Abstract
Bullous pemphigoid (BP) is a humoral autoimmune disease directed predominantly against the non-collagenous NC16A domain of the BP180 hemidesmosomal protein. Our laboratory has recently shown, using a mouse xenograft model, that passive transfer of IgE autoantibodies from BP sera induces a skin phenotype that recapitulates the early phases of the disease. Herein, we describe the development of a highly specific and sensitive ELISA to detect circulating IgE autoantibodies that recognize BP180-NC16A. Using this assay, we detect a high frequency (77%) of NC16A-specific IgE class autoantibodies in the sera of BP patients. This frequency, which is significantly higher than reported previously, is comparable to that of anti-NC16A IgG autoantibody production. In 3 BP patients monitored over time, the circulating NC16A-specific levels of both IgE and IgG were associated with clinical disease activity; however, patient sera did not always contain high levels of both isotypes. In conclusion, our ELISA provides a highly sensitive and specific tool for the detection of BP180-specific IgE in patient sera. Furthermore, we report that the majority of BP sera contain both IgE and IgG class autoantibodies specific for NC16A and suggest that screening for both isotypes of autoantibodies may provide a better diagnostic value than IgG alone.
Keywords: Autoimmunity, Autoantibody, IgE, IgG, Skin, Blister, ELISA
INTRODUCTION
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by tissue-bound and circulating autoantibodies specific for components of the epidermal basement membrane zone (BMZ). BP autoantibodies primarily recognize two protein constituents of the epidermal hemidesmosome, BP180 (BPAG2 or type XVII collagen) and/or BP230 (BPAG1) (Stanley et al., 1988; Giudice et al., 1992). The immuno-dominant epitopes associated with BP have been mapped to the NC16A region of BP180, a non-collagenous stretch of the protein’s extracellular linker domain (Giudice et al., 1993; Zillikens et al., 1997b). Passive transfer mouse models have shown that IgG autoantibodies that recognize the region of murine BP180 homologous with human NC16A elicit BP-like skin lesions in the host animals (Liu Z. et al., 1995). Recent passive transfer studies utilizing a humanized BP180 transgenic mouse line as the host confirm that NC16A-specific IgG autoantibodies are directly involved in the pathogenesis of BP (Nishie et al., 2007; Liu Zhi et al., 2008).
Historically, IgG class autoantibodies have been the focus of studies investigating BP pathogenesis, and disease severity has been shown to be correlated with BP180-specific autoantibody levels (Schmidt et al., 2000; Amo et al., 2001; Hofmann et al., 2002; Tsuji-Abe et al., 2005). In addition to IgG autoantibodies, IgE-class autoantibodies have been reported in the majority of BP patients (Christophoridis et al., 2000; Dopp et al., 2000; Dimson et al., 2003). These IgE-class autoantibodies also target the NC16A region of BP180 (Christophoridis et al., 2000; Dopp et al., 2000; Hofmann et al., 2002; Dimson et al., 2003; Fairley et al., 2005) and have been shown to have a pathogenic role in BP (Dimson et al., 2003; Fairley et al., 2007). Using a xenograft mouse model, our research group found that injection of BP IgE autoantibodies into human skin grafts resulted in lesions that reproduce the early phase of BP, including mast cell degranulation, eosinophil influx and spontaneous blister formation - key features that were lacking in passive transfer studies utilizing IgG (Fairley et al., 2007). Zone and colleagues (Zone et al., 2007) made similar observations after subcutaneous injection of a murine hybridoma producing BP180-specific IgE in SCID mice engrafted with human skin. Together, these findings suggest that IgE autoantibodies may be responsible for some of the early events leading to blister formation in BP.
The recognition of the pathogenic potential of IgE in BP underscores the need for the development of a sensitive and specific method for detection of these autoantibodies. The difficulty of this undertaking is compounded by the following factors: the level of IgE in serum is 1/10,000 that of IgG, a fraction of the total IgE is antigen specific, and BP sera contain IgE and IgG class autoantibodies targeting the same epitopes (Fairley et al., 2005). In the present study we utilized an NC16A-GST fusion protein to develop an IgE-specific ELISA that can easily and reliably measure autoantibody levels in patient sera. We show that this assay is highly specific and sensitive in the detection of these disease-associated autoantibodies. Furthermore, screening of a panel of well-characterized BP sera with our ELISA reveals that the majority of patients have NC16A-specific IgE in their sera.
METHODS
Sera and Clinical Assessment
Serum samples were collected from patients who met the clinical and immunologic, and histologic features consistent with BP (n=43), epidermolysis bullosa acquisita (EBA) (n=6), or systemic lupus erythematosus (n=21). All BP sera were from a panel of well characterized patients with histological subepidermal blistering and confirmatory direct immunofluorescence demonstrating IgG and/or C3 at the BMZ of perilesional skin. BP patients were of heterogeneous clinical disease severity and most were untreated when the samples were obtained. Sera were also obtained from age and gender-matched controls (n=55). Serial samples were obtained from 3 BP patients to examine the relationship of disease activity to NC16A IgE levels. Disease activity was assessed on a scale of 1 to 4. The score of 4 was assigned to generalized disease (often termed active or flaring, with multiple new blisters and erosions at multiple sites on the body), a score of 3 was assigned to localized active disease (few blisters or at limited areas of the body), a score of 2 was assigned if disease was controlled (no new lesions) while on immunosuppressive medications, and a score of 1 was assigned to the patients in the remission stage, no skin lesions in the absence of immunosuppressive therapy. This study was approved by the Institutional Review Board at the University of Iowa and was performed in adherence to the Declaration of Helsinki Guidelines.
Preparation of Recombinant Proteins
The expression and purification of glutathione S-transferase (GST) and the GST-NC16A fusion protein were described previously (Van den Bergh et al., 2006). Briefly, the control GST protein and GST-NC16A, consisting of the NC16A domain of human BP180 with a C-terminal collagen peptide [(Gly-Pro-Pro)10] and an N-terminal glutathione S-transferase (GST) moiety, were expressed in E. coli (Rosetta strain for GST-NC16A; DH5α strain for GST) using the pGEX-2T expression system (Pharmacia Biotech, Piscataway, NJ). Both proteins were purified by glutathione affinity chromatography (Novagen, EMD Chemicals, Inc., Gibbstown, NJ).
NC16A-specific IgE ELISA
To detect NC16A-specific IgE, immobilized GST-NC16A was probed with positive (a BP sera with high IgE reactivity to the epidermal BMZ by IIF and specific IgE reactivity to NC16A by Western blot) or negative (non autoimmune sera with low reactivity to GST) control sera in combination with a horseradish peroxidase-conjugated secondary antibody (Bethyl Laboratories, Montgomory, TX). As a control the same sera were probed with molar equivalent of GST. To determine the optimal working conditions of the assay, checkerboard titrations were performed with serial dilutions of antigen (100, 50, 10, 1, 0.1 and 0.05 μg/ml) and secondary antibody (5000, 10,000, 20,000, 40,000-fold) and a constant undiluted positive or negative control sera (described above). Analysis of the curves generated by plotting antigen concentration versus OD450 revealed that a 20,000-fold dilution of secondary antibody minimized the difference in absorbance between uncoated and GST-coated wells and maximized the difference in absorbance between NC16A-GST GST-coated wells in the assay. The specificity of the secondary antibody for IgE was confirmed by ELISA against IgG, IgA, IgM and IgE standards as previously described (Fairley et al., 2005).
In a similar fashion, checkerboard titrations were performed with serial dilutions of antigen (100, 50, 10 and 1 μg/ml) and positive and negative control sera (described above; undiluted, 2, 5 and 10-fold). Analysis of the plot of antigen concentration versus primary antibody dilution revealed that undiluted serum resulted in the highest level of discrimination. It was also determined that coating the wells overnight at 4°C, rather than 2 hr at room temperature (RT) or 37°C, resulted in optimal sensitivity of the assay.
The optimized ELISA was run using 96 well high bind ELISA (Nunc) plates coated with 50 μg/ml GST-NC16A or an equimolar concentration of GST diluted in 100 μl phosphate-buffered saline (PBS, pH 7.4) overnight at 4°C. Wells were washed a total of 3x by adding 250 μl PBST [PBS/0.05% Tween 20] per well. Non-specific protein binding was blocked by adding 200 μl blocking buffer [PBS/0.5% bovine serum albumin (BSA)] for 30 min at RT. Plates were washed as described above. Next, 50 μl of the positive and negative control sera (described above), henceforth referred to as high and low calibrators, respectively, PBS (blank) or undiluted samples were added to duplicate wells. Plates were sealed and incubated for 2 hr at RT. Plates were washed 5x. HRP-conjugated anti-human IgE was diluted 20,000-fold in blocking buffer and 100 μl was added to each well for 1 hr at RT. Plates were washed 5x. To develop the ELISA, 100 μl Ultra TMB (Pierce, Rockford, IL) was added and plates were placed in the dark at RT for 30 min. Finally, 100 μl stop solution (2M H2SO4) was added and the OD450 was determined.
Sample absorbances were calculated by subtracting the average GST absorbance value from the NC16A-GST absorbance value for each calibrator or sample and Index Units were calculated: index value = (sample absorbance − low calibrator absorbance)/(high calibrator absorbance − low calibrator absorbance) × 100. An index value below zero was observed with similar frequency in our BP and control groups and indicates that the serum exhibited a lower level of absorbance in NC16A-coated wells when compared with that of the low calibrator. Use of an index value limits the effect of plate-to-plate variability so multiple assays can be compared. All sera tested had an OD within the linear range of the plate reader (≤ 2.0).
BP180-specific IgG ELISA
All BP and control sera were screened using a commercially available ELISA for the presence of IgG specific for the NC16A region of BP180 according to manufacturer’s instructions (MBL International, Japan).
Western Blot
IgE and IgG reactivity with GST-NC16A or GST fusion proteins was assayed by immunoblot as previously described (Zillikens et al., 1997a) after optimization using our positive and negative laboratory control sera. Briefly, recombinant GST-NC16A (400 ng for IgE detection; 40 ng for IgG detection) or molar equivalent of GST protein were fractionated under reducing conditions on 10% SDS-PAGE and blotted (100V, 1hr) onto nitrocellulose membrane (Bio-Rad, USA). Filters were stained with fast green and cut into strips of identical size (3 mm × 8 mm) using the molecular weight markers as a guide. Strips were incubated with blocking solution [5% non-fat dry milk, 0.1% Tween 20 in 1 M Tris-buffered saline] for 1 hr at RT. The membrane strips were then incubated overnight at 4°C on a rocking platform in 0.5 ml of serum samples that were optimally diluted in blocking solution (10-fold and 100-fold for IgE and IgG detection, respectively). The membrane strips were washed 3x [0.1% Tween 20 in 1M Tris-buffered saline] and incubated for 1 hour at RT with optimized dilutions of HRP-conjugated isotype-specific anti-human antibody (Bethyl Laboratories, Montgomory, TX; 20,000-fold and 40,000-fold dilutions for IgE and IgG detection, respectively). After 3 washes, the strips were developed using enhanced chemiluminescence (Pierce ECL, Rockford, IL). Positivity was determined using densitometric comparison of NC16A and GST bands based on molecular weight and reactivity with positive control sera.
Immunofluorescent Testing
Circulating anti-BMZ autoantibodies were detected by indirect immunofluorescence (IIF) using 4 um cryosections normal human skin as a substrate and serial dilutions of the patient’s serum as a primary antibody as described previously (Dimson et al., 2003). For detection, FITC-conjugated anti-human IgG (1:2000) or IgE (1:500) (Bethyl Laboratories, Montgomery, TX) was used as a secondary antibody. Slides were viewed with a Nikon photomicroscope equipped for epifluorescence.
Statistical Analysis
Statistical analysis was performed in consultation with the University of Iowa Department of Biostatistics using GraphPad InStat 3.06, GraphPad Software (San Diego, CA). A receiver operating characteristic (ROC) curve was used to evaluate the ability of the ELISA to detect IgE autoantibodies against NC16A. The area under the curve is reported with the 95% confidence interval (95% CI). To assess the reproducibility of the ELISA results, coefficients of variance were calculated for the high calibrator. The inter-assay variability of the OD500 readings from 6 ELISAs, performed on different days was 7.2%. Analysis revealed that groups were not normally distributed, so non-parametric methods were applied. For comparison of control, SLE, EBA and BP sera, the Kruskal-Wallis test was followed by Dunn’s multiple comparisons. A Mann Whitney test was used to compare control to BP sera for both the IgE and IgG ELISA’s. An association between IgE and IgG positivity was conducted using the Spearman’s rank correlation test. In all cases p≤0.05 was considered significant. In the figures, each data point represents the mean determination of duplicate or triplicate samples.
RESULTS
Validation of NC16A-specific IgE ELISA
Conditions for the NC16A-specific IgE ELISA were optimized as described in Materials and Methods. Sera from BP patients or age- and gender-matched controls were tested for IgE reactivity to NC16A. The descriptive statistics for the BP and control groups are shown in Table I. The diagnostic performance of the ELISA was illustrated using ROC analysis, which is a plot of the test sensitivity versus 1-specificity (Figure 1). The performance of the test as measured by the area under the curve was determined to be 0.8763 (SE = 0.0426; 95% CI = 0.7974 to 0.9553) (p<0.0001).
Table I.
Descriptive measures for NC16A-specific IgE ELISA
| Measurea | Controlb | Bullous pemphigoid |
|---|---|---|
| Sample size | 55 | 43 |
| Mean (SE) | 0.992 (1.258) | 94.729 (25.382)c |
| Median | 1.000 | 42.000 |
| Minimum | −21.000 | −20.000 |
| Maximum | 31.000 | 751.000 |
test variable is expressed as Index Units based on internal positive and negative controls
matched by age (±3 years) and gender with no known autoimmune disease
p<0.001
Figure 1. Sensitivity and specificity of theNC16A-specific IgE ELISA.
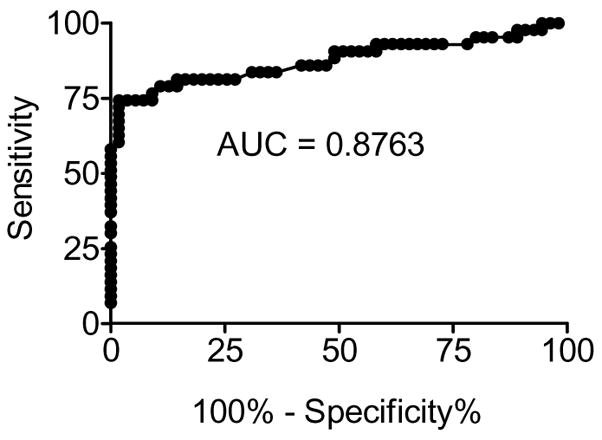
The diagnostic properties of the NC16A-specific IgE ELISA are depicted as a receiver operating characteristic curves for bullous pemphigoid (BP) sera. Sera of patients with active BP (n=43) and age/gender-matched normal controls (C; n=55) with no known autoimmune disease were screened for NC16A-GST or GST.
ROC analysis generates paired sensitivity and specificity values for selected cutoff values to ensure accurate diagnostic performance. Based on the maximization of the Youden’s index (J = sensitivity + specificity-1), the optimum cut-off point for a positive result was selected to be = 18.92 Index Units which corresponds to a Youden index of 0.726 (Table II). The selected cut-off resulted in a sensitivity of 74.42% and a specificity of 98.18%. For comparison, alternative threshold values and their corresponding J, sensitivity and specificity values are also shown (Table II). After cross-validation to reduce bias, analysis of sera using our selected cut-off revealed that 33 of 43 BP patients (77%) and 1 of 55 controls (1.8%) had detectable levels of BP180-specific IgE (Fig. 2).
Table II.
ELISA performance based on cut-off valuea
| threshold | sensitivity | specificity | Jb |
|---|---|---|---|
| 13.78 | 74.42 | 90.91 | 0.6533 |
| 16.14 | 74.42 | 94.55 | 0.6897 |
| 18.97c | 74.427 | 98.18 | 0.726 |
| 19.52 | 69.77 | 98.18 | 0.6795 |
| 22.50 | 65.12 | 98.18 | 0.633 |
The test variable is expressed as index value based on internal positive and negative calibrators.
Youden’s Index
Cut-off selected for data analysis
Figure 2. Diagnostic value of the NC16A-specific IgE ELISA.
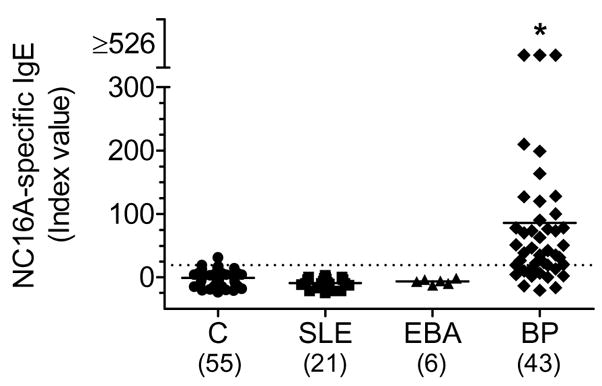
BP and control sera (from patients with systemic lupus erythematosus (SLE); epidermolysis bullosa acquisita (EBA); and normal healthy controls (C)) were incubated with immobilized NC16A-GST fusion protein or recombinant GST. Each serum was tested in duplicate with both proteins and the index value was calculated based on internal positive and negative controls. * = p<0.001 as determined by Kruskal-Wallis test and Dunn’s Multiple Comparsions post-test.
NC16A specific IgE in BP
Once the cut-off value for a positive test was established, a panel of sera from patients with other cutaneous (EBA) and systemic (SLE) autoimmune diseases was assayed for NC16A-specific IgE to confirm the diagnostic value of the NC16A-specific IgE ELISA (Fig. 2). In contrast to the BP sera, none of the sera from patients with a diagnosis of SLE or EBA were positive for NC16A-specific IgE by this ELISA. Non-parametric analysis revealed that the distribution of IgE index values obtained with BP sera was significantly different from those of the other groups (p<0.001).
Comparison of NC16A-specific IgE and IgG
Evaluation of circulating BP180-specific IgG using commercial ELISA kits suggest that autoantibody titres parallel the severity of clinical disease levels (Amo et al., 2001; Tsuji-Abe et al., 2005). For comparison, we assayed all of our BP (n=43) and control (n=55) sera for NC16A-specific IgG using the commercially available kit (Fig. 3A, B). As expected, a high percentage of BP sera (35 of 43, 81%) contained IgG autoantibodies that recognized NC16A, and nearly all of the BP sera in this study (41 of 43, 95%) were positive for NC16A immunoreactivity for at least one of the two isotypes (IgE or IgG). Of the control sera assayed, less than 2% and 7% had detectable levels of NC16A-specific IgE or IgG, respectively.
Figure 3. NC16A-specific IgE and IgG are present with similar frequency in BP sera.
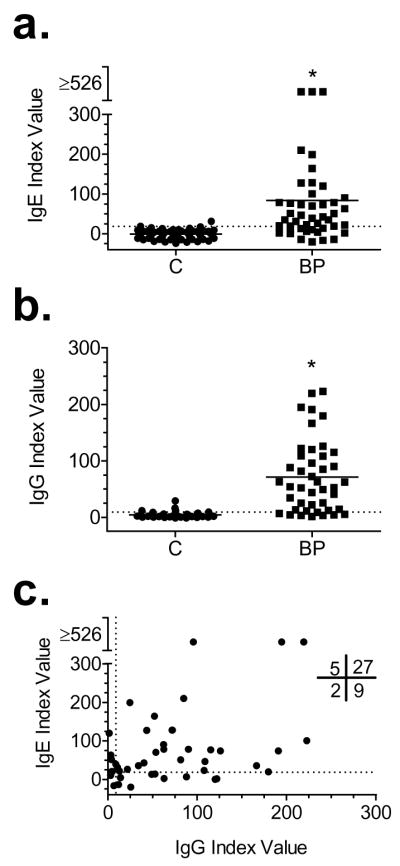
BP and control (C) sera were assayed by ELISA for IgE (panel A) and IgG (panel B) autoantibodies specific for NC16A. For IgE, reactivity to control GST fusion protein was also assayed and the difference of the means of OD450 with GPP-NC16A-GST and recombinant GST were determined. For both IgE and IgG Index values were calculated based on internal positive and negative controls. Correlation between NC16A-specific IgE and IgG index values (panel C). Each serum was tested in duplicate and data points represent the mean determination for each sample. The numbers in the quadrants indicate the numbers of patients positive or negative for the corresponding Abs. Mann-Whitney test p<0.0001 compared to control (C) for IgG or IgE.
Next, we determined if a correlation existed between the IgE and IgG reactivity to NC16A exhibited by BP sera. When our ELISA results are analyzed as a categorical data set (i.e., positive/negative, inset Fig. 3C), a there is a positive correlation (p =0.016) for the simultaneous detection of both IgE and IgG class autoantibodies in a given sera. In contrast, analysis of our scalar data set (index values) does not indicate a significant correlation (p=0.0537).
Comparison of autoantibody detection by ELISA, immunoblot or IIF
A subset of 13 BP sera were analyzed for the presence of both IgG and IgE autoantibodies using, in addition to the NC16A-specific ELISAs described above, two other immunological techniques, immunoblotting and IIF (Table III). In agreement with a previous study of BP IgG (Zillikens et al., 1997a), ELISA was the most sensitive of these methods, and this was true for both IgG and IgE detection. Twelve of these 13 BP sera were positive by ELISA for both IgE and IgG NC16A-specific autoantibodies. For IgG, the IIF and immunoblot methods each yielded positive results for 11 of the 13 patients’ sera. Only one BP serum was negative in both the IIF and immunoblot assays. For IgE, immunoblotting with recombinant NC16A was of intermediate sensitivity, yielding a positive result for 9 of the 13 BP sera. Using the IIF method on normal skin, less than half (5 of 13) of the BP sera were positive for IgE autoantibody reactivity with the BMZ. Interestingly, all of the patients who were positive for IgE autoantibodies by IIF or immunoblot also had IgG autoantibodies by that same method.
Table III.
Immunoassay profile of bullous pemphigoid patients
| Indirect IFa | Immunoblotb | ELISAc | ||||
|---|---|---|---|---|---|---|
| patient | IgE | IgG | IgE | IgG | IgE | IgG |
| 1000 | + | + | − | + | + | + |
| 1001 | + | + | + | + | + | + |
| 1002 | − | − | + | + | + | + |
| 1003 | − | + | − | + | − | + |
| 1004 | − | + | + | + | + | + |
| 1005 | − | + | + | + | + | + |
| 1006 | − | − | − | − | + | − |
| 1007 | − | + | − | − | + | + |
| 1008 | − | + | + | + | + | + |
| 1009 | + | + | + | + | + | + |
| 1010 | − | + | + | + | + | + |
| 1011 | + | + | + | + | + | + |
| 1012 | + | + | + | + | + | + |
| positive | 5/13 | 11/13 | 9/13 | 11/13 | 12/13 | 12/13 |
Indirect immunofluorescence conducted on normal human skin discarded from surgical procedures with FITC conjugated anti IgG or IgE compared to positive and negative control serum
Epidermal extract proteins were used for immunoblot in combination with HRP-conjugated anti IgG or IgE
reactivity to recombinant NC16A region of BP180
The effect of serum dilution on autoantibody detection
We and others have assayed for IgG reactivity to BP180 using patient and control sera diluted 100-fold (or greater), and have found specific reactivity in a high percentage of BP sera (Amo et al., 2001; Hofmann et al., 2002; Fairley et al., 2005; Ishiura et al., 2008; Iwata et al., 2008). Rigorous optimization of our IgE ELISA revealed that testing the BP and control sera undiluted resulted in the most reliable, sensitive and specific assay. As stated above, our ELISA detected BP180-specific IgE autoantibodies in about 74% of the BP patients in our study, a frequency that is much higher than most other reports.
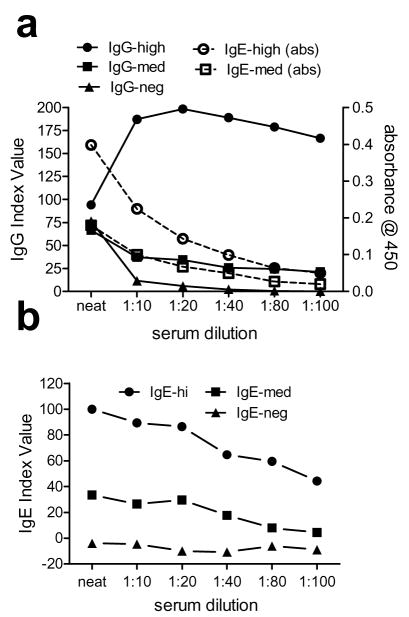
Based on these findings, we examined the effects of serum dilution on sensitivity and specificity in both the IgG and IgE ELISAs. Samples consisted of a BP serum with high levels of anti-NC16A autoantibodies of both isotypes, a BP serum with intermediate levels of both autoantibody isotypes and a negative control serum. The sera were tested undiluted (neat) or serially diluted 10-, 20-, 40-, 80- and 100-fold in assay diluent provided with the commercially available ELISA (Fig. 4).
Figure 4. Effect of serum dilution on ELISA sensitivity.
Samples consisted of a BP serum with high levels of both IgG and IgE, a BP serum with intermediate (med) levels of both antibody isotypes and a (neg) control. Sera were undiluted or serially diluted 1 to 10, 20, 40, 80 and 100. Index values are shown for the IgG (A) ELISA used as directed (closed symbols) and OD450 is shown for dilutions developed with anti-IgE secondary antibody (open symbols). For comparison, index values over a range of dilutions are shown for the IgE ELISA protocol described in this report (B). Results are representative of two independent experiments and each data point represents the mean of duplicate samples.
Using the commercially available ELISA plates, dilution of the BP serum containing a high level of NC16A-specific IgG did not negatively influence the results; however, dilution of sera containing intermediate levels of specific IgG resulted in decreased sensitivity of the assay (Fig. 4A). Likewise, when anti-IgE secondary was used in combination with the assay plates designed for IgG detection, sample dilution resulted in a dramatic loss of sensitivity (OD, open symbols, Fig. 4A). Interestingly, the undiluted negative control sample had detectable levels of IgG reactivity to GST that were reduced by sample dilution. Using the IgE ELISA protocol described in this report, dilution of sera also results in decreased assay sensitivity (Fig. 4B).
Decrease in NC16A-specfic IgE throughout treatment
The current study indicates that the presence of NC16A-specific IgE is an accurate predictor of BP, and cutaneous autoantibody levels are known to decline with standard immunosuppressive treatments. Therefore, NC16A-specific IgG and IgE were evaluated in serial serum samples obtained at different time points during the course of treatment for comparison to pre-treatment levels. At the time of the initial blood draw (day 0; Fig. 5), each of these patients had recently been diagnosed with BP and had not previously undergone any treatment. These patients were of heterogeneous clinical disease severity. Subsequent blood draws are indicated by the number of days elapsed from the first sample. Although the number of patients is limited, both NC16A-specific IgE and IgG decrease over the treatment course, when an improvement in disease severity is observed (Fig. 5, A, B), and autoantibody levels increase with increased disease severity (Fig. 5, C).
Figure 5. NC16A-specific IgE and IgG levels reflect clinical disease activity.
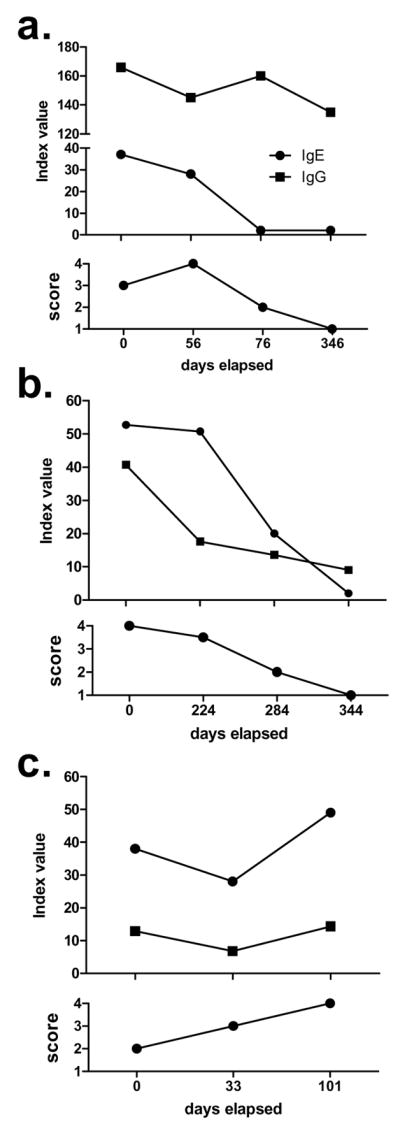
Serum IgE and IgG were assayed on serial blood draws obtained from untreated patients at the time of diagnosis and at various time points thereafter. All patients had active disease at the initial draw (time = 0 days) and responded to treatment and had not been previously treated. Disease activity was rate from 1–4: 4 = generalized disease with multiple new blisters and erosions at multiple sites on the body; 3 = localized active disease; 2 = controlled disease with immunosuppressive medications; 1 = no skin lesions in the absence of immunosuppressive therapy (remission).
DISCUSSION
In this paper, we report the development and characterization of a highly specific and sensitive ELISA for the detection of circulating IgE class autoantibodies to the NC16A domain of BP180. The need for such a protocol is underscored by the recent findings indicating that IgE autoantibodies play a role in BP pathogenesis (Dimson et al., 2003; Fairley et al., 2007). This assay utilizes a recombinant form of NC16A, which is known to contain the major antigenic sites recognized by both IgG and IgE class autoantibodies found in BP patient sera (Zillikens et al., 1997b; Fairley et al., 2005). Using this ELISA, IgE autoantibodies to NC16A were detectable in 33 of 43 (77%) of the BP sera tested. The specificity of this ELISA was calculated to be >98%.
In our hands, this ELISA was found to be more sensitive than immunoblotting or IIF for detecting IgE-class autoantibodies in BP sera. Similarly, in a previous study that compared IgG detection systems, an NC16A-specific ELISA was found to be more sensitive than immunoblotting or IIF (Zillikens et al., 1997a). However, others have reported that an NC16A-specific IgG ELISA was less sensitive than IIF on salt-split skin (Hata et al., 2000; Hofmann et al., 2002; Kobayashi et al., 2002; Kromminga et al., 2004; Mariotti et al., 2004; Sakuma-Oyama et al., 2004; Tsuji-Abe et al., 2005; Yoshida et al., 2006). There are two factors that might account for some of these differences. First, the process of salt-splitting skin creates a separation at the BMZ which is thought to increase antigen exposure, thereby increasing sensitivity of IIF. Second, NC16A is not the only target of BP autoantibodies – there are autoantibodies that react with other sites on BP180 and with another major BP autoantigen, BPAG1, that likely contribute to the IIF signal. In support of this latter point, a recent study that evaluated a small number of BP sera by immunoblotting found a high frequency of both IgG and IgE directed against the intracellular domain of BP180 (Dresow et al., 2009). Despite these factors, it is clear that IIF is inferior to our NC16A ELISA for detecting IgE autoantibodies in BP. We hypothesize that the sensitivity of IIF as a method for detecting BP IgE is compromised by the presence of higher levels of IgG autoantibodies directed against the same BMZ proteins.
Although BP disease activity does not correlate well with IF titers of patient sera (Ahmed et al., 1977; Zillikens et al., 1992), disease activity does show a good correlation with serum levels of anti-BP180 IgG autoantibodies detected by ELISA (Schmidt et al., 2000; Amo et al., 2001). In this study, careful protocol optimization resulted in an ELISA that can be used to easily and reliably screen large numbers of sera for IgE autoantibodies and that is proven in our hands to be more sensitive than IIF. It should be noted that although the majority of both IgG and IgE autoantibodies in BP are specific for the NC16A region of BP180, both IgG and IgE reactivity to sites other than NC16A has also been observed (Zillikens et al., 1997b; Dopp et al., 2000; Dimson et al., 2003; Fairley et al., 2005; Dresow et al., 2009). Thus, it is possible that an NC16A-specific ELISA could underestimate the frequency of BP180 reactivity associated with this disease.
Previous attempts to detect BP180-specific IgE in patient sera employed a commercially available IgG NC16A ELISA kit paired with an IgE-specific secondary antibody (Ishiura et al., 2008; Iwata et al., 2008). Using a similar strategy, Dopp and colleagues (Dopp et al., 2000) assayed for IgE by modifying an ELISA protocol that they had developed to detect NC16A-specific IgG. These other groups report a range of percentages of BP patients with NC16A-specific IgE (20–55%) that is substantially lower than what we report here (77%). These frequency differences are likely due to differences in the manner in which the assays have been optimized. For example, as has been previously shown with ELISA optimization (Sittampalam et al., 1996), dilution of patient sera was found to be a critical parameter in this IgE ELISA. Inadequate dilution of a very high titre sample could lead to an underestimate of antibody levels due to a limited access to antigen and, conversely, over-dilution of a low titer sample could result in loss of specific signal. In the aforementioned reports on NC16A ELISA’s, the patient sera were diluted as much as 50 to 100-fold for IgE detection, which, in our hands, results in decreased ability to detect IgE reactivity. A sub-optimal serum dilution could also account for the decreased sensitivity of IIF and immunoblot detection of circulating IgE, leading to an underestimate of the frequency of IgE class cutaneous autoantibodies.
Other reports have suggested that the presence of NC16A-specific IgE, in addition to IgG, identifies a subset of patients with a more severe form of BP that might require more aggressive therapeutic treatment (Delaporte et al., 1996; Dopp et al., 2000; Iwata et al., 2008). In the current study, NC16A-specific IgE and IgG are shown to be present in BP sera with similarly high frequencies and often simultaneously. Assuming that the majority of previous reports underestimated IgE reactivity frequencies due to sub-optimal assay sensitivities, we propose a modification of their conclusion: a more severe disease phenotype might be associated with high circulating IgE anti-NC16A levels. Moreover, our studies are in agreement with others (Dopp et al., 2000) suggesting that, similar to IgG, IgE autoantibody levels parallel disease activity and, thus, may provide more specific targets for therapeutic intervention in BP. Particularly noteworthy, our group has very recently published a report on the successful treatment of a steroid-unresponsive BP patient with omalizumab, a humanized monoclonal antibody that inhibits IgE binding to the high affinity IgE receptor, FcεR1 (Fairley et al., 2009). Furthermore, the lack of strong correlation in the magnitude of IgG and IgE index values in the current study suggests it is likely that a BP serum may have high levels of NC16A-specific IgE and low or undetectable levels of IgG, or vice versa. Taken together, these findings support the conclusion that both IgG and IgE-class autoantibodies are involved in the pathogenesis of BP.
The ELISA protocol presented in this report provides a convenient and sensitive method by which IgE autoantibodies directed against NC16A can be quantitated. The use of internal standards and calculation of an index value allow for comparison of IgE levels over time. Our data also suggest that evaluation of NC16A-specific IgE, in addition to IgG, may provide better a better indicator of disease severity and predictor of treatment efficacy than evaluation of either autoantibody isotype alone.
Acknowledgments
We would like to gratefully recognize Amber James and Marleen Janson for their technical assistance and Deb Brandt for serum collection and IRB assistance. This work was supported by the Biological Sciences Funding Program at the University of Iowa (KNM), a Merit Review Award from the Veterans Administration (JAF), NIH AR040410 (GJG), the University of Iowa CTSA NIH/NCRR grant 1UL1RR024979 (MHN) and the Doris Duke Charitable Foundation (MHN).
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Abbreviations used in this manuscript: BP, Bullous pemphigoid; NC16A, non-collagenous 16A domain of BP180; GST, glutathione-S-transferase; ELISA, enzyme-linked immunosorbent assay; EBA, epidermolysis bullosa acquisita; SLE, systemic lupus erythematosus; ROC, receiver operator curve; IF, immunofluorescence; IIF, indirect immunofluorescence.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed AR, Maize JC, Provost TT. Bullous pemphigoid. Clinical and immunologic follow-up after successful therapy. Archives of Dermatology. 1977;113:1043–1046. doi: 10.1001/archderm.113.8.1043. [DOI] [PubMed] [Google Scholar]
- Amo Y, Ohkawa T, Tatsuta M, Hamada Y, Fujimura T, Katsuoka K, Hashimoto T. Clinical significance of enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. Journal of Dermatological Science. 2001;26:14–18. doi: 10.1016/s0923-1811(00)00149-3. [DOI] [PubMed] [Google Scholar]
- Christophoridis S, Budinger L, Borradori L, Hunziker T, Merk HF, Hertl M. IgG, IgA and IgE autoantibodies against the ectodomain of BP180 in patients with bullous and cicatricial pemphigoid and linear IgA bullous dermatosis. British Journal of Dermatology. 2000;143:349–355. doi: 10.1046/j.1365-2133.2000.03661.x. [DOI] [PubMed] [Google Scholar]
- Delaporte E, Dubost-Brama A, Ghohestani R, Nicolas JF, Neyrinck JL, Bergoend H, Janin A, Capron M. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. Journal of Immunology. 1996;157:3642–3647. [PubMed] [Google Scholar]
- Dimson OG, Giudice GJ, Fu CL, Van den Bergh F, Warren SJ, Janson MM, Fairley JA. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. Journal of Investigative Dermatology. 2003;120:784–788. doi: 10.1046/j.1523-1747.2003.12146.x. [DOI] [PubMed] [Google Scholar]
- Dopp R, Schmidt E, Chimanovitch I, Leverkus M, Brocker EB, Zillikens D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in Bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. Journal of the American Academy of Dermatology. 2000;42:577–583. [PubMed] [Google Scholar]
- Dresow SK, Sitaru C, Recke A, Oostingh GJ, Zillikens D, Gibbs BF. IgE autoantibodies against the intracellular domin of BP180. British Journal of Dermatology. 2009;160:429–432. doi: 10.1111/j.1365-2133.2008.08858.x. [DOI] [PubMed] [Google Scholar]
- Fairley JA, Baum CL, Brandt DS, Messingham KAN. Pathogenicity of IgE in Autoimmunity: Successful Treatment of Bullous Pemphigoid with Omalizumab. Journal of Autoimmunity and Cellular Immunology. 2009;123:704–705. doi: 10.1016/j.jaci.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley JA, Burnett CT, Fu CL, Larson DL, Fleming MG, Giudice GJ. A Pathogenic Role for IgE in Autoimmunity: Bullous Pemphigoid IgE Reproduces the Early Phase of Lesion Development in Human Skin Grafted to nu//nu Mice. Journal of Investigative Dermatology. 2007;127:2605–2611. doi: 10.1038/sj.jid.5700958. [DOI] [PubMed] [Google Scholar]
- Fairley JA, Fu CL, Giudice GJ. Mapping the binding sites of anti-BP180 immunoglobulin E autoantibodies in bullous pemphigoid. Journal of Investigative Dermatology. 2005;125:467–472. doi: 10.1111/j.0022-202X.2005.23853.x. [DOI] [PubMed] [Google Scholar]
- Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. Journal of Investigative Dermatology. 1992;99:243–250. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. Journal of Immunology. 1993;151:5742–5750. [PubMed] [Google Scholar]
- Hata Y, Fujii Y, Tsunoda K, Amagai M. Production of the entire extracellular domain of BP180 (type XVII collagen) by baculovirus expression. Journal of Dermatological Science 23. 2000:183–190. doi: 10.1016/s0923-1811(00)00074-8. [DOI] [PubMed] [Google Scholar]
- Hofmann S, Thoma-Uszynski S, Hunziker T, Bernard P, Koebnick C, Stauber A, Schuler G, Borradori L, Hertl M. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. Journal of Investigative Dermatology. 2002;119:1065–1073. doi: 10.1046/j.1523-1747.2002.19529.x. [DOI] [PubMed] [Google Scholar]
- Ishiura N, Fujimoto M, Watanabe R, Nakashima H, Kuwano Y, Yazawa N, Echigo T, Okochi H, Tamaki K. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. Journal of Dermatological Science. 2008;49:153–161. doi: 10.1016/j.jdermsci.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Komura K, Kodera M, Usuda T, Yokoyama Y, Hara T, Muroi E, Ogawa F, Takenaka M, Sato S. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Archives of Dermatology. 2008;144:41–48. doi: 10.1001/archdermatol.2007.9. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, Hashimoto T, Shirakata Y, Hashimoto K, Nishikawa T. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. Journal of Dermatological Science. 2002;30:224–232. doi: 10.1016/s0923-1811(02)00109-3. [DOI] [PubMed] [Google Scholar]
- Kromminga A, Sitaru C, Hagel C, Herzog S, Zillikens D. Development of an ELISA for the detection of autoantibodies to BP230. Clinical Immunology. 2004;111:146–152. doi: 10.1016/j.clim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Liu Z, Diaz LA, Swartz SJ, Troy JL, Fairley JA, Giudice GJ. Molecular mapping of a pathogenically relevant BP180 epitope associated with experimentally induced murine bullous pemphigoid. Journal of Immunology. 1995;155:5449–5454. [PubMed] [Google Scholar]
- Liu Z, Sui W, Zhao M, Li Z, Li N, Thresher R, Giudice GJ, Fairley JA, Sitaru C, Zillikens D, Ning G, Marinkovich MP, Diaz LA. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. Journal of Autoimmunity. 2008;31:331–338. doi: 10.1016/j.jaut.2008.08.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti F, Grosso F, Terracina M, Ruffelli M, Cordiali-Fei P, Sera F, Zambruno G, Mastrogiacomo A, Di Zenzo G. Development of a novel ELISA system for detection of anti-BP180 IgG and characterization of autoantibody profile in bullous pemphigoid patients. British Journal of Dermatology. 2004;151:1004–1010. doi: 10.1111/j.1365-2133.2004.06245.x. [DOI] [PubMed] [Google Scholar]
- Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, Sakai K, Nakamura H, Olasz E, Yancey KB, Akiyama M, Shimizu H. Humanization of autoantigen. Nature Medicine. 2007;13:378–383. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- Olansky AJ, Briggaman RA, Gammon WR, Kelly TF, Sams WM., Jr Bullous systemic lupus erythematosus. Journal of the American Academy of Dermatology. 1982;7:511–520. doi: 10.1016/s0190-9622(82)70134-3. [DOI] [PubMed] [Google Scholar]
- Sakuma-Oyama Y, Powell AM, Oyama N, Albert S, Bhogal BS, Black MM. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. British Journal of Dermatology. 2004;151:126–131. doi: 10.1111/j.1365-2133.2004.06082.x. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Obe K, Brocker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Archives of Dermatology. 2000;136:174–178. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- Sittampalam GS, Smith WC, Miyakawa TW, Smith DR, McMorris C. Application of experimental design techniques to optimize a competitive ELISA. Journal of Immunological Methods. 1996;190:151–161. doi: 10.1016/0022-1759(95)00262-6. [DOI] [PubMed] [Google Scholar]
- Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. Journal of Clinical Investigation. 1988;82:1864–1870. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji-Abe Y, Akiyama M, Yamanaka Y, Kikuchi T, Sato-Matsumura KC, Shimizu H. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. Journal of Dermatological Science. 2005;37:145–149. doi: 10.1016/j.jdermsci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Van den Bergh F, Fu CL, Olague-Marchan M, Giudice GJ. The NC16A domain of collagen XVII plays a role in triple helix assembly and stability. Biochemical & Biophysical Research Communications. 2006;350:1032–1037. doi: 10.1016/j.bbrc.2006.09.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, Imamura K, Okamoto E, Yasumoto S, Hashimoto T. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. Journal of Dermatological Science. 2006;41:21–30. doi: 10.1016/j.jdermsci.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Zillikens D, Ambach A, Schuessler M, Dummer R, Hartmann AA, Burg G. The interleukin-2 receptor in lesions and serum of bullous pemphigoid. Archives of Dermatological Research. 1992;284:141–145. doi: 10.1007/BF00372706. [DOI] [PubMed] [Google Scholar]
- Zillikens D, Mascaro JM, Rose PA, Liu Z, Ewing SM, Caux F, Hoffmann RG, Diaz LA, Giudice GJ. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. Journal of Investigative Dermatology. 1997a;109:679–683. doi: 10.1111/1523-1747.ep12338088. [DOI] [PubMed] [Google Scholar]
- Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, Giudice GJ. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. Journal of Investigative Dermatology. 1997b;109:573–579. doi: 10.1111/1523-1747.ep12337492. [DOI] [PubMed] [Google Scholar]
- Zone JJ, Taylor T, Hull C, Schmidt L, Meyer L. IgE Basement Membrane Zone Antibodies Induce Eosinophil Infiltration and Histological Blisters in Engrafted Human Skin on SCID Mice. Journal of Investigative Dermatology. 2007;127:1167–1174. doi: 10.1038/sj.jid.5700681. [DOI] [PubMed] [Google Scholar]