Abstract
We designed a polymerase chain reaction (PCR) for amplifying the Helicobacter pylori gene encoding 16S rRNA. Primers for the specific detection of H. pylori were designed for areas of the 16S rRNA gene in which there is the least sequence homology between H. pylori and its closest relatives. The specificity of detection was confirmed by ensuring that the primers did not amplify DNA extracts from the campylobacters H. cinaedi, H. mustelae, and Wolinella succinogenes, which are the closest relatives of H. pylori, as determined by 16S rRNA sequencing. Serial dilution experiments revealed the detection of as little as 0.1 pg of DNA by PCR and 0.01 pg by nested PCR. H. pylori DNA was detected successfully in clinical paraffin-embedded and fresh gastric biopsy specimens from patients positive for the bacterium and also in fecal suspensions seeded with the organism. The DNA from the nonculturable coccoid form of H. pylori was also identified by the primers. Universal primers designed for highly conserved areas on the 16S rRNA gene enabled large amplification products to be produced for direct sequencing analysis. Gastric bacteria resembling H. pylori have been isolated from animals. DNA of these animal gastric bacteria amplified with H. pylori-specific primers yielded PCR products identical to those from human isolates of H. pylori, as confirmed by the use of a 20-base radiolabelled probe complementary to an internal sequence flanked by the H. pylori-specific primers. The results of PCR amplification and partial 16S rRNA gene sequence analysis strongly support the contention that the gastric organisms previously recovered from a pig, a baboon, and rhesus monkeys are H. pylori.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchard A., Barile M. F. Cloning of Ureaplasma urealyticum DNA sequences showing genetic homology with urease genes from gram-negative bacteria. Res Microbiol. 1989 May-Jun;140(4-5):281–290. doi: 10.1016/0923-2508(89)90020-x. [DOI] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. L., Pallen M. J., Kleanthous H., Wren B. W., Tabaqchali S. Nucleotide sequence of two genes from Helicobacter pylori encoding for urease subunits. Nucleic Acids Res. 1990 Jan 25;18(2):362–362. doi: 10.1093/nar/18.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. F. Progress in the pathology of gastritis and duodenitis. Curr Top Pathol. 1990;81:1–40. doi: 10.1007/978-3-642-74662-8_1. [DOI] [PubMed] [Google Scholar]
- Estrada I. C., Lamb F. I., Colston M. J., Cox R. A. Partial nucleotide sequence of 16S ribosomal RNA isolated from armadillo-grown Mycobacterium leprae. J Gen Microbiol. 1988 Jun;134(6):1449–1453. doi: 10.1099/00221287-134-6-1449. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Hamilton S. R., Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985 Jul 16;130(1):118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Blincow E. D., Warren J. R., Waters T. E., Sanderson C. R., Easton L. Evaluation of cultural techniques for isolating Campylobacter pyloridis from endoscopic biopsies of gastric mucosa. J Clin Pathol. 1985 Oct;38(10):1127–1131. doi: 10.1136/jcp.38.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Klein P. D., Evans D. J., Jr, Evans D. G., Alpert L. C., Opekun A. R., Boutton T. W. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet. 1987 May 23;1(8543):1174–1177. doi: 10.1016/s0140-6736(87)92145-3. [DOI] [PubMed] [Google Scholar]
- Gray S. F., Wyatt J. I., Rathbone B. J. Simplified techniques for identifying Campylobacter pyloridis. J Clin Pathol. 1986 Nov;39(11):1279–1279. doi: 10.1136/jcp.39.11.1279-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshina S., Kahn S. M., Jiang W., Green P. H., Neu H. C., Chin N., Morotomi M., LoGerfo P., Weinstein I. B. Direct detection and amplification of Helicobacter pylori ribosomal 16S gene segments from gastric endoscopic biopsies. Diagn Microbiol Infect Dis. 1990 Nov-Dec;13(6):473–479. doi: 10.1016/0732-8893(90)90079-b. [DOI] [PubMed] [Google Scholar]
- Jackson D. P., Lewis F. A., Taylor G. R., Boylston A. W., Quirke P. Tissue extraction of DNA and RNA and analysis by the polymerase chain reaction. J Clin Pathol. 1990 Jun;43(6):499–504. doi: 10.1136/jcp.43.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Lau P. P., DeBrunner-Vossbrinck B., Dunn B., Miotto K., MacDonnell M. T., Rollins D. M., Pillidge C. J., Hespell R. B., Colwell R. R., Sogin M. L. Phylogenetic diversity and position of the genus Campylobacter. Syst Appl Microbiol. 1987;9:231–238. doi: 10.1016/s0723-2020(87)80027-9. [DOI] [PubMed] [Google Scholar]
- Lee A., Hazell S. L., O'Rourke J., Kouprach S. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988 Nov;56(11):2843–2850. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
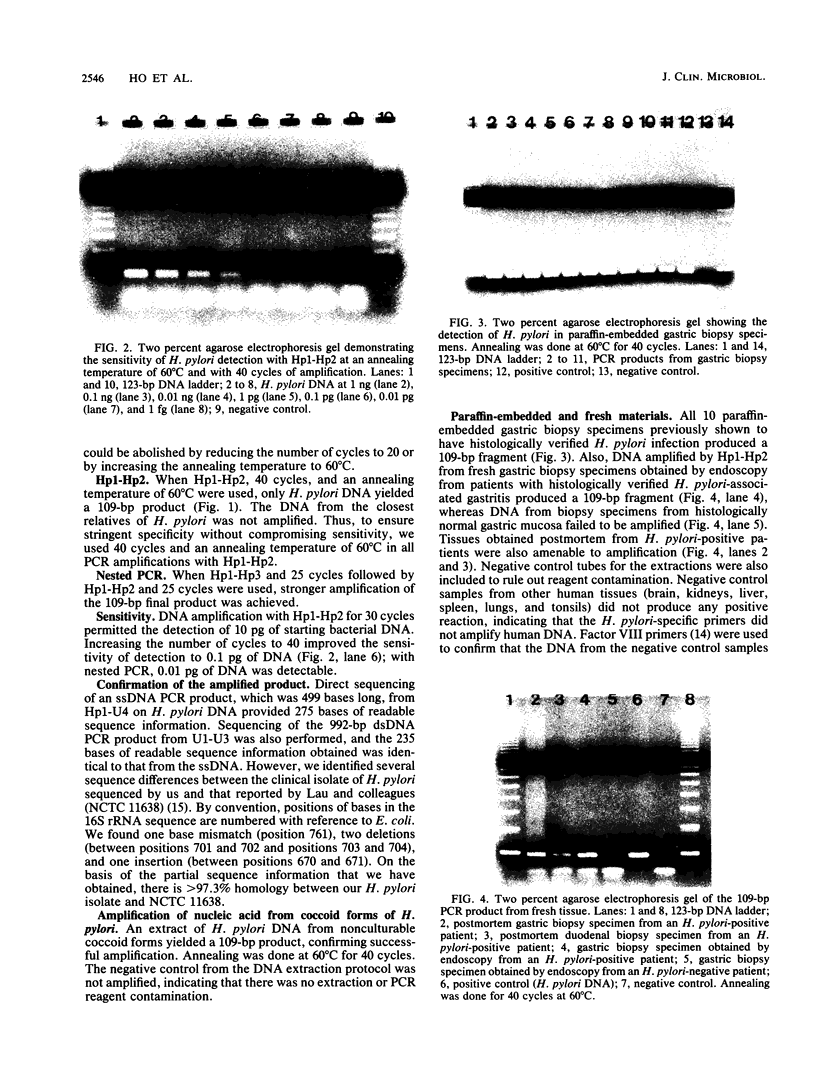
- Lo Y. M., Mehal W. Z., Fleming K. A. In vitro amplification of hepatitis B virus sequences from liver tumour DNA and from paraffin wax embedded tissues using the polymerase chain reaction. J Clin Pathol. 1989 Aug;42(8):840–846. doi: 10.1136/jcp.42.8.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy D. C., Nauman R. K., Paxton H. Detection of Borrelia burgdorferi using the polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1089–1093. doi: 10.1128/jcm.28.6.1089-1093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R., Francis G. J., Langton S. R., Goodwin C. S., Blincow E. D. Rapid urease test in the management of Campylobacter pyloridis-associated gastritis. Am J Gastroenterol. 1987 Mar;82(3):200–210. [PubMed] [Google Scholar]
- McNulty C. A., Dent J. C., Curry A., Uff J. S., Ford G. A., Gear M. W., Wilkinson S. P. New spiral bacterium in gastric mucosa. J Clin Pathol. 1989 Jun;42(6):585–591. doi: 10.1136/jcp.42.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotomi M., Hoshina S., Green P., Neu H. C., LoGerfo P., Watanabe I., Mutai M., Weinstein I. B. Oligonucleotide probe for detection and identification of Campylobacter pylori. J Clin Microbiol. 1989 Dec;27(12):2652–2655. doi: 10.1128/jcm.27.12.2652-2655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., Hudson M. J., Baskerville A. Isolation of a gastric campylobacter-like organism from the stomach of four rhesus monkeys, and identification as Campylobacter pylori. J Med Microbiol. 1988 Sep;27(1):41–44. doi: 10.1099/00222615-27-1-41. [DOI] [PubMed] [Google Scholar]
- Olive D. M. Detection of enterotoxigenic Escherichia coli after polymerase chain reaction amplification with a thermostable DNA polymerase. J Clin Microbiol. 1989 Feb;27(2):261–265. doi: 10.1128/jcm.27.2.261-265.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Lee A., Fox J. G., Dewhirst F. E., Tordoff L. A., Fraser G. J., O'Rourke J. L., Taylor N. S., Ferrero R. Phylogeny of Helicobacter felis sp. nov., Helicobacter mustelae, and related bacteria. Int J Syst Bacteriol. 1991 Jan;41(1):31–38. doi: 10.1099/00207713-41-1-31. [DOI] [PubMed] [Google Scholar]
- Porter-Jordan K., Rosenberg E. I., Keiser J. F., Gross J. D., Ross A. M., Nasim S., Garrett C. T. Nested polymerase chain reaction assay for the detection of cytomegalovirus overcomes false positives caused by contamination with fragmented DNA. J Med Virol. 1990 Feb;30(2):85–91. doi: 10.1002/jmv.1890300202. [DOI] [PubMed] [Google Scholar]
- Päbo S., Higuchi R. G., Wilson A. C. Ancient DNA and the polymerase chain reaction. The emerging field of molecular archaeology. J Biol Chem. 1989 Jun 15;264(17):9709–9712. [PubMed] [Google Scholar]
- Rauws E. A., Tytgat G. N. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990 May 26;335(8700):1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., Zoltowska B., Trust T. J., Lane D. J., Olsen G. J., Pace N. R., Stahl D. A. Campylobacter pylori, the spiral bacterium associated with human gastritis, is not a true Campylobacter sp. J Bacteriol. 1987 May;169(5):2137–2141. doi: 10.1128/jb.169.5.2137-2141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins D. S., Wyatt J. I., Rathbone B. J., West A. P. The characterization and pathological significance of gastric Campylobacter-like organisms in the ferret: a model for chronic gastritis? Epidemiol Infect. 1988 Oct;101(2):269–278. doi: 10.1017/s0950268800054182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J. L., Arthur R. R., Mobley H. L., Dick J. D. Detection of Helicobacter pylori by using the polymerase chain reaction. J Clin Microbiol. 1991 Apr;29(4):689–695. doi: 10.1128/jcm.29.4.689-695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary P. H., Andersen P. R., Green E., Hermon-Taylor J., McFadden J. J. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne's disease. J Clin Microbiol. 1990 May;28(5):933–937. doi: 10.1128/jcm.28.5.933-937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. M., Weller R., Bateson M. M. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature. 1990 May 3;345(6270):63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]