Abstract
Background:
Macrophage migration inhibitory factor (MIF) has recently emerged as an important cytokine possibly linking rheumatoid arthritis (RA) and atherogenesis. Because atherogenesis is accelerated in RA this study was conducted to investigate whether anti-tumour necrosis factor (TNF) therapy could lead to sustained downregulation of systemic MIF levels and improvement in lipid profiles.
Methods:
Fifty RA patients with active disease (disease activity score in 28 joints (DAS28) ⩾3.2), who started adalimumab therapy at 40 mg every other week, were included. At baseline, weeks 16 and 52 serum levels of MIF and lipids were assessed. In addition, the DAS28 and serum C-reactive protein (CRP) levels and erythrocyte sedimentation rate (ESR) were determined.
Results:
After 16 weeks of adalimumab therapy, both DAS28 and MIF levels were significantly decreased (p<0.001 and p = 0.020, respectively). This was sustained up to week 52 (p<0.001 and p = 0.012, respectively). CRP levels and ESR were significantly reduced after 16 and 52 weeks of adalimumab therapy (p<0.001). High-density lipoprotein cholesterol levels increased at week 16 (p<0.001), but returned to baseline at week 52. Apolipoprotein (apo) A-I levels increased at week 16 (p<0.001) and remained stable (p = 0.005). This resulted in an improved apo B/A-I ratio.
Conclusions:
The results underline the sustained downregulation of MIF as a potential new mechanism by which anti-TNF therapy might reduce vascular inflammation, and as such perhaps cardiovascular morbidity in RA patients. This hypothesis is supported by an improved apo B/A-I ratio as well as reduced CRP levels in these patients.
The atherosclerotic process is accelerated in patients with rheumatoid arthritis (RA), resulting in increased cardiovascular mortality when compared with the general population. It has been suggested that the chronic systemic inflammatory state in RA enhances atherogenesis1 over and above the presence of traditional risk factors (eg, diabetes, smoking, obesity, dyslipidaemia). Inflammatory mediators from the synovium and perhaps other sites can be released into the circulation where they can alter the function of various tissues, such as skeletal muscle, liver and vascular endothelium. This in turn may induce an array of proatherogenic changes, including insulin resistance, characteristic dyslipidaemia and endothelial dysfunction.2 Moreover, circulating inflammatory mediators may also stimulate leucocytes and smooth muscle cells within the atherosclerotic plaque thereby promoting plaque growth or rupture.3
Macrophage migration inhibitory factor (MIF) has emerged as a cytokine linking RA and atherogenesis.4 The association of coronary heart disease (CHD) with a haplotype containing the rs755622C allele, which has been reported before to increase the susceptibility to various inflammatory conditions, supports the notion that MIF plays a role in inflammation and atherogenesis, although there was no difference in MIF serum levels between patients with incident CHD and individuals without such disease during follow-up in a population-based case–cohort study.5 However, in another prospective population study in apparently healthy volunteers, elevated levels of MIF were associated with an increased risk of future coronary artery disease.6 The receptors CXCR2 on monocytes and CXCR4 on T cells have been identified as the functional receptors for MIF.7 Interaction of CXCR2 with MIF on aortic endothelial cells was shown to induce monocyte arrest. Similarly, the interaction of CXCR4 with MIF resulted in the arrest of T cells. MIF can also induce the secretion of tumour necrosis factor (TNF) by macrophages and, conversely, TNF is able to augment MIF production.8 In an animal model of atherosclerosis, MIF blockade reduced plaque infiltration by monocytes and T cells, and even led to plaque regression.7 Recent studies have demonstrated that MIF secretion by dendritic cells can be regulated by Toll-like receptors (TLR).9 In the atherosclerotic lesion, TLR4 in particular has been shown to be expressed by residing macrophages and dendritic cells.10 11 When TLR4 is triggered by its ligands (for example lipopolysaccharide), various cytokines, including TNF, IL-12, IL-23 and MIF, can be secreted, thereby further enhancing the inflammatory response.9 10 Together, the available data indicate that MIF exerts chemokine-like functions and is an important regulator of inflammatory cell recruitment and atherogenesis. It is thus conceivable that reducing MIF might be a potential therapeutic target for patients with atherosclerosis.
The notion that inflammation in RA and atherogenesis is linked is supported by data suggesting that reducing disease activity by adequate disease-modifying antirheumatic drug (DMARD) therapy may result in a decrease in cardiovascular mortality.12 13 TNF blockade could diminish the increased cardiovascular risk associated with RA by attenuating not only local but also systemic inflammation associated with atherogenesis.14 15
To explore the relationship between inflammation and factors involved in atherogenesis, we investigated the early and long-term effects of anti-TNF therapy on serum MIF levels and known risk factors such as C-reactive protein (CRP) levels and the lipid profile in RA patients.
PATIENTS AND METHODS
Patients
Fifty RA patients with active disease (disease activity score in 28 joints (DAS28) ⩾3.2) were included in the study. All patients received adalimumab 40 mg subcutaneously every other week in combination with methotrexate in a stable dose for at least 8 weeks. The concomitant use of prednisone (⩽10 mg/day) and non-steroidal anti-inflammatory drugs was allowed if stable for at least one month. Approval for this study was obtained from the institutional ethics review committee at the Academic Medical Center/University of Amsterdam. All participants gave written informed consent.
Clinical assessments
RA disease activity was assessed at baseline and weeks 16 and 52 after the start of adalimumab treatment using the DAS28. Clinical response was evaluated by the EULAR response criteria. For comparison of data between responders (good and moderate) and non-responders we used response measured at week 16. In addition, the presence of extra-articular manifestations (such as vasculitis, nodules and pleuritis) was noted before entry into the study.
Cardiovascular risk factor profiles
In the assessment of cardiovascular risk factors the following data were recorded: medical history including cardiovascular events, smoking (current smoker, ever smoker) and current medication, hypertension, dyslipidaemia, diabetes and body mass index (BMI; kg/m2).
Lipid profiles
Serum total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol, triglyceride and lipoprotein (a) (Lp(a)) levels were assessed by standard laboratory techniques. Apolipoprotein (apo) A-I and apo B levels were measured by an automated nephelometric assay using an array protein system nephelometer (Beckman, Mijdrecht, The Netherlands). In addition, erythrocyte sedimentation rate (ESR; mm/h) and CRP levels (mg/l) were determined. Blood was drawn from patients while fasting at baseline and at 16 and 52 weeks after initiation of adalimumab therapy. All values were determined by the GLP certified routine clinical chemistry laboratory at the Academic Medical Center in Amsterdam.
MIF ELISA
Natural serum MIF levels (pg/ml) were determined with a commercial quantitative sandwich-enzyme immunoassay (human MIF, DY289, R&D Systems Inc, Minneapolis, Minnesota, USA). The assay was performed according to the manufacturer’s instructions. Fasting serum samples were stored at −80°C and analysed all at once.
Statistical analysis
A paired t test or the Wilcoxon signed ranks test, whichever was appropriate, was used to determine significant changes from baseline. Probability values less than 0.05 were considered statistically significant in a two-tailed test. This exploratory study was not powered to correct for multiple comparisons by Bonferroni correction. Independent samples t tests were used for subanalysis to detect differences in baseline values or changes after treatment between groups. Correlations were assessed with the Pearson product-moment or Spearman rank-order correlation coefficients, whichever was appropriate. Stepwise backward multivariable linear regression analysis was used to identify possible baseline predictors of change in MIF levels at weeks 16 and 52. Because delta MIF levels had a skewed distribution, values were rank-transformed before linear regression analysis. Baseline variables included in the analysis were sex, BMI, MIF, CRP, triglyceride, total cholesterol and HDL levels. Values are expressed as the mean (SD) or median and interquartile range (IQR), whichever was appropriate. SPSS 12.0.2 for Windows was used.
RESULTS
Patients and clinical response
The baseline patient characteristics of 50 patients are shown in table 1. The DAS28 score decreased significantly after 16 weeks (DAS28 3.7, SD 1.2) and 52 weeks (3.4, SD 1.4) of adalimumab therapy compared with baseline (5.6, SD 1.1; both p<0.001). At week 16 all patients were evaluable for clinical response: 11 (22%) patients were EULAR non-responders, 25 (50%) were moderate responders and 14 (28%) were good responders. At week 52 there were 44 patients with an evaluable response, of whom six (14%) were non-responders, 18 (41%) were moderate responders and 20 (45%) were good responders. Six patients dropped out of the study between 16 and 52 weeks follow-up due to lack of efficacy in five patients and a serious adverse event in one patient.
Table 1. Baseline patient characteristics.
| All n = 50 | Responders n = 39 | Non-responders n = 11 | p Value | |
| Age, years (SD) | 51 (13) | 51 (12) | 47 (17) | 0.331 |
| Female (%) | 38 (76) | 28 (72) | 10 (91) | 0.190 |
| Disease duration, months (IQR) | 59 (33–145) | 61 (29–149) | 54 (34–142) | 0.935 |
| Erosive disease (%) | 33 (66) | 26 (67) | 7 (64) | 0.851 |
| Rheumatoid factor positive (%) | 36 (72) | 29 (74) | 7 (64) | 0.484 |
| Anti-CCP positive (%) | 35 (70) | 28 (72) | 7 (64) | 0.602 |
| Extra articular manifestations (IQR) | 14 (28) | 12 (31) | 2 (18) | 0.412 |
| DAS28 (SD) | 5.6 (1.1) | 5.7 (1.1) | 5.3 (0.8) | 0.225 |
| BMI, kg/m2 (SD) | 27 (6.3) | 27 (6.3) | 26 (6.7) | 0.557 |
| Smokers, current (%) | 12 (24) | 10 (26) | 2 (18) | 0.609 |
| Smokers, ever (%) | 31 (62) | 27 (69) | 4 (36) | 0.047* |
| SBP, mm Hg (SD) | 132 (15) | 133 (16) | 129 (12) | 0.606 |
| DBP, mm Hg (SD) | 80 (9) | 80 (9) | 83 (8) | 0.241 |
| ESR, mm/h (IQR) | 20 (11–35) | 20 (11–35) | 20 (15–36) | 0.824 |
| CRP, mg/l (IQR) | 17 (5–20) | 10 (4–22) | 8 (5–16) | 0.779 |
| Diabetes mellitus type 2 (%) | 4 (8) | 4 (10) | 0 (0) | 0.268 |
| Previous cardiovascular event (%) | 4 (8) | 3 (8) | 1 (9) | 0.687 |
| Statin use (%) | 6 (12) | 5 (13) | 1 (9) | 0.717 |
| Antihypertensive drug use (%) | 16 (32) | 13 (41) | 3 (27) | 0.704 |
| Methotrexate, mg/week (SD) | 18.4 (7.6) | 18.0 (7.7) | 19.5 (7.5) | 0.508 |
| Use of corticosteroids (%) | 16 (32) | 11 (28) | 5 (45) | 0.279 |
| Prednisone dose, mg/day (SD) | 7.8 (2.4) | 7.8 (2.4) | 8.0 (2.7) | 0.268 |
| Use of NSAID (%) | 36 (72) | 28 (78) | 8 (73) | 0.951 |
Mean values (SD), median and interquartile range (IQR) or percentages are shown.
*p Values <0.05 (two-sided) are significant.
BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C-reactive protein; DAS28, disease activity score in 28 joints; DBP, diastolic blood pressure; ESR, erythrocyte sedimentation rate; NSAID, non-steroidal anti-inflammatory drug; SBP, systolic blood pressure.
Pretreatment serum MIF levels
A large variability in MIF levels was observed between patients varying from the lowest detectable concentration of 60 pg/ml up to 6571 pg/ml. There was no significant relationship with the use of low-dose corticosteroids or the dosage of methotrexate, nor with clinical measures of disease activity at baseline (data not shown).
Pretreatment apo A-I and HDL levels are inversely correlated with systemic inflammation
There was a negative correlation between the pretreatment apo A-I and CRP levels (r = −0.338, p = 0.017) as well as ESR (r = −0.347, p = 0.014). Similarly, pretreatment HDL-cholesterol correlated inversely with CRP levels and ESR (r = −0.290, p = 0.041 and r = −0.340, p = 0.016, respectively).
Interestingly, higher pretreatment HDL levels correlated with lower MIF levels before the initiation of adalimumab therapy (r = −0.294, p = 0.040). As expected, baseline LDL-cholesterol was significantly lower in the six patients who used statins compared with the other 44 who did not use a statin (p = 0.007); other lipoproteins did not differ between these groups. All patients who used statins were known to have a history of hypercholesterolaemia. Four of the six patients had had a previous cardiovascular event, and all six were on concomitant antihypertensive drugs. One patient also had type 1 diabetes.
Sustained downregulation of MIF and inflammatory parameters, but not of HDL-cholesterol after adalimumab therapy
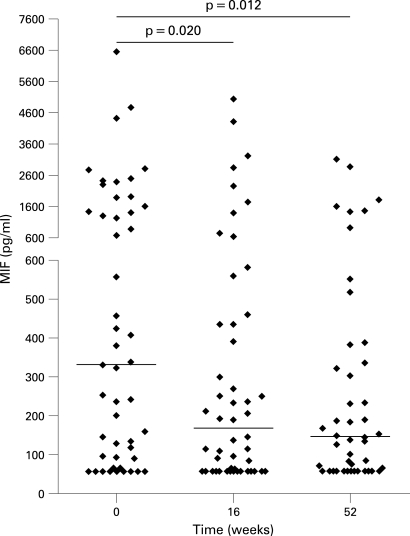
Serum MIF levels were significantly decreased 16 weeks after the initiation of adalimumab therapy (median 171 pg/ml, IQR 60–444) compared with baseline (median 333 pg/ml, IQR 93–1544, p = 0.020). This effect was sustained up to week 52 (median 145, IQR 60–335, p = 0.012; fig 1). CRP and ESR levels decreased significantly after 16 and 52 weeks of adalimumab therapy (both p<0.001, table 2).
Figure 1.
Serum macrophage migration inhibitory factor (MIF) levels before and 16 and 52 weeks after adalimumab therapy. The median values and ranges are shown for each time point. A large variability in MIF concentration was observed between patients. Some high pretreatment MIF concentrations may be due to the presence of MIF promoter polymorphisms in certain patients. The presence of such polymorphisms was not analysed in this study, the data, however, show that even high baseline MIF concentrations diminish significantly after anti-tumour necrosis factor alpha therapy.
Table 2. Changes in MIF levels and lipid profile over time.
| Week 0 (n = 50) | Week 16 (n = 50) | p Value | Week 52 (n = 44) | p Value | |
| MIF, pg/ml (IQR) | 333 (90–1544) | 171 (60–444) | 0.020* | 145 (60–335) | 0.012* |
| Total cholesterol, mmol/l (SD) | 4.86 (1.07) | 5.06 (1.16) | 0.053 | 4.98 (1.13) | 0.301 |
| HDL-cholesterol, mmol/l (SD) | 1.52 (0.38) | 1.66 (0.38) | <0.001* | 1.62 (0.39) | 0.061 |
| LDL-cholesterol, mmol/l (SD) | 2.88 (0.95) | 2.96 (1.01) | 0.392 | 2.93 (1.02) | 0.577 |
| Triglycerides, mmol/l (SD) | 1.01 (0.51) | 0.99 (0.54) | 0.513 | 0.94 (0.43) | 0.350 |
| Apo A-I, mmol/l (SD) | 1.46 (0.25) | 1.56 (0.22) | <0.001* | 1.56 (0.21) | 0.005* |
| Apo B, mmol/l (SD) | 0.99 (0.25) | 1.00 (0.27) | 0.465 | 0.99 (0.28) | 0.816 |
| Lp(a), mmol/l (IQR) | 198 (65–356) | 175 (65–377) | <0.001* | 171 (47–375) | 0.001* |
| Total cholesterol/HDL (SD) | 3.33 (0.93) | 3.15 (0.85) | 0.034* | 3.19 0.84) | 0.272 |
| Apo B/Apo A-I (SD) | 0.70 (0.21) | 0.65 (0.20) | 0.014* | 0.65 (0.20) | 0.050 |
| Glucose, mmol/l (SD) | 5.13 (1.97) | 4.95 (1.14) | 0.847 | 5.04 (1.34) | 0.930 |
| CRP, mg/l (IQR) | 8.8 (4.6–19.6) | 3.8 (1.6–9.1) | <0.001* | 2.7 (1.1–5.6) | <0.001* |
Values are represented as mean (SD) or median and interquartile range (IQR).
*p Values <0.05 (two-sided) are significant.
Apo, apolipoprotein; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp(a), lipoprotein (a); MIF, macrophage migration inhibitory factor.
The mean HDL-cholesterol levels increased at week 16 compared with baseline (p<0.001). However, HDL levels returned to nearly baseline at week 52 (table 2). LDL-cholesterol levels did not change after adalimumab treatment. Furthermore, Lp(a) levels decreased significantly at week 16 up to week 52 after treatment (both p = 0.001, table 2).
Improvement of the atherogenic index after adalimumab therapy
The mean apo A-I levels (high levels are thought to be cardioprotective) were significantly increased to 1.56 mmol/l (SD 0.22) at week 16 compared with 1.46 mmol/l (SD 0.25) at baseline (p<0.001) and remained significantly elevated up to week 52 (1.56 mmol/l, SD 0.22; p = 0.005). Of interest, apo B levels (an indicator of the total number of atherogenic particles) did not change over the course of 52 weeks adalimumab therapy. There was thus a significant decrease in the apo B/A-I ratio at week 16 (p = 0.014), which remained lowered up to week 52 (p = 0.050). The total cholesterol/HDL ratio showed a temporary improvement (p = 0.034), due to the increase in HDL levels at week 16, which had diminished again one year after the start of treatment (table 2).
Baseline MIF concentrations and gender predict changes in MIF after adalimumab treatment
With stepwise backward multivariable linear regression analysis we identified baseline predictors for change in MIF levels after treatment. Included in the analysis were the following baseline variables: sex, BMI, MIF, CRP, triglyceride, total cholesterol and HDL levels. No baseline predictors for change in MIF concentration over time were identified other than patient gender and pretreatment MIF concentrations. Baseline MIF concentration in combination with gender predicted 45% of the variance in change of the MIF concentration at week 16 (adjusted R2 = 0.453). The MIF concentration at baseline alone predicted 38% of the variance in change in the MIF concentration at week 52 (adjusted R2 = 0.383). A significantly larger decrease in the MIF concentration was seen in female compared with male patients at week 16 (p = 0.011), but no gender difference was observed at week 52. We observed no association between baseline levels of inflammation or lipid profile and changes in MIF levels after treatment.
Changes in MIF levels and lipid profile in relation to clinical response
We analysed whether changes in MIF concentration differ between EULAR responders compared with non-responders. We found no relationship between clinical response and changes in MIF concentration at week 16 nor at week 52. However, the decrease in the Lp(a) concentration was greater in responders than in non-responders at week 16 (p = 0.018), with a similar trend at week 52 (p = 0.087). Furthermore, in accordance with previous data, an increase in HDL levels at week 16 was associated with a decrease in the DAS28 score at the same time point (r = −0.308, p = 0.030).16 Similarly, HDL levels increased more in EULAR responders than in non-responders, although this difference did not reach statistical significance (p = 0.068). The increase in apo A-I was no different between response groups.
DISCUSSION
Both RA and atherosclerosis are related to chronic inflammation. There is increasing evidence that TNF and MIF are involved in these conditions and that the role of these cytokines is linked.4 8 17 In both RA and atherosclerosis enhanced MIF levels have been observed at the site of inflammation17 20 and MIF was shown to mediate leucocyte recruitment into the inflamed joint and vessel wall.18 21 Furthermore, MIF can mediate integrin activation and induce the expression of other inflammatory cytokines, such as IL-6, TNF and matrix metalloproteinases, associated with joint damage in RA and plaque instability in atherosclerosis.21–23 The role of TNF is supported by the observation that anti-TNF therapy may reduce the increased cardiovascular risk associated with RA by decreasing systemic inflammation. Previous work has shown that TNF blockade may influence lipid levels, insulin resistance, vascular adhesion molecule expression and endothelial function.16 24–27 We performed the present study to provide more insight into the mechanisms that could be involved in the effects of anti-TNF therapy on cardiovascular risk. The results confirm our hypothesis that adalimumab treatment leads to the downregulation of MIF, with potential beneficial consequences for vascular inflammation. Moreover, we show for the first time that long-term TNF blocking therapy with adalimumab has a favourable influence on the lipid profile of RA patients.
It has previously been suggested that chronic systemic inflammation in RA and subsequent atherogenesis are partly the result of chronic cytokine overflow from the inflamed joints into the circulation.2 Anti-TNF therapy has been shown to diminish local inflammation in the joints by decreasing synovial cell infiltration and the expression of adhesion molecules, chemokines and cytokines, which coincides with a reduction in acute phase reactants.19 25 28 29 A decrease in CRP levels was previously shown to be accompanied by a reduction in synovial MIF and TNF expression in the same patient when disease activity was reduced by conventional DMARD therapy.30 In light of these data we hypothesised that pro-inflammatory cytokine release from the inflamed joint could be diminished after adalimumab treatment, resulting in a decrease in systemic levels of cytokines, including MIF (fig 2). Consistent with this notion we found serum MIF levels to be significantly downregulated within 16 weeks after adalimumab therapy, an effect that was sustained up to one year after the initiation of treatment. Whether anti-TNF therapy reduces MIF in the atherosclerotic lesion in patients is as yet unknown, but beneficial effects of TNF inhibition on atherosclerotic lesions have been demonstrated in animal atherosclerosis models.31 A decrease in MIF expression could lead to reduced monocyte and T-cell influx into the inflamed vessel wall, hence arresting plaque formation.7
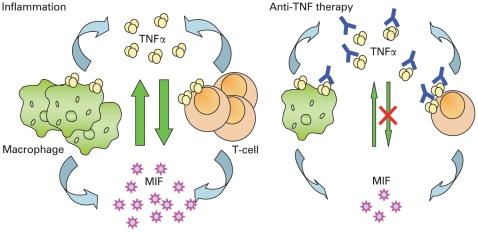
Figure 2.
Schematic view of the interaction between macrophage migration inhibitory factor (MIF) and tumour necrosis factor (TNF). Both macrophages and T cells as well as dendritic cells and fibroblast-like synoviocytes produce MIF and TNF. TNF induces the production of MIF, and vice versa. TNF production can be induced by MIF.8 17 In rheumatoid arthritis increased levels of MIF and TNF have been found locally in the synovial fluid and synovial tissue, which perpetuate the inflammatory process not only by inducing further cytokine secretion, but also by enhancing leucocyte migration towards the site of inflammation.18 With anti-TNF antibody therapy available bioactive TNF is neutralised. Furthermore, infiltration of the inflamed synovium by macrophages (main producers of TNF and MIF) was shown to diminish early after treatment.19 Therefore, both the number of MIF-producing cells as well as the concentration of bioactive TNF decreases after anti-TNF therapy, potentially leading to a decrease in systemic MIF levels.
HDL levels increased temporarily after treatment, resulting in an improved atherogenic index (total cholesterol/HDL-cholesterol) at week 16, which was no longer present after one year of adalimumab treatment. A brief rise in HDL-cholesterol, also known as an inverse phase reaction, is to be expected after reversing the inflammatory state and was previously reported in other studies with infliximab.16 32 In addition, we observed a sustained increase in apo A-I levels and thus an improvement of the apo B/A-I ratio. Based on these findings one can speculate that the apo B/A-I ratio may better reflect the cardiovascular risk profile after TNF blocking therapy than the traditional atherogenic index (total cholesterol/HDL), as differential effects of adalimumab therapy can be observed for apo A-I and HDL levels. Of interest, serum apo A-1 has previously been shown to inhibit T-cell contact-induced monocyte activation.33 As a result cytokine (TNFα and IL-1β) production by monocytes was inhibited while monocyte proliferation remained unaltered. These data indicate a novel anti-inflammatory mechanism of this apolipoprotein. Conceivably, the sustained increase of apo A-1 levels in our study might lead to the inhibition of T-cell-induced monocyte activation, both in the inflamed synovium and in the vessel wall.
Lp(a) has been demonstrated to have a spectrum of pathogenic activities among which are increased vascular adhesion molecule expression, chemotaxis of monocytes, foam cell formation, smooth muscle cell proliferation and increased platelet aggregation. Different clinical studies have shown Lp(a) levels to be an independent risk factor for developing CHD.34 35 The significant decrease in levels of Lp(a) both 16 and 52 weeks after adalimumab therapy could thus contribute to a decrease in the pro-atherogenic state. This could be a direct effect of anti-TNF therapy but may also be an indirect effect of the overall diminishment in inflammation.
The open-label rather than placebo-controlled design is obviously a limitation of this study, as we cannot conclude with complete certainty that the decease in MIF levels resulting from decreased inflammation was a direct effect of TNF blockade or merely a result of regression to the mean. However, the patients had persistent disease activity in spite of at least two conventional DMARD before inclusion in the study, suggesting that the reduction in inflammation was the result of TNF blockade. The data presented in this exploratory study thus provide the rationale for future studies with a controlled design to confirm the effects on lipid profiles and MIF levels in relation to cardiovascular endpoints.
In conclusion, TNF blocking therapy reduced systemic MIF levels, possibly reflecting a reduction in the atherogenic state. Apart from reduced systemic inflammation, as shown by reduced CRP and ESR levels, the sustained decrease in the apo B/apo A-I ratio suggests a favourable effect of adalimumab treatment on markers associated with atherogenesis.
Acknowledgments
The investigators would like to thank the research nurses Margot Colombijn and Mariane Anson for performing the clinical assessments and Laura Splint for performing the MIF ELISA.
Footnotes
Funding: This study was supported by Abbott Laboratories.
Competing interests: PPT is a member of the advisory board of Abbott and has received honoraria for lectures. The study sponsors had no involvement in the study design, the collection, analysis and interpretation of the data, writing the report, or the decision to submit the paper for publication.
Ethics approval: Approval for this study was obtained from the institutional ethics review committee at the Academic Medical Center/University of Amsterdam.
Patient consent: Obtained.
REFERENCES
- 1.van Leuven SI, Franssen R, Kastelein JJ, Levi M, Stroes ES, Tak PP. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology (Oxford) 2008;47:3–7 [DOI] [PubMed] [Google Scholar]
- 2.Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 2003;108:2957–63 [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–74 [DOI] [PubMed] [Google Scholar]
- 4.Morand EF, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov 2006;5:399–410 [DOI] [PubMed] [Google Scholar]
- 5.Herder C, Illig T, Baumert J, Muller M, Klopp N, Khuseyinova N, et al. Macrophage migration inhibitory factor (MIF) and risk for coronary heart disease: Results from the MONICA/KORA Augsburg case–cohort study, 1984–2002. Atherosclerosis 2008;200:380–8 [DOI] [PubMed] [Google Scholar]
- 6.Boekholdt SM, Peters RJ, Day NE, Luben R, Bingham SA, Wareham NJ, et al. Macrophage migration inhibitory factor and the risk of myocardial infarction or death due to coronary artery disease in adults without prior myocardial infarction or stroke: the EPIC–Norfolk Prospective Population study. Am J Med 2004;117:390–7 [DOI] [PubMed] [Google Scholar]
- 7.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 2007;13:587–96 [DOI] [PubMed] [Google Scholar]
- 8.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 1994;179:1895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popa C, van Lieshout AW, Roelofs MF, Geurts-Moespot A, van Riel PL, Calandra T, et al. MIF production by dendritic cells is differentially regulated by Toll-like receptors and increased during rheumatoid arthritis. Cytokine 2006;36:51–6 [DOI] [PubMed] [Google Scholar]
- 10.Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation 2007;116:2043–52 [DOI] [PubMed] [Google Scholar]
- 11.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 2001;104:3103–8 [DOI] [PubMed] [Google Scholar]
- 12.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 2002;359:1173–7 [DOI] [PubMed] [Google Scholar]
- 13.van Halm VP, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther 2006;8:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsson LT, Turesson C, Nilsson JA, Petersson IF, Lindqvist E, Saxne T, et al. Treatment with TNF blockers and mortality risk in patients with rheumatoid arthritis. Ann Rheum Dis 2007;66:670–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2007;56:2905–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popa C, van den Hoogen FH, Radstake TR, Netea MG, Eijsbouts AE, den Heijer M, et al. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis 2007;66:1503–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leech M, Metz C, Hall P, Hutchinson P, Gianis K, Smith M, et al. Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum 1999;42:1601–8 [DOI] [PubMed] [Google Scholar]
- 18.Gregory JL, Leech MT, David JR, Yang YH, Dacumos A, Hickey MJ. Reduced leukocyte-endothelial cell interactions in the inflamed microcirculation of macrophage migration inhibitory factor-deficient mice. Arthritis Rheum 2004;50:3023–34 [DOI] [PubMed] [Google Scholar]
- 19.Smeets TJ, Kraan MC, van Loon ME, Tak PP. Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum 2003;48:2155–62 [DOI] [PubMed] [Google Scholar]
- 20.Onodera S, Tanji H, Suzuki K, Kaneda K, Mizue Y, Sagawa A, et al. High expression of macrophage migration inhibitory factor in the synovial tissues of rheumatoid joints. Cytokine 1999;11:163–7 [DOI] [PubMed] [Google Scholar]
- 21.Schober A, Bernhagen J, Thiele M, Zeiffer U, Knarren S, Roller M, et al. Stabilization of atherosclerotic plaques by blockade of macrophage migration inhibitory factor after vascular injury in apolipoprotein E-deficient mice. Circulation 2004;109:380–5 [DOI] [PubMed] [Google Scholar]
- 22.Amin MA, Haas CS, Zhu K, Mansfield PJ, Kim MJ, Lackowski NP, et al. Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB. Blood 2006;107:2252–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radstake TR, Sweep FC, Welsing P, Franke B, Vermeulen SH, Geurts-Moespot A, et al. Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum 2005;52:3020–9 [DOI] [PubMed] [Google Scholar]
- 24.Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis 2005;64:765–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tak PP, Taylor PC, Breedveld FC, Smeets TJ, Daha MR, Kluin PM, et al. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum 1996;39:1077–81 [DOI] [PubMed] [Google Scholar]
- 26.Paleolog EM, Hunt M, Elliott MJ, Feldmann M, Maini RN, Woody JN. Deactivation of vascular endothelium by monoclonal anti-tumor necrosis factor alpha antibody in rheumatoid arthritis. Arthritis Rheum 1996;39:1082–91 [DOI] [PubMed] [Google Scholar]
- 27.Hurlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation 2002;106:2184–7 [DOI] [PubMed] [Google Scholar]
- 28.Taylor PC, Peters AM, Paleolog E, Chapman PT, Elliott MJ, McCloskey R, et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum 2000;43:38–47 [DOI] [PubMed] [Google Scholar]
- 29.Ulfgren AK, Andersson U, Engstrom M, Klareskog L, Maini RN, Taylor PC. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum 2000;43:2391–6 [DOI] [PubMed] [Google Scholar]
- 30.Morand EF, Leech M, Weedon H, Metz C, Bucala R, Smith MD. Macrophage migration inhibitory factor in rheumatoid arthritis: clinical correlations. Rheumatology (Oxford) 2002;41:558–62 [DOI] [PubMed] [Google Scholar]
- 31.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 2004;24:2137–42 [DOI] [PubMed] [Google Scholar]
- 32.Popa C, Barrea P, Netea MG, Stalenhoef AF, van der Meer JW. Anti-TNF therapy and plasma HDL cholesterol concentration. Atherosclerosis 2005;182:375. [DOI] [PubMed] [Google Scholar]
- 33.Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK III, Roux-Lombard P, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001;97:2381–9 [DOI] [PubMed] [Google Scholar]
- 34.Seman LJ, DeLuca C, Jenner JL, Cupples LA, McNamara JR, Wilson PW, et al. Lipoprotein(a)-cholesterol and coronary heart disease in the Framingham Heart Study. Clin Chem 1999;45:1039–46 [PubMed] [Google Scholar]
- 35.Luc G, Bard JM, Arveiler D, Ferrieres J, Evans A, Amouyel P, et al. Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis 2002;163:377–84 [DOI] [PubMed] [Google Scholar]