Abstract
Chronic alcohol intake leads to insulin resistance and alcoholic cardiomyopathy, which appears to be a result of the complex interaction between genes and environment. This study was designed to examine the impact of aldehyde dehydrogenase-2 (ALDH2) transgenic overexpression on alcohol-induced insulin resistance and myocardial injury. ALDH2 transgenic mice were produced using chicken β-actin promoter. Wild-type FVB and ALDH2 mice were fed a 4% alcohol or control diet for 12 wks. Cell shortening was evaluated using an edge-detection system. Western blot analysis was used to assess insulin signaling at the levels of receptor, IRS, Akt, GSK-3β, the transcription factors Foxo3a, c-Jun amino-terminal kinase (JNK) and c-Jun. Chronic alcohol intake led to glucose intolerance, reduced glucose uptake, cardiac hypertrophy and reduced cell shortening, the effects of which were alleviated by ALDH2. ALDH2 significantly attenuated alcohol-induced decrease in the insulin-stimulated tyrosine phosphorylation and increase in serine phosphorylation of IRS. Phosphorylation of Akt, GSK-3β and Foxo3a was reduced following alcohol intake, the effect of which was abrogated by ALDH2. Levels of JNK, c-Jun and their phosphorylation were elevated following chronic alcohol intake, which were obliterated by ALDH2. Transfection of H9C2 myoblast cells with Foxo3a adenovirus mimicked acetaldehyde-induced JNK activation and glucose uptake defect whereas the dominant negative Foxo3a ablated acetaldehyde-elicited insulin insensitivity. In addition, ALDH2 reversed alcohol-induced myocardial ER stress. These data revealed that ALDH2 overexpression antagonizes chronic alcohol intake-induced cardiac insulin insensitivity and contractile defect, possibly via improvement of insulin signaling at the levels of insulin receptor, IRS, Akt, Foxo3a and JNK.
Keywords: Alcohol, ALDH2, cardiomyocytes, insulin signaling
INTRODUCTION
While the light to moderate use of alcohol is known to display a beneficial effect for cardiovascular health, chronic consumption of large amount of alcohol results in devastating alcoholic organ damages including alcoholic cardiomyopathy manifested as cardiac hypertrophy and contractile dysfunction [1–3]. The chronic alcohol consumption-induced detrimental health consequences appear to be a result of complex interaction between genes and environment [3]. A number of clinical studies have revealed elevated levels of acetaldehyde, the first oxidized metabolite of ethanol, following chronic alcohol ingestion, especially in Asian and African American populations with defective aldehyde dehydrogenase (ALDH) [4;5]. More evidence has indicated that genetic polymorphism in the principal alcohol metabolizing enzymes [e.g., alcohol dehydrogenase (ADH) and ALDH] contributes to the differential blood acetaldehyde levels and risk of alcoholic complications following alcohol intake [3]. Acetaldehyde has been shown to directly compromise cardiac excitation-contraction coupling and sarco(endo)plasmic reticulum Ca2+ release [6–8], consistent with the observation of accentuated cardiac hypertrophy and contractile defect in mice with cardiac overexpression of ADH following chronic alcohol intake [4;9;10]. These data have prompted the “acetaldehyde toxicity” theory in the pathogenesis of alcoholic cardiomyopathy.
To better delineate the role of acetaldehyde and alcohol metabolism in alcoholic cardiomyopathy, transgenic mice with a systemic overexpression of the mitochondrial isoform of ALDH (ALDH2) were used to examine the impact of facilitated acetaldehyde detoxification on chronic alcohol intake-induced cardiac contractile dysfunction and insulin signaling. Elevated ALDH activity is known to decrease the circulating acetaldehyde levels by metabolizing it to acetate. Insulin receptor, insulin receptor substrate (IRS) and their phosphorylation, along with the downstream signaling molecule Akt, GSK-3β and the nuclear transcriptional factor Foxo3a were evaluated. Given the essential role of Foxo3a phosphorylation in myocardial Akt-GSK-3β signaling, survival and growth [11;12], ex vivo adenoviral transfection was performed with wild-type or dominant negative-Foxo3a virus to examine the role of Foxo3a in acetaldehyde-elicited effect on insulin signaling. We also evaluated the transcription factor c-Jun given its role in cardiac physiology and its likely activation by insulin resistance-induced oxidative stress [13]. Since c-Jun amino-terminal kinase (JNK) and endoplasmic reticulum (ER) stress are both known to interfere with insulin signaling and directly contribute to cardiac contractile dysfunction [14;15], JNK, JNK phosphorylation and the crucial ER stress protein markers such as eukaryotic translation initiation factor (eIF2α), inositol requiring enzyme-1 (IRE-1), GRP78 and gadd153 were also monitored in myocardium of ALDH and wild-type FVB mice following chronic alcohol intake. Our results revealed that ALDH2 overexpression significantly attenuated chronic alcohol intake-induced cardiac contractile dysfunction and loss of insulin sensitivity at the levels of insulin receptor, IRS, and post-receptor signaling including Akt, GSK-3β and Foxo3a. In addition, Foxo3a adenovirus mimicked acetaldehyde-induced JNK activation and glucose uptake defect whereas dominant negative Foxo3a ablated acetaldehyde-elicited insulin insensitivity. These data suggest a link between chronic alcohol intake and compromised myocardial insulin sensitivity, where acetaldehyde, the Foxo3a transcriptional factor and JNK signaling may play key roles.
MATERIALS AND METHODS
Generation of ALDH2 transgenic mice
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. The human ALDH2 gene was amplified by PCR from pT7–7-hpALDH2 (provided by Dr. Henry Weiner from Purdue University, Lafayette, IN) using the following primers: ALDH-F (5′-tcgaattctatgttgcgcgctgccgcccg) and ALDH-R (3′-cacggagtcttcttgagtattcttaaggc). The amplified ALDH2 fragment was digested with EcoRI and cloned into the EcoRI site of vector pBsCAG-2, where ALDH activity was increased using the chicken β-actin promoter to produce a systemic overexpression [16]. The ALDH2 insert (Fig. 1A) was excised, separated, purified and microinjected into a one-cell embryo of the inbred strain FVB. Around 30 microinjected embryos were implanted into each pseudopregnant female and allowed to come to term. After weaning, mice tail clips were collected for genotyping of DNA insertion of ALDH2 (Fig. 1B). All mice were housed in a temperature-controlled room under a 12hr/12hr-light/dark and allowed access to tap water ad libitum. Four month-old adult male FVB and ALDH2 (F8) mice were placed on a nutritionally complete liquid diet (Shake & Pour Bioserv Inc., Frenchtown, NJ) [17] for a one-week acclimation period. Upon completion of the acclimation period, half of the FVB and ALDH2 mice were maintained on the regular liquid diet and the remaining half began a 12-week period of isocaloric 4% (vol/vol) ethanol diet feeding. A pair-feeding regimen was employed to eliminate possible nutritional deficits [9]. (Detailed information is available on the online supplement)
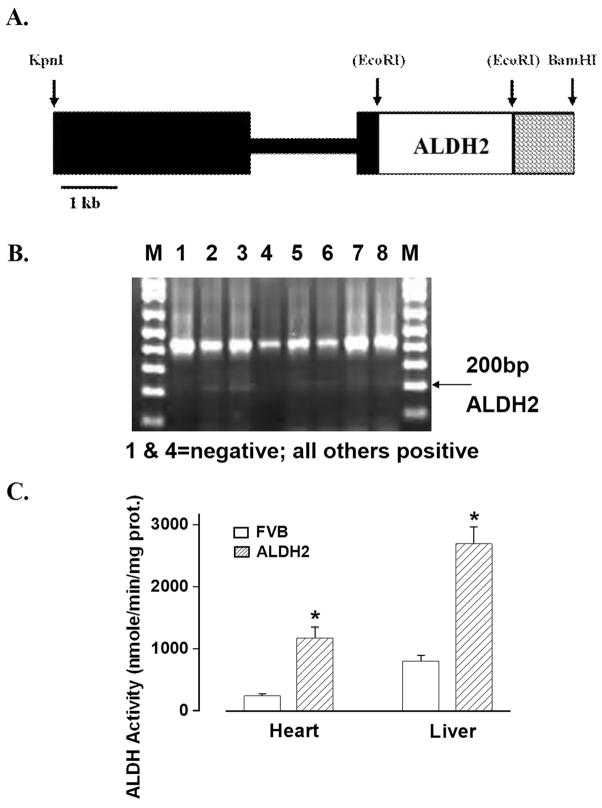
Fig. 1.
(A). Schematic of ALDH2 transgene: The black boxes include promoter of the chicken βactin gene. The open box contains the full length human ALDH2 cDNA. The cross hatched box contains the polyadenylation site of the rat insulin II gene. Some restriction sites used in construction are shown. Sites in parenthesis were destroyed in construction; (B). PCR identification of ALDH2 transgenic mice using genomic DNA isolated from 2 cm tail clips of 1-month-old mice. Lanes 1 and 4 are negative and the rest are positive for ALDH2 gene. M: marker. (C) ALDH activity from hearts and livers in FVB and ALDH2 mice. Mean ± SEM, n = 5 – 6 mice per group, *p < 0.05 vs. FVB group.
ALDH enzymatic activity
ALDH activity was measured in heart and liver homogenates at 25°C in 33 mM sodium pyrophosphate containing 0.8 mM NAD+, 15 μM propionaldehyde and 0.5 ml of tissue homogenates. Propionaldehyde, the substrate of ALDH2, was oxidized into propionic acid by ALDH2, while NAD+ was reduced to NADH to quantitatively indicate the ALDH activity. Production of NADH was determined by spectrophotometric absorbance at 340 nm. ALDH activity was expressed as nmole NADH/min/mg protein [18].
Intraperitoneal glucose tolerance test (IPGTT)
At the end of the 12-week feeding period, FVB and ALDH2 mice were fasted for 12 hrs and were given an intraperitoneal injection of glucose (2 g/kg b.w.). Blood samples were drawn from the tail immediately before the glucose challenge, as well as 15, 30, 60 and 120 min thereafter. Serum glucose levels were determined using an Accu-Chek III glucose analyzer [19].
Measurement of blood ethanol and acetaldehyde levels
On the last day (8–9 AM) of diet feeding, mice were sacrificed under anesthesia (ketamine/xylazine: 3:1, 1.32 mg/kg, i.p.). Blood was collected and was stored in sealed vials. A volume of 100 μl plasma from each sample was put into an autosampler vial. Six microliter of n-propanol and 194 μl H2O were then added to the vial. Following a 20-min incubation at 50°C, 50 μl aliquot of headspace gas was removed and transferred to an Agilent 6890 Gas Chromatograph (Agilent Technologies, Inc, Wilmington, DE) equipped with a flame ionization detector. Ethanol, n-propanol and other components such as acetaldehyde were separated on a 60 m VOCOL capillary column (Supelco Inc., Bellefonte, PA) with film of 1.8 μm in thickness and an inner diameter of 320 μm. The carrier gas was helium at a flow rate of 18.0 ml/min. Quantitation was achieved by calibrating peak areas against those from headspace samples of known ethanol standards [9].
Isolation of murine cardiomyocytes
After ketamine/xylazine sedation, hearts were removed and perfused with Krebs-Henseleit bicarbonate (KHB) buffer containing (in mM): 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES and 11.1 glucose. Hearts were digested with 223 U/ml collagenase D for 20 min. Left ventricles were removed and minced before being filtered. Only rod-shaped myocytes with clear edges were selected for mechanical study [9].
Cell shortening/relengthening
Mechanical properties of cardiomyocytes were assessed using an IonOptix™ soft-edge system (IonOptix, Milton, MA). Myocytes were placed in a chamber mounted on the stage of an Olympus IX-70 microscope and superfused (~2 ml/min at 25°C) with a KHB buffer containing 1 mmol/l CaCl2. Myocytes were field stimulated at 0.5 Hz unless otherwise stated. Cell shortening and relengthening were assessed including peak shortening (PS), time-to-PS (TPS), time-to-90% relengthening (TR90) and maximal velocities of shortening/relengthening (± dL/dt) [9]. To assess insulin-stimulated activation of insulin signaling cascade, a cohort of freshly isolated cardiomyocytes were exposed to 10 nM insulin for 15 min before protein was extracted.
Ex vivo wild-type (WT) and dominant negative (DN) Foxo3a transfection
For ex vivo Foxo3a transfection study, H9C2 myoblast cells (ATCC, Manassas, VA) were first incubated at 37°C in the DMEM medium (5.5 mM glucose and 10% fetal bovine serum) with or without WT-Foxo3a [multiplicity of infection (MOI): 60] or DN-Foxo3a (MOI: 30) adenovirus for 6 hrs before switching to a normal medium for an additional 12 hrs with or without acetaldehyde (200 μM). The dosage and treatment duration for Foxo3a virus and acetaldehyde were based on our previous experience [11;20]. The WT-Foxo3a and DN-Foxo3a virus were purchased from Vector Biolabs (Philadelphia, PA), the latter is a truncated version of Foxo3a (50 KD) which is devoid of the transactivation domain from the C-terminus (D256) of the full-length Foxo3a (97 KD). It was shown previously that the truncated Foxo may function as a dominant-negative inhibitor of transcription induced by Foxo3a [21]. Proteins were extracted from H9C2 cells as described [11].
Glucose uptake measurement
The cardiomyocytes were washed 3 times with Krebs-Ringer-N-[2-hydro-ethyl]-piperazine-N′-[2-ethanesulfonic acid] (HEPES) (KRH, 136 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 1.25 mM MgSO4, 10 mM HEPES, pH 7.4) buffer and incubated with 2 ml KRH buffer at 37°C for 30 min. Cardiomyocytes were exposed to insulin (10 nM) for 15 min prior to the initiation of glucose uptake assay with addition of 0.1 ml KRH buffer, 2-deoxy-D[3H] glucose (0.2 μCi/ml with a specific activity of 10 Ci/mmol) and 5 mM glucose. Glucose uptake was terminated 30 min later by washing the cells 3 times with cold PBS. Our earlier observation indicated that 2-deoxy-D-[3H] glucose uptake is still linear over the 30 min duration [19]. The cells were lysed overnight with 0.5 ml 0.5 M NaOH and 0.1% SDS (w/v). The radioactivity retained by cell lysates was determined by a scintillation counter (1 cpm = 0.888 × 10−12 Ci, Beckmann LC 6000IC) and was normalized to protein content [19].
Western blot analysis
Membrane proteins were separated on SDS-polyacrylamide gels and were transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% milk and then incubated overnight with specific antibodies. The antigens were detected by the luminescence method [19]. (Detailed information is available on the online supplement)
Data analysis
Data were presented as mean ± SEM. Statistical significance (p < 0.05) for each variable was estimated by analysis of variance (ANOVA) followed by a Tukey’s post hoc analysis.
RESULTS
General feature of mice, intraperitoneal glucose tolerance and glucose uptake
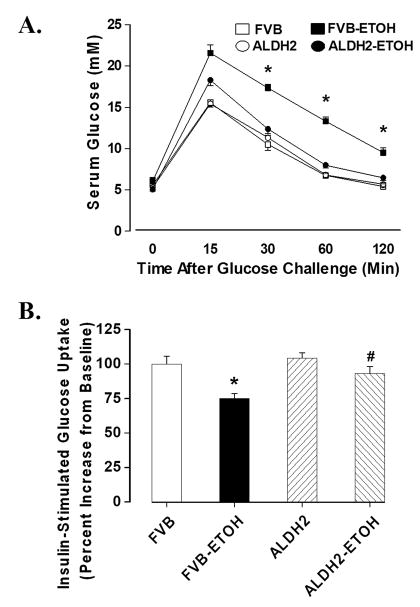
Transgenic overexpression of ALDH2 significantly enhanced the ALDH activity in both hearts and livers with a significant greater (~ 3.4-fold) activity in the livers compared with hearts from the wild-type FVB mice (Fig. 1C). Chronic alcohol feeding did not affect body weight although heart but not liver and kidney weights were significantly enhanced compared with non-alcohol consuming mice. ALDH2 did not affect body or organ weights in the absence of alcohol intake although it significantly alleviated alcohol-induced cardiac hypertrophy. Blood alcohol levels were significantly elevated equally in the alcohol consuming FVB and ALDH2 mice. ALDH2 significantly reduced chronic alcohol ingestion-induced elevation in blood acetaldehyde levels. The levels of blood alcohol and acetaldehyde were undetectable or minimal in non-alcohol consuming mice (Table 1). Following acute intraperitoneal glucose challenge, blood glucose levels in non-alcohol consuming mice started to drop after peaking at 15 min, and returned to nearly baseline value after 120 min. However, the post-challenge glucose levels maintained at higher levels from 15 to 120 min in alcohol consuming mice, the effect of which was significantly attenuated by ALDH2 overexpression (Fig. 2A). Basal and insulin-stimulated glucose uptake was determined to determine cardiac insulin sensitivity. While basal glucose uptake was comparable in all groups (data not shown), the insulin (10 nM, 15 min)-stimulated glucose uptake was significantly dampened in cardiomyocytes from the alcohol-consuming FVB mice, the effect of which was nullified by ALDH2 overexpression. ALDH2 itself elicited little effect on insulin-stimulated glucose uptake in the absence of alcohol intake (Fig. 2B).
Table 1.
Biometric parameters of mice fed an alcohol diet (4%) for 12 weeks
| Parameter | FVB | FVB-ETOH | ALDH | ALDH-ETOH |
|---|---|---|---|---|
| Body Weight (g) | 27.34 ± 0.61 | 27.37 ± 0.66 | 27.91 ± 0.52 | 27.36 ± 0.67 |
| Heart Weight (mg) | 184 ± 7 | 214 ± 7* | 180 ± 5 | 194 ± 5# |
| Heart/Body Weight (mg/g) | 6.73 ± 0.26 | 7.83 ± 0.22* | 6.48 ± 0.20 | 7.15 ± 0.24# |
| Liver Weight (g) | 1.45 ± 0.04 | 1.46 ± 0.04 | 1.42 ± 0.04 | 1.47 ± 0.05 |
| Liver/Body Weight (mg/g) | 53.9 ± 2.1 | 53.8 ± 1.9 | 51.2 ± 1.9 | 53.8 ± 1.6 |
| Kidney Weight (g) | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.02 | 0.39 ± 0.01 |
| Kidney/Body Weight (mg/g) | 14.4 ± 0.6 | 14.2 ± 0.04 | 14.2 ± 0.7 | 14.4 ± 0.3 |
| Blood Alcohol (μM) | Undetectable | 3422 ± 477* | Undetectable | 3885 ± 610* |
| Blood Acetaldehyde (μM) | 4.78 ± 4.12 | 73.22 ± 7.10* | 2.82 ± 1.98 | 38.88 ± 3.39*# |
ETOH = alcohol consuming; Mean ± SEM, n = 22 mice per group,
p < 0.05 vs. FVB group,
p < 0.05 vs. FVB-ETOH group.
Fig. 2.
(A). IPGTT in FVB and ALDH2 transgenic mice consuming control or alcohol diets for 12 weeks; (B). Insulin-stimulated glucose uptake shown as percent increase from baseline, Mean ± SEM, n = 5–6 mice per group, * p < 0.05 vs. FVB group, # p < 0.05 vs. FVB+ETOH group.
Cardiomyocyte contractile properties in FVB and ALDH2 mice fed with or without alcohol
Consistent with hypertrophied hearts in response to alcohol intake, chronic alcohol intake significantly enhanced the cross-sectional area. Moreover, alcohol intake significantly reduced peak shortening (PS) and ± dL/dt as well as prolonged TR90 but not TPS in FVB cardiomyocytes. Importantly, ALDH2 overexpression abolished chronic alcohol intake-induced enlargement in cell area and mechanical abnormalities without eliciting any effect in the absence of alcohol (Table 2).
Table 2.
Cardiomyocyte mechanical properties in mice fed an alcohol diet (4%) for 12 weeks
| Parameter | FVB | FVB-ETOH | ALDH | ALDH-ETOH |
|---|---|---|---|---|
| Cross-sectional area (μm2) | 2431 ± 58 | 2632 ± 58* | 2414 ± 48 | 2393 ± 59 # |
| PS (% cell length) | 5.53 ± 0.23 | 4.27 ± 0.22* | 5.14 ± 0.22 | 4.76 ± 0.26*, # |
| + dL/dt (μm/sec) | 127.8 ± 6.0 | 87.0 ± 5.1* | 119.8 ± 5.2 | 105.6 ± 06.1*, # |
| − dL/dt (μm/sec) | −112.3 ± 5.8 | −59.6 ± 4.9* | −103.2 ± 6.3 | −95.2 ± 7.2*, # |
| TPS (msec) | 108 ± 3 | 105 ± 3 | 100 ± 3 | 101 ± 4 |
| TR90 (msec) | 180 ± 8 | 271 ± 14* | 169 ± 8 | 182 ± 10 |
ETOH = alcohol consuming; PS = peak shortening; ± dL/dt = maximal velocity of shortening/relengthening; TPS = time-to-peak shortening; TR90 = time-to-90% relengthening; Mean ± SEM, n = 96 – 97 myocytes from 8 mice per group,
p < 0.05 vs. FVB group,
p < 0.05 vs. FVB-ETOH group.
Effect of chronic alcohol intake on insulin signaling molecules
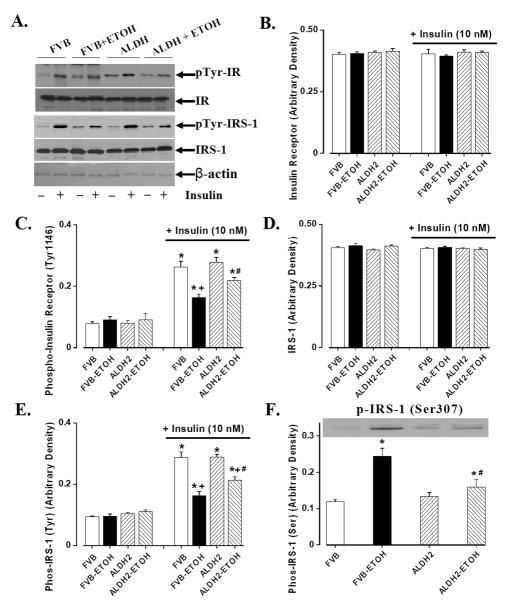
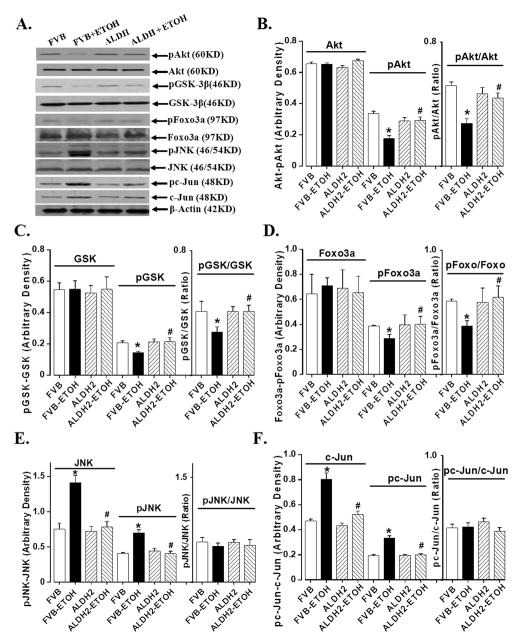
Chronic alcohol intake is associated with impaired insulin sensitivity [22;23]. Results shown in Fig. 3 indicate that neither alcohol nor ALDH2 overexpression affected expression of insulin receptor β and IRS-1 (with or without insulin stimulation), basal phosphorylation of insulin receptor (Tyr1146) and basal tyrosine phosphorylated IRS-1. Chronic alcohol ingestion significantly dampened insulin-stimulated tyrosine phosphorylation of both insulin receptor and IRS-1. ALDH2 overexpression attenuated alcohol-induced decrease in tyrosine phosphorylation of insulin receptor and IRS-1. To the contrary, alcohol ingestion significantly enhanced serine phosphorylation of IRS-1, the effect of which was reversed by ALDH2. ALDH2 itself did not affect insulin signaling at the receptor and IRS-1 levels in the absence of alcohol intake. Further scrutiny of the downstream post-insulin receptor signaling molecules including Akt, GSK-3β and Foxo3a revealed that chronic alcohol ingestion significantly reduced insulin-stimulated phosphorylation of Akt, GSk-3β and Foxo3a, the effect of which was nullified by ALDH2 overexpression. Total protein expression of Akt, GSk-3β and Foxo3a was not affected by either alcohol intake or ALDH2 overexpression. JNK has been considered a crucial mediator of insulin resistance and a potential therapeutic target for insulin resistance and type 2 diabetes [14]. Evidence from our group indicated that acetaldehyde may activate JNK [24]. Our data revealed enhanced JNK expression and its phosphorylation following chronic alcohol intake, which was abrogated by ALDH2. Our results further revealed enhanced expression and phosphorylation of c-Jun, a downstream transcription factor of JNK, following chronic alcohol ingestion. Consistent with its effect on JNK signaling, ALDH2 overexpression obliterated the chronic alcohol exposure-induced effects on c-Jun and its phosphorylation. Neither the pJNK/JNK nor the pc-Jun/c-Jun ratio was altered following chronic alcohol intake largely due to the elevated JNK and c-Jun protein levels. ALDH2 overexpression itself did not significantly affect the expression or phosphorylation of Akt, GSk-3β, Foxo3a, JNK and c-Jun in the absence of chronic alcohol intake (Fig. 4).
Fig. 3.
Basal and insulin-stimulated (10 nM, 15 min) insulin signaling from cardiomyocytes of FVB and ALDH2 mice consuming control or alcohol diet intake. (A). Representative gel blots depicting various proteins in the presence or absence of insulin stimulation; (B). Insulin receptor β; (C). Insulin receptor phosphorylation (tyrosine 1146); (D). IRS-1; (E). IRS-1 tyrosine phosphorylation; and (F). IRS-1 serine phosphorylation; Mean ± SEM, n = 7 mice per group, * p < 0.05 vs. non-insulin FVB group, + p < 0.05 vs. insulin-stimulated FVB group, # p < 0.05 vs. respective FVB+ETOH group.
Fig. 4.
Chronic alcohol (ETOH)-induced change in phosphorylation of Akt, GSK-3β, Foxo3a, JNK and c-Jun in myocardium from FVB and ALDH2 mice consuming control or alcohol diets. (A). Representative gels depicting total and phosphorylated proteins using respective antibodies; (B). Akt and pAkt; (C). GSK-3β and pGSK-3β; (D). Foxo3a and pFoxo3a; (E). JNK and pJNK; and (F). c-Jun and pc-Jun. Mean ± SEM, n = 6–7 mice per group, * p < 0.05 vs. FVB group, # p < 0.05 vs. respective FVB+ETOH group.
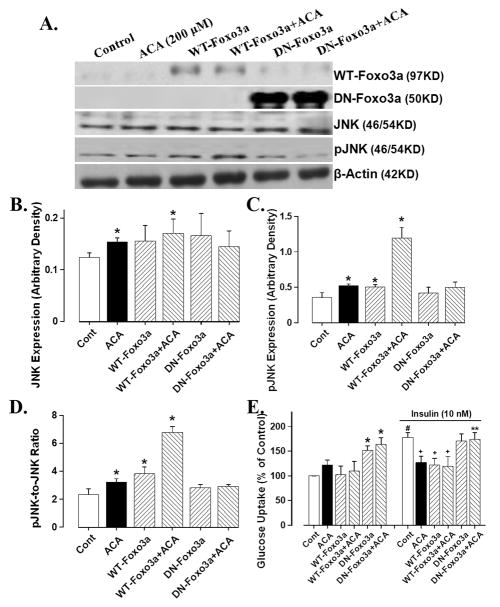
Effect of WT- and DN-Foxo3a adenovirus on acetaldehyde-induced response on JNK and glucose uptake
Our data shown in Fig. 4D depicted that ALDH2 restored chronic alcohol intake-induced reduction in phosphorylation of the transcriptional factor Foxo3a, suggesting a likely tie between acetaldehyde level and Foxo3a activity. To further examine the role of Foxo3a, a key Akt downstream signaling molecule responsible for myocardial survival and growth [11;12], in acetaldehyde-induced changes in insulin signaling, if any, ex vivo adenoviral transfection study was performed to transfect either WT- or DN-Foxo3a virus into the H9C2 myoblasts for 6 hrs before exposing the cells to acetaldehyde (200 μM) for 12 hrs. Our immunoblotting analysis revealed that acetaldehyde significantly upregulated the expression of JNK and pJNK (absolute value or pJNK-to-JNK ratio), an essential molecule known to interfere with insulin signaling [14]. Consistent with the JNK-pJNK data, acetaldehyde significantly dampened the insulin-stimulated glucose uptake without affecting basal glucose uptake. Interestingly, the WT-Foxo3a adenovirus mimiced or accentuated acetaldehyde-induced response on JNK-pJNK and glucose uptake whereas the DN-Foxo3a adenovirus ablated acetaldehyde-induced upregulation of the JNK-pJNK cascade and compromised insulin-stimulated glucose uptake (Fig. 5). These data suggested that the Foxo3a transcriptional factor plays a key role in the acetaldehyde-elicited detrimental effect on JNK signaling and insulin sensitivity.
Fig. 5.
Effect of viral transfection of wild-type (WT) and dominant negative (DN) Foxo3a on acetaldehyde-induced JNK phosphorylation and glucose uptake in H9C2 myoblasts. Cells were first incubated at 37°C in the DMEM medium with or without WT-Foxo3a or DN- Foxo3a (1:1000) adenovirus for 6 hrs before switching to normal medium for an additional 12 hrs in the absence or presence of acetaldehyde (ACA, 200 μM). (A). Representative gels depicting expression of WT-Foxo3a, DN-Foxo3a, JNK and phosphorylated JNK in control and acetaldehyde-treated cells with or without Foxo3a viral transfection. β-Actin was used as the loading control; (B). JNK expression; (C). Phosphorylated JNK (pJNK); (D). pJNK-to-JNK ratio; (E). basal- and insulin (10 nM, 15 min)-stimulated glucose uptake. Mean ± SEM, n = 6–7 per data point, * p < 0.05 vs. respective control group, # p < 0.05 vs. non-insulin-stimulated control group, + p < 0.05 vs. insulin-stimulated control group, ** p < 0.05 vs. respective acetaldehyde group.
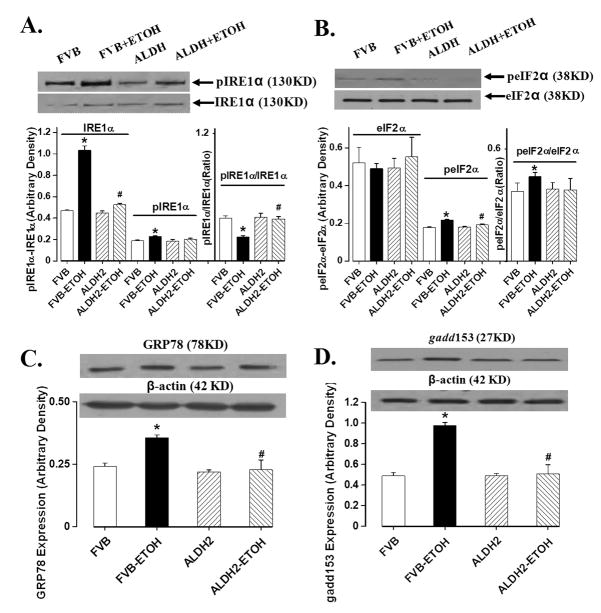
Effect of chronic alcohol intake on ER stress markers
Chronic ethanol intake leads to ER stress in liver and hearts [19;25]. To explore if ALDH2-protected myocardial protection was casually associated with changes in cardiac ER stress status, we evaluated the ER stress markers eIF2α, IRE1α, GRP78 and gadd153. Results shown in Fig. 6 revealed that IRE1α, phosphorylation of IRE1α and eIF2α, GRP78 and gadd153 were significantly elevated in myocardium following chronic alcohol intake. Total eIF2α expression was unaffected while the IRE1α-to-pIRE1α ratio was significantly decreased as a result of the drastically upregulated IRE1α level in response to chronic alcohol intake. Consistent with its effect on insulin signaling, ALDH2 overexpression significantly attenuated chronic alcohol intake-induced ER stress without eliciting any notable effect itself in the absence of alcohol drinking. This causal correlation between ALDH2-induced effects on insulin signaling and ER stress following alcohol consumption suggests a possible role of ER stress in chronic alcohol intake- and ALDH2 overexpression-induced effects on cardiac insulin signaling and contractile function.
Fig. 6.
Chronic alcohol (ETOH)-induced changes in the ER stress markers eIF2α, IRE1α, GRP78 and gadd153 in myocardium from FVB and ALDH2 transgenic mice. (A): IRE1α and phosphorylated IRE1α (pIRE1α); (B). eIF2α and phosphorylated eIF2α (peIF2α); (C): GRP78; and (D): gadd153. Inset: representative gels using specific antibodies. Mean ± SEM, n = 5–7 mice per group, * p < 0.05 vs. respective FVB group, # p < 0.05 vs. respective FVB+ETOH group.
DISCUSSION
ALDH2 activation was recently shown to protect the heart against ischemic damage [26]. Consistently, data from our current study revealed that ALDH2 alleviated chronic alcohol intake-induced cardiac dysfunction and insulin insensitivity, favoring a likely role of the alcohol metabolite acetaldehyde and insulin desensitization in alcohol-induced cardiac dysfunction. Up-to-date, several scenarios including ethanol toxicity, oxidative damage, lipid peroxidation and defective membrane integrity have been speculated in alcoholic tissue injury [27;28], although none was fully validated either experimentally or clinically. Recent clinical and epidemiological studies have reported altered susceptibility to the deleterious effects of alcohol in individuals with genetic polymorphism of ALDH2, suggesting a role of ALDH2 in alcohol-related tissue pathology [3;4;29]. Data from our current study further consolidate the key role of ALDH2 and its metabolic substrate acetaldehyde in alcohol-related organ and tissue pathology.
Pharmacokinetic and pharmacodynamic assessment have indicated that acetaldehyde, rather than ethanol, is primarily responsible for the observed alcohol sensitivity reactions in humans [29]. Allelic variation in the ALDH genes, particularly ALDH2, due to a point mutation in the active ALDH2*1 gene, significantly alters blood acetaldehyde levels and vulnerability for alcoholism [29;30]. Asian and African American populations commonly carry the mutant ALDH alleles (ALDH2*2/1 and ALDH2*2/2), leading to ~10-fold higher blood acetaldehyde levels following alcohol intake [31]. Although the mutant ALDH2*2/2 is capable of protecting against the development of alcohol dependence and alcohol-related injury due to the unpleasant physiological and psychological reactions of alcohol [29], functional ALDH2 gene protects against alcoholic complications via a faster elimination of acetaldehyde and lower accumulation of the toxin in circulation [4;29]. Our current finding that ALDH2 attenuates alcohol-elicited cardiac damage and insulin insensitivity is supported by our earlier reports of the elevated acetaldehyde accumulation, exaggerated cardiac hypertrophy, contractile dysfunction and oxidative damage in the cardiac-specific ADH transgenic mice following alcohol intake [4;9;10]. In our hand, the hepatic ALDH activity in FVB mice is approximately 3-fold greater than that of the cardiac tissues, consistent with the previous report [32]. This result has consolidated the notion that liver is the primary organ/site for acetaldehyde detoxification [3] and validated our rationale of overexpressing the ALDH2 gene systematically as oppose to cardiac-specifically.
In our study, chronic alcohol intake induced cardiac hypertrophy and contractile dysfunction associated with impaired insulin signaling at both insulin receptor and post-receptor (IRS-1, Akt, GSK-3β, Foxo3a, JNK and c-Jun) levels. ALDH2 facilitated acetaldehyde detoxification following chronic alcohol intake, which was associated with improved whole body glucose tolerance, cardiac glucose uptake and insulin signaling at the receptor and post-receptor levels. Binding of insulin to its receptor activates tyrosine kinase of the insulin receptor β subunit to phosphorylate IRS-1/IRS-2. To the contrary, serine phosphorylation of IRS counteracts the tyrosine phosphorylation of IRS, leading to dampened insulin sensitivity [13]. Tyrosine phosphorylation of IRS activates the phosphatidylinositol-3 kinase/Akt cascade to promote glucose transport and glycogen synthesis [13]. Our data revealed that ALDH2 overexpression attenuated alcohol-induced decrease in tyrosine phosphorylation of insulin receptor and IRS-1 while reversing alcohol-elicited increase in IRS-1 serine phosphorylation. Consistently, ALDH2 overexpression restored the alcohol-induced decline in insulin-stimulated phosphorylation of Akt, GSK-3β and Foxo3a. The Akt-GSK-3β-Foxo3a cascade has been considered as a sensor for myocardial oxidative stress, insulin signaling and cardiomyocyte stretch. The phosphorylation status of the Akt-GSK-3β-Foxo3a axis directly reflects the physiological condition of the hearts [33]. The reduced Foxo3a phosphorylation (or reduced Foxo3a inactivation) observed in our current study may render the hearts to apoptosis and compromised contractile function. The involvement of Foxo3a in chronic alcohol intake-induced cardiac defects is further testified by our Foxo3a adenovirus transfection study using the H9C2 myoblast cells. These data indicate that ALDH2 may alleviate chronic alcohol intake-compromised insulin signaling at the levels of insulin receptor and IRS-1 (reduced tyrosine- and increased-serine phosphorylation), as well as post-insulin receptor signaling (Akt, GSK-3β and Foxo3a).
JNK signaling is known to be activated by pro-inflammatory cytokines and free fatty acids, resulting in interrupted insulin signaling and development of insulin resistance [14]. In our study, both JNK and pJNK are upregulated following chronic alcohol intake, the effect of which may be alleviated by ALDH2 overexpression. These results in JNK and JNK phosphorylation are consistent with changes in the JNK downstream nuclear signaling c-Jun and c-Jun phosphorylation following chronic alcohol intake in both FVB and ALDH2 mice. Our observation that wild-type Foxo3a mimics whereas dominant negative Foxo3a counteracts acetaldehyde-elicited detrimental effects on JNK and glucose uptake favors a role of Foxo3a and JNK in acetaldehyde-triggered insulin insensitivity. It is worth mentioning that the increased JNK and pJNK levels in control H9C2 cells treated with acetaldehyde displayed a different extent of upregulation compared with mice fed an alcohol-diet. This apparent discrepancy may be ascribed to the difference between the in vivo and in vitro experimental settings and possibly levels of acetaldehyde available. Moreover, despite that dominant negative Foxo3a nullified acetaldehyde-elicited effects on JNK and glucose uptake, an additive effect appears to be present between acetaldehyde and wild-type Foxo3a in pJNK and glucose uptake. Although no precise explanation can be offered at this time, it is possible that acetaldehyde (at the concentration tested) possesses certain constitutive Foxo3a activity which is different from the wild-type Foxo3a adenovirus.
Our data suggested presence of ER stress in myocardium following chronic alcohol intake, consistent with our previous report [19]. ER is an intracellular membranous network responsible for Ca2+ storage, Ca2+ signaling, glycosylation and trafficking of membrane and secretory proteins [34]. Data from our study revealed upregulated and/or activated IRE1αand eIF2α, two of the major ER-resident transmembrane proteins sensing ER stress, following chronic alcohol ingestion. This is supported by the elevated unfolded protein response target pro-apoptotic protein gadd153, which is highly dependent upon the protein kinase RNA (PKR)-like ER kinase (PERK)/eIF2α pathway [34]. Our data also revealed upregulation of the ER chaperone GRP78 (also known as BiP) which directly interacts with the ER stress sensors, PERK/eIF2α, ATF6 and IRE1, and maintains them in inactive forms [15]. Upregulation of GRP78 is pivotal for cell survival to facilitate folding and assembly of ER proteins and prevent them from aggregation during ER stress [35]. The fact that ALDH2 ablated alcohol-induced changes in IRE1α, eIF2α, GRP78 and gadd153 suggests involvement of ER stress, which is known to trigger insulin resistance through activation of JNK and suppression of Akt [36;37], in alcohol-induced myocardial injury.
In summary, our data suggest that chronic alcohol intake may interrupt myocardial insulin signaling, the effect of which may be alleviated by ALDH2 overexpression. The concomitant effect of ALDH2 on insulin signaling and cardiomyocyte contractile dysfunction following alcohol intake suggests a role of insulin signaling in the alcohol-induced cardiac dysfunction and ALDH2-offered protection. Given that a tight correlation is present between insulin sensitivity and myocardial function [13;38], findings from our study should shed some lights towards a better understanding of the pathogenesis of alcoholic cardiomyopathy and the therapeutic potential of insulin sensitizers in the management of alcoholic cardiomyopathy.
Supplementary Material
Acknowledgments
This work was supported in part by American Diabetes Association (7-08-RA-130), NIH/NIAAA 1R01 AA013412 and NIH/NCRR 5P20RR016474 (JR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Preedy VR, Patel VB, Reilly ME, Richardson PJ, Falkous G, Mantle D. Oxidants, antioxidants and alcohol: implications for skeletal and cardiac muscle. Front Biosci. 1999 Aug 1;4:e58–e66. doi: 10.2741/A480. [DOI] [PubMed] [Google Scholar]
- 2.Siddiq T, Richardson PJ, Mitchell WD, Teare J, Preedy VR. Ethanol-induced inhibition of ventricular protein synthesis in vivo and the possible role of acetaldehyde. Cell Biochem Funct. 1993 Mar;11(1):45–54. doi: 10.1002/cbf.290110106. [DOI] [PubMed] [Google Scholar]
- 3.Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc. 2004 Feb;63(1):49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- 4.Ren J. Acetaldehyde and alcoholic cardiomyopathy: lessons from the ADH and ALDH2 transgenic models. Novartis Found Symp. 2007;285:69–76. doi: 10.1002/9780470511848.ch5. [DOI] [PubMed] [Google Scholar]
- 5.Tsukamoto S, Muto T, Nagoya T, Shimamura M, Saito M, Tainaka H. Determinations of ethanol, acetaldehyde and acetate in blood and urine during alcohol oxidation in man. Alcohol Alcohol. 1989;24(2):101–8. doi: 10.1093/oxfordjournals.alcalc.a044872. [DOI] [PubMed] [Google Scholar]
- 6.Ren J, Davidoff AJ, Brown RA. Acetaldehyde depresses shortening and intracellular Ca2+ transients in adult rat ventricular myocytes. Cell Mol Biol (Noisy -le-grand) 1997 Sep;43(6):825–34. [PubMed] [Google Scholar]
- 7.Ren J, Brown RA. Influence of chronic alcohol ingestion on acetaldehyde-induced depression of rat cardiac contractile function. Alcohol Alcohol. 2000 Nov;35(6):554–60. doi: 10.1093/alcalc/35.6.554. [DOI] [PubMed] [Google Scholar]
- 8.Brown RA, Jefferson L, Sudan N, Lloyd TC, Ren J. Acetaldehyde depresses myocardial contraction and cardiac myocyte shortening in spontaneously hypertensive rats: role of intracellular Ca2+ Cell Mol Biol (Noisy -le-grand) 1999 Jun;45(4):453–65. [PubMed] [Google Scholar]
- 9.Hintz KK, Relling DP, Saari JT, Borgerding AJ, Duan J, Ren BH, et al. Cardiac overexpression of alcohol dehydrogenase exacerbates cardiac contractile dysfunction, lipid peroxidation, and protein damage after chronic ethanol ingestion. Alcohol Clin Exp Res. 2003 Jul;27(7):1090–8. doi: 10.1097/01.ALC.0000075823.73536.DD. [DOI] [PubMed] [Google Scholar]
- 10.Duan J, McFadden GE, Borgerding AJ, Norby FL, Ren BH, Ye G, et al. Overexpression of alcohol dehydrogenase exacerbates ethanol-induced contractile defect in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2002 Apr;282(4):H1216–H1222. doi: 10.1152/ajpheart.00780.2001. [DOI] [PubMed] [Google Scholar]
- 11.Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol. 2008 Sep;295(3):H1206–H1215. doi: 10.1152/ajpheart.00319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006 Sep 12;114(11):1159–68. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang CX, Dong F, Ren BH, Epstein PN, Ren J. Metallothionein alleviates cardiac contractile dysfunction induced by insulin resistance: role of Akt phosphorylation, PTB1B, PPARgamma and c-Jun. Diabetologia. 2005 Nov;48(11):2412–21. doi: 10.1007/s00125-005-1940-y. [DOI] [PubMed] [Google Scholar]
- 14.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002 Nov 21;420(6913):333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 15.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006 Aug 25;313(5790):1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doser TA, Turdi A, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009 Aug; doi: 10.1161/CIRCULATIONAHA.108.823799. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Keane B, Leonard BE. Rodent models of alcoholism: a review. Alcohol Alcohol. 1989;24(4):299–309. doi: 10.1093/oxfordjournals.alcalc.a044916. [DOI] [PubMed] [Google Scholar]
- 18.Li SY, Gomelsky M, Duan J, Zhang Z, Gomelsky L, Zhang X, et al. Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogen-activated protein kinase. J Biol Chem. 2004 Mar 19;279(12):11244–52. doi: 10.1074/jbc.M308011200. [DOI] [PubMed] [Google Scholar]
- 19.Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol. 2008 Jun;44(6):992–1001. doi: 10.1016/j.yjmcc.2008.02.276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Fang CX, Yang X, Sreejayan N, Ren J. Acetaldehyde promotes rapamycin-dependent activation of p70(S6K) and glucose uptake despite inhibition of Akt and mTOR in dopaminergic SH-SY5Y human neuroblastoma cells. Exp Neurol. 2007 Jan;203(1):196–204. doi: 10.1016/j.expneurol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Skurk C, Maatz H, Kim HS, Yang J, Abid MR, Aird WC, et al. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004 Jan 9;279(2):1513–25. doi: 10.1074/jbc.M304736200. [DOI] [PubMed] [Google Scholar]
- 22.Ting JW, Lautt WW. The effect of acute, chronic, and prenatal ethanol exposure on insulin sensitivity. Pharmacol Ther. 2006 Aug;111(2):346–73. doi: 10.1016/j.pharmthera.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system research challenges and opportunities. J Am Coll Cardiol. 2005 Jun 21;45(12):1916–24. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 24.Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, et al. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006 Feb;40(2):283–94. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003 May;124(5):1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 26.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008 Sep 12;321(5895):1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cederbaum AI, Wu D, Mari M, Bai J. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic Biol Med. 2001 Dec 15;31(12):1539–43. doi: 10.1016/s0891-5849(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 28.Bailey SM, Pietsch EC, Cunningham CC. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic Biol Med. 1999 Oct;27(7–8):891–900. doi: 10.1016/s0891-5849(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 29.Chen YC, Peng GS, Wang MF, Tsao TP, Yin SJ. Polymorphism of ethanol-metabolism genes and alcoholism: correlation of allelic variations with the pharmacokinetic and pharmacodynamic consequences. Chem Biol Interact. 2009 Mar 16;178(1–3):2–7. doi: 10.1016/j.cbi.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Peng GS, Yin JH, Wang MF, Lee JT, Hsu YD, Yin SJ. Alcohol sensitivity in Taiwanese men with different alcohol and aldehyde dehydrogenase genotypes. J Formos Med Assoc. 2002 Nov;101(11):769–74. [PubMed] [Google Scholar]
- 31.Hashimoto Y, Nakayama T, Futamura A, Omura M, Nakarai H, Nakahara K. Relationship between genetic polymorphisms of alcohol-metabolizing enzymes and changes in risk factors for coronary heart disease associated with alcohol consumption. Clin Chem. 2002 Jul;48(7):1043–8. [PubMed] [Google Scholar]
- 32.Dipple KM, Crabb DW. The mitochondrial aldehyde dehydrogenase gene resides in an HTF island but is expressed in a tissue-specific manner. Biochem Biophys Res Commun. 1993 May 28;193(1):420–7. doi: 10.1006/bbrc.1993.1640. [DOI] [PubMed] [Google Scholar]
- 33.Baba HA, Stypmann J, Grabellus F, Kirchhof P, Sokoll A, Schafers M, et al. Dynamic regulation of MEK/Erks and Akt/GSK-3beta in human end-stage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res. 2003 Aug 1;59(2):390–9. doi: 10.1016/s0008-6363(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 34.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007 Jul;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 35.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007 Jul 31;581(19):3641–51. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and type 2 diabetes. Novartis Found Symp. 2007;286:86–94. doi: 10.1002/9780470985571.ch8. [DOI] [PubMed] [Google Scholar]
- 37.Yung HW, Korolchuk S, Tolkovsky AM, Charnock-Jones DS, Burton GJ. Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 2007 Mar;21(3):872–84. doi: 10.1096/fj.06-6054com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006 Jun;49(6):1434–46. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.