Abstract
Differentiation of neuronal stem cells into astrocytes or neurons is important in maintaining brain function. Oxidative stress and inflammation are now shown to bias differentiation toward astrocytes by modulating activity of the anti-ageing gene Sirt1. These findings link a longevity gene to the activity of neuronal stem cells and their response to stress.
Neuronal stem cells (neural progenitor cells, NPCs) can give rise to astrocytes, neurons and oligodendrocytes. Astrocytes perform many different functions, including providing structural and nutrient support for neurons, secreting signalling molecules, and uptake and metabolism of neurotransmitters. In response to brain injury, NPCs differentiate preferentially into astrocytes rather than neurons. Excessive astrocyte expansion, known as astrogliosis, can prevent growth of neurons and interfere with proper damage repair. Therefore, the ability to direct differentiation of NPCs may be useful in protecting the brain against inflammatory diseases, such as multiple sclerosis, which involve astrogliosis. In addition, the ability to direct differentiation of NPCs towards neurons may provide new therapeutic avenues for stroke, spinal cord injury and age-related cognitive conditions, such as Alzheimer’s and Parkinson’s diseases, which cause loss of neurons. On page 385 of this issue, Prozorovski et al. show that oxidative stress and inflammation promote differentiation of NPCs towards the astrocyte lineage and that this effect is mediated by the mammalian Sir2 orthologue Sirt1, through its ability to regulate expression of the Mash1 promoter1.
The NAD+-dependent deacetylase Sir2 and its homologues promote longevity in many organisms. Sirt1 has been shown to mediate metabolic adaptations to dietary changes by deacetylating metabolic regulators such as PGC-1α2 and LXR3. Sirt1 also mediates stress resistance in mammalian cells by deacetylating stress-response mediators, such as FOXO proteins and p53 (ref. 4). Sirt1 has been linked to neuroprotection in several systems, including both cell-based5 and in vivo rodent models6. Interestingly, the neuroprotection observed in Wallerian degeneration slow (Wlds) mice, caused by increased expression of an enzyme in the NAD+ biosynthesis pathway, was shown to involve Sirt1 (ref. 7).
A clue to the link between Sirt1 and neurogenesis came from the finding that it can mediate cellular responses to changes in redox state in several different cell types. Therefore, Prozorovski et al.1 began their study by further investigating the connection between the redox state of NPCs and their differentiation potential. They found that redox state does indeed affect the cell-fate decision of NPCs in vitro, with oxidizing conditions favouring differentiation into astrocytes, whereas reducing conditions favour neuron formation (Fig. 1). Next, they found that treatment of NPCs with the Sirt1-activating compound resveratrol mimicked oxidizing conditions and increased differentiation of NPCs towards astrocytes through a mechanism that requires Sirt1.
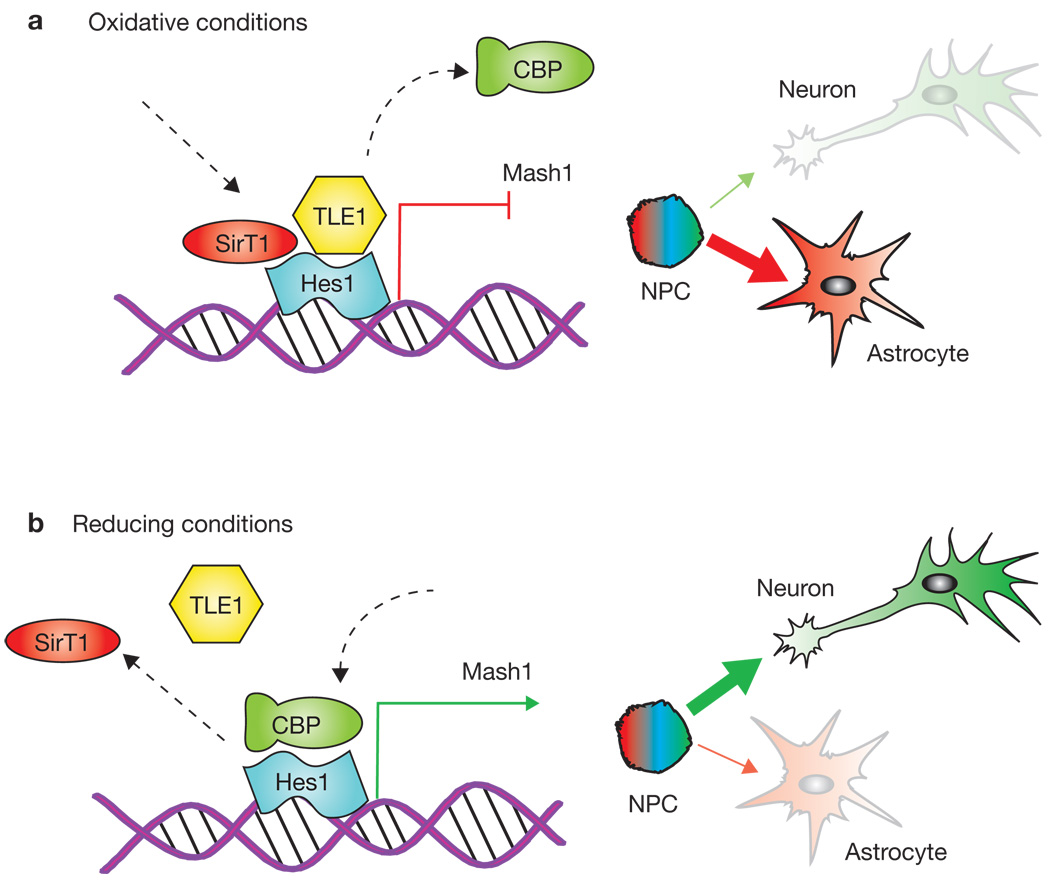
Figure 1.
Redox potential determines whether Sirt1 drives cells towards neuronal or astroglial fates. (a) In an oxidizing environment, Sirt1 forms a complex with Hes1 on the Mash1 promoter, deacetylates histones and recruits repressor proteins such as TLE1. This inhibits transcription of Mash1 and drives the cell towards an astrocyte fate. (b) In a reducing environment, Sirt1 does not bind to Hes1 on the Mash1 promoter. Instead, Hes1 recruits CBP, which activates Mash1 transcription and stimulates the stem cell to assume a neuronal fate.
To analyse the molecular mechanism underlying these effects, the authors investigated a gene called Hairy/enhancer of split 1 (Hes1), a transcriptional repressor of Mash1, which, in turn, is responsible for the activation of a neuron-specific transcription programme (Fig. 1). Prozorovski et al.1 found that under oxidizing conditions, Sirt1 and Hes1 form a complex that binds to and deacetylates histones at the Mash1 promoter, while recruiting co-repressors such as TLE1. Together, these events cause down-regulation of Mash1 expression and block neuronal differentation (Fig. 1a). In a reducing environment, the Hes1–Sirt1 complex is not observed. Instead, Hes1 recruits transcription activators such as CREB binding protein (CBP) to the Mash1 promoter, and this drives NPCs towards a neuronal fate (Fig. 1b). The influence of the redox state on NPC cell-fate decisions was eliminated by removal of Sirt1 activity either by RNAi or through the use of Sirt1 inhibitors. Thus, through its action at the Mash1 promoter, Sirt1 seems to act as a cell-fate decision switch in NPCs in vitro, with increased Sirt1 activity causing increased differentiation of NPCs into astrocytes at the expense of neurons.
Next the authors extended their findings by studying this mechanism in vivo, using healthy mice and a mouse model of multiple sclerosis. First, they found that injecting young mice with an oxidizing agent increased the number of newly differentiated astrocytes in the brain. Immunostaining of the early postnatal nervous system of untreated animals revealed that, although Mash1-positive and Sirt1-positive cells were plentiful in regions of the brain with active neurogenesis, relatively few cells were positive for both proteins, consistent with the proposed repression of Mash1 by Sirt1. In contrast, cells co-stained for Sirt1 and the astrocyte marker GFAP (glial fibrillary acidic protein) could be readily observed. To establish a causal role for Sirt1 in alterations in NPC cell-fate decisions, the authors performed in utero electroporation of GFP-marked RNAi constructs against Sirt1 to deplete Sirt1 in a small percentage of NPCs in otherwise wild-type animals. Cells in which Sirt1 had been knocked down gave rise to an increased proportion of Mash1-positive cells in postnatal animals treated with an oxidizing agent. Similarly, in young mice treated with a Sirt1 inhibitor, Mash1 transcription was increased in the sub-ventricular zone (SVZ), a region of the brain rich in NPCs. The authors also investigated Sirt1 expression in experimental autoimmune encephalomyelitis (EAE), a model of the autoimmune demyelination disease multiple sclerosis. EAE induces an oxidizing environment and reactive astroglyosis. The authors observed that, whereas Sirt1 expression was low in unaffected brain regions, inflamed areas with an abundance of invading leukocytes also contained an increased number of cells positive for both GFAP and Sirt1. Treatment of EAE animals with the Sirt1 activator resveratrol resulted in a modest increase in the number of astrocytes in the areas of inflammation. However, as the Sirt1-positive cells consisted of a relatively small fraction of the total GFAP-positive astrocytes localized to the EAE lesions, and because the effects of resveratrol treatment were modest, the importance of Sirt1 expression in EAE remains unclear.
This study provides an important foundation for the role of Sirt1 in differentiation of neuronal stem cells and many important issues have been raised. For example, with the exception of the EAE experiments, all of the in vitro and in vivo experiments relied on cells from either embryonic or early postnatal animals. Thus, the role of Sirt1 in NPCs from adult or aged mice is still unknown. Moreover, Sirt1 may have distinct effects on the differentiation of NPCs residing in different regions of the brain. For instance, the SVZ supplies neurons almost exclusively to the olfactory bulb, a region of the brain that is capable of repairing almost any type neuronal damage. Astrogliosis is never observed in this region of the brain and therefore the ability of Sirt1 to direct the differentiation of astrocytes in cells arising from the SVZ may be quite different from its effects in other regions of the brain, such as the cortex, where astrogliosis does occur. Other areas worth investigating include: 1) the influence of Sirt1 on the differentiation of stem cells in the hippocampus, where neurogenesis may have important implications for learning and memory; 2) effects of calorie restriction on the differentiation of stem cells in different brain regions, and whether any observed effects are mediated by Sirt1; 3) whether increased or decreased Sirt1 function affects the outcome in disease and injury models, such as experimental models of stroke or neurodegenerative disease.
How longevity-determining pathways affect brain function (both cognitive and metabolic)8, 9 is a new area of research and has been greatly enhanced by this study. Sirt1 seems to regulate numerous survival strategies of metabolic tissues in response to stressful environments. The regulation of neural stem-cell differentiation in the brain may be one such adaptation, and could offer new approaches for the treatment of declining brain function that accompanies ageing and disease.
References
- 1.Prozorovski, et al. Nature Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 2.Nemoto S, Fergusson MM, Finkel T. J. Biol. Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 3.Li X, et al. Mol. Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, et al. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, et al. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin W, et al. J. Biol. Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 7.Araki T, Sasaki Y, Milbrandt J. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 8.Bishop NA, Guarente L. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 9.Libert S, et al. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]