL-2Rα for nTreg isolation is an advantage, as it allows for capture of the Treg subset with broader and stronger suppressor functions than those mediated by CD4+CD25+ T cells.
We have determined that CD39 allows for the isolation of more than 90% of highly suppressive FOXP3+ Treg in the blood of NC as well as patients with cancer. However, cancer patients have a higher frequency of CD39+ Treg which mediate significantly higher levels of suppression than their counterparts isolated from the circulation of NC. This suggests that chronic antigenic stimulation, such as that existing in patients with cancer, leads to activation and expansion of CD39+ Treg. Similar results were previously reported by us for CD4+CD25high T cells (Strauss et al., 2007a, b). The overlapping expression of CD39 and CD25high markers on Treg in the peripheral circulation of NC and patients with cancer supports the conclusion that these CD4+ T cell subsets, although not identical, represent highly-enriched populations of suppressor T cells.
In aggregate, the presented data indicate that the immuno-bead method for human CD39+ Treg isolation from the peripheral blood of NC or patients with cancer consistently yields Treg with a high purity and strong suppressor function.
Acknowledgments
Support: Research described in this article was supported in part by PO-1 CA109688 to TLW. Dr. Mandapathil was supported by Philip Morris International.
Abbreviations
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- FSC
forward scatter
- GVHD
graft-versus-host disease
- HNSCC
head and neck squamous cell carcinoma
- nTreg
naturally occurring regulatory T cells
- Treg
regulatory T cells
- MFI
mean fluorescence intensity
- NC
normal controls
- PBMC
peripheral blood mononuclear cells
- PI
proliferation index
- S
suppressor cells
- SD
standard deviation
- RC
responder cells
- SSC
sideward scatter
Footnotes
Mandapathil M., Hilldorfer B., Szczepanski M.J., Czyatowska M., Szajnik M., Ren J, Lang S., Jackson E.K., Gorelik E., Whiteside T.L. 2009, Expression of ectonucleotidase and generation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Immunol, In Revision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadzdeh M, Aloisio FS, Heemskerk B, Powell DJ, Wunderlich JR, Merino MJ, Rosenberg SA. Foxp3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AL, Aixen Y, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2007;178:4062. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rötzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- Coghill JM, Carlson MJ, Moran TP, Serody JS. The biology and therapeutic potential of natural regulatory T-cells in the bone marrow transplant setting. Leuk Lymph. 2008;49:1860. doi: 10.1080/10428190802272684. [DOI] [PubMed] [Google Scholar]
- Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JR, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Hartigan-O’Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Jeal H. Isolation, Flow Cytometry Analysis, and Suppression Assay of CD4+CD25+ T regulatory Cells. Meth Mol Med. 2008;138:85. doi: 10.1007/978-1-59745-366-0_8. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, Barda AK, Gourgonlianis KI, Germenis AE. Foxp3 expression in human cancer cells. J Transl Med. 2008;6:19. doi: 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Roncarolo MG. Human CD25(+)CD4(+) T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203:1701. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in the peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- Morgan ME, van Bilsen JHM, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RRP, Huizinga TWJ, Ottenhoff THM, Toes REM. Expression of Foxp3 mRNA is not confined to CD4+CD25+ T regulatory Cells In Humans. Hum Immunol. 2005;66:13. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Mottet C, Goishayan D. CD4+CD25+Foxp3 regulatory T cells: from basic research to potential therapeutic use. Swiss Med Wkly. 2007;137:625. doi: 10.4414/smw.2007.11916. [DOI] [PubMed] [Google Scholar]
- Nagahama K, Nishimura E, Sakaguchi S. Induction of tolerance by adoptive transfer of Treg cells. Meth Mol Biol. 2007;380:431. doi: 10.1007/978-1-59745-395-0_27. [DOI] [PubMed] [Google Scholar]
- Peters JH, Preijers FW, Woestenenk R, Hilbrands LB, Koenen HJ, Joosten I. Clinical grade Treg: GMP isolation, improvement of purity by CD127 Depletion, Treg expansion, and Treg cryopreservation. PLoS ONE. 2008;3:e3161. doi: 10.1371/journal.pone.0003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S. Is Foxp3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38:901. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Kukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset: I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N. Regulatory T cells in immunologic self-tolerance and autoimmune disease. Int Rev Immunol. 2005;24:211. doi: 10.1080/08830180590934976. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Wing K, Miyara M. Regulatory T cells – a brief hstory and perspective. Eur J Immunol. 2007;37:S116. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007a;13:6301. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting IL-10 and TGF-β1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007b;13:4345. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- Woo E, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Prevalence of CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766. [PubMed] [Google Scholar]
- Zarek PE, Powell JD. Adenosine and anergy. Autoimmunity. 2007;40:425. doi: 10.1080/08916930701464939. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4+CD25+ T cells: multiple pathways on the road. Cell Physiol. 2007;211:590. doi: 10.1002/jcp.21001. [DOI] [PubMed] [Google Scholar]
- Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]


