Abstract
Nitric oxide (NO) produced by neuronal nitric oxide synthase (nNOS) has a role in synaptic plasticity, and evidence suggests its role in a range of effects produced by alcohol in the central nervous system. The aim of the current study was to investigate the role of the nNOS gene in the development of ethanol-induced conditioned place preference (CPP) in mice. The CPP paradigm is designed to investigate the reinforcing properties of drugs of abuse and the development of maladaptive behaviors, such as conditioned response to drug-associated stimuli, following repeated drug exposure. Adult male and female wild type (WT) and nNOS knockout (KO) mice on a mixed B6; 129S genetic background were trained by a morning saline session and afternoon ethanol (1, 2 and 3g/kg; intraperitoneally) session for four days. Place preference in a drug-free state was recorded on the following day. Results show that WT males and females developed robust CPP while nNOS KO mice did not (with the exception of female nNOS KO mice conditioned by 2g/kg ethanol). The differential response of WT and nNOS KO mice was not due to genotypic differences in motor behavior. To investigate if the absence of the nNOS gene causes specific impairment in processing the motivational effect of ethanol or an overall impairment in associative learning, WT and nNOS KO mice were trained by LiCl (150mg/kg) which causes conditioned place aversion (CPA). Results show that both WT and nNOS KO mice developed significant CPA. The findings that the absence of the nNOS gene impaired ethanol-induced CPP but not LiCl-induced CPA suggest that NO signaling has a specific role in processing the motivational effect of ethanol. Hence, inhibition of nNOS may attenuate the development of maladaptive behaviors associated with alcohol exposure.
Keywords: neuronal nitric oxide synthase (nNOS), ethanol, conditioned place preference (CPP) nNOS knockout mice, LiCl, conditioned place aversion (CPA)
Introduction
In the central nervous system (CNS) nitric oxide (NO) has a role in nonsynaptic communication between glutamatergic and monoaminergic neurons (Kiss and Vizi, 2001). NO synthase (NOS) is classified into three major isoforms, neuronal (nNOS), endothelial (eNOS) and inducible (iNOS). The first two are constitutive and calcium-dependent, and the third is present primarily in microglia and microphages (Bredt and Snyder, 1994). Three major alternative spliced nNOS transcripts have been found: nNOSα, β and γ (Brenman et al., 1996). The principal nNOSα accounts for the great majority of catalytic activity in the brain; mice with targeted deletion of nNOSα (nNOS knockout; KO) display 95% reduction in NOS catalytic activity (Huang et al., 1993). In brain, NO is produced by nNOS, primarily by activation of N-methyl-D-aspartate receptor (NMDAR) (Garthwaite and Boulton, 1995). The finding that nNOSα is linked to the NR2 subunit of the NMDAR via a postsynaptic density protein (PSD95) indicates a direct association between NMDAR activation and Ca+2 ions that enter the channel and stimulate the enzyme (Brenman and Bredt, 1997).
Increasing evidence suggests that NO has a major role in the various effects of alcohol (Davis and Syapin, 2005; review). Inhibition of NOS in rats attenuated ethanol consumption (Calapai et al., 1992; Revzvani et al., 1995) and, following chronic ethanol administration, subsequent tolerance (Wazlawik and Morato, 2002) and withdrawal symptoms (Adams et al., 1995; Adams and Cicero, 1998). Because most of these studies investigated NOS inhibitors that block all three NOS isoforms, it is difficult to ascertain the specific role of nNOS in these effects. The finding that nNOS KO mice consumed larger amounts of ethanol than wild type (WT) mice (Spanagel et al., 2002) challenged the notion that inhibition of nNOS attenuates alcohol drinking. However, intracerebroventricular (icv) administration of antisense oligos against nNOS decreased ethanol drinking in rats (Naassila et al., 2000). Thus, the exact role of nNOS in alcohol drinking in rodents is not clear.
In previous studies we investigated the effect of the relatively selective nNOS inhibitor 7-nitroindazole (7-NI) on ethanol-induced hyperlocomotion and conditioned place preference (CPP) in DBA/2J mice (Itzhak and Martin, 2000). The major findings were: First, daily intraperitoneal (ip) administration of ethanol to DBA/2J mice resulted in a progressive increase in locomotor activity (sensitization) which was blocked by co-administration of the nNOS inhibitor 7-NI. Second, ethanol-induced CPP was completely abolished by co-administration of the nNOS inhibitor. These findings support the role of nNOS in the development of behavioral sensitization and conditioned response to ethanol. More recently, we have shown that nNOS KO mice were resistant to ethanol-induced behavioral sensitization compared to WT mice (Itzhak and Anderson, 2008). This finding corroborates the pharmacological effect of the nNOS inhibitor 7-NI on ethanol-induced sensitization in DBA/2J mice (Itzhak and Martin, 2000).
The present study was undertaken to investigate the role of the nNOS gene in the development of ethanol-induced CPP. To consider the role of nNOS in processing the motivational effect of ethanol, the development of LiCl-induced conditioned place aversion (CPA) in WT and nNOS KO was also investigated. Most drugs of abuse that promote self-administration in rats and mice produce CPP (Bardo and Bevins, 2000). The development of a conditioned response to stimulus reward involves Pavlovian conditioning, which is implicated in the development of maladaptive behaviors including drug addiction. In a typical place conditioning experiment, the subject is paired repeatedly with an unconditioned stimulus (e.g., drug) in one compartment of the cage and with vehicle in a different compartment. A change in the affective state of the organism and associative learning and memory processes are involved in the formation of conditioned response to the drug-paired environment (White and Carr, 1985). The expression of CPP is viewed as a test for reactivity to drug-associated conditioned stimulus; thus, this test has face validity for cue-reactivity in human drug users (Sanchis-Segura and Spanagel, 2006).
We report that while WT male and female mice developed robust ethanol-induced CPP, nNOS KO mice did not (with the exception of female nNOS KO mice conditioned by 2g/kg ethanol). However, both WT and nNOS KO mice of both sexes developed significant LiCl place aversion, suggesting a particular role of the nNOS gene in processing the motivational effect of ethanol.
Materials and Methods
Animals
Mice purchased from Jackson Laboratories (Bar Harbor, Maine) were bred in our facilities at the University of Miami, Miller School of Medicine, Miami, FL as we described previously (Balda et al., 2006; Itzhak and Anderson, 2008). Both genotypes, WT and nNOS KO, were generated on a mixed B6;129S genetic background (Huang et al., 1993). For the experiments described herein, adult (7−8 weeks old) WT and nNOS KO mice of both sexes were used. Animals were housed in a temperature-(22±0.50C and humidity- (50%) controlled room and maintained on a 12-h light/dark schedule with free access to food and water. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, 1996) and approved by the University of Miami Animal Care and Use Committee.
Drugs
Ethanol was diluted in saline (0.9% NaCl) to yield doses of 1, 2 and 3g/kg. LiCl was dissolved in distilled water to yield a dose of 150mg/kg, a dose which induced CPA in Swiss Webster mice (Martin and Itzhak, 2000; Achat-Mendes et al., 2005). All drugs and vehicles were administered intraperitoneally in a volume of 0.1ml/10g.
Conditioning apparatus
Custom-designed Plexiglas cages (42 × 20 × 20 cm high; Opto-Max Activity Meter v2.16; Columbus Instruments, Columbus, OH) were used. A removable guillotine door separated the cage into two zones, one comprising four black walls with a smooth black floor and the other four white walls and a floor covered with sand paper (fine grit 150C, Norton). The differences between the two compartments consist of visual and tactile cues. Each cage was equipped with 2 horizontal sensors mounted alongside opposing lengths. The black and white zones (21 × 20 × 20 cm) were each scanned by 7 infrared beams at a rate of 10Hz (2.54cm intervals). A null zone 8 cm wide at the interface of the black and white zones was assigned to ensure that only full entry into each compartment was registered as ‘real’ time spent in each compartment.
Training
On the first day, mice were habituated to the conditioning apparatus (20min) and time spent in each compartment was recorded to determine preconditioning compartment-preference/aversion. To ensure a strictly unbiased CPP design, mice that showed initial preconditioning preference of more than 10−12% of the time (20min) to either compartment of the cage were discarded. For ethanol CPP studies the following were discarded: WT males: n=7 out of 35; nNOS KO males: n=9 out of 41; WT females: n=9 out of 36; nNOS KO females: n=7 out of 38. The number of mice per group trained by ethanol were WT males: n=28; nNOS KO males: n=32; WT females: n=27; nNOS KO females: n=31. For LiCl place aversion studies the following were discarded: WT males: n=2 out of 10; nNOS KO males: n=3 out of 13; WT females: n=3 out of 11; nNOS KO females: n=2 out of 12. The total number of mice trained by LiCl was n=36.
For the next 4 days (days 2−5) mice were conditioned by a morning vehicle (saline or water) session and, three hours later, an afternoon ethanol (10min) or LiCl (60min) session. For the unbiased design, training was counterbalanced: half of the subjects were trained with drug in the black compartment and the other half in the white compartment. The order of injections (saline-drug) was not counterbalanced because drug administration in the morning session may have a lingering effect into the afternoon session. Following each training session, the sandpaper was removed, the cage was thoroughly cleaned with diluted laboratory-grade detergent (Alconox) followed by water and then dried. On day 6 time spent in each compartment was recorded for 20min following vehicle injection. The test was conducted at midday during the same time period in which the pretraining habituation had been recorded. During the test session locomotor activity was recorded to determine if place preference is influenced by motor activity.
Statistical analysis
For each genotype and sex 4 groups were investigated, each containing n=8−12 mice. These were three groups for ethanol training (1, 2 and 3g/kg) and one group for LiCl training. Results are presented as mean ± SEM of the time spent in each compartment of the CPP cage pretraining and posttraining. For each dose of ethanol and LiCl, posttraining differences between times spent in drug- and vehicle-paired compartments were analyzed by paired Student t-test. The contributions of genotype, sex and dose of ethanol to the CPP results were analyzed by three-way ANOVA (genotype × sex × dose). Comparison between the magnitude of locomotor activity during the CPP test across the groups was analyzed by three-way ANOVA (genotype × sex × dose). The contributions of genotype and sex to the LiCl CPA results were analyzed by two-way ANOVA. Post hoc Bonferroni test was conducted to determine differences between specific groups. A difference was considered significant when the p value was less than 0.05. SPSS 16.0 software for Windows was used for the analyses.
Results
Ethanol CPP and LiCl CPA
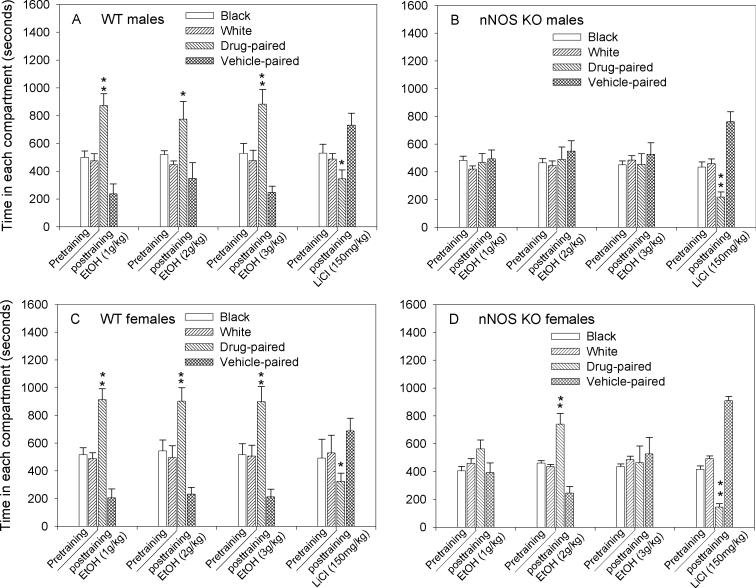
During the pretraining phase mice of both genotypes and sexes spent approximately equal times in the black and white compartments of the CPP cage (Fig. 1, columns labeled as “Pretraining”), suggesting the absence of biased preference for either color or floor texture. WT males developed significant preference for the ethanol-paired compartment over the saline-paired compartment (p<0.05) following training with all three doses of ethanol (1, 2 and 3g/kg; Fig. 1A). The magnitude of CPP across all three doses was similar (533−637 seconds), suggesting a lack of dose-response effect. As expected, training of WT males by LiCl (150mg/kg) induced aversion to the drug-paired compartment compared to the water-paired compartment (p<0.05; Fig. 1A). In contrast to WT males, nNOS KO males developed neither preference nor aversion to the ethanol-paired compartment at all three doses tested (Fig. 1B). However, nNOS KO males developed significant place aversion to the LiCl-paired compartment (p<0.01; Fig. 1B). WT females, like their male counterparts, developed both significant ethanol CPP and LiCl CPA (Fig. 1C). nNOS KO females showed differential sensitivity to ethanol compared to WT females. While the low (1g/kg) and the high (3g/kg) doses of ethanol had no effect, only the dose of 2g/kg resulted in reliable preference for the ethanol-paired compartment (p<0.01; Fig. 1D). Yet, the training by LiCl resulted in marked aversion from the LiCl-paired compartment (p<0.01; Fig. 1D).
Fig. 1.
nNOS KO mice show selective deficits in reward-related conditioning. Adult male and female WT and nNOS KO mice (n=8−12/group) were trained by various doses of ethanol (1,2 and 3g/kg) or LiCl (150mg/kg) for 4 days. Each pair of bars designated as “Pretraining: black & white”” shows the time spent in the black and white compartment of the conditioning apparatus during habituation (20min) the day before training began. All groups spent similar time in each compartment during the pretraining phase. A. WT males: all three doses of ethanol produced significant place preference for the ethanol-paired compartment over the vehicle (saline)-paired compartment (*p<0.05; **p<0.01). Conditioning by LiCl produced significant aversion for the drug-paired compartment compared to the vehicle (water)-paired compartment (*p<0.05). B. nNOS KO males: preference for the ethanol-paired compartment was not observed at any of the three training doses of the drug. However, training by LiCl induced significant place aversion for the drug-paired compartment compared to the vehicle-paired compartment (**p<0.01). C. WT females: all three doses of ethanol produced significant place preference for the ethanol-paired compartment over the vehicle-paired compartment (**p<0.01). LiCl produced significant aversion for the drug-paired compartment compared to the vehicle-paired compartment (*p<0.05). D. nNOS KO females: only the dose of 2g/kg ethanol produced significant preference for the drug-paired compartment over the vehicle-paired compartment (**p<0.01). LiCl induced significant place aversion for the drug-paired compartment compared to the vehicle-paired compartment (**p<0.01).
Results of ethanol CPP were further analyzed by three-way ANOVA. The fixed factors were genotype, sex and ethanol dose, and the dependent variable was time spent in the drug-paired compartment. There was a significant genotype effect F[1,106]=67.92; p<0.001, a significant sex effect F[1,106]=4.29; p=0.041 and no significant dose effect F[2,106]=2.14; p=0.08. No significant interactions between the factors were observed (p>0.05). Bonferroni post hoc analysis revealed significant differences between WT and nNOS KO males in their response to all three doses of ethanol( p<0.05). These findings suggest that regardless f the ethanol training dose, males exhibited genotypic differences in response to the ethanol-paired compartment. Among females, significant differences between WT and nNOS KO mice were observed in the groups that had been trained by 1 and 3g/kg ethanol (p<0.05) but not between WT and nNOS KO females trained by 2g/kg ethanol (p=0.14). These findings suggest dose-dependent genotypic differences in females’ response to the ethanol-paired compartment. That is, in females, only the dose of 2g/kg ethanol produced place preference in both genotypes (Fig. 1D).
Results of LiCl place aversion were analyzed by two-way ANOVA (genotype × sex). When the dependent variable was time spent in the LiCl-paired compartment, there was a significant genotype effect F[1,32]=16.63; p<0.001, no significant sex-effect F[1,32]<0.01; p=0.99, and no significant interaction F[1,32]=3.15; p=0.085. Bonferroni post hoc analysis showed a significant genotype effect within females (p<0.001) but not within males (p=0.11). Analysis of the results of time spent in the vehicle-(water) paired compartment showed a significant genotype effect F[1,32]=4.45; p=0.043, no significant sex effect F[1,32]=0.02; p=0.87 and a significant interaction F[1,32]=5.82; p=0.022. Bonferroni post hoc analysis showed a significant genotype effect within females (p=0.003) but not within males (p=0.81). Results suggest that the magnitude of conditioned place aversion in nNOS KO females was greater than in WT females (Fig. 1C and 1D).
Locomotor activity
During the CPP test (20min) on day 6, locomotor activity was also recorded. Results showed that locomotor activity during the CPP test was similar across the groups, regardless of genotype, ethanol training dose, or sex (Fig. 2). A three-way ANOVA (genotype × dose of ethanol × sex) revealed no significant genotype effect F[1,106]=0.17; p=0.84, no significant dose effect F[2,106]=0.22; p=0.64, and no significant sex effect F[1,106]=0.12; p=0.75. Results suggest that the differences between ethanol CPP in WT and nNOS KO mice were not due to differences in motor behavior.
Fig. 2.
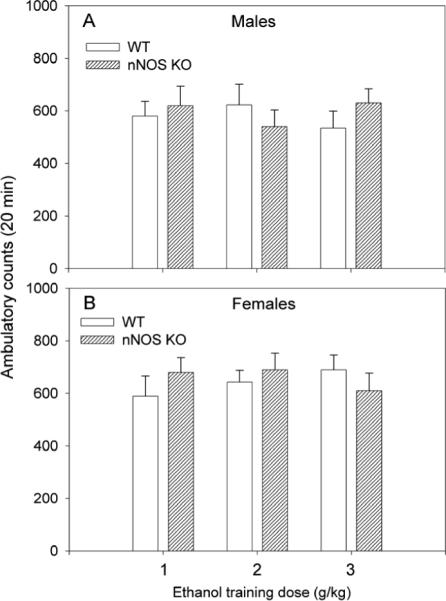
Locomotor activity during the CPP test. The day following training by various doses of ethanol, locomotor activity was recorded simultaneously during the CPP test, which was conducted in a drug-free state. The Y-axis represents mean ± SEM of ambulatory counts recorded during the 20 min test. The X-axis represents ethanol training doses. A three-way ANOVA (genotype × dose of ethanol × sex) revealed no significant genotype effect F[1,106]=0.17; p=0.84, no significant dose effect F[2,106]=0.22; p=0.64, and no significant sex effect F[1,106]=0.12; p=0.75.
Discussion
Most studies have used the 2-bottle choice test to deduce alcohol preference; fewer studies have investigated the motivational effects of ethanol in the CPP paradigm (Risinger et al., 2002). One reason could be that while ethanol CPP is usually observed in various mouse strains, in rats it has been more difficult to establish ethanol place preference. In rats, the route and dose of ethanol administration are some of the aspects which determine the development of ethanol place preference or aversion (Cunningham et al., 1993; Risinger et al., 2002; Walker and Ettenberg, 2007).
The major findings of the present study are: a) WT mice of both sexes developed significant ethanol CPP across three doses of ethanol, 1, 2 and 3g/kg. b) nNOS KO males did not develop ethanol CPP, while nNOS KO females developed CPP only following training by an intermediate dose of ethanol (2g/kg). c) Despite having deficits in ethanol CPP, nNOS KO mice developed a typical LiCl place aversion as had WT counterparts. d) The differential response of WT and nNOS KO mice to ethanol CPP was not due to differences in motor behavior.
We have previously found that DBA/2J mice developed significant ethanol CPP, and that the administration of the nNOS inhibitor 7-NI 30min prior to training by ethanol inhibited the development of CPP (Itzhak and Martin, 2000). Moreover, 7-NI alone had neither appetitive nor aversive properties (Itzhak and Martin, 2000). Likewise we have shown that the nNOS inhibitor 7-NI did not affect the development of LiCl CPA in Swiss Webster mice (Martin and Itzhak, 2000). Together these findings suggest that the pharmacological blockade of the nNOS enzyme and the deletion of the nNOS gene resulted in a similar outcome: ethanol CPP was abolished while LiCl CPA remained unaffected. Hence, it appears that the absence of the nNOS gene causes specific impairment in processing the motivational effect of ethanol rather than an overall impairment in associative learning. The findings that no genotypic differences in either basal locomotor activity (Balda et al., 2006) or during CPP testing were observed (present study) suggest that the differential response of WT and nNOS KO mice to ethanol CPP was not due to motor behavior. Likewise, we assume that the genotypic differences in ethanol CPP were not due to difference in blood ethanol concentration (BEC). We recently showed that neither genotype- nor sex-dependent differences in BEC arise following administration of 1.5g/kg ethanol to WT and nNOS KO mice (Itzhak and Anderson, 2008). Similarly, no differences in BEC between WT and nNOS KO adult males were observed following administration of a higher dose of ethanol (3.5g/kg) (Spanagel et al., 2002).
The findings that nNOS KO males were resistant to ethanol-induced a) behavioral sensitization (Itzhak and Anderson, 2008) and b) CPP (present study) suggest that similar neuroadaptations may underlie the development of behavioral sensitization and conditioned response to ethanol-associated conditioned stimuli. We found that administration of saline to ethanol-experienced mice in the context of ethanol administration resulted in context-dependent hyperlocomotion in WT but not nNOS KO males (Itzhak and Anderson, 2008). Context-dependent hyperlocomotion in mice and rats usually develops after the administration of psychostimulants (cocaine and amphetamine). This behavioral phenotype is context-specific and is an index for arousal in response to cues associated with drugs of abuse (Itzhak, 1997; Wolf and Khansa, 1991). The expression of CPP is also a manifestation of cue-reactivity to drug-associated conditioned stimulus. The findings that nNOS KO males developed neither ethanol-induced context-dependent hyperlocomotion (Itzhak and Anderson, 2008) nor ethanol CPP support the role of the nNOS gene in processing the motivational effect of ethanol.
Gonadal hormones, neurosteroids, and stress hormones may have major roles in sex-dependent differences in response to alcohol (Witt, 2007). In the present study, three-way ANOVA (genotype × dose × sex) revealed not only a significant genotype effect but also an overall significant sex effect (p=0.042). However, Bonferroni pair-wise comparisons between males and females for each genotype trained by a particular dose of ethanol did not reveal significant sex-dependent differences. Yet, results presented in Fig. 1D show that nNOS KO females, in contrast to male counterparts, developed significant place preference following training by 2g/kg ethanol. This observation was confirmed by the post hoc Bonferroni test that followed the three-way ANOVA. Within females, significant differences between WT and nNOS KO mice were observed in the groups that had been trained by 1 and 3g/kg ethanol (p<0.05) but not between WT and nNOS KO females trained by 2g/kg ethanol (p=0.14). That is, in females, only the dose of 2g/kg ethanol produced place preference in both genotypes (Fig. 1C and D). The reason for this “bell shape” dose-response is unclear; we had expected that female nNOS KO mice would not develop CPP at any of the three doses of ethanol.
While the effect of the estrus cycle on ethanol CPP is unclear, results of the effect of the estrus cycle on ethanol consumption are mixed. When rats were allowed to cycle freely, the estrus cycle had no effect on ethanol consumption (Roberts et al., 1998). Other studies suggest that rat's ethanol drinking is the lowest on the proestrus day (Ford et al., 2002). In turn, repeated exposure to ethanol caused irregularity of rat's estrus cycle (Krueger et al., 1982; Sanchis et al., 1985). Hence, in the present study the estrus cycle was not determined. The finding that the magnitude of ethanol CPP in WT males and females was similar suggests that sex hormones play no major role in ethanol CPP. The difference, however, between nNOS KO males and females suggests that sex hormones may have a more pronounced effect on ethanol CPP in the absence of the nNOS gene. That is, nNOS KO females may be less resistant to ethanol's rewarding effect compared to male counterparts. Notably, we found that the nNOS gene has a major role in the development of cocaine CPP in nNOS KO males but not females (Balda et al., 2006). Likewise we found that nNOS KO males are resistant to cocaine-induced behavioral sensitization (Balda et al., 2008), while nNOS KO females developed sensitization to cocaine as had WT counterparts (Balda et al., 2009). Taken together these findings suggest that sex-dependent difference in the responses to ethanol and cocaine may be more conspicuous in the absence than in the presence of the nNOS gene.
The question to what extent ethanol drinking in rodents relates to the reinforcing effects of ethanol was a topic of a recent review (Green and Grahame, 2008). Based on analysis of several studies in mice, the authors conclude that there is a modest positive relationship between ethanol drinking and ethanol CPP. With some exceptions, mice that have shown high free-choice ethanol drinking also developed ethanol CPP (Green and Graham, 2008). However, this relationship may not exist in nNOS KO mice. The finding that nNOS KO mice consumed larger amounts of ethanol compared to WT mice (Spanagel et al., 2002) seems to contrast the present findings that nNOS KO mice did not develop ethanol CPP. Notably, however, it has been reported that icv administration of antisense oligos against nNOS decreased alcohol drinking in rats (Naassila et al., 2000). The study by Spanagel et al. (2002) should be considered with caution because: a) Mice had been backcrossed with C67BL/6J mice and hence had a predominance of the C57BL/6J background. In our study the genetic background of WT and KO mice was a mixed B6;129S in a 1:1 ratio. b) The nNOS KO mice consumed not only more ethanol than WT mice, but also larger amounts of sweetened solution. Given that reduced alcohol consumption is directly correlated with reduced saccharine-solution consumption (Blednov et al., 2008), two questions arise: 1) Was the increased alcohol drinking in nNOS KO mice due to the role of NO-signaling in the motivational effect of ethanol or to their preference for sweetened solution? 2) Is the 2-bottle choice test appropriate for investigating the motivational effect of ethanol in nNOS KO mice? While we do not have answers to these questions, we posit that the CPP paradigm may be relevant for correlating between behavioral sensitization and “drug-seeking behavior” as determined in CPP studies. For instance, male nNOS KO mice had deficits in expression of cocaine-induced psychomotor sensitization (Balda et al., 2008; Itzhak et al., 1998a) and cocaine-induced CPP (Balda et al., 2006; Itzhak et al. 1998b). Likewise, the inability of nNOS KO males to acquire ethanol CPP is in agreement with the finding that these mice are resistant to ethanol-induced behavioral sensitization (Itzhak and Anderson, 2008). However, a correlation between the magnitudes of behavioral sensitization to ethanol and ethanol-induced CPP is not always apparent and may be due to genotype-dependent variations in response to ethanol (Cunningham, 1995).
In summary, the present study suggests that the nNOS gene is required for ethanol-reward, but not LiCl-aversive, associative learning. The findings of the present study, and those from our previous studies on psychostimulants, support the hypothesis that NO has a role of a downstream signaling molecule in neuroadaptations consequent to repeated exposure to alcohol and cocaine. Further studies are required to determine if manipulation of the NO signaling pathway may be beneficial for the management of alcohol misuse disorders, particularly in male subjects.
Acknowledgements
This work was supported in part by R01 DA019107 from the National Institute on Drug Abuse, National Institutes of Health, USA (YI) and Programa Nacional de Formación de Profesorado Universitario (AP2006−04048), MICINN, and MICINN (PSI2008−00101 and Red de Trastornos Adictivos, RD06/001/0016), Spain (CRS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achat-Mendes C, Ali SF, Itzhak Y. Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacology. 2005;30:1128–1137. doi: 10.1038/sj.npp.1300675. [DOI] [PubMed] [Google Scholar]
- Adams ML, Cicero TJ. Alcohol intoxication and withdrawal: the role of nitric oxide. Alcohol. 1998;16:153–158. doi: 10.1016/s0741-8329(97)00185-7. [DOI] [PubMed] [Google Scholar]
- Adams ML, Sewing BN, Chen J, Meyer ER, Cicero TJ. Nitric oxide-related agents alter alcohol withdrawal in male rats. Alcohol. Clin. Exp. Res. 1995;19:195–199. doi: 10.1111/j.1530-0277.1995.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Differential role of the nNOS gene in the development of behavioral sensitization to cocaine in adolescent and adult B6;129S mice. Psychopharmacology. 2008;200:509–519. doi: 10.1007/s00213-008-1228-2. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Development and persistence of long-lasting behavioral sensitization to cocaine in female mice: Role of the nNOS gene. Neuropharmacology. 2009;56:709–715. doi: 10.1016/j.neuropharm.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Bredt DS. Synaptic signaling by nitric oxide. Curr. Opin. Neurobiol. 1997;7:374–378. doi: 10.1016/s0959-4388(97)80065-7. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Calapai G, Squadrito F, Altavilla D, Zingarelli B, Campo GM, Cilia M, Caputi AP. Evidence that nitric oxide modulates drinking behaviour. Neuropharmacology. 1992;31:761–764. doi: 10.1016/0028-3908(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology. 1995;120:28–41. doi: 10.1007/BF02246142. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Noble D. Species difference in sensitivity to ethanol's hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- Davis RL, Syapin PJ. Interactions of alcohol and nitric-oxide synthase in the brain. Brain Res. Rev. 2005;49:494–504. doi: 10.1016/j.brainresrev.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol. Clin. Exp. Res. 2002;26:635–643. [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu. Rev. Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Modulation of cocaine- and methamphetamine-induced behavioral sensitization by inhibition of brain nitric oxide synthase. J. Pharmacol. Exp. Ther. 1997;282:521–527. [PubMed] [Google Scholar]
- Itzhak Y, Anderson KL. Ethanol-induced behavioral sensitization in adolescent and adult mice: role of the nNOS gene. Alcohol. Clin. Exp. Res. 2008;32:1839–1848. doi: 10.1111/j.1530-0277.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Blockade of alcohol-induced locomotor sensitization and conditioned place preference in DBA mice by 7-nitroindazole. Brain Res. 2000;858:402–407. doi: 10.1016/s0006-8993(00)01940-5. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF, Martin JL, Black MD, Huang PL. Resistance of neuronal nitric oxide synthase-deficient mice to cocaine-induced locomotor sensitization. Psychopharmacology. 1998a;140:378–386. doi: 10.1007/s002130050779. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Black MD, Huang PL. The role of neuronal nitric oxide synthase in cocaine-induced conditioned place preference. Neuroreport. 1998b;9:2485–2488. doi: 10.1097/00001756-199808030-00011. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001;24:211–215. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- Krueger WA, Bo WJ, Rudeen PK. Female reproduction during chronic ethanol consumption in rats. Pharmacol. Biochem. Behav. 1982;17:629–631. doi: 10.1016/0091-3057(82)90335-5. [DOI] [PubMed] [Google Scholar]
- Martin JL, Itzhak Y. 7-Nitroindazole blocks nicotine-induced conditioned place preference but not LiCl-induced conditioned place aversion. Neuroreport. 2000;11:947–949. doi: 10.1097/00001756-200004070-00010. [DOI] [PubMed] [Google Scholar]
- Naassila M, Beauge F, Sebire N, Daoust M. Intracerebroventricular injection of antisense oligos to nNOS decreases rat ethanol intake. Pharmacol. Biochem. Behav. 2000;67:629–636. doi: 10.1016/s0091-3057(00)00407-x. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Grady DR, Peek AE, Pucilowski O. Inhibition of nitric oxide synthesis attenuates alcohol consumption in two strains of alcohol-preferring rats. Pharmacol. Biochem. Behav. 1995;50:265–270. doi: 10.1016/0091-3057(94)00310-f. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL, Bevins RA, Holloway FA. Place conditioning: what does it add to our understanding of ethanol reward? Alcohol. Clin. Exp. Res. 2002;26:1444–1452. doi: 10.1097/01.ALC.0000029582.19240.14. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol. Clin. Exp. Res. 1998;22:1564–1569. [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Sanchis R, Esquifino A, Guerri C. Chronic ethanol intake modiifies estrous cyclicity and alters prolactin and LH levels. Pharmacol. Biochem. Behav. 1985;23:221–224. doi: 10.1016/0091-3057(85)90560-x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Siegmund S, Cowen M, Schroff KC, Schumann G, Fiserova M, Sillaber I, Wellek S, Singer M, Putzke J. The neuronal nitric oxide synthase gene is critically involved in neurobehavioral effects of alcohol. J. Neurosci. 2002;22:8676–8683. doi: 10.1523/JNEUROSCI.22-19-08676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. Intracerebroventricular ethanol-induced conditioned place preference are prevented by fluphenazine infusion into the nucleus accumbens of rats. Behav. Neurosci. 2007;121:401–410. doi: 10.1037/0735-7044.121.2.401. [DOI] [PubMed] [Google Scholar]
- Wazlawik E, Morato GS. Effects of intracerebroventricular administration of 7-nitroindazole on tolerance to ethanol. Brain Res. Bull. 2002;57:165–170. doi: 10.1016/s0361-9230(01)00736-5. [DOI] [PubMed] [Google Scholar]
- White NM, Carr GD. The conditioned place preference is affected by two independent reinforcement processes. Pharmacol. Biochem. Behav. 1985;23:37–42. doi: 10.1016/0091-3057(85)90127-3. [DOI] [PubMed] [Google Scholar]
- Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol. Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Khansa MR. Repeated administration of MK-801 produces sensitization to its own locomotor stimulant effects but blocks sensitization to amphetamine. Brain Res. 1991;562:164–168. doi: 10.1016/0006-8993(91)91202-c. [DOI] [PubMed] [Google Scholar]