Abstract
A major limitation to the application of stem-cell therapy to repair ischemic heart damage is the low survival of transplanted cells in the heart, possibly due to poor oxygenation. We hypothesized that hyperbaric oxygenation (HBO) can be used as an adjuvant treatment to augment stem-cell therapy. Therefore, the goal of this study was to evaluate the effect of HBO on the engraftment of rat bone-marrow-derived mesenchymal stem cells (MSCs) transplanted in infarct rat hearts. Myocardial infarction (MI) was induced in Fisher-344 rats by permanently ligating the left-anterior-descending coronary artery. MSCs, labeled with fluorescent superparamagnetic iron oxide (SPIO) particles, were transplanted in the infarct and peri-infarct regions of the MI hearts. HBO (100% oxygen at 2 ATA for 90 min) was administered daily for 2 weeks. Four MI groups were used: untreated (MI); HBO; MSC; MSC+HBO. Echocardiography, electro-vectorcardiography, and magnetic resonance imaging were used for functional evaluations. The engraftment of transplanted MSCs in the heart was confirmed by SPIO fluorescence and Prussian-blue staining. Immunohistochemical staining was used to identify key cellular and molecular markers including CD29, troponin-T, connexin-43, VEGF, α-smooth-muscle actin, and von-Willebrand factor in the tissue. Compared to MI and MSC groups, the MSC+HBO group showed a significantly increased recovery of cardiac function including left-ventricular (LV) ejection fraction, fraction-shortening, LV wall-thickness, and QRS vector. Further, HBO treatment significantly increased the engraftment of CD29-positive cells, expression of connexin-43, troponin-T and VEGF, and angiogenesis in the infarct tissue. Thus, HBO appears to be a potential and clinically-viable adjuvant treatment for myocardial stem-cell therapy.
Keywords: Mesenchymal stem cell, hyperbaric oxygen, myocardial infarction, stem-cell therapy
Introduction
Cell transplantation shows great promise for repair and restoration of heart function following myocardial infarction [1, 2]. However, the potential of cell-based therapy for myocardial tissue repair is limited by the death of transplanted cells, mostly within the first few days after transplantation in the infarct tissue, likely from a combination of ischemia (deprivation of nutrients and oxygen), inflammation, and apoptosis [3]. Several interventions, including the use of cells over-expressing pro-survival/anti-apoptotic proteins [4, 5], and pharmacological agents [6], have been reported to augment survival, function, and homing of the transplanted cells in the hostile ischemic environment [7]. Laflamme et al used a “cocktail” of pro-survival factors to inhibit death-promoting pathways and upregulate survival-signaling. They reported a higher proportion of engraftment when compared to simpler interventions [8]. Recently, we have shown the beneficial effect of preconditioning cells with trimetazidine, an anti-ischemic drug, prior to cardiac cell therapy [9]. Most of the above mentioned approaches involved manipulation of the cells prior to, or during, transplantation in the infarct heart. However, to date, no post-transplantation strategies have been considered for promoting cellular engraftment.
Hypoxia is a formidable factor in the ischemic tissue that can lead to the production of oxygen free radicals. The hostile environment with persistent oxidative stress ultimately leads to apoptosis of the majority of the transplanted cells. Therefore, we considered a straightforward physiological approach to reduce the severity of hypoxia by hyperoxygenating the infarct tissue following cell transplantation. Hyperbaric oxygenation (HBO) is a safe, clinically-viable treatment that has been used as a primary therapy in patients with decompression sickness, arterial gas embolism and carbon-monoxide poisoning [10]. It is also used as an adjuvant therapy to promote wound healing[11], and for the treatment of various conditions, including ischemic injury [12]. HBO involves inhalation of 100% oxygen under greater-than-one atmospheric absolute (ATA) pressure. Such doses of oxygen have a number of beneficial biochemical, cellular, and physiologic effects [13]. HBO, administered at the onset of reperfusion in an open-chest rabbit model of myocardial ischemia-reperfusion injury, showed a significant reduction in infarct size [14]. More recent studies have shown that HBO attenuates myocardial injury via nitric-oxide signaling [13], improves cardiac function in patients with acute myocardial infarction [15], and helps mobilization of stem cells by enhancing CXCR4 and VEGFR-2 in humans [16]. However, the efficacy of HBO as an adjuvant to cell therapy has not yet been studied.
We therefore hypothesized that HBO, when applied in conjunction with stem-cell therapy, would improve oxygenation in the infarct heart, leading to increased cell engraftment and cardiac function. Using a rat model of myocardial infarction, induced by permanent ligation of the left-anterior-descending (LAD) coronary artery, followed by transplantation of rat bone marrow-derived mesenchymal stem-cells (MSCs), we have demonstrated a substantial increase in cell engraftment, reduction in infarct size, recovery of myocardial function, restoration of electrophysiological normalcy, and angiogenesis in cell-transplanted hearts subjected to HBO treatment.
Materials and Methods
Reagents
Dulbecco’s Modified Eagle medium (DMEM), fetal bovine serum, penicillin, streptomycin, trypsin, sodium pyruvate, and phosphate-buffered saline (PBS) were purchased from Gibco BRL (Grand Island, NY). MTT (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) colorimetric assay kit, lactate dehydrogenase (LDH) assay kit, Nuclear Fast Red (Kernechtrot) solution were obtained from Sigma (St. Louis, MO). DAPI (4,6-diamino-2-phenylindole) was from Invitrogen. Superparamagnetic iron oxide (SPIO) particles (average size, 0.9 μm) were purchased from Bangs Laboratories (Fishers, IN). The particles contained magnetite cores encapsulated with styrene/divinyl benzene and coated with Dragon-green fluorophore.
Cell culture
Rat (Fisher 344) MSCs were procured from Chemicon (Billerica, MA). The cells were thawed and cultured using DMEM with GlutaMax (4500 mg glucose/l) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Accutase (Chemicon, Billerica, MA), a cell-detachment solution containing proteolytic and collagenolytic enzymes, was used for separation of adherent cells. MSCs of passage 2 were used for experiments. The cells were grown at 37°C in a humidified environment of 5% CO2/95% air.
Labeling of MSCs with SPIO particles
SPIO particles (0.9-μm diameter, 1.25×105/ml of medium; Bangs Laboratories, IN) were incubated with MSCs (1×106 cells in 15 ml medium) in a T75 flask for 24 hours. After incubation, the cells were washed off to remove the medium with un-internalized SPIOs and resuspended in fresh media. Images were taken using an inverted fluorescence microscope (Nikon TE2000, Japan) equipped with a FITC filter-set to confirm the presence of SPIO particles (Dragon-green fluorescence: λEx=480 nm, λEm=520 nm) in the cell.
Cytotoxicity studies
Cytotoxicity was measured on MSCs labeled with SPIO particles and kept in culture for 24 h. The following assays were performed as described [17]. Nuclear viability using PI-binding assay, mitochondrial viability using MTT assay, membrane damage using LDH assay, and cell proliferation using BrdU-incorporation assay. All assays were performed in 3 parallels and repeated 3 times.
Induction of MI
Fisher-344 rats (150–200g body weight; Charles River Laboratories) were used in the study. All the procedures were performed with the approval of the Institutional Animal Care and Use Committee of the Ohio State University and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 86-23, Revised 1996). MI was created by permanently occluding the left-anterior-descending coronary artery (LAD). An oblique 12-mm incision was made 8 mm away from the left sternal border toward the left armpit. The chest cavity was opened with scissors by a small incision (10-mm-long) at the level of the third or fourth intercostal space, 2 to 3 mm from the left sternal border. The LAD was visualized as a pulsating bright-red spike running through the midst of the heart wall from underneath the left atrium toward the apex. The LAD was ligated 2-mm below the tip of the left auricle using a tapered needle and a 6-0 polypropylene ligature passed underneath the LAD, and a double knot was made to occlude the LAD permanently. Occlusion was confirmed by a sudden change in color (pale) of the anterior wall of the LV and an ST elevation on the EKG trace (vide infra). Following transplantation of the cells (vide supra), the chest cavity was closed by bringing together the third and fourth ribs with 4-0 silk suture. The layers of muscle and skin were then closed with 4-0 polypropylene suture.
Transplantation of MSCs in the ischemic heart
MSCs were transplanted in the rat hearts after 30 min of LAD ligation. Three intramyocardial injections (one in the infarct and two in the peri-infarct regions) of MSCs labeled with SPIO using a total of 0.5×106 cells in 100 μl of serum-free medium were given to the infarct and peri-infarct regions of the hearts 30 min after LAD ligation.
HBO treatment
The rats were subjected to HBO treatment by placing them inside a custom-built small-animal hyperbaric chamber (Polyfab; Boston Plastics Manufacturing, Wilmington, MA) that was connected to a compressed gas cylinder containing 100% oxygen. HBO was administered daily (100% O2, 2 ATA, 90 min) for three days prior to cell transplantation. After a break for three days post-transplantation to allow the animals recover from surgical trauma, HBO was administered daily for 2 weeks. The animals were placed 1 per cage with up to 3 cages at a time in the chamber. After the chamber was closed, a fill-valve was opened to slowly allow the pressure within the chamber to reach 2 ATA (atmospheric pressure absolute) over 5–10 minutes. The valve was closed to seal the chamber and the rats were allowed to remain in the unit for the next 90 minutes under constant observation. After this period, a safety pressure-release valve was manually activated to slowly depressurize the unit to normal atmospheric pressure over the next 5–10 minutes. No adverse effect was noticed during the entire course of treatments.
Experimental groups
The following groups of rats (6 rats/group) were used: (i) MI only, where the animals received medium only; (ii) HBOT, where the animals received medium only and later subjected to HBO regimen; (iii) MSC, where the animals received MSCs (labeled with SPIO), but not subjected to HBO treatment; (iv) MSC+HBO, where the animals received MSCS (labeled with SPIO) followed by HBO regimen. In addition, experiments were done in rats (4 rats/group) to measure myocardial oxygenation in the non-infarct (sham) and infarct (MI) after HBO regimen.
Measurement of myocardial pO2 by EPR oximetry, in vivo
Myocardial pO2 was measured in the closed chest of rat hearts using in vivo EPR oximetry as described [9, 18]. Measurements were done at least 3 days after placement of the oximetry probe in the tissue to allow time for the animal to recover from the thoracic surgical procedures. The pO2 measurements were done before as well as immediately after HBO treatment in control (non-infarct) and infarct (MI) hearts.
Electrocardiography (EKG) measurements, in vivo
Animals were anaesthetized using isoflurane (1.5% in air) and placed on a heated gel pack (Braintree Scientific, Braintree, MA) designed to maintain normo-thermia during data acquisition. EKG recordings were made with adhesive electrodes attached to all four paws and chest with the rat in a supine position. Electrocardiograms were digitally recorded using a physiologic data acquisition system (PowerLab, AD Instruments, CO), with a sampling rate of 1 kHz. The EKGs were signal-averaged (from lead II) over 150–200 beats per acquisition for determination of the P wave duration, P–R interval, and QT interval.
Evaluation of the orientation of the mean QRS vector in the frontal plane was done by the average precession of the depolarization wave through the ventricles. For most mammals the orientation of the mean spatial QRS vector in the frontal plane is tail-ward, leftward and slightly dorsal. However, with occlusion of the LAD and subsequent death of cardiac tissue within the region, the left anterior region of the ventricular mass is electrophysiologically removed from the activation sequence, and the resulting procession is dominated by the left-ventricular free-wall, activated in a cranio-caudal (tail-ward) orientation. Thus, the orientation of the mean QRS in the frontal plane would be oriented tail-ward. The orientation of the QRS vector in all hearts was tracked over time to assess changes in the left-ventricular depolarization wave in the infarcted region.
Assessment of cardiac function by echocardiography, in vivo
M-mode transthoracic echocardiography was performed in rats as reported [19].
MR imaging of hearts, in vivo
The MRI imaging was performed using a 9.4 T horizontal-bore imaging system (Bruker BioSpin, Billerica, MA). Rats were anaesthetized initially with 3.5% isoflurane mixed with carbogen (1 liter/min) and maintained with 1–1.5% isoflurane during MR imaging. Physiologic parameters such as the EKG and respiration the mouse were monitored using a monitoring system (Model 1025, Small Animal Instruments, Stony Brook, NY). The EKG signal was obtained by placing subcutaneous EKG leads on the right forepaw and leg of the animal. A pneumatic pillow was used to monitor the respiration of the animal. For gated cardiac imaging, the animal was secured to an animal bed and placed at the center of the MRI scanner. Short-axis images of mouse heart were acquired so as to cover the whole left ventricle (FLASH sequence; parameters: TR = up to 180, TE = 1.4 ms; matrix = 256 × 256; FOV = 4.5 × 4.5 cm; slice thickness = 1 mm, 20 frames per cardiac cycle).
Assessment of fibrosis in cardiac tissue
Hearts were quickly excised, washed with cold PBS and fixed in formalin. After 12 hours, the heart sections were embedded in paraffin and left ventricular (LV) cross-sections from apex, mid-LV, and base were stained with Masson-trichrome. Images of left ventricular area (LVA) of each slide were taken by Nikon Model C-PS (objective × 20) with Spot Insight camera (Diagnostic). Fibrosis was defined as the sum of the epicardial and endocardial infarct circumference divided by the sum of the total LV epicardial and endocardial circumferences using computer-based planimetry Fibrosis and total LV area in each image was quantified using MetaVue image analysis software (Molecular Devices, Downingtown, PA). The percentage of the fibrosis was calculated as (fibrosis area/total LVA) × 100.
Prussian-blue staining of cells and cardiac tissue
Cells labeled with SPIO particles were identified using Prussian-blue staining. Cells were incubated for 30 minutes with 2% potassium ferrocyanide in 6% hydrochloric acid and then counterstained with Nuclear Fast Red. This indicates the presence of iron (blue color) within the cells. Similarly, in cardiac tissue sections, SPIO particles appeared as blue precipitates in the cytoplasm and pink color in the nucleus.
Immunohistochemical staining of cardiac tissue for CD29, α-SMA, vWF, and VEGF expression
Hearts were fixed in formalin and embedded in paraffin. Six-micron sections were cut and used for Masson-trichrome staining for fibrosis. For immunofluorescence staining, the fixed tissue sections were serially rehydrated in 100%, 95%, and 80% ethanol after deparaffinization with xylene. Slides were kept in steam for 30 min and then washed in PBS (pH 7.4) three times for 5 min each. The tissue sections were then incubated with 2% goat serum and 5% bovine-serum albumin in PBS to reduce nonspecific binding. The sections were then incubated for 4 h with mouse anti-CD29, anti-α-smooth muscle actin (α-SMA) or anti-von Willebrand factor (vWF) monoclonal antibody or anti-VEGF. The sections were then incubated with appropriate anti-mouse secondary antibodies (1:1000 dilution) conjugated to Texas red (CD29, α-SMA), FITC (vWF and VEGF). Nuclei were counterstained with hardest DAPI (Vector Labs). The tissue slides were visualized using an inverted Nikon fluorescence microscope. Separate sections were also stained without primary antibodies to examine nonspecific binding. Blood vessels staining positive for α-SMA and vWF were counted in both infarct and peri-infarct regions of the heart.
Immunohistochemistry for connexin-43 and troponin-T
Heart tissues were fixed in Histochoice fixative (Amresco, Solon, OH) and paraffin-embeded. Immunohistochemistry was performed as follows. Paraffin-embedded tissue sections were deparaffinized with xylene and gradually rehydrated with histograde ethanol. The presence of aldehydes in the fixative were removed by dipping the slides in 0.2-M glycine for 30 min followed by the treatment with peroxidazed-H2O2/methanol solution (Biocare Medical, Walnut Creek, CA) for 10 min to remove any endogenous peroxidase. Antigen retrieval was performed by placing the slides in a boiling sodium citrate buffer (10-mM sodium citrate, 0.05% Tween 20, pH 6) for 20 min and allowed the slides to cool for another 20 min in the same buffer. At the end of every step, the slides were washed three times in 1× PBS for 3 min each. Tissue sections were incubated overnight at 4°C with mouse anti-connexin 43 monoclonal antibody at a dilution of 1:300 (Millipore, Billercia, MA) or mouse monoclonal antibody to cardiac troponin-T at a concentration of 2 μg/ml (Abcam, Cambridge, MA), whereas negative control slides were incubated with antigen diluent buffer, Van-Gogh Yellow (Biocare Medical, CA). The slides were gently washed in PBS, and incubated with Alexa Flour-594 goat anti-mouse biotinylated secondary antibody for connexin-43 detection (Molecular Probes, CA) and Alexa Fluor-488 goat anti-mouse secondary antibody for the localization of troponin-T. For troponin-T localization after the second antibody incubation, the specimens were gently rinsed in 1× PBS, air-dried and mounting medium with DAPI (Vector labs, CA) was applied topically and slides were viewed under fluorescence microscope (Nikon TE 2000, Japan).
Data analysis
The statistical significance of the results was evaluated using ANOVA and a Student’s t-test. Repeated-measures ANOVA and Bonferroni t-test were used for multiple comparisons of pO2 data. The values were expressed as mean±SD. A p value of <0.05 was considered to be significant.
Results
Effect of SPIO labeling on MSCs
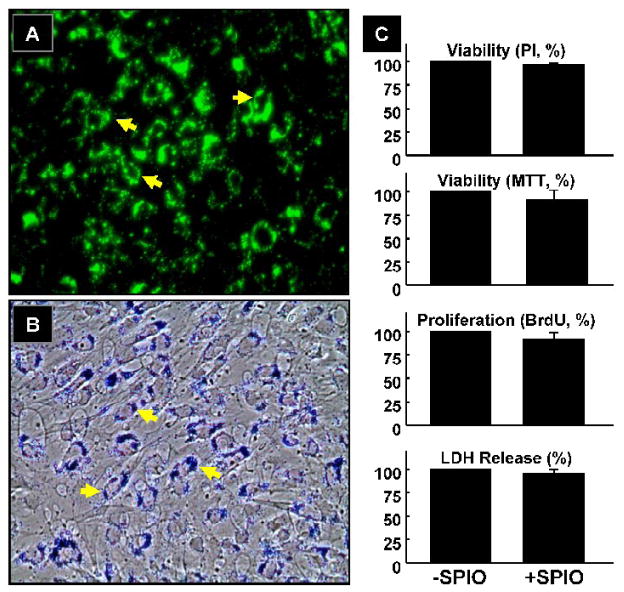
MSCs were labeled with Dragon-green fluorescence-conjugated SPIO particles by co-incubation in culture for 24 hours. Fluorescence microscopy and phase-contrast optical images confirmed uptake of SPIO particles by MSCs (Figure 1). The cells were then examined for possible cytotoxicity induced by the uptake of SPIO particles. The labeled cells were kept in culture for 24 hours, and subjected to standard cytotoxicity tests including PI-binding assay for nuclear viability, MTT assay for mitochondrial function, BrdU-incorporation assay for proliferation, and LDH-release assay for membrane integrity. The results (Figure 1C) showed that labeling of MSCs with SPIO had no significant effect upon cellular viability and proliferation.
Figure 1. Effect of SPIO labeling on MSCs.
Labeling was performed by incubating MSCs with SPIO particles (0.9 μm) conjugated with Dragon-green fluorescence microspheres for 24 hours in culture. (A) Fluorescence image (100x). (B) Phase-contrast image (100x) of MSCs stained with Prussian blue, a marker for iron. The SPIO particles are indicated by arrows. (C) Cytotoxicity as determined by PI-staining (nuclear viability), MTT assay (mitochondrial viability), anti-BrdU assay (proliferation), and LDH assay (membrane damage) in cells labeled with SPIO particles. No significant toxicity was observed after SPIO labeling.
Hyperbaric oxygenation and myocardial pO2, in vivo
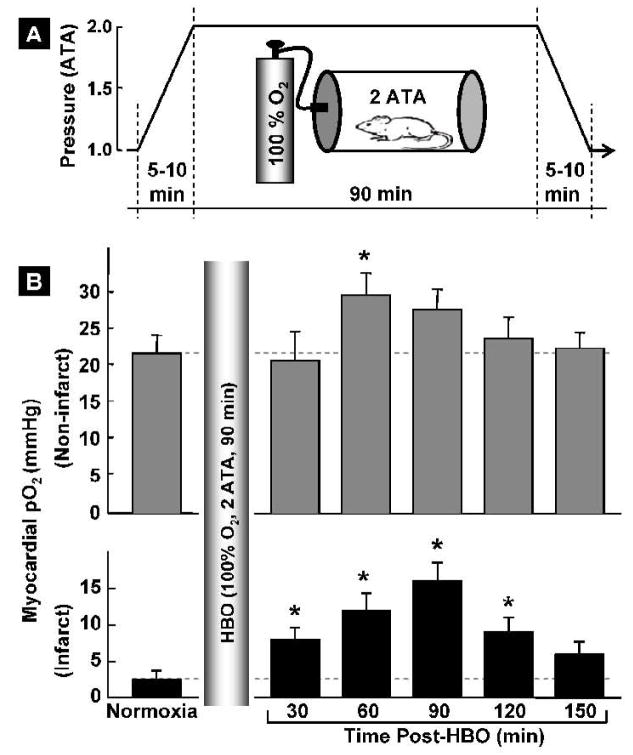
Myocardial pO2 was measured by in vivo EPR oximetry in healthy (non-infarcted) and infarcted hearts of rats subjected to HBO. The mean baseline pO2 in healthy hearts before HBO was 21.1±2.2 mmHg (Figure 2). Immediately following HBO, there was a decrease in myocardial pO2 (at 10 min, data not shown); however, the pO2 recovered and showed a significant increase, to 30.0±3.2 mmHg or 42.9% beyond the baseline level, at 60 min after termination of HBO. The pO2 level returned to baseline value in about two hours after HBO. Similarly, in HBO-treated infarct rat hearts the myocardial pO2 was substantially elevated; for example, at 90-min post-HBO the pO2 level was significantly higher (15.7±3.1 mmHg) compared to the baseline (2.7±0.8 mmHg). The myocardial pO2 remained significantly elevated up to 2 h in the infarcted region after HBO treatment. The pO2 results indicated that exposure of rats to HBO temporarily enhanced myocardial oxygenation in the normal and infarcted myocardium
Figure 2. Hyperbaric oxygenation and myocardial pO2, in vivo.
(A) The HBO protocol used 5-min compression, 90-min maintenance of hyperbaric oxygenation (100% O2 at 2 ATA), followed by 5-min decompression using a custom-built small-animal chamber. (B) Myocardial pO2 in (non-infarcted and infarct) rat hearts measured before (normoxia) and after HBO. Data represent mean±SD obtained from 4 rats. The results show an increase in oxygenation levels reaching significance at 60 min followed by returning to baseline in about 2.5 hours in the non-infarcted hearts. In the infarcted hearts the oxygenation levels are significantly higher up to 2 hours post-treatment. It is interesting to note that a 5.8-fold increase in oxygenation is achieved in the infarct hearts after 90 min of HBO.
Left-ventricular cardiac function in MI hearts treated with MSC and HBO
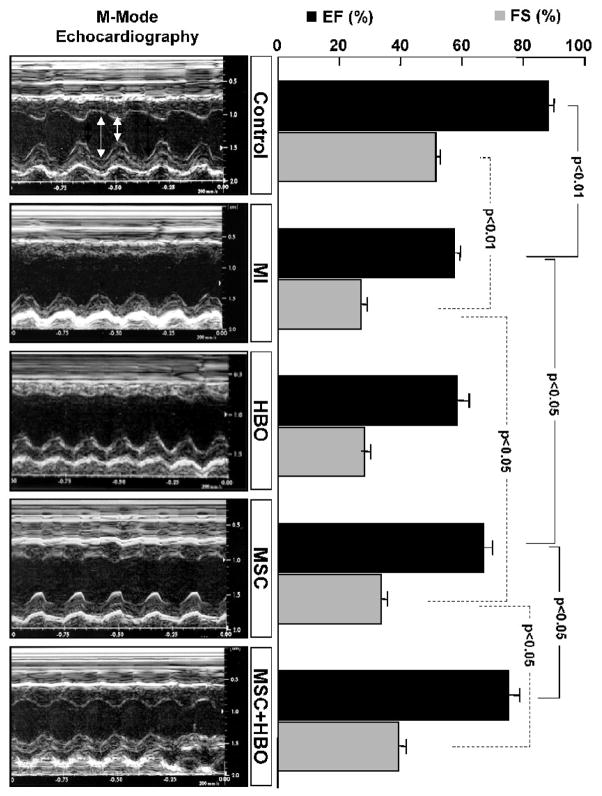
M-mode echocardiography was performed in rats at 2 weeks after treatment of infarcted hearts with MSC alone, or MSC followed by HBO. The results, LV ejection fraction (EF) and fraction shortening (FS), are summarized in Figure 3. There was a significant decrease in both EF and FS in the MI group when compared to control group (p<0.01). MSC treatment significantly restored the cardiac functions. However, HBO by itself had no significant effect on the recovery. In contrast, HBO in combination with MSC treatment significantly improved both the functions when compared to MSC-alone treatment. The echocardiography results indicated that HBO augmented the recovery of cardiac function by MSC treatment.
Figure 3. Left-ventricular cardiac function as measured by M-mode echocardiography in MI hearts treated with MSC and HBO.
Transthoracic echocardiography was used to measure cardiac function in hearts 2 weeks after transplanted with MSCs. Representative M-mode echocardiograms obtained from hearts with no infarction (Control), infarction (MI), infarction subjected to HBO treatment (HBO), infarction subjected MSC transplantation (MSC), and infarction subjected MSC and HBO treatment (MSC+HBO). Shown in the right panel are mean±SD values of LV ejection fraction (EF) and fractional shortening (FS) obtained from 6 hearts. The MSC+HBO group shows a significantly improved functional recovery when compared to MSC group.
Ventricular depolarization in MI hearts treated with MSC and HBO
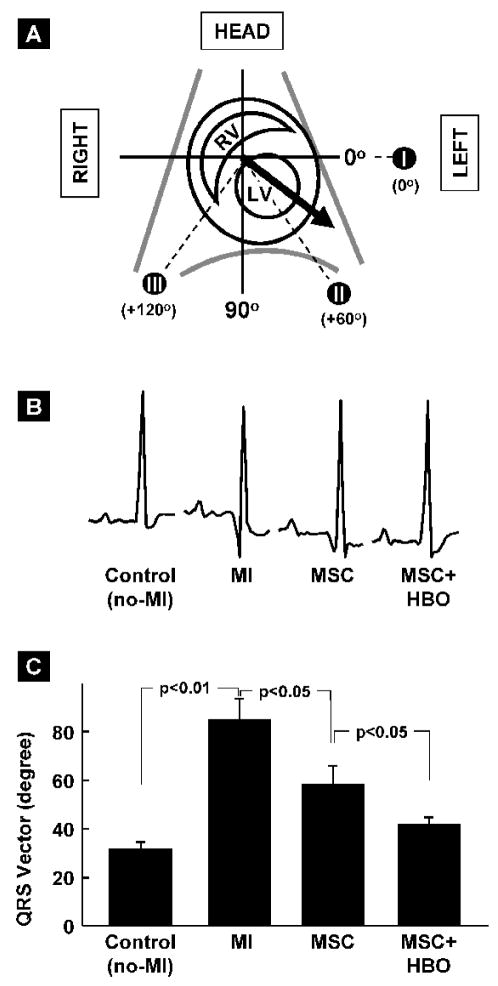
The QRS vector is used as a measure of abnormalities in ventricular depolarization that may occur as result of loss of functional cardiomyocytes in MI heart [20]. It represents the net orientation of electrical impulse propagation during ventricular depolarization. The mean QRS vector is obtained from signal averaged QRS complex EKG data. A schematic illustration of the QRS vector with reference to cardiac anatomy and traditional reference axis system is shown in Figure 4A. The values of mean QRS vector obtained from the different group of hearts are shown in Figure 4B. The mean QRS vector in non-infarct control hearts was 31.7±2.9°. A very significant right-shift was observed in MI hearts (85.1±8.9°; p<0.01 versus Control). While MSC treatment significantly blunted the right-shift (58.4±7.4°; p<0.5 versus MI), MSC followed by HBO showed further restoration of the QRS vector (41.6±3.0°; p<0.05 versus MSC) towards electrophysiological normalcy. The vectorcardiography results indicated that HBO worked in conjunction with MSCs to protect MI hearts by normalizing ventricular depolarization and restoring electrical integrity.
Figure 4. Ventricular depolarization as measured by vectorcardiography (VCG) in MI hearts treated with MSC and HBO.
Signal-averaged QRS vector (mean electrical axis) in the frontal plane of rat hearts was obtained from EKG data measured (see methods for a description) 2 weeks after MSC transplantation. (A) Schematic representation of the QRS vector (bold arrow) in the context of heart chambers and axial reference system (I, II & III). (B) Representative signal-averaged EKG tracings (Lead II) after MSC transplantation at 2 weeks. (C) Orientation of QRS vector in the hearts of treatment groups, as indicated. Data represent mean±SD obtained from 6 hearts. MI hearts show a very significant rightward deviation from Control hearts. MSC+HBO group shows significantly increased leftward shift (restoration) when compared to MSC group
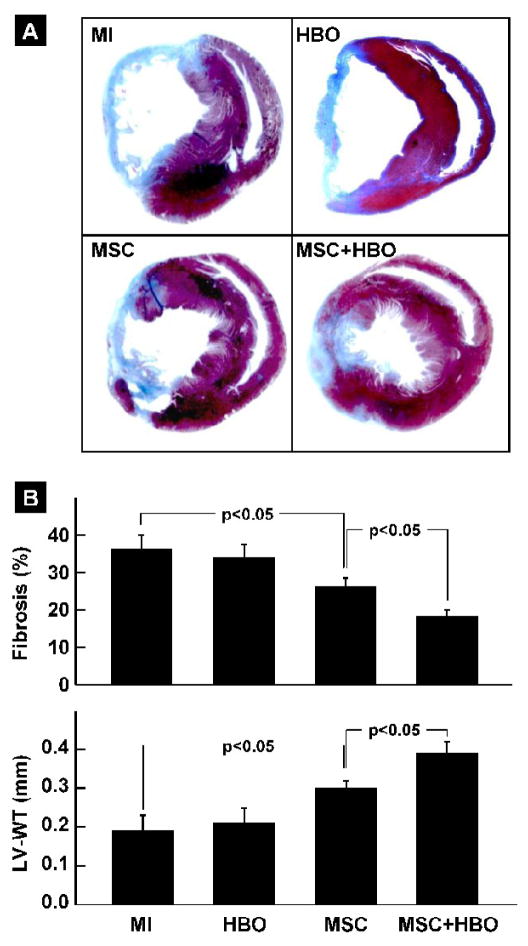
Cardiac tissue fibrosis and remodeling in MI hearts treated with MSC and HBO
The effect of HBO and MSC transplantation on myocardial fibrosis and left-ventricular wall thickness (LV-WT) was assessed in hearts excised 2 weeks after stem-cell therapy. The results (Figure 5) showed a significant reduction in tissue fibrosis and recovery of LV-WT in both the MSC and MSC+HBO groups when compared to MI group. Combined treatment of MSC and HBO resulted in a significant attenuation of fibrosis and restoration of LV-WT when compared to MSC alone treatment.
Figure 5. Cardiac tissue fibrosis and remodeling in MI hearts treated with MSC and HBO.
Masson-trichrome staining of heart sections was performed in infarcted hearts (MI), and infarcted hearts treated with HBO (HBO), MSC (MSC) or MSC followed by HBO (MSC+HBO) at 2 weeks after transplantation of MSCs. (A) Representative images of heart sections stained with Masson-trichrome. (B) Percentage of fibrosis and LV wall thickness (LV-WT), as determined by computer planimetry. Data represent mean±SD obtained from 6 hearts per group. The MSC+HBO group exhibits a significant reduction in the fibrosis and improvement in LV-WT when compared to MSC group.
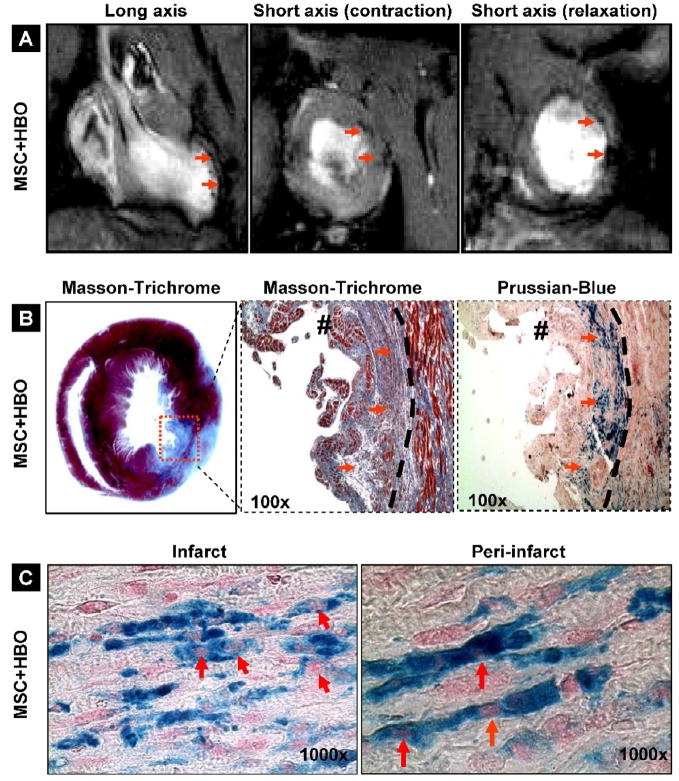
Engraftment of MSCs in the infarcted hearts
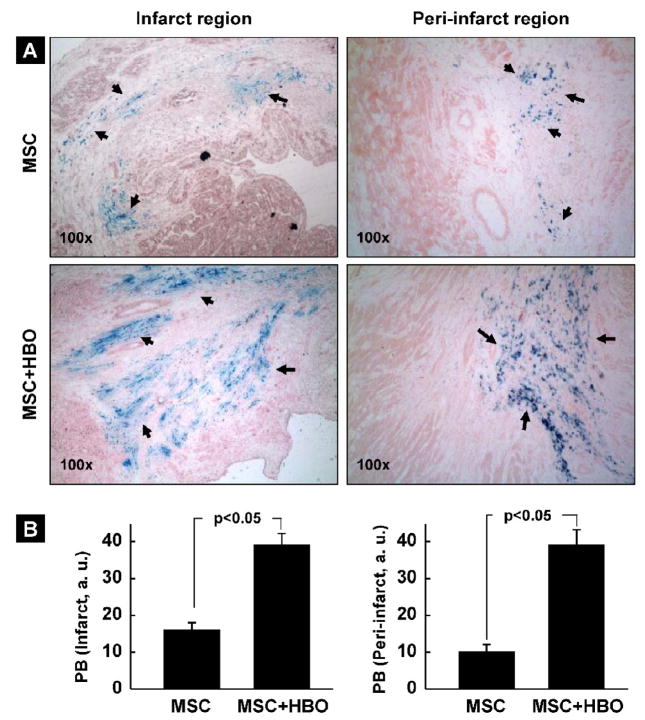
The retention and distribution of SPIO-labeled cells in the infarct heart at 2 weeks post-transplantation was verified by in vivo MR imaging as well as by histochemical staining using Prussian blue. Two weeks after intramyocardial injection of MSCs labeled with SPIO. MR imaging was performed using a 9.4 T MRI system. Large, hypointense areas were detected at the site where SPIO-labeled MSCs were injected (Figure 6A). Masson-trichrome staining (for fibrosis) and Prussian-blue staining (for SPIO) in the heart tissue harvested 2 weeks post-transplantation showed positive staining for engrafted cells (Figure 6B). The images further revealed a substantial distribution of the Prussian-blue-stained cells in the peri-infarct region as well (Figure 6C). We estimated the relative amounts of engrafted cells in the infarct and peri-infarct regions in the MSC and MSC+HBO group of hearts. Figure 7A shows representative histological slides stained with Prussian blue. A substantial increase in the staining was observed in both the infarct and peri-infarct regions of the MSC+HBO group. Quantitative analysis of the Prussian-blue staining revealed significantly higher levels of cell engraftment up on HBO treatment (Figure 7B). Taken together, the in vivo MRI and histology results clearly suggested engraftment of the transplanted cells in the infarcted heart and further that HBO enhanced cell engraftment both in the infarct as well peri-infarct regions. However, the above results do not identify the type of cell being stained.
Figure 6. Engraftment of MSCs in the infarcted hearts at 2 weeks after transplantation.
(A) MR images of a rat heart transplanted with SPIO-labeled MSCs. The images were acquired as described in the methods section. The long- and short-axis images show hypointense (black) regions (pointed by arrows) indicating the distribution of the SPIO particles in the LV wall. (B) Masson-trichrome staining showing fibrosis and Prussian-blue staining showing the engraftment of SPIO-labeled cells (arrows) in the infarct heart. The dashed curve shows the demarcation between infarct (fibrotic) and peri-infarct regions. (C) Prussian-blue staining in the infarct and peri-infarct regions showing cells (blue) and nucleus (pink, indicated by arrows). The results indicate a substantial engraftment of the SPIO-labeled cells in the infarct and peri-infarct regions.
Figure 7. Effect of HBO on the engraftment of MSCs in the infarcted hearts at 2 weeks after transplantation.
Prussian-blue staining was used to image MSCs labeled with SPIO in the tissue sections obtained 2 weeks after transplantation. (A) Representative histological images showing Prussian-blue (PB) staining (indicated by arrows) for cells in the infarct and peri-infarct regions of MSC and MSC+HBO groups. (B) Quantification of PB staining in the infarct and peri-infarct regions of the heart. Data represent mean±SD obtained from 6 hearts. The staining is significantly increased in the MSC+HBO group when compared to MSC group suggesting that HBO has significantly enhanced cell retention/survival in the heart.
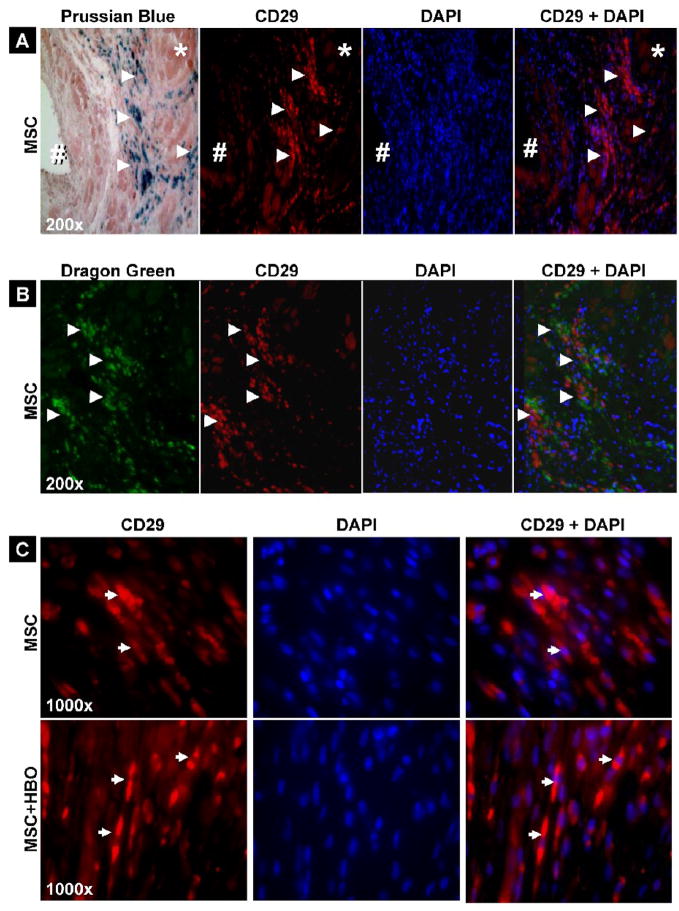
Identification of CD29-positive cells in the infarct hearts transplanted with MSCs
To identify whether the cells stained by Prussian blue (vide supra) are of MSC-type, we used immunohistochemical staining for CD29 (integrin β1, a specific marker for MSC) in heart tissue sections obtained 2 weeks after transplantation of MSCs labeled with SPIO (tagged with Dragon-green microspheres). Most of the cells stained CD29-positive were clearly identified in the tissue sections which correlated well with the Prussian-blue staining (Figure 8A) and Dragon-green fluorescence (Figure 8B). We further noted that the significantly higher levels of engrafted cells observed up on HBO treatment (vide supra) were CD29-expressing cells (Figure 8C). The co-imaging results clearly demonstrated the presence of MSC-type cells in the infarct hearts at 2 weeks post-transplantation. The results, however, do not preclude possible other transformations occurring to the transplanted cells.
Figure 8. CD29-positive cells in the infarct hearts 2 weeks after MSC transplantation.
Prussian-blue staining or Dragon-green fluorescence image was compared with DAPI and CD29-positive staining for identification of cells and their origin. (A) Prussian-blue staining is shown (separately) with CD29 and DAPI overlay images. (B) Overlay of Dragon-green fluorescence, CD29-positive, and DAPI images correlated with SPIO-labeled MSCs in cardiac tissue. (C) Overlay of CD29-positive and DAPI images obtained from hearts treated with MSCs or MSCs followed HBO. The images show that CD-29-positive cells in the cardiac tissues. Arrow heads indicate CD-29 positive cells and SPIO-labeled MSCs in corresponding panels.
Expression of connexin-43 by the engrafted cells in the infarct heart
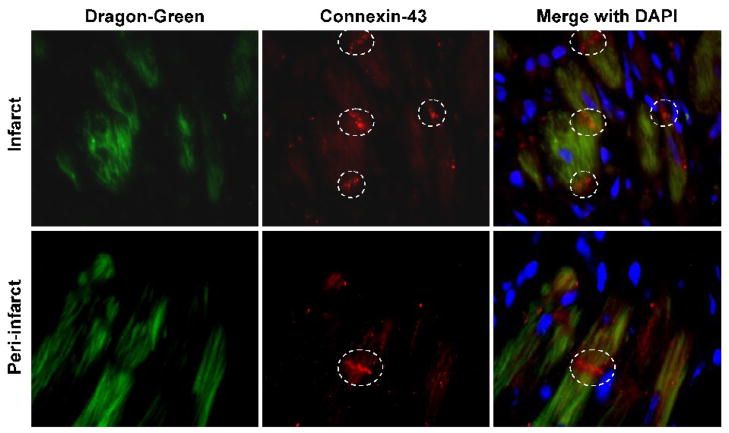
We next examined whether the CD29-positive cells expressed cardiac muscle-specific protein connexin-43 in the infarct heart. Connexin-43 is the major gap-junction protein whose expression in the engrafted stem cells is important to the restoration electrophysiological normalcy in the infarct heart. Connexin-43 protein in the heart tissues obtained at 2-weeks post-MI was imaged by immunofluorescence and merged with SPIO Dragon-green fluorescence image (Figure 9). SPIO-labeled MSCs expressed connexin-43 protein in both the infarct and peri-infarct regions of the heart. These results support the electrocardiographic vector analysis findings which showed increased recovery of QRS vector in the MSC+HBO group.
Figure 9. Expression of connexin-43 in the engrafted cells 2 weeks after transplantation.
Overlay images of Dragon-green fluorescence (green), connexin-43 immunofluorescence (red), and DAPI staining (blue) in the infarct and peri-infarct sections of a heart from MSC+HBO group. White broken circles indicate positive staining for connexin-43 expression.
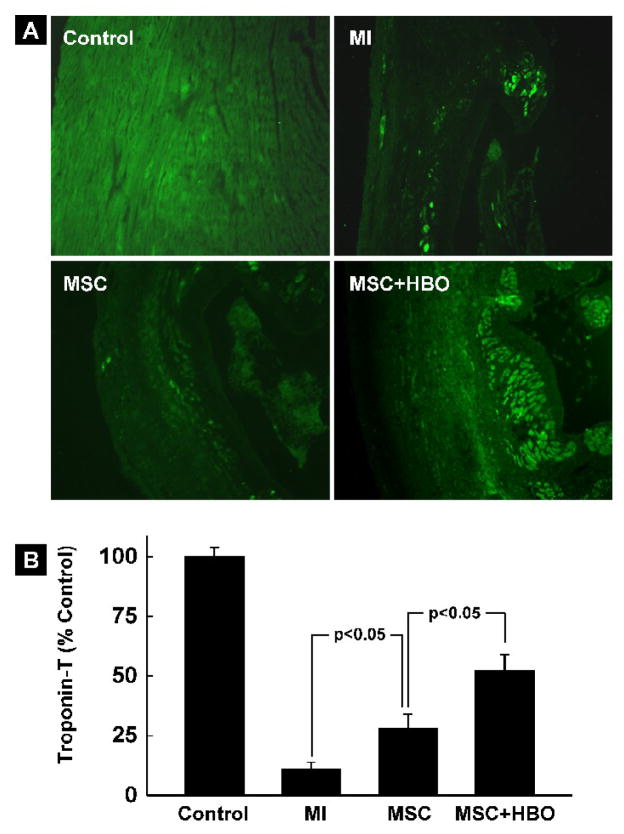
Cardiac troponin-T in the MI hearts treated with MSC and HBO
Troponin-T is a cardiac-specific contractile protein which is implicated in the functional impairment of ischemic hearts. In order to evaluate whether the enhanced functional recovery of MI hearts treated with MSC and HBO was associated with the troponin-T levels in the heart, we performed immunohistochemical analysis using a troponin-T antibody. Figure 10A shows troponin-T fluorescence (green) image in tissue sections from non-infarct (Control), MI, MSC and MSC+HBO group of hearts. The results (Figure 10B) show substantially increased levels of troponin-T in hearts treated with MSC and MSC+HBO when compared to MI hearts.
Figure 10. Cardiac troponin-T expression in the MI heart at 2 weeks after transplantation of MSC.
(A) Overlay images of anti-troponin-T immunofluorescence (green) and DAPI staining (blue) in heart sections in the experimental groups including a non-MI (Control) group. (B) Quantification of troponin-T levels in the cardiac tissues. Data represent mean±SD from 4hearts per group. The troponin-T level is significantly higher in the MSC+HBO group when compared to the MSC group.
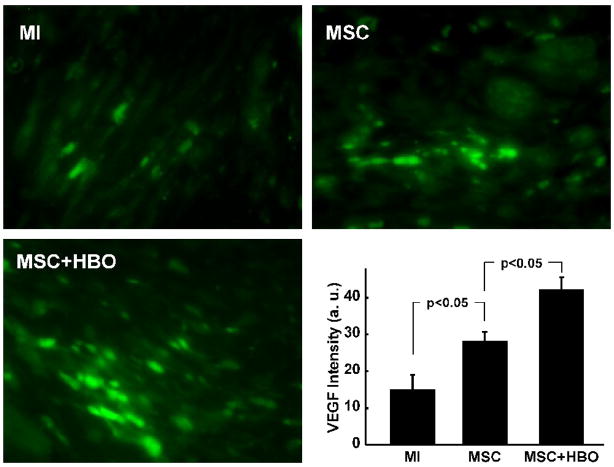
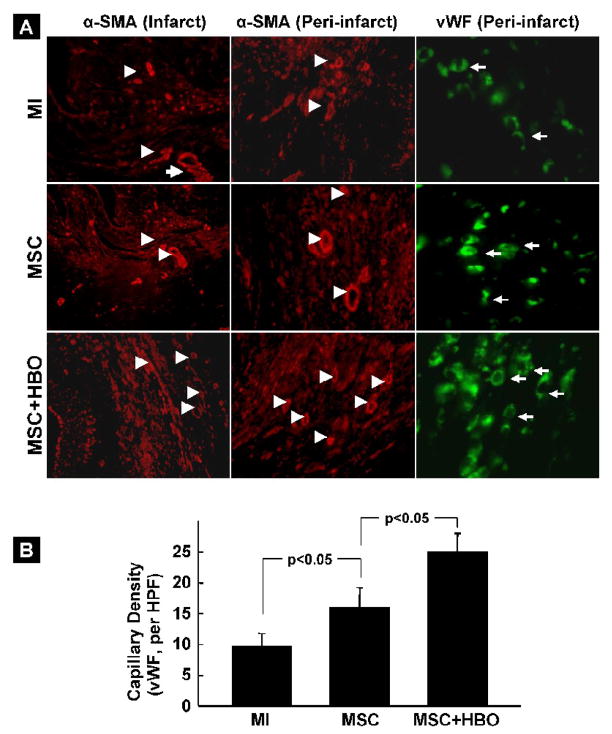
VEGF expression and angiogenesis in the heart tissue after MSC transplantation
Immunostaining for VEGF expression was performed in heart sections at 2 weeks post-MSC transplantation. The images showed higher levels of VEGF expression in the infarct tissue of the MSC and MSC+HBO groups when compared to the MI group (Figure 11). The VEGF expression was significantly higher in the MSC+HBO group when compared to MSC group. Since the increased VEGF expression group may enhance angiogenesis and new blood vessel formation in the HBO-treated hearts, we further evaluated the blood vessels and capillary density in heart sections at 2 weeks post-MSC transplantation. Cardiac tissue sections were stained using anti-α-SM-actin and anti-vWF antibodies to identify mature blood vessels and microcapillaries, respectively. The results showed increased vasculogenesis and microcapillary formation in the MSC+HBO group (Figure 12), in both infarct and peri-infarct areas, when compared to the MI and MSC groups.
Figure 11. VEGF expression in the infarcted heart tissues 2 weeks after MSC transplantation.
Heart sections were stained with a VEGF antibody to identify the VEGF expression. Shown are VEGF immunofluorescence images (green) in sections from MI, MSC, and MSC+HBO hearts and quantitative assessment of the VEGF fluorescence intensity. Data represent mean±SD obtained from 3 hearts. The VEGF level is significantly higher in the MSC+HBO group when compared to the MSC group.
Figure 12. Blood vessel and capillary densities in the infarct hearts 2 weeks after MSC transplantation.
Blood vessels and capillaries in the tissues sections were imaged by using antibodies for α-smooth-muscle actin (α-SMA) and von Willebrand Factor VIII (vWF), respectively. (A) Representative immunofluorescence images of tissue sections showing α-SMA (red, arrow heads) and vWF (green, arrows) in MI, MSC, and MSC+HBO groups. (B) Quantitative assessment of the total number of capillaries in the peri-infarct region of the heart. The images show a significantly increased number of capillaries in the peri-infarct region of MSC+HBO group when compared to MSC group.
Discussion
The results of the present study indicated the beneficial effects of HBO treatment in enhancing the engraftment of MSCs transplanted into an ischemic heart, leading to significant improvements in cardiac function, restoration of electrophysiological normalcy, and increased angiogenesis. MSCs are pluripotent, adult stem cells residing primarily within the bone marrow. Under in vitro conditions, MSCs can be induced to differentiate into cardiomyocyte-like cells by treating with 5-azacytidine [21]. MSCs have been shown to be capable of engraftment in host myocardium, expression of muscle-specific proteins, and attenuation of contractile dysfunction and pathologic thinning in a swine model of left-ventricular wall infarction [22]. Autologous bone marrow-derived MSCs used in a rat model of hind-limb ischemia induced therapeutic angiogenesis through VEGF production [23]. Orlic et al. showed the therapeutic potential of intramyocardially-delivered bone marrow-derived cells which were hematopoietic lineage-negative and c-kit-positive. These cells provided not only a functional benefit, but appeared to generate de novo myocardium in the scar area [24]. Our results using rat bone marrow-derived MSCs clearly established the engraftment of CD29-positive cells expressing cardiac markers, including troponin-T and connexin-43, and induction of angiogenesis through VEGF production. The engraftment and differentiation of MSCs were markedly augmented by adjuvant HBO.
HBO is emerging as a potential therapy for cardiovascular diseases [12]. In a randomized multicenter study using patients with acute MI, adjunctive HBO with thrombolytic agents (recombinant tissue plasminogen activator or streptokinase) resulted in a decrease in creatine phosphokinase levels, rapid resolution of pain and an improvement in ejection fraction [15, 25]. Preconditioning of rats with HBO (2.5 ATA, 60 min, twice daily for two days) has been shown to alleviate the injury to ischemic myocardium induced by LAD coronary artery ligation [26]. The results of the present study did not show any significant effect of HBO on cardiac function (Figure 3) or infarct size (Figure 5) measured 2 weeks after induction of infarction. Tritto et al. used a rat model of permanent coronary artery occlusion to show that HBO could only delay the progression of ischemic cell death, without affecting the ultimate extent of necrosis measured after 3 weeks [27]. Our results also seemed to indicate that HBO alone could not provide any beneficial effect in the long term, although HBO was administered throughout the two-week period.
HBO significantly increased the recovery of cardiac function and reduced infarct size in hearts transplanted with MSCs. The beneficial effects of adjuvant HBO were significantly greater than that provided by MSCs alone. The synergistic effect could stem from a HBO-mediated increase in CD29-positive graft survival (Figure 8), leading to an increase in VEGF production (Figure 11) and angiogenesis (Figure 12). Hyperbaria, as well as hyperoxia, have been shown to limit infarct size and attenuate ischemia-reperfusion injury [14, 28]. Pre-exposure of rats to one-time, 60-min hyperbaric oxygenation, which includes both hyperoxia and hyperbaria, was shown to be protective of hearts isolated and then subjected to ischemia-reperfusion injury [29]. The study concluded that the protective effect was mediated by nitric oxide synthase and dependent on the oxygen availability (partial pressure of oxygen) rather than hyperbaria alone. In contrast, in our present study using in vivo hearts we did not observe any significant reduction in infarct size in the HBO group. The difference could be attributed to the long period (2 weeks) after the induction of MI in our study. However, when HBO was used as an adjuvant with stem-cell therapy, we observed a significant reduction in myocardial fibrosis and wall thinning.
The HBO-mediated recovery of the QRS vector in the MSC+HBO group of hearts (Figure 4) is reflective of connexin-43 expression in the grafted cells (Figure 9). Taken together, the results seem to suggest that HBO works in conjunction with transplanted MSCs, although by itself may not be beneficial in the long term. It has been reported that HBO mobilized bone marrow-derived stem/progenitor cells in humans and animals by stimulating nitric oxide synthase and that this mobilization of cells remained elevated in human blood over the course of 20 HBO treatments [16]. While the contribution of a similar effect to mobilize endogenous stem cells by HBO cannot be ruled out in our model, the observed results do not suggest any significance in their contribution.
We measured myocardial tissue oxygenation by implanting an oxygen-sensitive EPR probe in the myocardium as reported previously [18, 19]. Immediately after HBO administration, there was a substantial increase in myocardial oxygenation in the infarct hearts, and it remained significantly elevated up to 2.5 h following termination of the HBO session. Although the magnitude (absolute value) of increase was similar to that in non-infarct hearts, the hyperoxygenated infarct hearts showed a 5.8-fold increase when compared to the normoxic baseline. The in vivo oximetry measurements clearly established that HBO increases myocardial tissue oxygenation in the infarct tissue. The observed improvements in cell engraftment and cardiac function indicated by HBO suggest that the hyperoxic post-conditioning could play a major role in enhancing graft survival and retention.
There is an increasing body of evidence that MSCs transfected with VEGF improves cardiac function and increases angiogenesis in the infarct heart [30]. In addition to its potent angiogenic functions, VEGF can stimulate proliferation, delay senescence, suppress apoptosis and promote survival of various cells [31]. Different oxygen tensions appear to activate different signaling pathways to stimulate VEGF expression for angiogenesis [32]. Hypoxia enhances reactive oxygen species (ROS) production, which potently induces VEGF expression via a hypoxia-inducible factor-1 (HIF-1)-independent pathway [32]. A recent study showed that HBO reduces liver injury in regenerating rat liver after a partial hepatectomy through enhanced VEGF expression [33]. Similarly, the results from our study show that there was a significant increase in VEGF expression in the MSC+HBO hearts at 2 weeks post-transplantation. A possible mechanism responsible for the increased angiogenesis observed in the MSC+HBO group in our study could be this increase in VEGF expression. Thus, there is strong evidence to support our contention that HBO, used in conjunction with MSC cardiomyoplasty, enhances the engraftment and survival of transplanted cells through increased VEGF expression and angiogenesis.
Electrocardiography remains one of the most important and non-invasive means of identifying and quantifying the severity of myocardial infarction A typical frontal plane QRS loop for carnivores and primates is oriented inferiorly and leftward, because the prevailing portion of the QRS loop is produced by waves of depolarization traversing the normally-dominant left-ventricular free-wall in an endocardial to epicardial direction. When a left anterior descending coronary artery is ligated, an anteroseptal infarct is produced, and that region of myocardium does not contribute to the QRS loop. In the rat viewed from the front (from the ventral surface of the torso of a quadruped) surface, the QRS loop would be displaced from the left, caudal (tail-ward) surface of the torso. Thus, instead of the QRS loop being inscribed leftward and caudal in a usually clockwise manner, its direction of inscription becomes counter-clockwise having been deviated from the region of infarction. If the regenerated tissue is capable of conducting cardiac electrical signals it will become depolarized and the orientation of the QRS loop tends to return towards electrophysiologic normalcy. The significant restoration of the mean QRS vector orientation in the MSC+HBO group of hearts (Figure 4) indicates not only the regenerative capability of transplanted stem cells, but also their capability to express connexin-43 to restore electrical integrity in the infarct myocardium.
The differentiation of MSCs into fully-functional cardiomyocytes is still controversial. A recent study by Haider, et al. showed that MSCs have the ability to engraft in the ischemic heart and differentiate to adopt a cardiac phenotype [34]. The differentiating MSCs showed a spectrum of morphological changes that were characteristic of developing myofibers. Immunostaining for connexin-43, along with cardiac actin showed that the neofibers also developed intercellular communication junctions [34]. The tendency of MSCs to display plasticity towards a cardiac lineage has been reported [35]. Bone marrow-derived MSCs, co-cultured with rat embryonic cardiomyocytes, exhibited expression of troponin-T, troponin-I, α-actin, and connexin-43 [35]. The induction of MSCs towards the cardiomyocyte lineage was attributed to local cell-to-cell contacts and/or the presence of soluble factors in the culture medium. Similarly, our study demonstrated that the engrafted MSCs expressed both troponin-T and connexin-43. Connexin-43 expression was mostly observed in MSCs present in the peri-infarct regions of the heart. The tendency towards connexin-43 expression was relatively higher in the MSC+HBO group.
Overall, the present study demonstrated that HBO could serve as an effective adjuvant to augment MSC-based cardiomyoplasty. HBO treatment significantly enhanced the survival of transplanted MSCs in the infarct heart, leading to improvements in cardiac function. In addition, MSC transplantation, along with HBO, resulted in increased VEGF and connexin-43 expressions, as well as angiogenesis in the ischemic tissue. Thus, HBO appears to be an effective and clinically-applicable method to improve the survival and retention of stem cells used in the treatment of myocardial infarction and/or heart failure, thereby improving therapeutic efficacy and overall clinical outcome.
Acknowledgments
This study was supported by grants from AHA (SDG 0930181N to MK) and NIH (R01 EB006153 to PK).
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- ECHO
Echocardiography
- EF
Ejection fraction
- EKG
Electrocardiography
- EPR
Electron paramagnetic resonance
- HBO
Hyperbaric oxygenation
- LAD
Left anterior descending coronary artery
- MI
Myocardial infarction
- MRI
Magnetic resonance imaging
- MSC
Mesenchymal stem cell
- SPIO
Superparamagnetic iron oxide
- VCG
Vectorcardiography
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murry CE, Field LJ, Menasche P. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112:3174–83. doi: 10.1161/CIRCULATIONAHA.105.546218. [DOI] [PubMed] [Google Scholar]
- 2.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–16. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 3.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–81. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–27. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 5.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 6.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–42. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 7.Seeger FH, Zeiher AM, Dimmeler S. Cell-enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med. 2007;4 (Suppl 1):S110–3. doi: 10.1038/ncpcardio0734. [DOI] [PubMed] [Google Scholar]
- 8.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 9.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, et al. Pharmacological preconditioning of mesenchymal stem cells with Trimetazidine protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Therap. 2009 doi: 10.1124/jpet.109.150839. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–8. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- 11.Thackham JA, McElwain DL, Long RJ. The use of hyperbaric oxygen therapy to treat chronic wounds: A review. Wound Repair Regen. 2008;16:321–30. doi: 10.1111/j.1524-475X.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 12.Yogaratnam JZ, Laden G, Madden LA, Seymour AM, Guvendik L, Cowen M, et al. Hyperbaric oxygen: a new drug in myocardial revascularization and protection? Cardiovasc Revasc Med. 2006;7:146–54. doi: 10.1016/j.carrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Yogaratnam JZ, Laden G, Guvendik L, Cowen M, Cale A, Griffin S. Pharmacological Preconditioning With Hyperbaric Oxygen: Can This Therapy Attenuate Myocardial Ischemic Reperfusion Injury and Induce Myocardial Protection via Nitric Oxide? J Surg Res. 2007 doi: 10.1016/j.jss.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Sterling DL, Thornton JD, Swafford A, Gottlieb SF, Bishop SP, Stanley AW, et al. Hyperbaric oxygen limits infarct size in ischemic rabbit myocardium in vivo. Circulation. 1993;88:1931–6. doi: 10.1161/01.cir.88.4.1931. [DOI] [PubMed] [Google Scholar]
- 15.Dekleva M, Neskovic A, Vlahovic A, Putnikovic B, Beleslin B, Ostojic M. Adjunctive effect of hyperbaric oxygen treatment after thrombolysis on left ventricular function in patients with acute myocardial infarction. Am Heart J. 2004;148:E14. doi: 10.1016/j.ahj.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290:H1378–86. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- 17.Wisel S, Chacko SM, Kuppusamy ML, Pandian RP, Khan M, Kutala VK, et al. Labeling of skeletal myoblasts with a novel oxygen-sensing spin probe for noninvasive monitoring of in situ oxygenation and cell therapy in heart. Am J Physiol Heart Circ Physiol. 2007;292:H1254–61. doi: 10.1152/ajpheart.01058.2006. [DOI] [PubMed] [Google Scholar]
- 18.Khan M, Kutala VK, Wisel S, Chacko SM, Kuppusamy ML, Kwiatkowski P, et al. Measurement of oxygenation at the site of stem cell therapy in a murine model of myocardial infarction. Adv Exp Med Biol. 2008;614:45–52. doi: 10.1007/978-0-387-74911-2_6. [DOI] [PubMed] [Google Scholar]
- 19.Khan M, Kutala VK, Vikram DS, Wisel S, Chacko SM, Kuppusamy ML, et al. Skeletal myoblasts transplanted in the ischemic myocardium enhance in situ oxygenation and recovery of contractile function. Am J Physiol Heart Circ Physiol. 2007;293:H2129–39. doi: 10.1152/ajpheart.00677.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamlin RL. The QRS electrocardiogram, epicardiogram, vectorcardiogram and ventricular excitation of swine. Am J Physiol. 1960;198:537–42. doi: 10.1152/ajplegacy.1960.198.3.537. [DOI] [PubMed] [Google Scholar]
- 21.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–25. doi: 10.1016/s0003-4975(02)03517-8. discussion 26. [DOI] [PubMed] [Google Scholar]
- 23.Al-Khaldi A, Al-Sabti H, Galipeau J, Lachapelle K. Therapeutic angiogenesis using autologous bone marrow stromal cells: improved blood flow in a chronic limb ischemia model. Ann Thorac Surg. 2003;75:204–9. doi: 10.1016/s0003-4975(02)04291-1. [DOI] [PubMed] [Google Scholar]
- 24.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 25.Stavitsky Y, Shandling AH, Ellestad MH, Hart GB, Van Natta B, Messenger JC, et al. Hyperbaric oxygen and thrombolysis in myocardial infarction: the ‘HOT MI’ randomized multicenter study. Cardiology. 1998;90:131–6. doi: 10.1159/000006832. [DOI] [PubMed] [Google Scholar]
- 26.Han C, Lin L, Zhang W, Zhang L, Lv S, Sun Q, et al. Hyperbaric oxygen preconditioning alleviates myocardial ischemic injury in rats. Exp Biol Med (Maywood) 2008;233:1448–53. doi: 10.3181/0801-RM-8. [DOI] [PubMed] [Google Scholar]
- 27.Tritto I, Ambrosio G, Capelli-Bigazzi M, Perrone-Filardi P, Lepore S, Tifano R, et al. Effects of hyperbaric oxygen therapy on experimental infarct size: Salvage versus delay of myocardial necrosis. J Appl Cardiol. 1991;6:359–66. [Google Scholar]
- 28.Tahep IdP, Valen G, Starkopf J, Kairane C, Zilmer M, Vaage J. Pretreating rats with hyperoxia attenuates ischemia-reperfusion injury of the heart. Life Sci. 2001;68:1629–40. doi: 10.1016/s0024-3205(01)00964-x. [DOI] [PubMed] [Google Scholar]
- 29.Cabigas BP, Su J, Hutchins W, Shi Y, Schaefer RB, Recinos RF, et al. Hyperoxic and hyperbaric-induced cardioprotection: role of nitric oxide synthase 3. Cardiovasc Res. 2006;72:143–51. doi: 10.1016/j.cardiores.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Zhou W, Zheng W, Ma Y, Lin L, Tang T, et al. Effects of myocardial transplantation of marrow mesenchymal stem cells transfected with vascular endothelial growth factor for the improvement of heart function and angiogenesis after myocardial infarction. Cardiology. 2007;107:17–29. doi: 10.1159/000093609. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 32.Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem. 2002;277:33284–90. doi: 10.1074/jbc.M203391200. [DOI] [PubMed] [Google Scholar]
- 33.Ijichi H, Taketomi A, Yoshizumi T, Uchiyama H, Yonemura Y, Soejima Y, et al. Hyperbaric oxygen induces vascular endothelial growth factor and reduces liver injury in regenerating rat liver after partial hepatectomy. J Hepatol. 2006;45:28–34. doi: 10.1016/j.jhep.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-Overexpressing Mesenchymal Stem Cells Accelerate Bone Marrow Stem Cell Mobilization via Paracrine Activation of SDF-1{alpha}/CXCR4 Signaling to Promote Myocardial Repair. Circ Res. 2008;103:1300–8. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 35.Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–92. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]