Abstract
Objective
To investigate the relationship between antimullerian hormone (AMH) and steroidogenesis in follicles of normoandrogenic ovulatory women undergoing FSH therapy for IVF-ET.
Design
Prospective cohort.
Setting
Institutional/private practice.
Patients
26 normoandrogenic ovulatory women. All women received GnRH analog and ovarian stimulation for IVF-ET.
Interventions
Follicle fluid was aspirated at oocyte retrieval from the first follicle of each ovary.
Main Outcome Measures
Follicle fluid was assayed for AMH, E2, P, androstenedione, T, dihydrotestosterone, insulin and FSH.
Results
Intrafollicular AMH levels positively and negatively correlated with E2 and FSH concentrations in follicles, respectively, causing a positive relationship between follicle fluid AMH levels and E2/FSH ratios as a measure of follicle sensitivity to FSH. A positive relationship also existed in follicles between AMH levels and E2/androgen ratios as a marker of aromatase activity.
Conclusions
AMH levels in follicles of IVF patients positively correlate with follicle sensitivity to FSH.
Key terms: antimullerian hormone, mullerian-inhibiting substance, intrafollicular steroidogenesis, E2, IVF-ET
INTRODUCTION
As a homodimeric glycoprotein of the transforming growth factor-β (TGF- β) superfamily, antimullerian hormone (AMH) is emerging as an important regulator of mammalian follicle development (1,2). Produced by granulosa cells of growing follicles after birth (3), AMH levels normally are low in primary follicles, increase to maximal levels in large preantral and small antral stages, and then decline during final follicular maturation, becoming restricted to cumulus cells surrounding the oocyte (4). Serum AMH concentrations correlate with the numbers of antral follicles before and with the ovarian response to gonadotropin therapy for IVF-ET (5,6), and diminish after oophorectomy to those of ovary-intact postmenopausal women (1). Within the mammalian ovary, moreover, AMH inhibits follicle recruitment and FSH-dependent follicle growth as well as selection (1,2), thereby suppressing aromatase activity during early folliculogenesis (7).
These inhibitory actions of AMH on early follicle development are overcome by FSH therapy during IVF-ET, which raises circulating FSH levels above the threshold sensitivity of follicles to FSH. Following gonadotropin therapy for IVF-ET, AMH concentrations in small (8–12 mm in diameter) and large (16–20 mm in diameter) antral follicles positively correlate with granulosa cell responsiveness to FSH and negatively correlate with intrafollicular P levels (8). Moreover, AMH levels in these large antral follicles predict enhanced embryo implantation and successful pregnancy outcome, suggesting a role for AMH in oocyte development (6). Since appropriate ovarian steroidogenesis is a prerequisite for optimal oocyte development (9), the present study investigates the relationship between AMH and steroidogenesis in follicles of normoandrogenic ovulatory women undergoing recombinant human (rh) FSH therapy for IVF-ET.
MATERIALS AND METHODS
Experimental Subjects
Institutional Review Board approval was obtained before initiation of the study and fully informed consent was obtained from 26 normoandrogenic ovulatory women undergoing gonadotropin therapy for IVF-ET. Normoandrogenic ovulatory women received assisted reproduction for non-ovarian indications (male factor [n=15], endometriosis [n=3], tubal factor [n=4] and multifactor infertility [n=4]). General inclusion criteria were age less than 38 years, normal serum prolactin levels and normal thyroid function studies. No woman had galactorrhea, endometriomas, or ovarian cysts greater than 18 mm in diameter.
All normoandrogenic ovulatory women had regular menstrual cycles occurring every 21–35 days, luteal serum P values (>3 ng/mL [SI Conversion, 3.18 nmol/L]) and absence of hyperandrogenism, as previously described (10, 11). None had polycystic ovaries by transvaginal ultrasound (TVUS) (12). Fourteen women had a body mass index (BMI) < 25 kg/m2, while the remaining 12 individuals had a BMI ≥ 25 kg/m2. The patient/IVF-ET cycle characteristics and follicle fluid hormone determinations of these individuals previously have been reported (10, 11).
Gonadotropin stimulation for IVF-ET
Briefly, all women began midluteal leuprolide acetate (Lupron, TAP Pharmaceuticals) therapy at a dose of 1.0 mg subcutaneously (sc) each day until pituitary down-regulation (e.g., no ovarian cysts larger than 18 mm in diameter and serum E2 <50 pg/mL), after which the dose was reduced to 0.5 mg daily until the day of hCG administration. Recombinant human (rh) FSH (Gonal-F, Serono Laboratories, Madrid, Spain) was started at 225 IU daily SC for 3 days, followed by daily changes in dosage, as necessary. Human chorionic gonadotropin (10,000 IU, IM) was administered when at least two dominant follicles reached ≥ 18 mm in diameter and serum E2 levels reached approximately 300 pg/mL/dominant follicle. At oocyte retrieval, follicular fluid was aspirated from the first follicle of each ovary, which was selected by accessibility and size of at least 16 mm in mean diameter, as calculated by 3 independent follicular measurements in perpendicular planes. These measurements were used to calculate the size of the follicle in which AMH predicts enhanced oocyte quality (6), while also controlling for follicle size as a confounding variable (8).
Follicle fluid AMH determinations
Only fluid uncontaminated by blood from the study follicle of each ovary was individually assayed for AMH (total follicle number: N=36), as described below. All follicle fluid samples were stored at −80 C for later hormone determinations.
Hormone assays
All AMH, sex steroid, insulin and FSH analysis were performed in the National Primate Research Center (NPRC) Hormone Assay Services Laboratory (10, 11). Follicular fluid AMH was measured by enzymeimmunoassay (13) and the intra- and interassay CVs for AMH were 1.9% and 8.3%, respectively. The lower level of AMH detection was 0.025 ng/mL. The AMH enzymeimmunoassay measures total AMH since both its capture and detection antibodies recognize epitopes in the pro-region of the AMH molecule (14). As both N-terminal domain and C-terminal domain circulate in non-covalent attachment, and such attachment is required for full bioactivity (15), measurement of total AMH provides relevant quantitation of biologically active AMH. Follicular fluid FSH, E2, androstenedione (A4) and insulin were measured by RIA. The intraassay coefficients of variation (CVs) were: FSH, 3.6%, E2, 5.7%, A4, 4.9% and insulin, 4.6%; the interassay CVs were: FSH, 6.9%, E2, 18.6%, A4, 17.2% and insulin, 7.9%. P, T and dihydrotestosterone (DHT) were measured by an enzyme immunoassay, as previously described (10, 11). The intraassay CVs were: P, 3.6%, T, 1.8% and DHT, 3.2%; the interassay CVs were: P, 20.0%, T, 19.0% and DHT, 18.3%.
Statistical analysis
Logarithmic transformations were performed when necessary to meet assumptions in regression modeling. Regression models with estimation by generalized estimating equations (16) were used to estimate associations between AMH and hormone levels in the same follicle, while adjusting for intra-subject correlations owing to more than one follicle per patient (10, 11). Follicle fluid hormone values were adjusted a priori for the effects of patient age and BMI on AMH production (1, 17), and were expressed as ng/mL rather than ng/mg protein because protein content in follicular fluid increased with follicle size (data not shown). P<0.05 is considered significant.
RESULTS
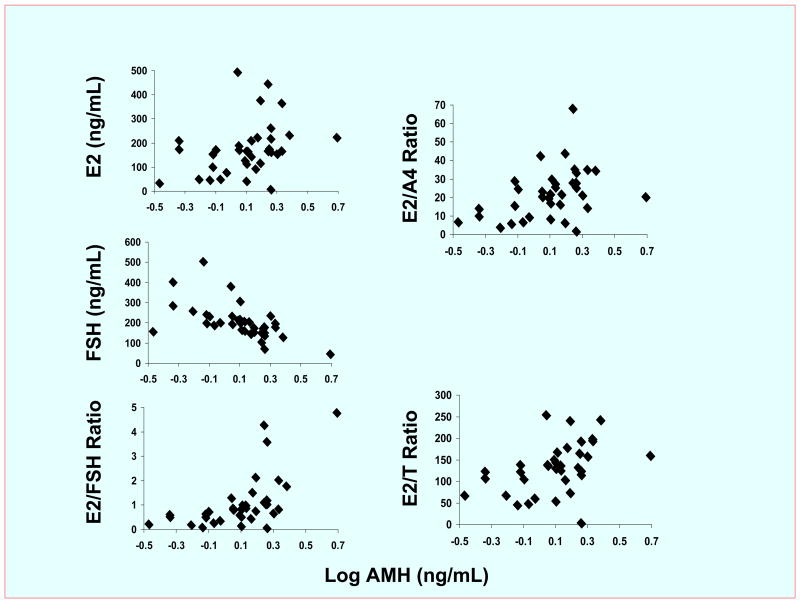
Adjusting for BMI and age, AMH levels positively correlated with E2 concentrations (R=+0.26; P<0.025) and negatively correlated with FSH (R=−0.74; P<0.0001) concentrations in all follicles, causing a positive relationship in follicular fluid between the amounts of AMH and the proportions of E2 relative to FSH (R=+0.70; P<0.0001, Figure 1). Moreover, although intrafollicular AMH and androgen levels were unrelated (A4, P=0.9; T, P=0.1), a positive relationship also existed in follicles between the amounts of AMH and the ratios of E2/A4 (R=+0.26; P<0.025) and of E2/T (R=+0.31; P<0.0005). In these same follicles, AMH concentrations were unrelated to the levels of DHT (P=0.6), P (P=0.5) and insulin (P=0.4), or the ratios of DHT/T (P=0.9) and P/E2 (P=0.1).
Figure 1.
Correlations between AMH and hormone concentrations in follicles of 26 normoandrogenic ovulatory women undergoing IVF-ET. AMH levels significantly correlated with E2 (R=+0.26; P<0.025) and FSH (R=−0.74; P<0.0001) concentrations as well as E2/FSH (R=+0.70; P<0.000), E2/A4 (R=+0.26; P<0.025) and E2/T (R=+0.31; P<0.0005) ratios. Conversion to SI units, AMH* 7.14 pmol/L. The first follicle of each ovary was selected by size (at least 16 mm in diameter) and accessibility to replicate the follicle size in which AMH previously has been shown to predict enhanced oocyte development (6).
DISCUSSION
Produced predominantly by granulosa cells of preantral and small antral follicles (4), AMH inhibits follicle recruitment and attenuates the FSH-dependent increase in aromatase activity during early follicle development (1, 2, 7). In follicles of women receiving GnRH analog/rhFSH therapy followed by hCG administration, however, our study shows that AMH levels positively and negative correlate with E2 and FSH concentrations, respectively. Adjusting for patient age and BMI, the consequent positive relationship between intrafollicular AMH concentrations and E2/FSH ratios parallels a positive correlation between AMH levels and E2/androgen ratios (as estimates of aromatase activity) in the same follicles, suggesting that AMH inhibition of FSH-dependent aromatase activity in early folliculogenesis is overcome when the threshold sensitivity of the follicle to FSH is exceeded by exogenous FSH administration (7, 18)
One reason why AMH inhibition of FSH action is attenuated in IVF patients is that intrafollicular AMH concentrations decline by two to three orders of magnitude during gonadotropin stimulation for IVF-ET (1, 19, 20). In support of this, follicle fluid AMH concentrations in our study (mean, 1.4 ng/mL) and other IVF studies (mean, 1.2–2.8 ng/mL) are markedly lower than those reported in small human antral follicles (< 8–9 mm; mean, 790 ng/mL) (20, 21). Within small follicles, moreover, AMH is expressed exclusively by granulosa cells with mitotic activity (22, 23), presumably because it interacts with mitogenic growth factors during follicle development (i.e., epidermal growth factor, transforming growth factor-β, and insulin-like growth factor-I) (24, 25). Therefore, our findings of a positive relationship between intrafollicular AMH concentrations and the ratios of E2/FSH and E2/androgens in the same follicles may represent the degree of granulosa cell proliferation during FSH-stimulated follicle growth, which could counteract AMH inhibition of FSH-dependent aromatase activity during folliculogenesis (6, 8, 20, 21). An alternate explanation for our findings is that decreased AMH bioactivity from polymorphisms in AMH or the AMH type II receptor might simultaneously increase AMH production and decrease AMH inhibition of follicle sensitivity to FSH (26). In rats, AMH and AMH type II receptor messenger ribonucleic acids (mRNAs) are primarily coexpressed in preantral and small antral follicles (27), supporting an autocrine action for AMH during early follicular development, although AMH type II receptor mRNA expression is low in human preantral follicles (28) and is unknown during later human folliculogenesis.
Our study does not confirm previous observations of an inverse relationship between AMH and P levels in similarly-sized follicles of IVF-ET patients (6, 8). Absence of a relationship between AMH and follicle luteinization (i.e., follicle fluid P level and P/E2 ratio) may be unique to our exclusive use of GnRH analog/rhFSH therapy, rather than the use of other gonadotropin therapies that utilize GnRH antagonist or combined FSH/LH (6, 8). During ovarian stimulation for IVF-ET, GnRH analog administration induces differences in granulosa cell cycle kinetics associated with AMH alterations in follicular fluid (29), while LH supplementation alters ovarian steroidogenesis (30), suggesting complex interactions between AMH production and follicle luteinization by type of ovarian stimulation protocol. Luteinizing hormone, but not FSH, also tends to elevate AMH release from cultured granulosa cells of small human antral follicles (greater than 10mm in diameter) (13), further complicating interpretation of AMH results from ovarian stimulation protocols that combine FSH/LH.
In this study, large preovulatory follicles (>15 mm in diameter) alone were examined because AMH levels in follicles of this size predict enhanced oocyte developmental competence, as defined by improved embryo implantation and successful pregnancy outcome by IVF-ET (6). Furthermore, human cumulus cells contain AMH protein, raising the possibility that AMH in follicles of IVF-ET patients has a cumulus cell origin (4). Such a hypothesis agrees with the observation that granulosa cells lose their AMH expression at the 6–8 mm stage of follicle development (4, 13), although high intraovarian AMH levels from multiple preantral and small antral follicles might also diffuse into large follicles during gonadotropin therapy for IVF-ET.
Despite the same starting dose of rhFSH in all study participants, the FSH concentration in the follicle is directly proportional to the total amount of rhFSH administered (18), raising the possibility that differences in intrafollicular FSH availability might have affected AMH production. In female immature rats treated with GnRH antagonist, for example, rhFSH administration down-regulates AMH mRNA expression in preantral and small antral follicles (27). Nevertheless such an inverse relationship between FSH availability and AMH production in the rodent ovary may be less relevant in the primate ovary since GnRH antagonist administration to adult female marmoset monkeys decreases AMH protein expression in preantral and early antral follicles (31), while FSH stimulation of human granulosa cells in vitro has no effect on AMH secretion (13).
Other limitations of this study include the relatively small number of patients, which precludes examining the effects of patient age and BMI on intrafollicular AMH levels (1, 17), including the controversial role of insulin resistance in AMH regulation (32). Lacking sufficient statistical power, however, our analysis adjusted data a priori for both age and BMI, given their possible associations with AMH production (1, 17). In addition, the exclusive use of GnRH analog/rhFSH therapy, while controlling for type of ovarian stimulation, does not allow extrapolation of our data to other ovarian stimulation protocols that, by eliminating GnRH analog or adding LH supplementation, might affect AMH production differently (29, 30). Importantly, the existence of AMH protein in human cumulus cells (4) raises questions as to whether cumulus cell-oocyte signaling mediated by AMH affects human oocyte development, given its interactions with oocyte-derived factors to regulate meiosis in animals (25, 33, 34, 35), and its abilities in follicle fluid to predict successful oocyte fertilization and improved pregnancy outcome by IVF-ET (6, 36, 37).
Acknowledgments
The authors thank Rebekah R. Herrmann for her assistance in manuscript preparation. Supported by NIH Grants U01 HD044650 as part of the National Institute of Child Health and Human Development National Cooperative Program on Female Health and Egg Quality, R01 RR 013635, Mayo Clinical Research Grant 2123-01, Mayo Grant M01-RR-00585, Grant P51 RR 000167 (to the National Primate Research Center, University of Wisconsin, Madison, a facility constructed with support from Research Facilities Improvement Program Grants RR15459-01 and RR020141-010), and Serono and Ferring Pharmaceuticals. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Presented at a meeting: Annual Meeting of the American Society for Reproductive Medicine, Washington, DC, October 13–17, 2007, Abstract O-71.
References
- 1.Seifer DB, MacLaughlin DT. Mullerian Inhibiting Substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88:539–46. doi: 10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Knight PG, Glister C. Local roles of TGF- β superfamily members in the control of ovarian follicle development. Anim Reprod Sci. 2003;78:165–83. doi: 10.1016/s0378-4320(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 3.Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–6. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- 4.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 5.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468–71. doi: 10.1016/s0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 6.Fanchin R, Mendez Lozano DH, Frydman N, Gougeon A, di Clemente N, Frydman R, et al. Anti-mullerian hormone concentrations in the follicle fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796–1802. doi: 10.1210/jc.2006-1053. [DOI] [PubMed] [Google Scholar]
- 7.Visser JA, de Jong FH, Laven JSE, Themmen APN. Anti-mullerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 8.Fanchin R, Louafi N, Lozano DHM, Frydman N, Frydman R, Taieb J. Per-follicle measurements indicate that anti-mullerian hormone secretion is modulated by the extent of follicular development and luteinization and may reflect qualitatively the ovarian follicular status. Fertil Steril. 2005;84:167–73. doi: 10.1016/j.fertnstert.2005.01.115. [DOI] [PubMed] [Google Scholar]
- 9.Tesarik J, Mendoza C. Nongenomic effects of 17B-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–43. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- 10.Phy JL, Conover CA, Abbott DH, Zschunke MA, Walker DL, Session DR, et al. Insulin and Messenger Ribonucleic Acid Expression of Insulin Receptor Isoforms in Ovarian Follicles From Nonhirsute Ovulatory Women and Polycystic Ovary Syndrome Patients. J Clin Endocrinol Metab. 2004;89:3561–6. doi: 10.1210/jc.2003-031888. [DOI] [PubMed] [Google Scholar]
- 11.Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome (PCOS) patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91:2327–33. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- 12.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 13.Pellat L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, et al. Granulosa cell production of anti-mullerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–5. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- 14.Al-Qahtani A, Muttukrishna S, Appasamy M, Johns J, Cranfield M, Visser JA, et al. Development of a sensitive enzyme immunoassay for anti-Müllerian hormone and the evaluation of potential clinical applications in males and females. Clin Endocrinol (Oxf) 2005;63:267–73. doi: 10.1111/j.1365-2265.2005.02336.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson CA, di Clemente N, Ehrenfels C, Pepinsky RB, Josso N, Vigier B, et al. Mullerian inhibiting substance requires its N-terminal domain for maintenance of biological activity, a novel finding within the transforming growth factor-beta superfamily. Mol Endocrinol. 1993;7:247–57. doi: 10.1210/mend.7.2.8469238. [DOI] [PubMed] [Google Scholar]
- 16.Liang K-Y, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 17.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LCL, Strauss JF., III Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87:101–6. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 18.Foong SC, Abbott DH, Lesnick TG, Session DR, Walker DL, Dumesic DA. Diminished intrafollicular estradiol (E2) levels in women with reduced ovarian responsiveness to recombinant human follicle stimulating hormone (FSH) therapy for in vitro fertilization (IVF) Fertil Steril. 2005;83:1377–83. doi: 10.1016/j.fertnstert.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 19.di Clemente N, Goxe B, Remy JJ, Cate RL, Josso N, Vigier B, et al. Inhibitory effect of AMH upon aromatase activity and LH receptors of granulosa cells of rat and porcine immature ovaries. Endocrine. 1994;2:553–8. [Google Scholar]
- 20.Andersen CY, Byskov AG. Estradiol and regulation of anti-mullerian hormone, inhibin-A, and inhibin B secretion: analysis of small antral and preovulatory human follicles’ fluid. J Clin Endocrinol Metab. 2006;91:4064–9. doi: 10.1210/jc.2006-1066. [DOI] [PubMed] [Google Scholar]
- 21.Grossman MP, Nakajima ST, Fallat ME, Siow Y. Mullerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cells. Fertil Steril. doi: 10.1016/j.fertnstert.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 22.Hirobe S, He WW, Lee MM, Donahoe PK. Mullerian inhibiting substance messenger ribonucleic acid expression in granulosa and sertoli cells coincides with their mitotic activity. Endocrinology. 1992;131:854–62. doi: 10.1210/endo.131.2.1639028. [DOI] [PubMed] [Google Scholar]
- 23.Hirobe S, He WW, Gustafson ML, Maclaughlin DT, Donahoe PK. Mullerian inhibiting substance gene expression in the cycling rat ovary correlates with recruited or graafian follicle selection. Bio Reprod. 1994;50:1238–43. doi: 10.1095/biolreprod50.6.1238. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Seibel MM, MacLaughlin DT, Donahoe PK, Ransil BJ, Hametz PA, et al. The inhibitory effects of mullerian-inhibiting substance on epidermal growth factor induced proliferation and progesterone production of human granulosa-luteal cells. J Clin Endocrinol Metab. 1992;75:911–7. doi: 10.1210/jcem.75.3.1517385. [DOI] [PubMed] [Google Scholar]
- 25.May JV, Frost JP, Schomberg DW. Differential effects of epidermal growth factor, insulin-like growth factor, and transforming growth factor beta on porcine granulosa cell DNA synthesis and cell proliferation. Endocrinology. 1988;123:168–79. doi: 10.1210/endo-123-1-168. [DOI] [PubMed] [Google Scholar]
- 26.Kevenaar ME, Themmen AP, Laven JS, Sonntag B, Fong SL, Uitterlinden AG, et al. Anti-mullerian hormone and anti-mullerian hormone type II receptor polymorphisms are associated with follicular phase estradiol levels in normo-ovulatory women. Hum Reprod. 2007;22:1547–54. doi: 10.1093/humrep/dem036. [DOI] [PubMed] [Google Scholar]
- 27.Baarends WM, Uilenbroek JTJ, Kramer P, Hoogerbrugge JW, van Leeuwen ECM, Themmen APN, et al. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrus cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–62. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- 28.Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-mullerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab. 2007;92:1034–40. doi: 10.1210/jc.2006-1697. [DOI] [PubMed] [Google Scholar]
- 29.Seifer DB, MacLaughlin DT, Penzias AS, Behrman HR, Asmundson L, Donahoe PK, et al. Gonadotropin-releasing hormone agonist-induced differences in granulosa cell kinetics are associated with alterations in follicular fluid mullerian-inhibiting substance and androgen content. J Clin Endocrinol Metab. 1993;76:711–4. doi: 10.1210/jcem.76.3.8445031. [DOI] [PubMed] [Google Scholar]
- 30.Smitz J, Andersen AN, Devroey P, Arce JC the MERIT Group. Endocrine profile in serum and follicular fluid differs after ovarian stimulation with HP-hMG or recombinant FSH in IVF patients. Hum Reprod. 2007;22:676–87. doi: 10.1093/humrep/del445. [DOI] [PubMed] [Google Scholar]
- 31.Thomas FH, Telfer EE, Fraser HM. Expression of anti-mullerian hormone protein during early follicle development in the primate ovary in vivo is influenced by suppression of gonadotropin secretion and inhibition of vascular endothelial growth factor. Endocrinology. 2007;148:2273–81. doi: 10.1210/en.2006-1501. [DOI] [PubMed] [Google Scholar]
- 32.Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820–6. doi: 10.1093/humrep/deh850. [DOI] [PubMed] [Google Scholar]
- 33.Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-mullerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266:201–8. doi: 10.1016/j.ydbio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi M, Koide SS, Donahoe PK. Mullerian inhibiting substance as oocyte meiosis inhibitor. Mol Cell Endocrinol. 1986;47:225–34. doi: 10.1016/0303-7207(86)90116-4. [DOI] [PubMed] [Google Scholar]
- 35.Ueno S, Manganaro TF, Donahoe PK. Human recombinant mullerian inhibiting substance inhibition of rat oocyte meiosis is reversed by epidermal growth factor in vitro. Endocrinology. 1988;123:1652–9. doi: 10.1210/endo-123-3-1652. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi C, Fujito A, Kazuka M, Sugiyama R, Ito H, Isaka K. Antimullerian hormone substance from follicle fluid is positively associated with success in oocyte fertilization during in vitro fertilization. Fertil Steril. 2008;89:586–91. doi: 10.1016/j.fertnstert.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 37.Wunder DM, Guibourdenche J, Birkhäuser MH, Bersinger NA. Anti-Müllerian hormone and inhibin B as predictors of pregnancy after treatment by in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2008 Feb 19; doi: 10.1016/j.fertnstert.2007.10.078. Epub ahead of print. [DOI] [PubMed] [Google Scholar]