Abstract
Endothelial nitric oxide synthase (NOS3) regulates the functional response to β-adrenergic (β-AR) stimulation via modulation of the L-type Ca2+ current (ICa). However, the NOS3 signaling pathway modulating ICa is unknown. This study investigated the contribution of soluble guanylate cyclase (sGC) and phosphodiesterase type 5 (PDE5), a cGMP-specific PDE, in the NOS3-mediated regulation of ICa. Myocytes were isolated from NOS3 knockout (NOS3−/−) and wildtype (WT) mice. We measured ICa (whole-cell voltage-clamp), and simultaneously measured Ca2+ transients (Fluo-4 AM) and cell shortening (edge detection). Zaprinast (selective inhibitor of PDE5), decreased β-AR stimulated (isoproterenol, ISO)-ICa, and Ca2+ transient and cell shortening amplitudes in WT myocytes. However, YC-1 (NO-independent activator of sGC) only reduced ISO-stimulated ICa, but not cardiac contraction. We further investigated the NOS3/sGC/PDE5 pathway in NOS3−/− myocytes. PDE5 is mislocalized in these myocytes and we observed dissimilar effects of PDE5 inhibition and sGC activation compared to WT. That is, zaprinast had no effect on ISO- stimulated ICa, or Ca2+ transient and cell shortening amplitudes. Conversely, YC-1 significantly decreased both ISO-stimulated ICa, and cardiac contraction. Further confirming that PDE5 localizes NOS3/cGMP signaling to ICa; YC-1, in the presence of zaprinast, now significantly decreased ISO-stimulated Ca2+ transient and cell shortening amplitudes in WT myocytes. The effects of YC-1 on ICa and cardiac contraction were blocked by KT5823 (a selective inhibitor of the cGMP-dependent protein kinase, PKG). Our data suggests a novel physiological role for PDE5 in restricting the effects of NOS3/sGC/PKG signaling pathway to modulating β-AR stimulated ICa, while limiting effects on cardiac contraction.
INTRODUCTION
Nitric oxide (NO), produced by NO synthase (NOS), is an important modulator of cardiac contractility [1]. Endothelial NOS (NOS3) is constitutively expressed within cardiac myocytes and is an important regulator of β-adrenergic (β-AR) signaling. For example, in vivo investigations examining cardiac performance (left ventricle pressure, pressure/volume loops) demonstrated that mice with genetic deletion of NOS3 had a greater contractile response to β-AR stimulation [2–4]. Conversely, mice with cardiac myocyte specific overexpression of NOS3 exhibited a blunted functional response to β-AR stimulation [5, 6]. We have previously found that myocytes isolated from NOS3 knockout (NOS3−/−) mice also had an enhanced contractile response (i.e., augmented shortening and Ca2+ transient amplitudes) to β-AR stimulation [7]. We further demonstrated that this effect was due to an increase in the L-type Ca2+ current (ICa).
Cyclic guanosine 3′, 5′-monophosphate (cGMP) is an important second messenger and is hypothesized to be the signaling molecule of NOS3 [8]. Yet this pathway has not been completely characterized in cardiac myocytes. Previous studies have shown that exogenous cGMP or cGMP analogs are able to decrease the β-AR stimulated ICa [9, 10]. In mammalian cardiac myocytes, it appears that activation of the cGMP-dependent protein kinase (PKG) is the main transducer of cGMP signaling [11]. As with cGMP, exogenously applied PKG can also inhibit β-AR stimulated ICa [12, 13]. However, it is unknown whether the regulation of the L-type Ca2+ channel by NOS3 is via the cGMP/PKG pathway in cardiac myocytes.
An important characteristic of signaling pathways, such as the β-AR pathway, is their spatial localization [14]. Phosphodiesterases, which degrade cyclic nucleotides, are key proteins for compartmentalization by restricting the spread of the second messengers [15, 16]. Phosphodiesterase type 5 (PDE5) is the main isozyme for compartmentation of cGMP produced via soluble guanylate cyclase (sGC) [17]. We have previously shown that inhibition of PDE5 is able to decrease β-AR stimulated ICa via stimulation of PKG [18]. Interestingly, a study demonstrated that the anti-adrenergic effect of PDE5 inhibition was absent in NOS3−/− mice [19]. These data suggest a functional interaction exists between NOS3 and PDE5. In addition, PDE5 inhibitors may also be utilized as a potential therapy for cardiovascular patients, but limited data exists on the mechanism for this protective effect [20]. Thus, the purpose of our study is to further examine if PDE5 and PKG are involved in the NOS3-mediated regulation of ICa. We hypothesize that the regulation of ICa by NOS3 signaling involves a tightly regulated signaling microdomain involving sGC/PKG/PDE5.
MATERIALS AND METHODS
Isolation of ventricular myocytes
Ventricular myocytes were isolated from NOS3−/− and their corresponding wildtype (C57BL/6J) mice (Jackson Laboratories, Bar Harbor, Maine), as previously described [7]. Briefly, the heart was mounted on a Langendorff apparatus and perfused with Ca2+ free normal Tyrode solution. Blendzyme Type II (0.077 mg/ml) (Roche Applied Science, Indianapolis, IN) was then added to the perfusate. After 5–10 minutes, the heart was taken down, the ventricles minced, and myocytes dissociated by trituration. Subsequently the myocytes were filtered, centrifuged, and resuspended in normal Tyrode solution containing 200 μmol/L Ca2+. Myocytes were used within 6 hours of isolation. All the animal protocols and procedures were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University.
Measurement of L-type Ca2+ current (ICa)
ICa was measured using the whole cell voltage-clamp technique with an Axopatch-200B amplifier and pClamp 8.1 software (Axon Instrument, Foster City, CA.), as described previously [7, 18]. Electrodes (borosilicate glass tubing), with a resistance of 1.5–3 MΩ, were filled with (in mmol/L): 120 CsCl, 6 MgCl2, 10 EGTA, 10 HEPES, 2 MgATP, pH 7.2 adjusted with CsOH. The bath solution consisted of (in mmol/L): 120 NaCl, 4 CsCl, 1 MgCl2, 1 CaCl2, 10 glucose, 5 HEPES, 1 L-arginine, pH 7.4 adjusted with CsOH or HCl. ICa was elicited by 200 ms pulses to 0 mV from a holding potential of −80 mV (following a pre-pulse to −40 mV) at a frequency of 0.2 Hz. This procedure isolates ICa by inactivation of the Na+ current with the pre-pulse, and replacement of K+ with Cs+ eliminates K+ current. Measurements were performed at room temperature.
Simultaneous measurement of Ca2+ transients and cell shortening
Functional measurements were performed as previously described [21]. Briefly, myocytes were loaded at room temperature with Fluo-4 AM (10 μmol/L, Molecular Probes, Eugene, OR) for 30 min, and then 30 min were allowed for intracellular de-esterification. The instrumentation used for cell fluorescence measurements was a Cairn Research Limited (Faversham, UK) epifluorescence system. [Ca2+]i was measured by Fluo-4 epifluorescence with excitation at 480±20 nm and emission at 535±25 nm. The illumination field was restricted to collect the emission of a single cell. Data were expressed as ΔF/F0, where F is the fluorescence intensity and F0 is the intensity at rest. Simultaneous measurement of shortening was also performed using an edge detection system (Crescent Electronics, Sandy, UT). Data were expressed as % of resting cell length (%RCL). Measurements were performed at room temperature.
Solution and drugs
Normal Tyrode (NT) solution consisted of (in mmol/L): 140 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 glucose, 5 HEPES, 1 L-arginine, pH 7.4 adjusted with NaOH or HCl. Isoproterenol (ISO, 1 μmol/L, a non-selective β-AR agonist); zaprinast (ZAP, 10 μmol/L, a selective inhibitor of PDE5) [18, 22]; YC-1 (10 μmol/L, NO-independent activator of sGC) [23]; T-0156 (1 μmol/L, a selective inhibitor of PDE5 [24, 25], Tocris Bioscience, Ellisville, MO, USA); L-NIO (10 μmol/L, selective NOS3 inhibitor [7, 26], Calbiochem, La Jolla, CA, USA); KT 5823 (0.05 μmol/L, selective inhibitor of PKG [27] Calbiochem, La Jolla, CA, USA) were prepared fresh each experimental day. All chemicals were from Sigma except where indicated.
Statistics
Data were presented as mean±SEM. Differences between groups were evaluated for statistical significance (P<0.05) by paired or unpaired Student’s t tests.
RESULTS
Inhibition of PDE5 Decreased β-AR Stimulated ICa and Cardiac Contraction in WT Myocytes
We tested the effects of zaprinast (ZAP, 10 μmoll/L), a specific PDE5 inhibitor, in wildtype (WT) myocytes during β-AR stimulation. We first examined if ZAP was able to modulate ICa. Figure 1A shows representative current traces (left) and time plot (right) of the effects of β-AR stimulation (isoproterenol, a non-selective β-AR agonist, ISO, 1 μmol/L) and PDE5 inhibition (ZAP). Summary data are shown in Figure 1B. After the ISO response reached steady state, the solution was switched to ISO plus ZAP, resulting in a significant reduction in ICa ( NT: 3.2±0.5 -pA/pF; ISO: 4.0±0.5 -pA/pF; ISO+ZAP: 3.5±0.5 -pA/pF, P<0.05 vs ISO). ZAP is a selective PDE5 inhibitor; however it can also inhibit other PDE isoforms. Therefore, we corroborated our results with another PDE5 inhibitor, T-0156. As shown in Figure 1C, T-0156 also significantly reduced the β-AR stimulated I Ca (NT: 2.5±0.3 -pA/pF; ISO: 4.3±0.6 -pA/pF; ISO+T-0156: 3.2±0.4 -pA/pF, P<0.05 vs ISO). The inhibition of PDE5-induced decrease in current was not due to “run down” since ICa started to increase upon washout of the inhibitor (i.e., ISO alone) as shown in the time plot (Figure 1A, right). In addition, ZAP had no effect on basal ICa (9±7 % Δ from control, P=NS, n=6 myocytes/3 hearts). Thus, PDE5 inhibition is able to blunt β-AR stimulated ICa.
Figure 1.
PDE5 inhibition reduced the response to β-adrenergic (isoproterenol, ISO) stimulation in WT myocytes. A) Representative traces (left) and time plot (right) of ICa with ISO (1 μmol/L) and zaprinast (ZAP, 10 μmol/L) in a WT myocyte. B) Summary data (mean±SEM) of the effect of ISO and ZAP on ICa. C) Summary data (mean±SEM) of the effect of ISO and T-0156 on ICa. D) Representative traces of shortening and Ca2+ transient with ISO and ZAP in a WT myocyte. D) Summary data (mean±SEM) of the effects of ISO and ZAP on shortening and Ca2+ transient amplitudes. *P<0.05 vs ISO alone; n=6–16 cells/4 hearts.
We then tested if ZAP was able to modulate myocyte contraction. We simultaneously measured Ca2+ transients and cell shortening. Figure 1D shows representative shortening (left) and Ca2+ transient (right) traces of the effects of β-AR stimulation (ISO) and PDE5 inhibition (ZAP). Summary data are shown in Figure 1E. Consistent with previous reports, PDE5 inhibition resulted in a significant reduction in the β-AR stimulated shortening amplitude ( NT: 5.0±0.6 %RCL; ISO: 10.0±1.4 %RCL; ISO+ZAP: 8.5±1.2 %RCL, P<0.05 vs ISO). Not previously observed, our data shows that PDE5 inhibition also significantly blunted β-AR stimulated Ca2+ transient amplitude ( NT: 0.8±0.1 F/F0; ISO: 1.2±0.1 ΔF/F0, ISO+ZAP: 1.1±0.1 ΔF/F0, P<0.05 vs ISO). ZAP had no effect on basal shortening (16±12 % Δ from control, P=NS) and Ca2+ transient (4±4 % Δ from control, P=NS, n=5 myocytes/2 hearts) amplitudes. There was a trend that ZAP increased basal shortening, which is consistent with previous results [19]. Thus, besides blunted ISO-stimulated myocyte shortening, PDE5 inhibition also decreases Ca2+ transient amplitude, in part via decreased ICa in WT myocytes.
Activation of sGC Decreased β-AR Stimulated ICa but not Cardiac Contraction in WT Myocytes
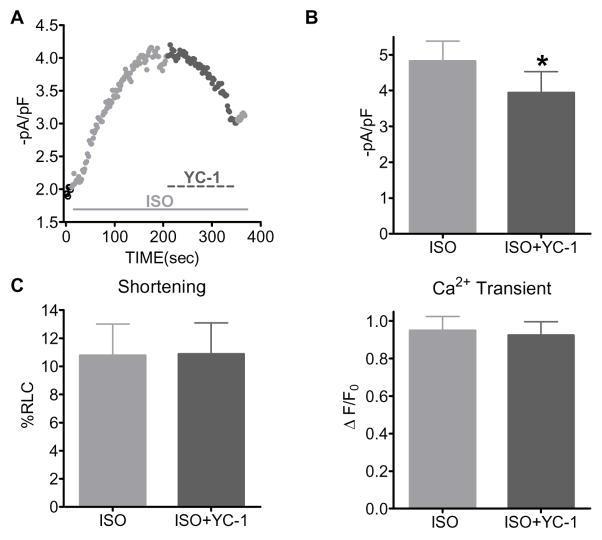
We continued investigating the cGMP signaling pathway by using YC-1, (10 μmol/L), a NO independent stimulator of sGC. We first examined the effects of YC-1 on ICa. Shown in Figure 2A is a representative time plot of the effects of β-AR stimulation (ISO, 1 μmol/L) and sGC activation (YC-1). Summary data are shown in Figure 2B. After the ISO response reached steady state, the solution was switched to ISO plus YC-1, resulting in a significant reduction in ICa ( NT: 1.8±0.1 -pA/pF; ISO: 4.8±0.6 -pA/pF; ISO+ZAP: 3.9±0.6 -pA/pF, p<0.05 vs ISO). YC-1 had no effect on basal ICa (0.4±3 % Δ from control, P=NS, n=5 myocytes/2 hearts). Thus, sGC activation is able to blunt β-AR stimulated ICa.
Figure 2.
Activation of sGC with YC-1 only decreased ISO-stimulated ICa, but not cardiac contraction in WT myocytes. A) Representative time plot of ICa with ISO (1 μmol/L) and YC-1(10 μmol/L) in a WT myocyte. B) Summary data (mean±SEM) of the effect of ISO and YC-1 on ICa. C) Summary data (mean±SEM) of the effect of ISO and YC-1 on shortening and Ca2+ transient amplitudes. *P<0.05 vs ISO alone; n=7–11 cells/2–3 hearts.
We then tested if YC-1 was able to modulate myocyte contraction. Summary data are shown in Figure 2C. Contrary to the effect observed with PDE5 inhibition, we observed no effect of YC-1 on β-AR stimulated myocyte contraction. That is, YC-1 had no effect on shortening ( NT: 4.9±0.9 %RCL; ISO: 10.8±2.2 %RCL; ISO+ZAP: 10.9±2.2 %RCL, P=NS vs ISO; left panel) or Ca2+ transient ( NT: 0.6±0.1 ΔF/F0; ISO: 0.9±0.1 ΔF/F0; ISO+ZAP: 0.9±0.1 ΔF/F0, P=NS; right panel) amplitudes. YC-1 also had no effect on basal shortening (19±11 % Δ from control, P=NS) and Ca2+ transient (−7±4 % Δ from control, P=NS, n=6 myocytes/3 hearts) amplitudes. These data suggest that activation of sGC is only able to reduce β-AR stimulated ICa but not cardiac contraction in WT myocytes.
Inhibition of PDE5 had no effect on β-AR Stimulated ICa or Cardiac Contraction in NOS3−/− Myocytes
Since we observed different functional effects with PDE5 inhibition and sGC activation in WT myocytes, we further investigated the cGMP signaling pathway in myocytes isolated from NOS3−/− hearts. We chose this model because it is known that PDE5 localization is altered leading to a lack of an effect on myocyte contraction [19]. Consistent with our previous study [7], NOS3−/− myocytes had a larger increase in ICa with β-AR stimulation compared to WT myocytes (72±16% vs 32±6% of control). We tested if ZAP (10 μmol/L) was able to modulate ICa in NOS3−/− myocytes. Figure 3A (left) shows a time plot of the effects of β-AR stimulation (ISO) and PDE5 inhibition (ZAP) in a NOS3−/− myocyte. Summary data are shown in Figure 3A (right). After the ISO response reached steady state, the solution was switched to ISO plus ZAP, resulting in no further change in ICa ( NT: 2.3±0.5 -pA/pF; ISO: 3.9±0.4 -pA/pF; ISO+ZAP: 3.9±0.5 -pA/pF, P=NS vs ISO). This is in contrast to what was observed in WT myocytes. Figure 3B shows that ZAP had a much larger effect in reducing the β-AR stimulated ICa in WT vs NOS3−/− myocytes (−22±5 vs 1±7 % Δ from ISO, P<0.05). These data suggest that NOS3 is essential for PDE5 inhibition to be effective. We further tested this hypothesis by acutely inhibiting NOS3 with the specific inhibitor L-NIO in WT myocytes. In the presence of L-NIO, ZAP had no effect on β-AR stimulated ICa (NT: 2.1±0.3 -pA/pF; ISO: 3.5±0.4 -pA/pF; ISO+ZAP: 3.6±0.4 -pA/pF, P=NS vs ISO, Fig 3B). Thus, the effect of PDE5 inhibition on ICa was also ineffective in WT myocytes with acute NOS3 inhibition (1±3 % Δ from ISO, Figure 3B). ZAP was also without effect on basal ICa (−4±6 % Δ from control, P=NS, n=5 myocytes/2 hearts). The absence of an anti-adrenergic effect of ZAP with NOS3 knockout or acute inhibition indicates that the blunted ICa caused by PDE5 inhibition is NOS3 dependent.
Figure 3.
PDE5 inhibition with zaprinast (ZAP) had no effect in NOS3−/− myocytes. A) Representative time plot (left) and summary data (mean±SEM) (left) of ICa with ISO (1 μmol/L) and ZAP (10 μmol/L) in NOS3−/− myocytes. B) Summary data (mean±SEM) of the effect of ZAP on ICa in WT, NOS3−/−, and WT with acute NOS3 inhibition (WT +L-NIO) myocytes. C) Summary data (mean±SEM) of the effect of ZAP on ISO-stimulated shortening and Ca2+ transient amplitudes in NOS3−/− myocytes. D) Summary data (mean±SEM) of the effects of ZAP on shortening and Ca2+ transient amplitudes in WT and NOS3−/− myocytes. *P<0.05 vs WT; n=7–13 cells/3–4 hearts.
We then tested if ZAP was able to modulate myocyte contraction. Consistent with our previous study [7], NOS3−/− myocytes had a larger increase in cardiac contraction with β-AR stimulation compared to WT myocytes (shortening: 196±47% vs 108±21% of control; Ca2+ transient: 63±9% vs 51±9% of control). We observed that ZAP had no effect on ISO-stimulated cell shortening ( NT: 3.6±0.6 %RCL; ISO: 10.8±2.4 %RCL; ISO+ZAP: 9.8±1.8 %RCL, P=NS vs ISO; Figure 3C, left) and Ca2+ transient ( NT: 0.5±0.05 ΔF/F0; ISO: 0.8±0.1 ΔF/F0; ISO+ZAP: 0.8±0.1 ΔF/F0, P=NS vs ISO; Figure 3C, right) amplitudes in NOS3−/− myocytes. This different effect of PDE5 inhibition between WT and NOS3−/− myocytes can be more clearly seen in figure 3D. ZAP decreased WT myocyte shortening significantly more than in NOS3−/− myocytes (−15±3 vs 3±6 % Δ from ISO, P<0.05). The same result was also observed in Ca2+ transient amplitude (−7±2 vs −1±1 % Δ from ISO, P<0.05). ZAP also had no effect on basal shortening (14±7 % Δ from control, P=NS) and Ca2+ transient (3±4 % Δ from control, P=NS, n=7 myocytes/2 hearts) amplitudes. Thus, similar to PDE5 inhibition having no effect on NOS3−/− myocyte β-AR stimulated ICa, ZAP was also without effect on β-AR stimulated myocyte contraction. Thus, the anti-adrenergic effects of PDE5 inhibition are dependent on NOS3.
Activation of sGC Decreased both β-AR Stimulated ICa and Cardiac Contraction in NOS3−/− Myocytes
We also tested the effects of YC-1 (10 μmol/L) in NOS3−/− myocytes. Shown in Figure 4A (left) is a time plot of the effects of β-AR stimulation (ISO) and sGC activation (YC-1) on ICa in a NOS3−/− myocyte. Summary data is shown in Figure 4A (right). Activation of sGC with YC-1 dramatically decreased ISO-stimulated ICa in NOS3−/− myocytes ( NT: 2.1±0.1 -pA/pF; ISO: 4.2±0.1 -pA/pF; ISO+ZAP: 2.3±2.2 -pA/pF, P<0.05 vs ISO). Additionally, Figure 4B shows that there was a much greater reduction of ICa with YC-1 in NOS3−/− myocytes compared to WT myocytes (− 47±5 vs − 18±4 % Δ from ISO, P<0.05). YC-1 had no effect on basal ICa (−0.4±4 % Δ from control, P=NS, n=5 myocytes/2 hearts).
Figure 4.
Activation of sGC with YC-1 significantly decreased ISO-stimulated ICa, shortening and Ca2+ transients in NOS3−/− myocytes. A) Representative time plot (left) and summary data (mean±SEM) (right) of ICa with ISO (1 μmol/L) and YC-1 (10 μmol/L) in NOS3−/− myocytes. B) Summary data (mean±SEM) of the effect of YC-1 on ICa in WT and NOS3−/− myocytes. C) Summary data (mean±SEM) of the effect of YC-1 on ISO-stimulated shortening and Ca2+ transient amplitudes in NOS3−/− myocytes. D) Summary data (mean±SEM) of the effects of YC-1 on shortening and Ca2+ transient amplitudes in WT and NOS3−/− myocytes. *P<0.05 vs ISO alone, ** P<0.05 vs WT; n=7–14 cells/3–4 hearts.
Interestingly, knockout of NOS3 abolished the differential regulation of YC-1 between ICa and cardiac contraction. That is, in NOS3−/− myocytes, YC-1 now significantly decreased ISO-stimulated cell shortening ( NT: 3.9±0.7 %RCL; ISO: 10.6±1.5 %RCL; ISO+ZAP: 7.9±1.4 %RCL, P<0.05 vs ISO) and Ca2+ transient ( NT: 0.6±.1 Δ F/F0; ISO: 1.1±0.1 Δ F/F0; ISO+ZAP: 0.9±0.1 Δ F/F0, P<0.05 vs ISO) amplitudes, and these data are summarized in Figure 4C. The distinct effects of sGC activation on cardiac contraction between WT and NOS3−/− myocytes can be seen in Figure 4D (cell shortening: WT 3±4 vs NOS3−/− − 24±6.0 % Δ from ISO, P<0.05; Ca2+ transient: WT − 2±1 vs NOS3−/− −11±2 % Δ from ISO, P<0.05). YC-1 also had no effect on basal shortening (66±35 % Δ from control, P=NS) and Ca2+ transient amplitude (23±14 % Δ from control, P=NS, n=8 myocytes/2 hearts). There was a trend that YC-1 increased basal contraction, consistent with previous results [28]. These data suggest that PDE5 restricts NOS3/cGMP signaling pathway to the L-type Ca2+ channel. When PDE5 is mislocalized, as in NOS3−/− myocytes, this compartmentalized effect is lost and now cGMP is able to modulate cardiac contraction.
Inhibition of PDE5 released cGMP to Regulate Cardiac Contraction in WT myocytes
We further investigated the hypothesis that PDE5 restricts cGMP signaling to the L-type Ca2+ channel by repeating the YC-1 contraction experiments in WT myocytes with PDE5 inhibition. As hypothesized, in the presence of ZAP, YC-1 now significantly decreased shortening ( NT: 3.9±0.7 %RCL; ISO: 8.7±1.1 %RCL; ISO+YC-1+ZAP: 6.2±0.9 %RCL, P<0.05 vs ISO) and Ca2+ transient ( NT: 0.9±0.1 Δ F/F0; ISO: 1.4±0.1 ΔF/F0; ISO+YC-1+ZAP: 1.2±0.1 ΔF/F0, P<0.05 vs ISO) amplitudes. This is in contrast to the effects of YC-1 alone in WT myocytes. As shown in Figure 5A, YC-1, in the presence of ZAP had a much greater effect in reducing ISO-stimulated cell shortening (− 31±3 % Δ from ISO, P<0.05 vs YC-1 alone) and Ca2+ transients (−12±1 % Δ from ISO, P<0.05 vs YC-1 alone). Interestingly, these reductions of cardiac contraction in WT myocytes are similar to the levels observed in NOS3−/− myocytes (Figure 5B). YC-1+ZAP had no effect on basal shortening (20±19 % Δ from control, P=NS) and Ca2+ transient (10±4 % Δ from control, P=NS, n=4 myocytes/1 heart) amplitudes. There was a trend that YC-1+ZAP increased basal myocyte contraction, consistent with our YC-1 and ZAP alone results. These data suggest that upon PDE5 inhibition (or mislocalization with NOS3 knockout) the NOS3/cGMP signaling pathway “spills over” and modulates cardiac contraction.
Figure 5.
Inhibition of PDE5 released the restriction of sGC signaling on cardiac contraction. A) Summary data (mean±SEM) of the effect of YC-1 (10 μmol/L) in WT myocytes (± ZAP) on shortening and Ca2+ transient amplitudes. B) Summary data (mean±SEM) of the effects of YC-1 + ZAP in WT myocytes and YC-1 in NOS3−/− myocytes on shortening and Ca2+ transient amplitudes. C) Left: Summary data (mean±SEM) of the effect of YC-1 in WT myocytes (± ZAP) on ICa . Right: Summary data (mean±SEM) of the effects of YC-1 + ZAP in WT myocytes and YC-1 in NOS3 −/− myocytes on ICa. *P<0.05 vs −ZAP. n=5–17 cells/2–5 hearts.
Since YC-1 had a greater inhibitory effect on β-AR stimulated ICa in NOS3−/− vs WT myocytes, we also repeated the YC-1 experiments on ICa in the presence of ZAP. In WT myocytes, YC-1 in the presence of ZAP decreased ISO- stimulated ICa (NT: 2.6±0.3 –pA/pF; ISO: 4.5±0.5 –pA/pF; ISO+YC-1+ZAP: 2.9±0.5, P<0.05 vs ISO). As shown in Figure 5C (left panel), the co-infusion of YC-1 and ZAP resulted in a much larger reduction in ISO-stimulated ICa (−36±8 % Δ from ISO, P<0.05 vs YC-1 alone). As with the effects on contraction, this reduction of ICa in WT myocytes is similar to the levels observed in NOS3 −/− myocytes (Figure 5C, right panel). YC-1+ZAP had no effect on basal ICa (0.4±4 % Δ from control, P=NS, n=5 myocytes/3 hearts).
PKG is involved in the NOS3/sGC signaling pathway
We investigated if PKG was the downstream effecter of the NOS3/sGC signaling pathway by investigating the effects of KT5823 (a specific PKG inhibitor, 0.05 μmol/L) on the YC-1 induced changes in β-AR stimulated ICa and contraction. Figure 6A shows that the effect of YC-1 to reduce ICa was eliminated in the presence of KT5823 (WT: 3±7, NOS3−/−: −5±5 % Δ from ISO, both P<0.05 vs YC-1 alone). KT5823 had no effect on basal ICa in either WT (control: 2.1±0.3 -pA/pF; KT5823: 2.1±0.4 -pA/pF, P=NS, n=6 myocytes/4 hearts) or NOS3−/− –myocytes (control: 2.1±0.2 -pA/pF; KT5823: 2.1±0.4 -pA/pF, P=NS, n=5 myocytes/2 hearts). We observed the same phenomenon in NOS3−/− myocyte contraction (Figure 6B). That is, in the presence of KT5823, the reduction of shortening (−5±4 % Δ from ISO, P<0.05 vs YC-1 alone) and Ca2+ transient (−5±1 % Δ from ISO, P<0.05 vs YC-1 alone) amplitudes by YC-1 was greatly blunted. KT5823 also had no effect on basal shortening (WT: 21±16 % Δ from control, P=NS; NOS3−/−: −3±9 % Δ from control, P=NS) and Ca2+ transient (WT: 10±5 % Δ from control, P=NS; NOS3−/−: 0±3 % Δ from control, P=NS, n=4–7 myocytes/1–2 hearts) amplitudes. There was a trend that PKG increased basal shortening in WT myocytes. Thus, PKG is the main signaling molecule of the anti-adrenergic effects of the cGMP signaling pathway.
Figure 6.
PKG is the primary protein targart for the NOS3/sGC signaling pathway. A) Summary data (mean±SEM) of the effect of KT5823 (0.05 μmol/L) on the YC-1 response on ICa in WT and NOS3−/− myocytes B) Summary data (mean±SEM) of the effects of KT5823 on the YC-1 response on shortening and Ca2+ transient amplitudes in NOS3−/− myocytes. *p<0.05 vs –KT5823. n= 7–16 cells/2–4 hearts.
DISCUSSION
We have previously shown that NOS3 is an important modulator of ICa. However, the NOS3 signaling pathway has not been completely characterized. Our data demonstrates that in WT myocytes, activation of sGC via YC-1 only decreased β-AR stimulated ICa (but not cardiac contraction) and this effect was prevented by PKG inhibition with KT5823. When PDE5 was inhibited by ZAP, sGC activation with YC-1 now reduced β-AR stimulated cardiac contraction. These results reveal a novel physiological role for PDE5 in limiting the functional effects of NOS3/sGC signaling to modulate β-AR stimulated ICa. Further, sGC signaling (YC-1) was drastically altered in NOS3−/− myocytes in that there was an anti-adrenergic effect on both ICa and cardiac contraction. These effects were prevented by PKG inhibition. In addition, PDE5 inhibition (ZAP) had no effect on ICa or cardiac contraction in NOS3−/− myocytes. These differing effects of YC-1 and ZAP in WT and NOS3−/− myocytes may be due the mislocalization of PDE5 in NOS3−/− myocytes [19]. With PDE5 inhibition (or NOS3 knockout) the spatially restricted cGMP now “spills over” and is able to modulate cardiac contraction. Thus, our data demonstrates that NOS3/sGC modulates β-AR stimulated ICa via activation of PKG, and this signaling pathway exists in a microdomain formed by PDE5.
NOS3-mediated regulation of ICa
Our data demonstrates that inhibition of the cGMP-specific PDE (PDE5) via ZAP decreases ICa in WT (Figure 1) but not in NOS3−/− (Figure 3) myocytes. These data suggest that NOS3 must be present to lead to the production of cGMP which will blunt the β-AR stimulation of ICa. In addition to inhibiting PDE5, ZAP is also a PDE1 inhibitor. While PDE5 is specific for hydrolyzing cGMP, PDE1 hydrolyzes both cAMP and cGMP. Previous studies have shown that specific PDE1 inhibition in cardiac myocytes increased cAMP levels by ~20% [29]. In a previous study, we found that ZAP (at the same concentration used in the current study) increased cGMP levels, but had no effect on basal or ISO-stimulated cAMP levels in isolated myocytes [18]. In addition, a previous study has shown that specific PDE1 inhibition (MIMX) had no effect on basal ICa, but significantly increased ISO-stimulated ICa (the opposite effect of ZAP) [29]. Furthermore, we used another selective inhibitor of PDE5 (T-0156) [24, 25], and observed similar results as with ZAP (Figure 1). Hence, at the concentration used, ZAP is a selective inhibitor of PDE5 in cardiac myocytes.
We further explored the cGMP pathway by using YC-1, a NO-independent stimulator of sGC. Similar to PDE5 inhibition, YC-1 decreased β-AR stimulated ICa in WT myocytes (Figure 2). Unlike PDE5 inhibition, YC-1 was able to blunt the β-AR stimulated ICa in NOS3−/− myocytes (Figure 4). Thus, the cGMP pathway is still able to modulate ICa in NOS3−/− myocytes. NOS3 is localized to caveolae, along with superoxide dismutase, a superoxide anion scavenger [30, 31]. Superoxide dismutase will prevent the superoxide anion from reacting with NO, making NOS3 signaling more likely to be via the cGMP-dependent pathway [8]. Thus, we may not have observed an effect of PDE5 inhibition in NOS3−/− myocytes most likely due to the lack of sGC activation and resultant cGMP production.
We also tested if PKG was involved in the NOS3 signaling pathway since PKG mediates the majority of the cGMP-dependent effects [11]. We observed that PKG inhibition did indeed prevent the reduction in ICa caused by YC-1 in WT and NOS3−/− myocytes (Figure 6). Previous studies have shown that PKG is able to phosphorylate the α1C and β2a subunits of the L-type Ca2+ channel [32, 33]. We have also previously shown in guinea pig cardiac myocytes that PDE5 inhibition decreased ICa through a PKG-dependent mechanism [18]. However, the involvement of NOS3 was not investigated in these studies. Our data demonstrate that the NOS3-induced activation of sGC is crucial for the cGMP mediated regulation of ICa. Thus, increasing cGMP levels either by NOS3-induced sGC activation or by inhibiting hydrolysis with PDE5 inhibition (in the presence of NOS3) will decrease β-AR stimulated ICa via PKG. In addition, we do not believe that NOS3−/− myocytes have reduced basal PKG phosphorylation. Many studies have shown that PKG phosphorylation leads to a reduction in ICa [12, 13, 27]. However, our previous [7] and current results demonstrate that there is no difference in basal ICa between WT and NOS3−/− myocytes, and PKG inhibition (KT5823) had no effect on basal ICa. These data suggest no difference in basal ICa PKG phosphorylation levels.
A previous study found that there was no difference in PDE5 expression or basal levels of PKG activity in myocytes isolated from WT and NOS3−/− mice[19]. However, PDE5 inhibition was not able to increase PKG activity in NOS3−/−-myocytes, while it significantly increased PKG in WT myocytes [19]. The failure to increase PKG activity in NOS3−/− myocytes was not due to changes in PKG expression [34]. Specifically, in the presence of acute NOS inhibition in WT myocytes (thus PKG expression will not be altered), PDE5 inhibition was unable to increase PKG activity. This is consistent with our data. PDE5 inhibition with ZAP decreased β-AR stimulated ICa in WT myocytes through a PKG-dependent pathway (Figures 1 and 6). However, in the presence of acute, specific inhibition of NOS3 with L-NIO in WT myocytes, ZAP was now ineffective in blunting β-AR stimulated ICa (Figure 3). These data suggest that, in the presence of NOS3 inhibition, PDE5 inhibition is unable to lead to the activation of PKG, even though expression is unaltered. Further, activation of sGC with YC-1 had a much larger functional effect in NOS3−/− myocytes compared to WT myocytes (Figure 4). This effect was via activation of PKG (Figure 6). These data indicate that PKG is functional in NOS3−/− myocytes; however it is not able to be activated by PDE5 inhibition with ZAP. Thus, we believe that the difference in response to PDE5 inhibition in WT vs NOS3−/− myocytes is not due altered PDE5 and/or PKG expression but a difference in the NOS3/sGC/PDE5/PKG signaling cascade.
These data could have important clinical implications as there is increasing evidence which suggests the potential utility of PDE5 inhibitors to treat a variety of cardiovascular diseases [20]. These beneficial effects have been observed in protecting ischemic myocardium [35, 36] and not only preventing, but also reversing cardiac hypertrophy [37]. The protective effects of PDE5 inhibition have also been shown to, in part, precondition cardiac myocytes against necrosis and apoptosis [38], reduce ventricular fibrillation [35], and blunt calcineurin, NFAT, and ERK1/2 activation [37]. As discussed, PDE5 inhibition also reduced β-AR stimulated ICa in WT myocytes (Figure 1). Our studies reveal another important end target of PDE5 inhibition, L-type Ca2+ channels. Modulation of ICa could be a key contributor to the protective mechanisms observed with PDE5 inhibition. Our previous data showed that NOS3 knockout or acute inhibition increased β-AR stimulated ICa resulting in higher incidences of spontaneous activity (early and delayed afterdepolarizations) in myocytes [7], supporting the antiarrhythmic effects of PDE5 inhibition. It is also known that Ca2+ influx via ICa activates calcineurin, leading to NFAT dephosphorylation and the activation of hypertrophic signaling. Exogenous NO-mediated inhibition of ICa can inhibit this calcineurin activation [39]. Excessive Ca2+ influx via ICa also leads to apoptosis [40]. Thus, increased Ca2+ influx via β-AR stimulated ICa can be detrimental to the myocyte. Hence, decreasing β-AR stimulated ICa by PDE5 inhibition should be beneficial for the treatment of heart disease.
PDE5 Restricts NOS3/sGC Signaling
Our data and others [19, 41] demonstrate that the effects of PDE5 inhibition are dependent on NOS3 and sGC activation. That is, PDE5 inhibition decreased β-AR stimulated ICa, and Ca2+ transient and shortening amplitudes in WT myocytes (Figure 1), but not in NOS3−/− myocytes (Figure 3). However, when we activated sGC with YC-1 in WT myocytes, we only observed a reduction in β-AR stimulated ICa, but not cardiac contraction (Figure 2). Taken together, our data indicates that PDE5 restricts NOS3/sGC/PKG signaling to modulate β-AR stimulated ICa. PDEs are key controllers in limiting the spread of cGMP, thus shaping intracellular signaling microdomains [16]. Castro et al. demonstrated that PDE5 controls the cGMP pool produced by sGC, whereas cGMP produced via the particulate isoform is under the exclusive control of PDE2 [17]. Thus, our data is consistent with this scheme, since we observed that PDE5 restricts NOS3/sGC/PKG signaling to modulate β-AR stimulated ICa. Further evidence supporting this signaling microdomain is that NOS3, along with L-type Ca2+ channels, are localized to the caveolae [31, 42]. A previous publication has shown that there is a marked co-localization of sGC with NOS3 [43]. Since, NOS3 is localized to the caveolae along with the L-type Ca2+ channel [31, 42], it appears that sGC is localized to the same region. In addition, activation of sGC, but not the particulate isoform, activates PKG [34]. PKG phosphorylation also increases cGMP binding affinity [44] and the activity of PDE5 [45]. Thus, this NOS3/sGC/PKG/PDE5 signaling regulates ICa activity in a spatially localized microdomain (Figure 7A).
Figure 7.
NOS3/sGC signaling is compartmentalized via PDE5. A) In WT myocytes, the effect of NOS3/sGC signaling is localized to modulate ICa. B) With NOS3 knockout or PDE5 inhibition, compartmentation of NOS3/sGC signaling is lost allowing for modulation of cardiac contraction. ATP= SR Ca2+ ATPase, PLB= phospholamban, RyR= ryanodine receptor. See text for other abbreviations.
However, this pathway is altered in NOS3−/− myocytes. A study showed that PDE5 inhibition was without effect on cardiac contraction in NOS3−/− myocytes [19]. Our data is consistent in that we also observed no effect of PDE5 inhibition on contraction in NOS3−/− myocytes (Figure 3). We further showed that PDE5 inhibition also had no effect on ICa (Figure 3). These data suggest that NOS3 is essential for PDE5 to be effective. That is, NOS3-derived NO will activate sGC to produce cGMP, and this specific pool of cGMP would be hydrolyzed by PDE5. This pathway is also important for PDE5 localization. In NOS3−/− myocytes, PDE5 is no longer localized to the z-bands. This is due to the knockout of NOS3 and not some compensatory adaptation since adenoviral mediated expression of NOS3 in these myocytes restores the localization of PDE5 [19]. This effect was also observed with chronic NOS inhibition [41]. With PDE5 mislocalization, NOS3−/− myocytes had a markedly different response to sGC activation. Not only did YC-1 result in a greater reduction of β-AR stimulated ICa compared to WT myocytes, it also resulted in depressed β-AR stimulated cardiac contraction (Figure 4), which was prevented by PKG inhibition (Figure 6). Thus, the resultant effects of YC-1 on NOS3−/− myocytes may be due to cGMP “spilling out” of the compartment to decrease myofilament Ca2+ sensitivity via PKG phosphorylation of troponin I [19, 46]. Unlike previous studies, we also observed decreased Ca2+ transient amplitude, which could be due to the reduced ICa. We further tested if PDE5 is indeed responsible for restricting sGC signaling in WT myocytes. In the presence of PDE5 inhibition, superfusion with YC-1 now blunted β-AR stimulated cardiac contraction (Figure 5). In fact, the cumulative effect of YC-1 and ZAP in WT myocytes on cardiac contraction and ICa was similar to the effects of YC-1 alone in NOS3−/− myocytes (Figure 5). Thus, with NOS3 knockout or PDE5 inhibition, cGMP will no longer be restricted and will “spill over” and affect cardiac contraction via troponin I and additional ICa phosphorylation (Figure 7B).
Previous studies have demonstrated that the L-type Ca2+ channel (Cav1.2) exists as a preassembled macromolecular complex. That is, many of the kinases and phosphatases that regulate Cav1.2 activity are found as a macromolecular signaling complex [47, 48]. This has been shown for PKA (as well as related signaling components- β2-adrenergic receptor, Gsα, Giα, adenylate cyclase, and A-kinase anchoring protein), protein phosphatase 2a, and PKC. Further, this PKA/β2 receptor complex has been found to exist in the caveolae of ventricular myocytes [42]. Thus, we speculate that there is also a macromolecular complex with PKG and its signaling components (sGC, PDE5 and NOS3), but this remains to be determined.
In conclusion, our results demonstrate a novel physiological role for PDE5 within cardiac myocytes. That is, PDE5 confines the NOS3/sGC/PKG signaling pathway in a microdomain that regulates the L-type Ca2+ channel, while limiting the effects on cardiac contraction.
Acknowledgments
This work was supported by The American Heart Association (Postdoctoral Fellowship 0725560B, HW; Pre-doctoral Fellowship 0715159B, MJK) and the National Institutes of Health (K02 HL094692, R01 HL079283, MTZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ziolo MT, Kohr MJ, Wang H. Nitric oxide signaling and myocardial function. J Mol Cell Cardiol. 2008;45(5):625–32. doi: 10.1016/j.yjmcc.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyurko R, Kuhlencordt P, Fishman MC, Huang PL. Modulation of mouse cardiac function in vivo by eNOS and ANP. Am J Physiol Heart Circ Physiol. 2000;278(3):H971–81. doi: 10.1152/ajpheart.2000.278.3.H971. [DOI] [PubMed] [Google Scholar]
- 3.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416(6878):337–9. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 4.Champion HC, Georgakopoulos D, Takimoto E, Isoda T, Wang Y, Kass DA. Modulation of in vivo cardiac function by myocyte-specific nitric oxide synthase-3. Circ Res. 2004;94(5):657–63. doi: 10.1161/01.RES.0000119323.79644.20. [DOI] [PubMed] [Google Scholar]
- 5.Brunner F, Andrew P, Wolkart G, Zechner R, Mayer B. Myocardial contractile function and heart rate in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Circulation. 2001;104(25):3097–102. doi: 10.1161/hc5001.101966. [DOI] [PubMed] [Google Scholar]
- 6.Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res. 2004;94(9):1256–62. doi: 10.1161/01.RES.0000126497.38281.23. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Kohr MJ, Wheeler DG, Ziolo MT. Endothelial nitric oxide synthase decreases beta-adrenergic responsiveness via inhibition of the L-type Ca2+ current. Am J Physiol Heart Circ Physiol. 2008;294(3):H1473–80. doi: 10.1152/ajpheart.01249.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziolo MT. The fork in the nitric oxide road: cyclic GMP or nitrosylation? Nitric Oxide. 2008;18(3):153–6. doi: 10.1016/j.niox.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi RC, Alloatti G, Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea pig ventricular myocytes. Pflugers Arch. 1989;413(6):685–7. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- 10.Wahler GM, Rusch NJ, Sperelakis N. 8-Bromo-cyclic GMP inhibits the calcium channel current in embryonic chick ventricular myocytes. Can J Physiol Pharmacol. 1990;68(4):531–4. doi: 10.1139/y90-076. [DOI] [PubMed] [Google Scholar]
- 11.Wegener JW, Nawrath H, Wolfsgruber W, Kuhbandner S, Werner C, Hofmann F, et al. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res. 2002 Jan 11;90(1):18–20. doi: 10.1161/hh0102.103222. [DOI] [PubMed] [Google Scholar]
- 12.Mery PF, Lohmann SM, Walter U, Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci U S A. 1991;88(4):1197–201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumii K, Sperelakis N. cGMP-dependent protein kinase regulation of the L-type Ca2+ current in rat ventricular myocytes. Circ Res. 1995;77(4):803–12. doi: 10.1161/01.res.77.4.803. [DOI] [PubMed] [Google Scholar]
- 14.Bers DM, Ziolo MT. When is cAMP not cAMP? Effects of compartmentalization. Circ Res. 2001;89(5):373–5. [PubMed] [Google Scholar]
- 15.Mongillo M, Zaccolo M. A complex phosphodiesterase system controls beta-adrenoceptor signalling in cardiomyocytes. Biochem Soc Trans. 2006;34(Pt 4):510–1. doi: 10.1042/BST0340510. [DOI] [PubMed] [Google Scholar]
- 16.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, et al. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99(8):816–28. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 17.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113(18):2221–8. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziolo MT, Lewandowski SJ, Smith JM, Romano FD, Wahler GM. Inhibition of cyclic GMP hydrolysis with zaprinast reduces basal and cyclic AMP-elevated L-type calcium current in guinea-pig ventricular myocytes. Br J Pharmacol. 2003;138(5):986–94. doi: 10.1038/sj.bjp.0705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96(1):100–9. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 20.Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res. 2007;101(11):1084–95. doi: 10.1161/CIRCRESAHA.107.162511. [DOI] [PubMed] [Google Scholar]
- 21.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases {beta}-adrenergic stimulation in cardiomyocytes. Cardiovasc Res. 2008;77(2):353–61. doi: 10.1093/cvr/cvm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotera J, Fujishige K, Akatsuka H, Imai Y, Yanaka N, Omori K. Novel alternative splice variants of cGMP-binding cGMP-specific phosphodiesterase. J Biol Chem. 1998;273(41):26982–90. doi: 10.1074/jbc.273.41.26982. [DOI] [PubMed] [Google Scholar]
- 23.Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84(12):4226–33. [PubMed] [Google Scholar]
- 24.Mochida H, Takagi M, Inoue H, Noto T, Yano K, Fujishige K, et al. Enzymological and pharmacological profile of T-0156, a potent and selective phosphodiesterase type 5 inhibitor. Eur J Pharmacol. 2002;456(1–3):91–8. doi: 10.1016/s0014-2999(02)02590-6. [DOI] [PubMed] [Google Scholar]
- 25.Mochida H, Noto T, Inoue H, Yano K, Kikkawa K. T-0156, a novel phosphodiesterase type 5 inhibitor, and sildenafil have different pharmacological effects on penile tumescence and electroretinogram in dogs. Eur J Pharmacol. 2004;485(1–3):283–8. doi: 10.1016/j.ejphar.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 26.Wolff DJ, Lubeskie A, Gauld DS, Neulander MJ. Inactivation of nitric oxide synthases and cellular nitric oxide formation by N6-iminoethyl-L-lysine and N5-iminoethyl-L-ornithine. Eur J Pharmacol. 1998;350(2–3):325–34. doi: 10.1016/s0014-2999(98)00267-2. [DOI] [PubMed] [Google Scholar]
- 27.Wahler GM, Dollinger SJ. Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. Am J Physiol. 1995;268(1 Pt 1):C45–54. doi: 10.1152/ajpcell.1995.268.1.C45. [DOI] [PubMed] [Google Scholar]
- 28.Langer M, Luttecke D, Schluter KD. Mechanism of the positive contractile effect of nitric oxide on rat ventricular cardiomyocytes with positive force/frequency relationship. Pflugers Arch. 2003;447(3):289–97. doi: 10.1007/s00424-003-1187-8. [DOI] [PubMed] [Google Scholar]
- 29.Verde I, Vandecasteele G, Lezoualc’h F, Fischmeister R. Characterization of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br J Pharmacol. 1999;127(1):65–74. doi: 10.1038/sj.bjp.0702506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brahmajothi MV, Campbell DL. Heterogeneous basal expression of nitric oxide synthase and superoxide dismutase isoforms in mammalian heart: implications for mechanisms governing indirect and direct nitric oxide-related effects. Circ Res. 1999;85(7):575–87. doi: 10.1161/01.res.85.7.575. [DOI] [PubMed] [Google Scholar]
- 31.Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes. Implications for the autonomic regulation of heart rate. J Biol Chem. 1998;273(46):30249–54. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- 32.Jiang LH, Gawler DJ, Hodson N, Milligan CJ, Pearson HA, Porter V, et al. Regulation of cloned cardiac L-type calcium channels by cGMP-dependent protein kinase. J Biol Chem. 2000;275(9):6135–43. doi: 10.1074/jbc.275.9.6135. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res. 2007;101(5):465–74. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- 34.Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, et al. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115(16):2159–67. doi: 10.1161/CIRCULATIONAHA.106.643536. [DOI] [PubMed] [Google Scholar]
- 35.Das S, Maulik N, Das DK, Kadowitz PJ, Bivalacqua TJ. Cardioprotection with sildenafil, a selective inhibitor of cyclic 3′,5′-monophosphate-specific phosphodiesterase 5. Drugs Exp Clin Res. 2002;28(6):213–9. [PubMed] [Google Scholar]
- 36.du Toit EF, Rossouw E, Salie R, Opie LH, Lochner A. Effect of sildenafil on reperfusion function, infarct size, and cyclic nucleotide levels in the isolated rat heart model. Cardiovasc Drugs Ther. 2005;19(1):23–31. doi: 10.1007/s10557-005-6894-2. [DOI] [PubMed] [Google Scholar]
- 37.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214–22. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 38.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280(13):12944–55. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 39.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99(17):11363–8. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005 Nov 11;97(10):1009–17. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 41.Nagayama T, Zhang M, Hsu S, Takimoto E, Kass DA. Sustained soluble guanylate cyclase stimulation offsets nitric-oxide synthase inhibition to restore acute cardiac modulation by sildenafil. J Pharmacol Exp Ther. 2008;326(2):380–7. doi: 10.1124/jpet.108.137422. [DOI] [PubMed] [Google Scholar]
- 42.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103(19):7500–5. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brahmajothi MV, Campbell DL. Heterogeneous expression of NO-activated soluble guanylyl cyclase in mammalian heart: implications for NO- and redox-mediated indirect versus direct regulation of cardiac ion channel function. Channels (Austin) 2007;1(5):353–65. doi: 10.4161/chan.5189. [DOI] [PubMed] [Google Scholar]
- 44.Corbin JD, Turko IV, Beasley A, Francis SH. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur J Biochem. 2000;267(9):2760–7. doi: 10.1046/j.1432-1327.2000.01297.x. [DOI] [PubMed] [Google Scholar]
- 45.Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem. 2002;277(5):3310–7. doi: 10.1074/jbc.M106562200. [DOI] [PubMed] [Google Scholar]
- 46.Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540(Pt 2):457–67. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, et al. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293(5527):98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, et al. Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280(1):207–14. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]