Abstract
Background
Analysis of the composition of muscle fibers constituent to a cleft palate could provide significant insight into the cause of velopharyngeal inadequacy. The authors hypothesized that levator veli palatini muscle dysfunction inherent to cleft palates could affect the timing and outcome of cleft palate repair.
Methods
Single, permeabilized muscle fibers from levator veli palatini muscles of three normal (n = 19 fibers) and three chemically induced congenital cleft palates (n = 21 fibers) of 14-month-old goats were isolated, and contractile properties were evaluated. The maximum isometric force and rate constants of tension redevelopment (ktr) were measured, and the specific force and normalized power were calculated for each fiber.
Results
The ktr measures indicate that cleft fibers are predominantly fast-fatigable; normal fibers are slow fatigue-resistant: after a 10-minute isometric contraction, fibers from cleft palates had a loss of force 16 percent greater than that from normal palates (p = 0.0001). The cross-sectional areas of the fibers from cleft palates (2750 ± 209 μm2) were greater (p = 0.05) than those from normal palates (2226 ± 143 μm2). Specific forces did not differ between the two groups. Maximum normalized power of fibers from cleft palates (11.05 ± 1.82 W/l) was greater (p = 0.0001) than fibers from normal palates (1.60 ± 0.12 W/l).
Conclusions
There are clear physiologic differences in single muscle fibers from cleft palates and normal palates: cleft palate fibers are physiologically fast, have greater fatigability, and have greater power production. Detection of functional and/or fiber type differences in muscles of cleft palates may provide preoperative identification of a patient's susceptibility to velopharyngeal inadequacy and permit early surgical intervention to correct this clinical condition.
The rate of cleft palate has been reported to be as high as 6.8 per 10,000 live births,1,2 which makes cleft palate the second most common congenital disability in the world. Although corrective surgery has been well described, 15 to 25 percent of cleft palate repairs still require an additional surgical procedure to correct persistent velopharyngeal insufficiency.3,4 Although the variability in both surgical technique and the type of repair may help to explain inconsistent rates of success,5 previous studies have shown that intrinsic differences in cleft palate muscles before surgical intervention could potentially play a significant role in determining surgical outcomes. Cohen et al. observed disorganization in fiber orientation in levator veli palatini muscle of cleft palates.6 Schendel et al. found that isolated human cleft palate muscle contained a mixture of type I and type II fibers; however, they did not compare their results to normal palatal musculature.7 Lindman et al. compared levator veli palatini muscle obtained from cleft palate infants to those obtained from levator veli palatini muscle of normal adults. They observed a larger variation in fiber size, with an overall smaller mean fiber diameter, an increase in the proportion of type II fibers, and an increase in the amount of fast myosin heavy chain composition in the levator veli palatini muscles from infant cleft palates compared with those of normal adults. In the congenital cleft palate model, Weinzweig et al. observed degeneration of myofibrils and Z-bands.8 We hypothesize that intrinsic differences in the levator veli palatini muscle alter the functional state of the muscles and that those differences may play a more crucial role in the differential outcomes following surgery than the surgical procedure itself.
Skeletal muscles with predominately fast (type II) fibers are more fatigable and more prone to contraction-induced injury compared with slow (type I) fibers.9,10 Therefore, the increase in fast fibers observed in the cleft muscle may lead to increased fatigability and injury to the levator veli palatini during speech and contribute to the conditions of persistent velopharyngeal inadequacy observed in a portion of cleft palate patients following surgical intervention. Using electromyography to monitor muscle activity, Tachimura et al. observed a decline in muscle activity in the levator veli palatini muscles of repaired cleft palate subjects with velopharyngeal inadequacy compared with normal speakers. They concluded that the subjects with velopharyngeal inadequacy were displaying increased levator veli palatini muscle fatigue during speech compared with subjects who had normal palates.11 However, other explanations, such as the subjects’ willingness to perform the task, may have influenced measures of muscle fatigue. Therefore, we used single permeabilized fibers to study the intrinsic properties of the muscle without extrinsic neuronal and hormonal influences.
The congenital cleft goat model has been well characterized by Weinzweig et al.12 According to the authors, the Nicotiana glauca slurry diminishes the movement of the fetal tongue, with prolonged maintenance of the tongue between the palatal shelves during the period of shelf closure. Disruption of palatal shelf closure is believed to be the mechanical mechanism responsible for the resultant palatal clefting. The authors have not observed any direct teratogenic effect of the anabasine on the maxillary musculoskeletal region. The effect of the anabasine on musculoskeletal motion is transient and resolves shortly after administration of the teratogen is completed. Complete clefting of the secondary palate occurs in 97 percent of the fetuses.
In all cases, the cleft extended from the posterior aspect of the alveolar ridge to the uvula; the majority of these clefts were bilateral, with complete detachment of the vomer. Morphologically, these clefts were similar to human clefts.12
The purpose of this study was to use the single permeabilized muscle fiber technique to study the intrinsic properties of muscle, such as force, power, and fatigability, specifically, of levator veli palatini muscles obtained from congenitally cleft palates and normal palates of Spanish goats.
MATERIALS AND METHODS
Data were collected on single permeabilized fibers from the levator veli palatini muscle of six 14- to 15-month-old, female, Spanish goats with body masses of 25 to 56 kg. Three of these goats had unrepaired, teratogenically induced palatal clefts; the other three had normal, noncleft palates. The cleft palate goat model, developed by Weinzweig et al., used Nicotiana glauca to induce the palatal clefts. Pregnant goats were gavaged with a N. glauca plant slurry, containing the natural teratogen anabasine, twice daily from days 32 to 41 of gestation because palatal shelf closure occurs on day 40 of gestation. Complete clefting of the secondary palate is achieved with a success rate of 97 percent.12
Operative Procedures
Each goat was killed with an overdose of Beuthanasia (Schering-Plough Animal Health Corp., Kenilworth, N.J.), and within 15 minutes, the muscle was harvested. Muscle biopsy specimens were transferred immediately into skinning solution composed of 125 mM potassium propionate, 20 mM imidazole, 5 mM ethylene glycol-bis (b-aminoethyl ether)-N′ N′-tetraacetic acid (EGTA), 2 mM MgCl2, and 2 mM adenosine triphosphate at 4°C and separated into bundles in less than 20 minutes. Bundles were then placed in skinning solution containing the nonionic detergent Brij 58 (ICI Americas, Inc., Wilmington, Del.) (0.5 weight/volume) at 4°C for 30 minutes, to enhance the permeabilization of the fibers. After 30 minutes, the bundles were transferred into storage solution composed of skinning solution with glycerol substituted for 50 percent of the water volume at 4°C for 24 hours, after which they were transferred to fresh storage solution and kept at −20°C until bundles were removed to extract single fibers.
Procedure to Obtain Single Permeabilized Fibers
To extract single fibers, the bundles were thawed to 4°C and then moved to a relaxation solution (pCa 9.0; pH 7.1) composed of 90 mM HEPES, 10.3 mM magnesium (total), 1.0 mM magnesium ion, 50 mM EGTA, 8.0 mM adenosine triphosphate, 10.0 mM C-reactive protein, 1.0 mM sodium azide, 36 mM sodim (total), 125 mM potassium (total). Individual fibers were teased out of the bundle using fine forceps. Care was taken to ensure the individual fibers were not stretched during extraction.
Single Fiber Contractile Properties
Each single fiber was then transferred to a 15°C chamber containing relaxation solution. One end of the fiber was secured to a force transducer (Model 403A; Aurora Scientific, Inc., Aurora, Ontario, Canada) with two 10−0 monofilament nylon sutures, and the other end was secured to the lever arm of a servomotor (Model 403A; Aurora Scientific) with the same technique. The solution-changing system (Model 802A; Aurora Scientific) consisted of three separate glass-bottom wells machined into a movable, temperature-controlled, stainless-steel plate. Movement of the plate with respect to the fiber was achieved by remote control of two stepper motors: one lowered and raised the wells and the other moved the wells laterally to place the fiber in a new well position. Sarcomere length was set at 2.5 μm by projecting the laser diffraction pattern produced by the fiber onto a calibrated target screen. Fiber length (Lo) was measured by a calibrated micrometer on the microscope eyepiece. Fiber diameters were estimated at Lo based on two high-magnification digital images obtained from both top and side views of the fiber. Side views were obtained with a prism embedded in the side of the bath. Five pairs of width and depth diameter measurements were obtained at approximately 100-μm intervals along the midsection of each fiber. Fiber cross-sectional areas were calculated for each diameter pair assuming an elliptical cross-section, and overall cross-sectional area was estimated by averaging the five individual areas. Each fiber was then transferred to a chamber containing a low-[Ca2+] preactivation solution (pH 7.1) composed of 90 mM HEPES, 8.50 mM magnesium (total), 1.0 mM magnesium ion, 50 mM EGTA, 50 mM Ca2+ (total), 8.0 mM adenosine triphosphate, 10.0 mM C-reactive protein, 1.0 mM sodium azide, and 36 mM sodium (total) and allowed to equilibrate for 3 minutes. The preactivating solution is weakly buffered for Ca2+, which allows for a very rapid activation and force development on introduction of the fiber into the activating solution.13 Fibers were then moved into a chamber containing the activation solution (pCa 4.5; pH 7.1) composed of 90 mM HEPES, 8.12 mM magnesium (total), 1.0 mM magnesium ion, 50 mM EGTA, 50 mM Ca2+ (total), 8.0 mM adenosine triphosphate, 10.0 mM C-reactive protein, 1.0 mM sodium azide, 36 mM sodium (total), and 125 mM potassium (total). Fibers were allowed to contract until they reached their maximum force production. Each fiber was then stretched to Lo + 10 percent and cycled through a series of step-ramp shortening movements while the fibers were fully activated. The size of the step ranged from 3 to 5 percent of Lo. The speeds of the ramps used varied according to fiber type. Data were collected with a Labview 7.1 program (National Instruments Corp., Austin, Texas).
Power Calculations
The velocity of each ramp was plotted versus the force (F0) generated during each ramp. A rectangular hyperbola was fitted to these data points. Each value for velocity represented by the curve was multiplied by the value for F0 and a power curve was calculated. The peak of the curve was recorded as the maximum power-generating capacity (Pmax). Pmax was then divided by the fiber volume (Lf × cross-sectional area) to obtain normalized power (nPmax).
Determination of Fiber Type
Fiber type was assigned to each fiber by determining their rate constants of tension redevelopment (ktr) following one cycle of activation. The ktr can be used as an indirect measure of fiber type14 because the ktr is indicative of the kinetics of attachment and detachment reactions of cross-bridges.15 Burton et al. have shown that double exponential equations best describe the tension redevelopment of a single permeabilized fiber during chemical activation.15,16 Therefore, tension redevelopment versus time data were fitted to a double exponential using analysis software (Signo; Alamedo Applied Sciences, San Leandro, Calif.) and a least-squares fit to the following equation:
where F is tension at time t, Ffmax is the maximal tension for the fast component, krf is the rate constant of the fast component, Fsmax is the maximal tension for the slow component, krs is the rate constant of the slow component, and Fres represents the residual tension present immediately after the release and restretch maneuver. Using either krf or krs, one can determine fiber type. In this study, we used krf as the rate constant to categorize fiber type.
Fatigue during Isometric Contractions
Single, permeabilized muscle fibers were activated and allowed to generate maximum force. Fibers were cycled, as described by Brenner, to ensure the fatigue was not caused by the inability for permeabilized fibers to keep their sarcomeres aligned after prolonged activation.17 Once fibers reached their maximum force, the servomotor arm moved in at a rate of 1000 L0/second, causing the fiber to become completely slack for 80 μsec, and then the arm moved back at the same rate to return the fiber to L0. This maneuver unloaded the fiber, thus allowing sarcomeres time to reset. This cycle was repeated for 10 minutes, and the percentage of force maintained by each fiber at the end of the protocol was calculated.
Statistical Analysis
Values are presented as means ±SE. Statistical analysis was performed by using Jump In 5.1 (SAS Institute, Inc., Cary, N.C.). A nested one-way analysis of variance was conducted to compare the differences between cleft and normal levator veli palatini muscle. When the variance was not evenly distributed, the nonparametric Mann-Whitney analysis was conducted. Differences were considered significant at p < 0.05.
RESULTS
Fiber Structure and Type
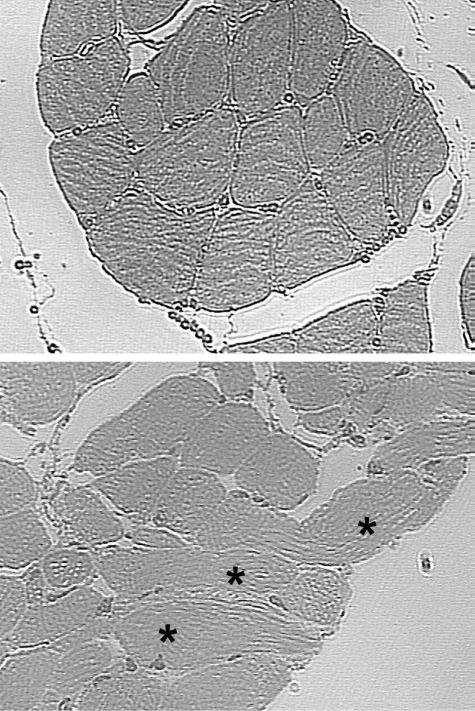
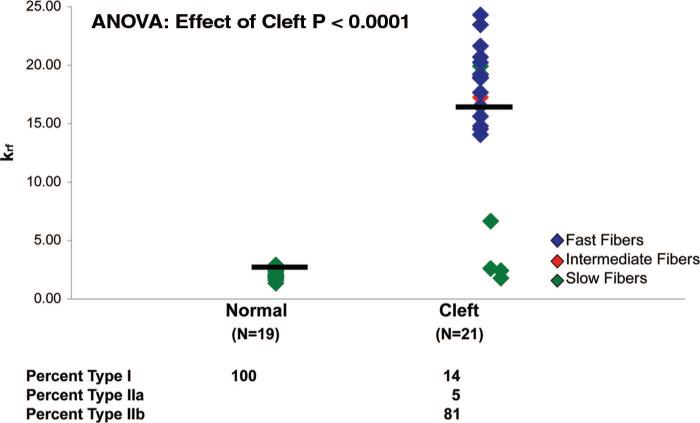
Compared with normal levator veli palatini muscle (Fig. 1, above), the cleft levator veli palatini muscle (Fig. 1, below) exhibits disorganization of muscle fibers and bundles. The highly organized hierarchical pattern of myofibers aligned in parallel and organized into discrete fascicles does not exist in the cleft palate muscle. Instead, we observed fibers running perpendicular and at various angles to each other. Characterization of the fiber type and fiber speed (krf) of single permeabilized muscle fibers from the levator veli palatini of 14-month-old goats in both the cleft (n = 21) and normal (n = 22) group are presented in Figure 2. Seventeen of the 21 muscle fibers from the cleft group were fast (type II) fibers and the remaining four consisted of one intermediate fiber and three slow (type I) fibers. In contrast, each of the 22 fibers from the normal group was a slow (type I) fiber.
Fig. 1.
Compared with normal levator veli palatini muscle (above), the cleft levator veli palatini muscle (below) exhibits disorganization of muscle fibers and bundles. The highly organized hierarchical pattern of myofibers aligned in parallel and organized into discrete fascicles does not exist in the cleft palate muscle. Instead, we observed fibers running perpendicular and at various angles to each other (asterisks).
Fig. 2.
Fiber type was assigned to each fiber by determining their rate constants of tension redevelopment (ktr) following one cycle of activation. Using krf, normal levator veli palatini consists of 100 percent slow (type I) fibers, whereas the congenitally cleft palate consists of 14 percent slow (type I), 5 percent intermediate (type IIa), and 81 percent fast (type IIb) fibers. Each symbol represents one fiber and the bars denote the mean values.
Cross-Sectional Area
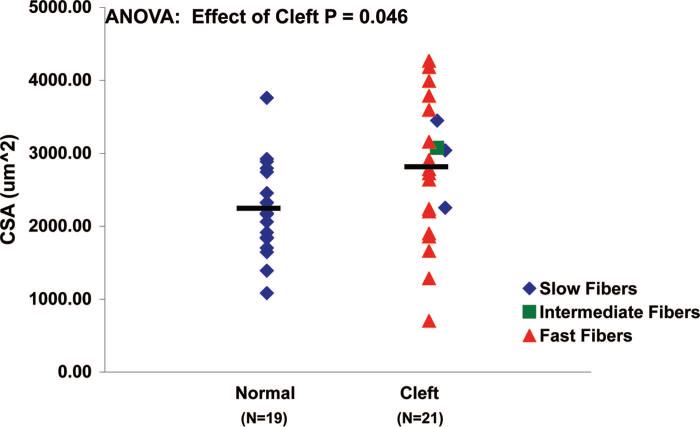
The cross-sectional areas of single muscle fibers from cleft palates were larger than those from the normal palates (p = 0.05). The average ± SE cross-sectional area of single muscle fibers from normal palates was 2226 ± 143 μm2, whereas the single muscle fibers from cleft palates had an average cross-sectional area of 2750 ± 209 μm2 (Fig. 3). All of the values for cross-sectional area are 20 to 50 percent larger than the values for intact fibers, because permeabilized fibers become swollen because of their storage in the permeabilization solution.
Fig. 3.
Fiber cross-sectional area in normal and cleft palate muscle fibers. The cross-sectional areas of single muscle fibers from cleft palates were greater than those from normal palates (p = 0.046). The average cross-sectional area of single muscle fibers from normal palates was 2226 ± 143 μm2, whereas the single muscle fibers from cleft palates had an average cross-sectional area of 2750 ± 209 μm2. Each symbol represents one fiber and the bars denote the mean values.
Contractile Properties
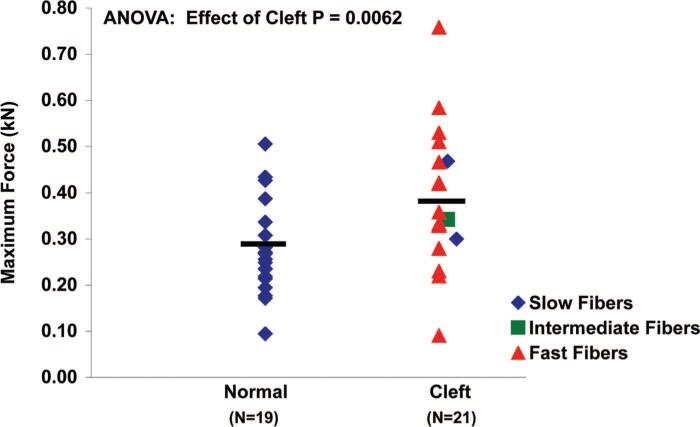
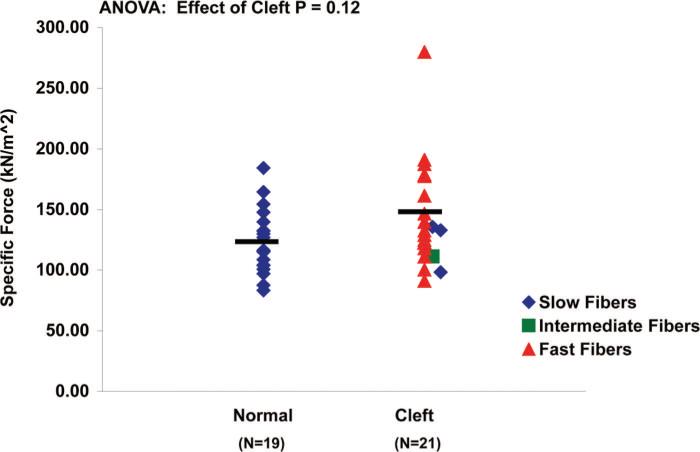
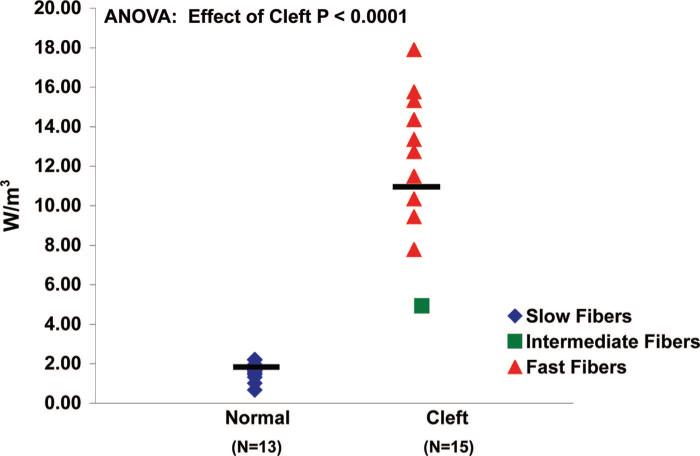
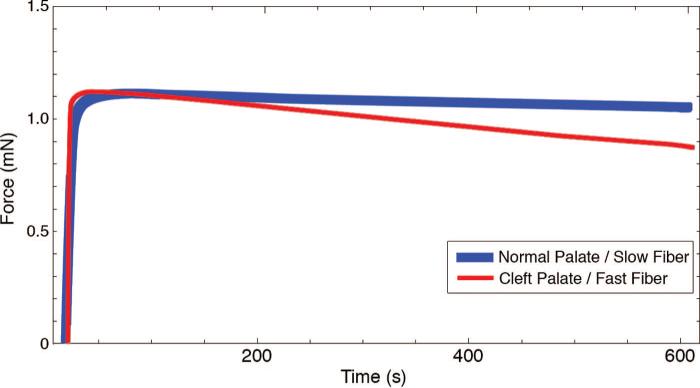
Single muscle fibers from the cleft palate group generated more maximum isometric force (F0) (0.38 ± 0.03 kN) than those from the normal palate group (0.28 ± 0.02 kN) (Fig. 4) (p = 0.014). Muscle fibers from the cleft palate group did not significantly differ in their ability to produce specific force (sF0) (143 ± 9.34 μN/m2) compared with fibers from normal palate group (123 ± 6.09 μN/m2) (Fig. 5) (p = 0.10). Single muscle fibers from cleft palates had greater nPmax than those from normal palates (p < 0.0001). The average nPmax of single fibers from cleft palates was found to be 11.05 ± 1.82 W/l compared with 1.60 ± 0.12 W/l for those from normal levator veli palatini muscles (Fig. 6). Muscle fibers from the normal palates (type I) were able to develop a higher percentage (95 percent) of their Fmax by the end of a 10-minute isometric contraction (Fig. 7). Each of the fibers from the cleft palates analyzed for fatigue were type II, and these fibers were able to develop only 79 percent of their maximum force production after a 10-minute isometric contraction. The fibers from the cleft palates were much more prone to sarcomere disruption without the use of Brenner cycling, maintaining only 80 percent of their force after a 5-minute isometric contraction. Few to no differences were observed when comparing the effects of Brenner cycling on the fibers from normal palates.
Fig. 4.
Maximum isometric force in normal and cleft palate muscle fibers. Muscle fibers from the cleft palate group generated more maximum isometric force (0.38 ± 0.03 kN) than those from the normal palate group (0.28 ± 0.02 kN) (p = 0.0062). Each symbol represents one fiber and the bars denote the mean values.
Fig. 5.
Specific force in normal and cleft palate muscle fibers. Muscle fibers from the cleft palate group did not generate more specific force (143 ± 9.34 kN/m2) than those from the normal palate group (123 ± 6.09 kN/m2)(p = 0.12). Each symbol represents one fiber and the bars denote the mean values.
Fig. 6.
Maximum normalized power (nPmax) in normal and cleft palate muscle fibers. The average nPmax of muscle fibers from normal palatal muscle was greater than that of muscle fibers from cleft palate muscle (p = 0.0001). The average nPmax of single fibers from cleft palates was found to be 11.05 ± 1.82 W/l compared with 1.60 ± 0.12 W/l found in single fibers from normal levator veli palatini muscle. Each symbol represents one fiber and the bars denote the mean values.
Fig. 7.
Isometric fatigue tracings of a muscle fiber from a normal and a cleft palate. The fiber from the cleft palate, unlike that from the normal palate, rapidly loses its ability to maintain its Fmax. By the end of the 10-minute contraction, the fiber from the cleft palate generates 16 percent less force than the fiber from the normal palate. This trend was consistent among all the fibers from the respective groups.
DISCUSSION
This study examined the contractile properties and fatigability of single permeabilized muscle fibers obtained from the levator veli palatini muscles from a congenital cleft palate model developed in goats by Weinzweig et al.12 and from age-matched normal goat palates. Our data are consistent with previous studies in humans that have shown an increase in type II fibers in levator veli palatini muscles of cleft compared with normal palates.7,18 In the goat model, normal levator veli palatini consists of 100 percent slow (type I) fibers, whereas the congenitally cleft palate consists of 14 percent slow (type I), 5 percent intermediate (type IIa), and 81 percent fast (type IIb) fibers. In addition to the shift in fiber type, the single permeabilized fibers from the levator veli palatini muscle of clefted palates had increased fatigability compared with fibers from normal goats, which may have contributed to the velopharyngeal incompetence observed in cleft palate patients after surgical repair.
The shift in fiber type composition of cleft muscle toward an increase in fast fatigable fibers, as observed in cleft muscle of the goat model, has also been observed in samples obtained from humans with cleft palates.7,18 Schendel et al. obtained muscle samples from 2- to 10-month-old human patients with cleft palate during primary palatoplasty and found a mixed fiber type (56.7 percent type I and 43.3 percent type II) in the levator veli palatini muscles of cleft palates.7 However, Schendel et al. did not have normal tissue for comparison and, as a result, this mixed fiber type in the cleft palates may not have been different from that seen in normal palates. Lindman et al. characterized the palatal muscle of human infants (age range, 18 to 21 months) with cleft palates and compared them to levator veli palatini muscles obtained from five adults (age range, 46 to 75 years). They observed 36.9 percent type I and 63.1 percent type II in the infant cleft versus 74.9 percent type I and 25.1 percent type II in the normal adult levator veli palatini muscle.18 Once again, there was no comparison to age-matched, normal levator veli palatini muscle and, as a result, the clinical significance of these fiber type transformations was difficult to define. These studies nicely identified the differences in normal and cleft palate levator veli palatini muscle, leading to the hypothesis that inherent differences in the levator veli palatini muscle of cleft and normal palates contributes to velopharyngeal inadequacy following cleft palate repair. Using single muscle fibers in a congenital cleft palate model, we were able to evaluate the functional differences between levator veli palatini muscles from cleft and normal palates in age-matched experimental groups. Although the shifts in the percentages of fibers were not identical to those seen in humans, the goat cleft palate model developed by Weinzweig et al. serves as a dependable model for studying additional characteristics of single fiber functional properties and other general properties of the palatal musculature for comparison with human cleft palates.
During normal muscle development, a muscle fiber begins as a fast fiber and either remains fast or shifts to a slow fiber following complete innervation.19 Because neither the study by Schendel et al. nor the one by Lindman et al. compared their cleft palate muscles to age-matched normal palate muscle, one cannot conclude from these studies whether the increased type II fiber population is normal for a developing levator veli palatini muscle or whether the increased type II fiber population is the result of the cleft palate condition. Our study compared palatal muscle from age-matched (14 months old) cleft and normal goats that have passed the developmental stage for muscle. Unlike the levator veli palatini muscle from normal human palate, we found 100 percent slow fibers in normal goat levator veli palatini muscle. In addition, we found a greater shift toward the type II fiber in the levator veli palatini muscles of the cleft goats, 86 percent compared with the 43 percent and 63 percent fast fibers observed in cleft levator veli palatini in human studies. It is also possible that these fibers are in a state of arrested development and have not completely shifted from type I to type II fibers, or that there is a postmaturational alteration in muscle function causing the shift in fiber population. A postdevelopmental shift in fiber type from slow to fast can occur in response to lack of tension development and/or lack of innervation.20 Further characterization of proteins expressed during muscle regeneration and reinnervation should be performed to assess the mechanism for the fiber type shift in the cleft muscle.
In addition to the shift in fiber type, the cleft muscle also exhibits disorganization of muscle fibers and bundles. The highly organized hierarchical pattern of myofibers aligned in parallel and organized into discrete fascicles does not exist in the cleft palate muscle. Instead, we observed fibers running perpendicular and at various angles to each other. Because of the disorganized array of muscle fibers found in the cleft muscle, we found it impossible to monitor the contractile function of the entire levator veli palatini muscle. Therefore, we used a single permeabilized muscle fiber model to characterize the function of the levator veli palatini muscle. Obviously, this muscle fiber disorganization in the levator veli palatini muscle from cleft palates can compromise the function of the whole levator veli palatini muscle and potentially contribute to velopharyngeal inadequacy even after cleft palate repair.
Single permeabilized muscle fibers obtained from the levator veli palatini muscle of cleft palates generated greater F0 than those from normal palates. Many studies have shown that fast fibers have increased F0.21 In addition, our study observed higher nPmax from levator veli palatini muscle fibers from the cleft palates. Widrick et al. also observed type II fibers producing five to 10 times more normalized power than type I fibers.22 The profile of a malfunctioning fiber is diminished specific force. Therefore, these data suggest that the fast fibers of cleft palates are functioning normally.
Fast fibers fatigue more readily than slow fibers. After a 10-minute isometric contraction protocol, single permeabilized fibers from cleft palates maintained less of their initial Fmax compared with normal palates, which maintained on average 96 percent of their Fmax. Gonzalez and Delbono used a similar protocol and found that fibers from the extensor digitorum longus muscle of rats, which is composed of 95 percent type II fibers, maintained less of their maximum tension than fibers from the soleus muscle of rats, which is composed of 98 percent type I fibers.23 Once again, at the single fiber level, the muscle fibers obtained from the cleft palate appear to be functioning as normal type II fibers. The levator veli palatini muscle is used primarily during speech. Increased fatigability of the levator veli palatini muscle in clefted patients may explain the inability to lift the soft palate enough to provide velopharyngeal closure, which results in hypernasal speech. Tachimura et al. used nasoendoscopy to evaluate velopharyngeal closure during speech in patients with velopharyngeal inadequacy following cleft palate repair.11 Tachimura et al. observed a decline in muscle activity in the levator veli palatini muscles of cleft palate repair subjects with velopharyngeal inadequacy compared with normal speakers. They concluded that the muscles of the subjects with velopharyngeal inadequacy were displaying increased levator veli palatini muscle fatigue during speech compared with subjects with normal palates.
CONCLUSIONS
The major finding in this study is that single permeabilized muscle fibers from the cleft palates of goats are functioning like normal type II fibers in that they have increased cross-sectional area, F0, nPmax, and fatigability compared with type I fibers obtained from normal palates. The continuance of the large population of fast-fatigable fibers or the inability to convert to the slow fiber type may entirely explain the decreased muscle function of the levator veli palatini muscle during velopharyngeal incompetence, which occurs in approximately 25 percent of cleft palate patients following cleft palate repair. Future studies will be conducted to resolve the mechanism involved in the shift from slow to fast fibers and to optimize surgical interventions specific to the preoperative morphology to optimize the reversal of fiber type back to slow fibers following the intervention to diminish the incidence of postsurgical velopharyngeal inadequacy.
ACKNOWLEDGMENTS
The single muscle fiber preparation and testing were supported by Nathan Shock Center of Excellence in Basic Biology of Aging grant P30 AG 13283. D. Yu was supported by additional fellowships (National Institutes of Health Regenerative Sciences Training grant T90 DK-070071, the Academic Scholar of American Association of Plastic Surgeons, and National Institutes of Health Training Grant in Trauma, Burn, and Wound Healing Research grant T32 GM008616-06A1).
REFERENCES
- 1.Forrester MB, Merz RD. Descriptive epidemiology of oral clefts in a multiethnic population, Hawaii, 1986−2000. Cleft Palate Craniofac. J. 2004;41:622. doi: 10.1597/03-089.1. [DOI] [PubMed] [Google Scholar]
- 2.Elahi MM, Jackson IT, Elahi O, et al. Epidemiology of cleft lip and cleft palate in Pakistan. Plast. Reconstr. Surg. 2004;113:1548. doi: 10.1097/01.prs.0000117184.77459.2b. [DOI] [PubMed] [Google Scholar]
- 3.Becker DB, Grames LM, Pilgram T, et al. The effect of timing of surgery for velopharyngeal dysfunction on speech. J. Craniofac. Surg. 2004;15:804. doi: 10.1097/00001665-200409000-00020. [DOI] [PubMed] [Google Scholar]
- 4.McWilliams BJ, Randall P, LaRossa D, et al. Speech characteristics associated with the Furlow palatoplasty as compared with other surgical techniques. Plast. Reconstr. Surg. 1996;98:610. doi: 10.1097/00006534-199609001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Shaw WC, Dahl E, Asher-McDade C, et al. A six-center international study of treatment outcome in patients with clefts of the lip and palate: Part 5. General discussion and conclusions. Cleft Palate Craniofac. J. 1992;29:413. doi: 10.1597/1545-1569_1992_029_0413_asciso_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SR, Chen LL, Burdi AR, et al. Patterns of abnormal myogenesis in human cleft palates. Cleft Palate Craniofac. J. 1994;31:345. doi: 10.1597/1545-1569_1994_031_0345_poamih_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 7.Schendel SA, Cholon A, Delaire J. Histochemical analysis of cleft palate muscle. Plast. Reconstr. Surg. 1994;94:919. doi: 10.1097/00006534-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Weinzweig J, Panter KE, Spangenberger A, et al. The fetal cleft palate: III. Ultrastructural and functional analysis of palatal development following in utero repair of the congenital model. Plast. Reconstr. Surg. 2002;109:2355. doi: 10.1097/00006534-200206000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Macpherson PC, Schork MA, Faulkner JA. Contraction-induced injury to single fiber segments from fast and slow muscles of rats by single stretches. Am. J. Physiol. 1996;271:C1438. doi: 10.1152/ajpcell.1996.271.5.C1438. [DOI] [PubMed] [Google Scholar]
- 10.Macpherson PC, Dennis RG, Faulkner JA. Sarcomere dynamics and contraction-induced injury to maximally activated single muscle fibres from soleus muscles of rats. J. Physiol. 1997;500:523. doi: 10.1113/jphysiol.1997.sp022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tachimura T, Nohara K, Satoh K, et al. Evaluation of fatigability of the levator veli palatini muscle during continuous blowing using power spectra analysis. Cleft Palate Craniofac. J. 2004;41:320. doi: 10.1597/02-038.1. [DOI] [PubMed] [Google Scholar]
- 12.Weinzweig J, Panter KE, Pantaloni M, et al. The fetal cleft palate: I. Characterization of a congenital model. Plast. Reconstr. Surg. 1999;103:419. doi: 10.1097/00006534-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Moisescu DG, Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J. Physiol. 1978;275:241. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahr PA, Rall JA. Role of calcium and cross bridges in determining rate of force development in frog muscle fibers. Am. J. Physiol. 1997;272:C1664. doi: 10.1152/ajpcell.1997.272.5.C1664. [DOI] [PubMed] [Google Scholar]
- 15.Burton K, White H, Sleep J. Kinetics of muscle contraction and actomyosin NTP hydrolysis from rabbit using a series of metal-nucleotide substrates. J. Physiol. 2005;563:689. doi: 10.1113/jphysiol.2004.078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swartz DR, Moss RL. Influence of a strong-binding myosin analogue on calcium-sensitive mechanical properties of skinned skeletal muscle fibers. J. Biol. Chem. 1992;267:20497. [PubMed] [Google Scholar]
- 17.Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys. J. 1983;41:99. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindman R, Paulin G, Stal PS. Morphological characterization of the levator veli palatini muscle in children born with cleft palates. Cleft Palate Craniofac. J. 2001;38:438. doi: 10.1597/1545-1569_2001_038_0438_mcotlv_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 19.Wigmore PM, Evans DJ. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int. Rev. Cytol. 2002;216:175. doi: 10.1016/s0074-7696(02)16006-2. [DOI] [PubMed] [Google Scholar]
- 20.Ohira Y, Yoshinaga T, Nomura T, et al. Gravitational unloading effects on muscle fiber size, phenotype and myo-nuclear number. Adv. Space Res. 2002;30:777. doi: 10.1016/s0273-1177(02)00395-2. [DOI] [PubMed] [Google Scholar]
- 21.Widrick JJ, Trappe SW, Blaser CA, et al. Isometric force and maximal shortening velocity of single muscle fibers from elite master runners. Am. J. Physiol. 1996;271:C666. doi: 10.1152/ajpcell.1996.271.2.C666. [DOI] [PubMed] [Google Scholar]
- 22.Widrick JJ, Trappe SW, Costill DL, et al. Force-velocity and force-power properties of single muscle fibers from elite master runners and sedentary men. Am. J. Physiol. 1996;271:C676. doi: 10.1152/ajpcell.1996.271.2.C676. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez E, Delbono O. Age-dependent fatigue in single intact fast and slow fibers from mouse EDL and soleus skeletal muscles. Mech. Ageing Dev. 2001;122:1019. doi: 10.1016/s0047-6374(01)00229-9. [DOI] [PubMed] [Google Scholar]