Abstract
Background
For clinical trials of interventions that could affect mortality or major morbidity, Data Monitoring Committees have an important role in safeguarding patient interests and enhancing trial integrity and credibility. In trials overseen by an independent DMC it is widely recognized that interim data should remain confidential to the DMC and to the statistical group preparing reports. However, we have found that the principle of confidentiality is not always followed in practice, particularly where the interim data include complete results on a short-term outcome measure.
Purpose
To discuss the reasoning and evidence supporting the principle of confidentiality of interim data with emphasis on the setting where the interim data include complete results on a short-term outcome.
Methods
We review the reasons why wider access to interim data can increase the risk of false positive or false negative conclusions and discuss the types of harm which can occur. We provide illustrations and insights from recent experiences and discuss the level of consensus in the research community.
Results
The arguments in favor of early release of interim data include the need to provide reliable data in a timely manner to patients and physicians, the potential to increase the enthusiasm of trial investigators, and to restore equipoise. However interim data, even where these include complete results on a short-term outcome measure, provide an unreliable and biased assessment of the overall benefit-to-risk profile of the trial treatments. Pre-judgment based on over-interpretation of such interim data can affect recruitment, treatment delivery, and follow-up, risking the ability of the trial to achieve its goals.
Conclusions
In order to preserve the integrity of a trial and safeguard the interests of patients, interim data, including complete data on short-term outcomes, should remain confidential to the DMC and the statistical group responsible for preparing interim reports until the trial has achieved its primary objectives.
Introduction
Independent Data Monitoring Committees (DMCs) play an important role in safeguarding the interests of study participants and in enhancing the integrity and credibility of clinical trials. This is especially important for Phase 2b or Phase 3 trials conducted in settings where study interventions could readily affect mortality or serious morbidity, or where the trial is studying vulnerable populations, such as children or patients with impaired cognitive function.
It is widely recognized in the literature [1,2] and in guidelines [3–6] that unblinded access to interim efficacy and safety data should only be available to the DMC and the statistical group responsible for providing the reports to the DMC. However, we have found that this practice is not consistently followed, particularly in settings where the interim data include complete results on a short-term outcome measure.
Example 1: Comparison of laparoscopic and open colectomy for cancer
In the 1990s, four multicenter trials comparing laparoscopic colectomy to open colectomy in patients with colon cancer were initiated [7–10]. Laparoscopic surgery was believed likely to provide short-term benefits in terms of quicker recovery and improved quality of life, but there were concerns about a longer term increased risk of cancer-related death relative to open colectomy. All four trials included cancer-free survival as a primary endpoint (three-year for the first three trials and five-year for ALCCaS). The COST, COLOR and CLASICC trials all published short-term outcome results before data on the influential measures of long-term disease recurrence or long-term mortality were available. The COST study group was the first to publish, and commented:
‘To meet the ethical obligations to fully inform patients considering enrollment in this and similar ongoing studies, the North Central Cancer Treatment Group External Data Monitoring Committee and the investigators chose to release the short-term QOL results while the trial was ongoing.’
Example 2: Clinical endpoint ‘validation trials’ that also provide biomarker data for earlier regulatory review under the FDA Accelerated Approval (AA) process.
In 1992, the US Congress created a regulatory process, often referred to as ‘accelerated approval.’ Under this process, a sponsor could receive temporary marketing approval from the FDA for a new treatment regimen that appears to address unmet needs for patients having a life threatening disease, if clinical trials establish the regimen has compelling effects on a biomarker and if such effects are ‘reasonably likely to predict clinical benefit.’ Once AA is achieved, a ‘validation trial’ must be completed in a timely manner to determine whether this new regimen truly provides meaningful benefit on clinical endpoints, i.e., on tangible measures of clinical benefit [11]. To ensure the validation trial will be completed in a timely manner after AA has been granted, a recent practice in the oncology setting has been to design trials with the dual objective of (i) providing interim data on biomarker effects that, if compelling, would be released to sponsors and FDA for consideration of AA; and (ii) serving as the ‘validation trial’ through continued enrollment and follow-up to provide a definite evaluation of effects on tangible measure(s) of clinical benefit. Recognizing that the principal evidence regarding the benefit to risk profile of the experimental regimen is obtained from the longer term second objective, early release of the biomarker data is a breach of the confidentiality principle concerning interim data that can compromise the integrity of the validation trial.
In this study we consider the issues regarding the practice of maintaining confidentiality of interim data. We review the reasons why wider access to interim data can lead to increased risks of false positive or false negative conclusions and the types of risks to trial integrity resulting from failure to maintain confidentiality of interim results. We provide insights from recent experiences, and discuss the level of consensus in the research community regarding the need to maintain confidentiality of interim data.
Interpreting interim results: why the need for such caution?
Much of the literature discussing the need for confidentiality of interim results focuses on estimates of treatment effect based on interim data on a long-term primary endpoint, such as survival. Many authors have documented the substantial fluctuations that arise over time in such interim trial results. If one reviews outcome data repeatedly during the conduct of the trial, these repeated analyses of treatment effect would lead to substantial increases in the rate of false positive or false negative conclusions unless proper monitoring guidelines are used that take into account the need for caution in interpreting interim data [1,12–17].
Furthermore, conducting repeated analyses over the course of the trial and focusing on interim data that provide the most favorable impression of the benefit-to-risk profile can also lead to obtaining misleading estimates of treatment effect. This bias (a form of ‘regression to the mean’) occurs because there is true signal and random noise in every estimate of treatment effect and, when many analyses are conducted, there is a tendency for those results that appear to be most favorable to be due, at least in part, to random overestimates of true effect.
Where the interim data take the form of complete results on a short-term outcome measure, there are still substantial risks to trial integrity. If the primary objective of a trial is to evaluate disease outcome over several years, it is implicitly recognized that treatment decisions should not be based upon short-term outcomes unless the short-term data provide a convincing answer to the research question and the trial is terminated early. Interim data on the short-term outcome measure, even if complete, can provide a very biased view of the longer term benefit-to-risk profile, and release of such data runs a serious risk of engendering pre-judgment of trial results. This can lead to particular problems where the long-term endpoint is the primary outcome and the short-term outcomes are expected to favor the experimental treatment.
In the setting of the Phase 2b or Phase 3 trials that are described in the Introduction, in order to reduce the risk of misleading conclusions, access to interim results on efficacy and safety of study interventions should be limited to the statistical group preparing the interim reports and to members of an independent DMC who are guided in data interpretation by proper group sequential monitoring guidelines. As asserted by Ellenberg et al. [1],
‘This principle is justified by the need to minimize the risk of widespread prejudgment of unreliable results based on limited data … this prejudgment could adversely impact rates of patient accrual, continued adherence to trial regimens, and ability to obtain unbiased and complete assessment of trial outcome measures. This prejudgment could also result in publications of early results that might be very inconsistent with final study data on the benefit-to-risk profile of the study interventions.’
Illustrations and insights from recent experiences
The main arguments that have been made in favor of early release of interim results relate to the need to provide reliable data in a timely manner to inform the decision making of patients and physicians. Secondary arguments include the need to restore equipoise after results of similar trials have been released, the potential to increase enthusiasm for the trial among investigators, and the lack of relevance and influence of trial results if release is delayed. We address these arguments below.
Will early release of interim data increase enthusiasm of participating investigators?
The United Kingdom NHS Health Technology Assessment Program commissioned the ‘Data Monitoring Committees: Lessons, Ethics, Statistics Study Group’ (DAMOCLES) to investigate existing processes of monitoring accumulating data and to identify ways of improving the DMC process. Following extensive research, the DAMOCLES group provided an overview document [18]. With respect to this question, the report concluded,
‘The current prevailing view is that the trial investigators should not see the unblinded interim results, and that the argument that releasing interim results would aid enthusiasm and accrual is false.’
Green et al. [19] provides documentation of these important risks to trial integrity and credibility when interim data are known to the sponsor, the investigators, the investment and scientific communities, or the general public. In their overview, these authors documented that early access to interim data provided substantial risk for prejudgment of unreliable early results, resulting in greatly diminished enthusiasm in enrolling patients into ongoing trials. This led not only to increases in duration of enrollment but also, in some instances, to termination of trials that were no longer able to reach accrual targets. Early access also led to some publications of premature positive results that were inconsistent with final results from those trials or other major trials.
The Prince Margaret Hospital trial [20] evaluating efficacy of pre-operative radiation therapy in the setting of rectal cancer provides a specific illustration of such prejudgment. In that trial, interim data were routinely provided to participating investigators, and these early results suggested lack of benefit. In the publication of study results, the authors reported that the trial was terminated after enrolment of only 125 patients because ‘the absence of any trend in survival during the early years caused the study to die a natural death.’ A further trial was then required which provided much more reliable evidence for lack of benefit of pre-operative radiation therapy, both in the overall rectal cancer setting as well as in a subgroup of Duke’s C patients where Princess Margaret investigators had claimed benefit following a post-hoc analysis of data from their trial [21].
In addition to affecting accrual, access to interim data can lead to reduced adherence to trial regimens. In a trial conducted in the FDA accelerated approval setting discussed in Example 2 in the Introduction, sorafenib was evaluated in patients with renal cell carcinoma [22]. Interim data revealed a strong biomarker effect, with sorafenib delaying the increase of disease burden. Following release of this interim data control patients were allowed to cross into the sorafenib arm in spite of the recognition that the interim data did not provide a definitive assessment of the effect on the primary clinical endpoint, overall survival. The final results from the trial failed to provide interpretable information about the effects of sorafenib on overall survival, demonstrating the harm to scientific integrity resulting from the breach of confidentiality, (sorafenib/placebo survival relative risk=0.88, p=0.146; American Society of Clinical Oncology Abstract #5023, May 2007).
Once recruitment and protocol-prescribed treatment are complete, these obviously cannot be adversely impacted by release of interim results. However the same prejudgments, caused by over-interpretation of unreliable interim results can affect delivery of secondary treatments. It may also lead to biased assessment of outcome measures, and could adversely affect the effort to ensure accuracy and completeness of follow-up.
Release of interim data may provide positive feedback to trial investigators and a return for their investment in the trial; however in order to protect the scientific integrity of the trial, interim publications should be restricted to data which do not provide clues to the benefit-to-risk profiles of the trial treatments.
Will early release of data provide more timely access to reliable insights?
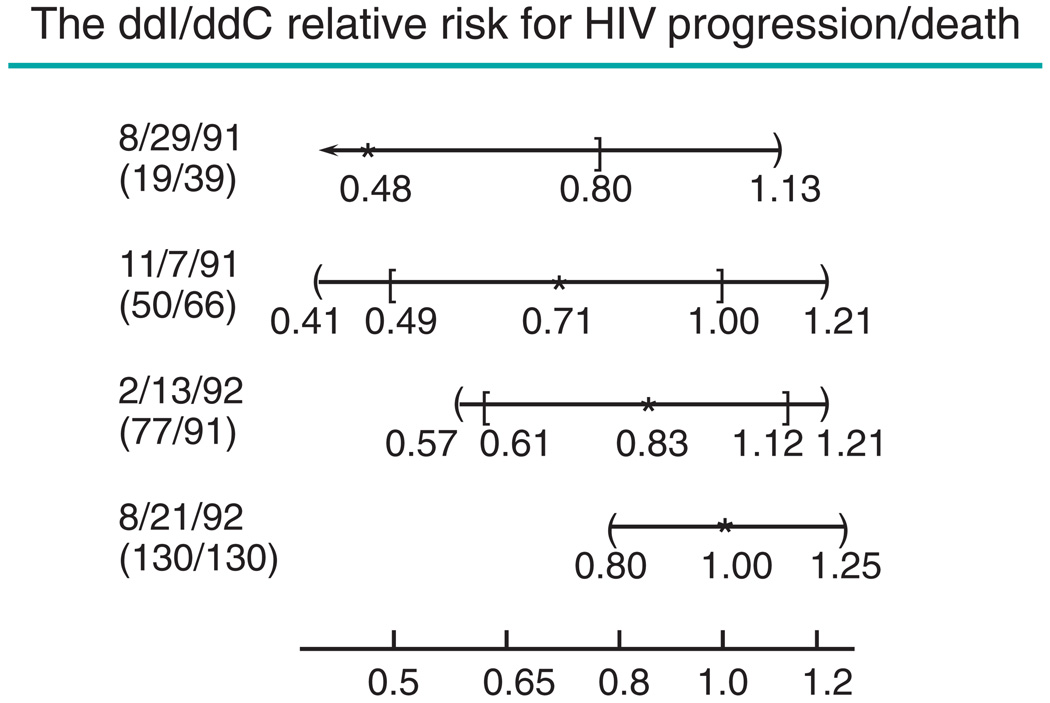
While early release of interim data certainly provides timely access, such insights usually are not reliable. Those experienced in serving on DMCs can provide numerous examples where final trial results were inconsistent with interim data. Ellenberg et al. [1] use the Community Programs for Clinical Research on AIDS (CPCRA) #002 trial [23] (see Figure 1) to illustrate the striking changes that can occur in estimates of treatment effect during the course of the study. In this non-inferiority trial, the first two interim analyses would lead to a false claim that zalcitabine(ddC) is inferior (if the standard 95% confidence interval is used), and even the third interim analysis would yield a misleadingly unfavorable impression about the relative efficacy of the two regimens. Only at the final analysis do data reflect the non-inferiority of zalcitabine(ddC). The CPCRA #002 trial does not support the claim by Korn [24] that ‘Early release of data from non-inferiority trials will effectively guide clinical practice.’
Figure 1.
Estimating the relative efficacy of didanosine (ddI) and zalcitabine (ddC) in patients with HIV. Estimates (*) and confidence intervals (CIs) for the relative risks for progression-free survival, by the date of interim analysis. Appearing on the left is the date of the interim analysis and the number of events on ddI and ddC arms at that time. Square brackets are 95% CIs; curly brackets are proper group sequential repeated CIs
The 15,290-patient VALUE trial [25], comparing an angiotensin receptor blocker, Valsartan, with a calcium channel blocker, Amlodipine, was conducted in hypertensive patients at high cardiovascular risk. Interim data indicated Amlodipine was preferable due to considerably lower rates of stroke, myocardial infarction (MI), and overall risk of death (see Table 1). However, final trial results available several years later indicated that these short-term relative risks were not consistent with longer term relative risks, and that Valsartan also led to a substantially lower rate of diabetes.
Table 1 .
Interim and final data on key outcome measures from the VALUE Trial evaluating Valsartan and Amlodipine. The randomization to the two regimens was 1:1
| Hypertensive patients at high cardiovascular risk | ||||
|---|---|---|---|---|
| May’98 to August’00 | n=15,290 | May’98 to December’03 | n=15,245 | |
| Valsartan/Amlodipine | Valsartan/Amlodipine | |||
| Outcome measure | Events | RR | Events | RR |
| All deaths | 178/141 | 1.25 | 841/818 | 1.02 |
| All myocardial infarctions | 102/76 | 1.33 | 369/313 | 1.17 |
| All strokes | 124/92 | 1.34 | 322/281 | 1.14 |
| Hospitalization for heart failure | 104/112 | 0.92 | 354/400 | 0.88 |
| New onset diabetes | No data | – | 690/845 | 0.81 |
Access to interim results by those outside the DMC would likely have done irreparable harm to these two studies, and would have resulted in temporary if not long-term unfavorable impressions about effects of important interventions (i.e., ddC and Valsartan) relative to the much more reliable insights that successfully completed trials were able to provide.
The recent experience in the NIH sponsored ADAPT trial [26] illustrates the risks to trial integrity even when the trial’s steering committee is the only body beyond the DMC to receive access to interim data. The ADAPT trial provided a placebo controlled evaluation of naproxen and a cox-2 inhibitor, celecoxib. While the trial was targeting prevention of Alzheimer’s disease, concerns were raised about excess cardiovascular risks of celecoxib in December 2004 when the ‘Adenoma Prevention with Celecoxib’ trial revealed celecoxib, like other cox-2 inhibitors, increased the rate of cardiovascular death/MI/stroke events. Even though previous randomized trials did not implicate naproxen, the ADAPT steering committee reviewed interim data and made a unilateral decision, contrary to the views of the ADAPT DMC (personal communication), to stop ADAPT and report concerns with the cardiovascular safety of naproxen as well as of celecoxib. This was done even though the excess of cardiovascular death/MI/stroke events on the naproxen arm was entirely consistent with random chance. This early release of unreliable data led to substantial misinterpretation about the trial’s evidence regarding safety of naproxen, and in turn led to strong response from the public against its use that was not justified by those data.
In the laparoscopic colectomy setting (Example 1 in the Introduction) complete results on short-term outcome measures were published without longer term data on cancer risk [7–9]. Published data included short-term quality of life, morbidity and mortality, resection margins, numbers of lymph nodes removed, analgesic use and length of hospital stay. While evidence of serious short-term risk with laparoscopic colectomy may have been sufficient to answer the trial questions, the very modest gain in short-term benefit demonstrated in the publications could easily be offset by a relatively small adverse effect on long-term survival.
When interim data are published there is substantial risk that proper cautionary statements will not be provided and that authors and editors will overstate the reliability of the available evidence. The COST trial [7] provided cautionary statements in their initial publication of the short-term outcome data (‘until ongoing trials establish that laparoscopic assisted colectomy is as effective as open colectomy in preventing recurrence and death from colon cancer, this procedure should not be offered to patients with colon cancer’) but the CLASICC trial [8] claimed ‘Laparoscopic-assisted surgery for cancer of the colon is as effective as open surgery in the short-term and is likely to produce similar long-term outcomes.’ The accompanying Lancet editorial claimed ‘in appropriately selected patients who are operated on by experienced surgeons, laparoscopic surgery for colorectal cancer may be the new gold standard.’ Going even a step further the COLOR trial [9] claimed ‘Laparoscopic surgery can be used for safe and radical resection of cancer in the right, left and sigmoid colon.’
Contrary to the above overstatements, longer follow-up is essential before reliable assessments can be made about whether the benefit-to-risk profile of laproscopic colectomy is non-inferior to that of open colectomy. Publishing short-term (secondary endpoint) data alone, without long-term primary endpoint data, could result in prejudgment of the overall benefit-to-risk profile of laparoscopic colectomy. Since the publication of the short-term results from the COST, COLOR, and CLASICC trials, the rates of laparoscopic colectomy carried out outside a trial setting have increased substantially, suggesting fairly widespread prejudgment did occur.
Will waiting to study completion to release results render a trial non-influential, especially if data have been released from related concurrent trials?
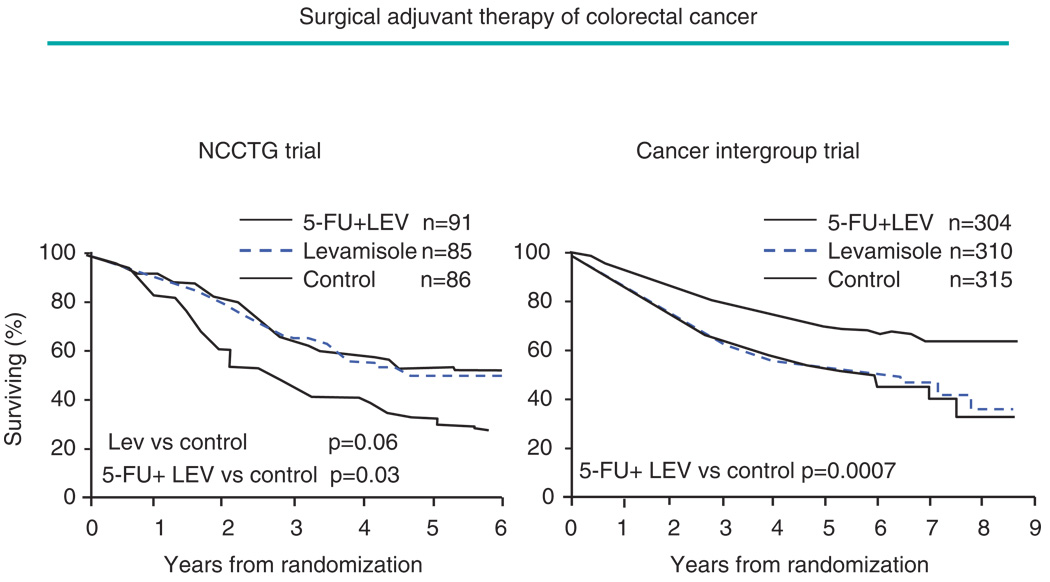
Clinical trials can have significant impact on clinical practice, even when the results of a long-term study are not released until they are definitive. The trials evaluating 5-fluorouracil and levamisole for adjuvant treatment of stage 3 colon cancer provide an illustration [27,28], see Figure 2. The first of two pivotal trials was initiated in 1977 but the benefit of this adjuvant treatment was not considered to be established until the results of the long-term confirmatory Cancer Intergroup Trial were reported for the first time in 1990 after they were judged to be reliably positive. These trials resulted in the first FDA regulatory approval of an agent for adjuvant treatment of colon cancer. The trials also illustrate that confirmatory trials can reduce the risk of false positive conclusions. The earlier North Central Cancer Treatment Group trial had indicated benefit from use of levamisole alone. This evidence is inconsistent with the results from other randomized trials evaluating levamisole as a single agent in the advanced and adjuvant colorectal disease settings, including the much larger confirmatory Cancer Intergroup Trial.
Figure 2.
Estimating the effect on patient survival of 5-fluorouracil (5FU) and levamisole (LEV) following surgery in patients with stage 3 colorectal cancer: The smaller North Central Cancer Treatment Group (NCCTG) Trial suggested a similar benefit from 5FU+LEV, and LEV alone. However the subsequent and larger NCI Cancer Intergroup Trial indicated benefit from combination treatment only
Given the importance of confirmatory trials, it is difficult to understand how release of data from one trial would render a second trial non-influential, if the latter study is designed and conducted with quality. The Syntex 1654 [29] and the Community Program for Clinical Research in AIDS (CPCRA) 023 [30] trials evaluated oral gancyclovir in prevention of symptomatic cytomegalovirus (CMV) disease in HIV infected patients. In July 1994, the Syntex trial was published with claims of highly significant reductions in occurrence of CMV disease and borderline significant effects on mortality. Interim data from CPCRA #023 were not consistent with the Syntex trial results, see Table 2. It was also recognized that the Syntex trial included not only symptomatic but also non-symptomatic CMV disease events through regular review of funduscopic exams by ophthalmologists. Because of inconsistent results in the two trials and the importance of having a reliable understanding of effects on symptomatic events, the CPCRA trial was continued. Upon its completion in July 1995, it provided evidence against clinically meaningful benefit from oral gancyclovir in prevention of CMV symptomatic disease.
Table 2.
Final data from the Syntex #1654 trial, and interim and final data from the Community Program for Clinical Research in AIDS (CPCRA) #023 trial evaluating effects of oral gancyclovir versus placebo on prevention of symptomatic cytomegalovirus disease (CMV) in HIV-infected patients. RR denotes relative risk
| Release of data from a concurrent companion trial | ||||||
|---|---|---|---|---|---|---|
| CPCRA 023 Trial: April 1993–July 1995 | ||||||
| Oral Gancyclovir in the prevention of CMV symptoms | ||||||
| July 1994 Syntex #1654 | July 1994 CPCRA #023 | July 1995 CPCRA #023 | ||||
| Oral gancyclovir | Placebo | Oral gancyclovir | Placebo | Oral gancyclovir | Placebo | |
| No. of patients | 486 | 239 | 646 | 327 | 662 | 332 |
| CMV events | 76 | 72 | 40 | 23 | 101 | 55 |
| RR=0.45, p=0.0001 | RR=0.87, p=0.60 | RR=0.92, p=0.60 | ||||
| Deaths | 109 | 68 | 58 | 23 | 222 | 132 |
| RR=0.71, p=0.052 | RR=1.27, p=0.34 | RR=0.83, p=0.09 | ||||
Berlex conducted European (EU) [31] and North American (NA) [32] clinical trials evaluating effects of betaseron on progression of disease, using the Expanded Disability Status Scale (EDSS) in patients with secondary-progressive multiple sclerosis. Both trials were of four year duration, with the EU trial initiated two years before the NA trial. The NA trial was initiated in February 1996 and at its midpoint in 1998, the EU data were released reflecting a significant 1/3 reduction in the rate of progression of disease, see Table 3. The NA trial, as seen in the table, suggested lack of benefit at that time. The NA trial was completed in 2000 and continued to reveal no benefit of betaseron (see Figure 2 in [32]).
Table 3.
Final data from the European (EU) trial, and interim and final data from the North American (NA) trial evaluating the effects of betaseron versus placebo on Expanded Disability Status Scale (EDSS) progression in patients with secondary-progressive multiple sclerosis
| Betaseron in secondary progressive MS patients | ||||||
|---|---|---|---|---|---|---|
| Berlex North America (NA) Trial: February 1996–February 2000 | ||||||
| Number and percent with confirmed EDSS progression | ||||||
| October 1998 EU Trial | October 1998NA Trial | February 2000NA Trial | ||||
| Bertaseron | Placebo | Betaseron | Placebo | Betaseron | Placebo | |
| No. of patients | 360 | 358 | 631 | 308 | 631 | 308 |
| EDSS progression | ||||||
| Number | 148 | 178 | 119 | 57 | 227 | 106 |
| Percent | 38.9 | 49.7 | 18.9 | 18.5 | 36.0 | 34.4 |
| OR=0.64, 2p=0.005 | OR=1.03, 2p=0.90 | OR=1.07, 2p=0.64 | ||||
Clinical trials, if designed to address important clinical questions and if conducted with high quality, provide important insights whether or not results have been released earlier from related clinical studies. The CPCRA #023 and NA clinical trials provided influential information to the clinical and regulatory communities. FDA awaited the data from these two studies before making regulatory decisions. The Agency did not license either gancyclovir or betaseron in these clinical settings, even though earlier trials had provided considerable evidence for benefit of these therapies.
Should interim data be released to restore equipoise when equipoise has been disturbed by release of data from other related trials?
When data are released from similar trials beliefs regarding equipoise may be disturbed. Where accruing data in an ongoing trial show a contradictory picture, DMCs and investigators may be tempted to believe that the trial will have the best chance of achieving its objectives if interim data are released with the aim of restoring equipoise. The COST investigators were fairly circumspect about the short-term outcome data they provided in their initial publication – they published quality of life data showing minimal improvement with laparoscopic surgery, which, given their cautionary statements may well have been intended to counter pre-judgment in favor of laparoscopic surgery. However, rather than restoring equipoise and successfully encouraging surgeons to wait until the results on cancer risk were available, it in fact set a precedent for publishing short-term outcome data and thus led to increased levels of pre-judgment.
The clinical trials discussed in an earlier section provide important insights about this issue of releasing some interim data to restore equipoise. Results of the Syntex 1654 trial were published, claiming to have established benefit of ganciclovir on the basis of a reduction in CMV disease and death. As discussed, the early results from the similarly designed CPCRA 023 trial suggested only a small effect of oral ganciclovir on prevention of CMV disease, and the mortality trend was in the wrong direction. The DMC thought achieving continued compliance to the control regimen during the remaining 12 months of the trial may be difficult. To restore a sense of equipoise the CPCRA 023 DMC recommended making an immediate limited disclosure of key current results. Letters were sent to study patients, their physicians, and institutional review boards summarizing the Syntex results and stating that the 023 results ‘did not support the conclusions found in the Syntex study.’
The experience from the EU and NA trials in secondary-progressive multiple sclerosis patients indicated that even such limited release may well not be necessary. As discussed in an earlier section, the EU trial was completed two years before the closely related NA trial and reported that the experimental regimen provided highly significant benefits. The DMC for the NA trial recognized that their trial would provide very significant insights beyond what had been reported by the authors of the EU trial, if the NA trial could be successfully completed in a blinded manner. The DMC for the NA trial decided that a letter should be sent to investigators indicating this judgment. The DMC also decided that it was not appropriate or necessary to release any data at that time to ‘restore equipoise.’ Subsequent events established their judgment to be correct. The trial was successfully completed in a timely manner (contrary to the claims of some that investigators and patients would be unwilling to continue their participation in the NA trial after being informed about the EU data), and the completed results provided very substantial insights that meaningfully altered the clinical community’s earlier views that had been based on the EU trial alone.
Consensus in the research community regarding confidentiality of interim data
In a clinical trial sponsored by the Canadian Institutes of Health Research which evaluated the efficacy and safety of the addition of warfarin to aspirin in patients with peripheral arterial disease, the sponsor requested indirect access to relative efficacy information during the trial to inform a decision about whether to continue funding. This access was to a calculation of ‘conditional power’ which the sponsor specified to be the probability the trial would be positive conditionally given the current data, and where the current data also would be used as the alternative hypothesis under which the conditional power would be calculated. The request for indirect access to interim estimates regarding treatment effect was denied by the trial’s DMC, and this denial was supported by the Steering Committee chair and the Principal Investigator of the study. These latter two individuals surveyed experienced clinical trialists, asking them, ‘Do you think that in a large randomized clinical trial, in which there is an independent DMC made up of reputable clinical trialists and biostatisticians who carefully monitor the trial, interim data such as conditional power should be given to the sponsor when requested?’ More than two dozen respondents unanimously replied, ‘No’ (personal communication by Sonia Anand, Janet Wittes and Salim Yusuf). This collective opinion is consistent with the DAMOCLES document [33] which states
‘There is near unanimity that the interim data and the deliberations of the DMC should be absolutely confidential … At the end of the meeting, the DMC will make a recommendation to the steering committee, but the DMC will not discuss the actual data with the steering committee or anyone else. Breaches of confidence are to be treated extremely seriously.’
A dissenting opinion is provided by Lilford et al. [34], stating ‘Why should data arising in a trial be secret … setting up a system that perpetuates ignorance violates Kant’s injunction that people should not be used as a “mere” means to an end.’ However, withholding unreliable interim data does not ‘perpetuate ignorance’ and therefore does not violate individual ethics. Lilford’s opinion also does not recognize that clinical trials must be conducted in a manner to address both collective and individual ethics. Addressing collective ethics includes achieving the goal of a timely and reliable evaluation of the overall benefits and risks of an intervention for the benefit of all patients. Furthermore, many patients join clinical trials in part due to altruistic interests in achieving this same goal, so failure to maintain trial integrity violates individual as well as collective ethics. A referee observed that in addition to the widely understood first principle of clinical equipoise (that there should exist an initial substantial lack of clinical community consensus for the trial to be ethical), the second principle is that anything that jeopardizes the trial’s ability to perturb the initial state of clinical equipoise is to be avoided.
What is the consensus within the research community regarding this confidentiality issue? In addition to the DAMOCLES document and a detailed summary of confidentiality issues in monitoring of clinical trials in Chapter 5 of Ellenberg et al. [1], several consensus documents have been developed by scientific and regulatory bodies regarding the scientific and ethical issues in monitoring clinical trials, including issues of confidentiality. The following excerpts reflect the consensus that has emerged. In these excerpts, note in some instances that Data and Safety Monitoring Board (DSMB) is used as alternative terminology to DMC.
The NIH policy for Data and Safety Monitoring [3] states:
‘Confidentiality must be maintained during all phases of the trial including monitoring, preparation of interim results, review, and response to monitoring recommendations … usually only voting members of the DSMB should see interim analyses of outcome data. Exceptions may be made under circumstances where there are serious adverse events, or whenever the DSMB deems it appropriate.’
The WHO Operational Guidelines for the Establishment and Functioning of Data and Safety Monitoring Boards [4] states:
‘The DSMB should ensure confidentiality and proper communication to enhance the integrity and credibility of the study. It is recommended that each DSMB meeting be divided into two sessions: an open and a closed session. This will enable the DSMB to interact with groups and individuals who assume responsibilities for the study while ensuring the independence and integrity of the Board’s recommendations.’
The European Medicines Agency (EMEA) Guideline on Data Monitoring Committees [5] states:
‘A critical point in all DMC activities is to ensure the integrity and credibility of the ongoing trial. Thus, it is within the responsibilities of the DMC and the sponsor to have appropriate policies in place to ensure the integrity of the study. As an example, policies to avoid the dissemination of interim study results prior to unblinding have to be in place.’
The FDA Guidance on the Establishment and Operation of Clinical Trials DMCs [6] states:
‘Knowledge of unblinded interim comparisons from a clinical trial is not necessary for those conducting or those sponsoring the trial … Therefore, the interim data and the results of interim analyses should generally not be accessible by anyone other than DMC members. Sponsors should establish procedures to ensure the confidentiality of the interim data.’
Conclusions
Unless a DMC has judged that interim data conclusively answer the principal questions the trial was designed to address, such data should be recognized to be unreliable. Release of unreliable interim data induces a significant risk of inappropriate pre-judgment and threatens the ability of the trial to achieve its goals. These risks also apply where the interim data provide complete results on short-term outcome measures where the trial was designed to evaluate effects of an intervention on long-term safety and efficacy, especially when the long-term outcomes are critical to the overall benefit-to-risk profile of the intervention. Examples include short-term quality of life outcomes in the colectomy trials or biomarkers used to determine whether temporary marketing of regimens should be granted under the FDA accelerated approval process.
The consensus documents referenced in the previous section and numerous articles relating to the science of monitoring clinical trials reflect scientific and public endorsement of the need for confidentiality of interim data in Phase 2b and Phase 3 trials, especially for studies that are evaluating interventions that could impact the risk of serious morbidity or mortality. Only the DMC and the reporting statistical group should have unblinded access to interim efficacy and safety data. Ensuring this is of critical importance in enabling the DMC to achieve its role of safe-guarding patient interests and preserving the integrity and credibility of clinical trials.
Acknowledgments
The authors are members of the DMC for the ALCCAS trial and are independent of the trial’s research team. This DMC is funded by the Health Research Council of New Zealand. The authors thank Paul Gallo, Timon Bogumil, Henry Fuchs, Sonia Anand, and Salim Yusuf for their assistance. The source of financial support for research described in this article is an NIH/NIAID grant entitled ‘Statistical Issues in AIDS Research’ (R37 AI 29168).
References
- 1.Ellenberg SS, Fleming TR, DeMets DL. Data Monitoring Committees in Clinical Trials: A Practical Perspective. Chichester, West Sussex, England: John Wiley & Sons, Ltd; 2002. [Google Scholar]
- 2.Grant AM, Altman DG, Babiker AB, et al. Damocles study group. Issues in data monitoritng and interim analyses of trials. Health Technology Assessment (Winchester, England) 2005;9 doi: 10.3310/hta9070. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health. [accessed 19 February 2007];Policy for Data and Safety Monitoring. http://grants.nih.gov/grants/guide/notice-files/not98-084.html.
- 4.World Health Organisation. [accessed 19 February 2007];Operational Guidelines for the Establishment and Functioning of Data and Safety Monitoring Boards. http://www.who.int/tdr/publications/publications/pdf/operat_guidelines.pdf.
- 5.European Medicines Agency. [accessed 19 February 2007];Committee for Medicinal Products for Human Use. Guideline on Data Monitoring Committees. doi: 10.1002/sim.2585. http://www.emea.eu.int/pdfs/human/ewp/587203en.pdf. [DOI] [PubMed]
- 6.FDA. [accessed 19 February 2007];Guidance for clinical trial sponsors: On the establishment and operation of clinical trial data monitoring committees. http://www.fda.gov/OHRMS/DOCKETS/98fr/o1d-0489-gdl0003.pdf.
- 7.Weeks JC, Nelson HN, Gelber S, et al. for the Clinical Outcomes of Surgical Therapy (COST) Study Group. Short term quality-of-life outcomes following laparoscopic-assisted versus open colectomy for colon cancer. JAMA. 2002;287:321–328. doi: 10.1001/jama.287.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Guillou PJ, Quirke P, Thorpe H, et al. for the MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multi-centre, randomized controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 9.The COLon cancer Laparoscopic or Open Resection Study Group. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomized trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 10.Australasian Laparoscopic Colon Cancer Study. [accessed 19 February 2007]; http://clinicaltrials.gov/ct/show/NCT00202111.
- 11.Fleming TR. Surrogate endpoints and FDA’s accelerated approval process: The challenges are greater than they seem. Health Affairs. 2005;24:67–78. doi: 10.1377/hlthaff.24.1.67. [DOI] [PubMed] [Google Scholar]
- 12.Armitage P, McPherson CK, Rowe BC. Repeated significance tests on accumulating data. Journal of the Royal Statistical Society, Series A. 1969;132:235–244. [Google Scholar]
- 13.McPherson K. Statistics: the problem of examining accumulating data more than once. New England Journal of Medicine. 1974;290:501–502. doi: 10.1056/NEJM197402282900907. [DOI] [PubMed] [Google Scholar]
- 14.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. British Journal of Radiology. 1971;44:793–797. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 15.Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;64:191–199. [Google Scholar]
- 16.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 17.Fleming TR, Harrington DP, O’Brien PC. Designs for group sequential tests. Controlled Clinical Trials. 1984;5:348–361. doi: 10.1016/s0197-2456(84)80014-8. [DOI] [PubMed] [Google Scholar]
- Grant AM, Altman DG, Babiker AB, et al. DAMOCLES Study Group. Issues in data monitoring and interim analyses of trials. Health Technology Assessment (Winchester, England) 2005;9:25. doi: 10.3310/hta9070. [DOI] [PubMed] [Google Scholar]
- 19.Green SJ, Fleming TR, O’Fallon JR. Policies for study monitoring and interim reporting of results. Journal of Clinical Oncology. 1987;5:2477–2484. doi: 10.1200/JCO.1987.5.9.1477. [DOI] [PubMed] [Google Scholar]
- 20.Rider WD, Palmer JA, Mahoney LJ, Robertson CT. Preoperative irradiation in operable cancer of the rectum: Report of the Toronto trial. Canadian Journal of Surgery. 1977;20:335–338. [PubMed] [Google Scholar]
- 21.Medical Research Council Working Party. The evaluation of low-dose preoperative x-ray therapy in the management of operable rectal cancer: results of a randomly controlled trial. British Journal of Surgery. 1984;71:21–25. doi: 10.1002/bjs.1800710107. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Eisen T, Stadler WM, et al. the TARGET Study Group. Sorafenib in advanced clear-cell renal cell carcinoma. New England Journal of Medicine. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 23.Abrams D, Goldman A, Launer C, et al. A comparative trial of didanosine or zalcitabine after treatment with zidovudine in patients with human immunodeficiency virus infection. New England Journal of Medicine. 1994;330:657–662. doi: 10.1056/NEJM199403103301001. [DOI] [PubMed] [Google Scholar]
- 24.Korn EL, Hunsberger S, Freidlin B, et al. Preliminary data release for randomized clinical trials of noninferiority: a new proposal. Journal of Clinical Oncology. 2005;23:5831–5836. doi: 10.1200/JCO.2005.02.105. [DOI] [PubMed] [Google Scholar]
- 25.Julius S, Kjeldsen SE, Weber M, et al. The VALUE trial group, Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 26.ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized controlled Alzheimer’s disease anti-inflammatory prevention trial (ADAPT) PLoS Clinical Trials. 2006;1:e 33. doi: 10.1371/journal.pctr.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurie JA, Moertel CG, Fleming TR, et al. Surgical adjuvant therapy of large bowel carcinoma: An evaluation of levamisole and the combination of leucovorin and fluorouracil. J Clin Oncol. 1989;7:1447–1456. doi: 10.1200/JCO.1989.7.10.1447. [DOI] [PubMed] [Google Scholar]
- 28.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. New England Journal of Medicine. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 29.Spector SA, McKinley GF, Collins G, et al. Oral gancyclovir for the prevention of cytomegalovirus disease in patients with AIDS. New England Journal of Medicine. 1996;334:1491–1497. doi: 10.1056/NEJM199606063342302. [DOI] [PubMed] [Google Scholar]
- 30.Brosgart CL, Louis TA, Hillman DW, et al. A randomized placebo-controlled trial of the safety and efficacy of oral gancyclovir for prophylaxis of cytomegalovirus disease in HIV-infected individuals. Terry Beirn Community Programs for Clinical Research on AIDS. AIDS. 1998;12:269–277. doi: 10.1097/00002030-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 31.European Study Group on Interferon β-1b in Secondary Progressive MS. Placebo-controlled multicenter randomized trial of Interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352:1491–1497. [PubMed] [Google Scholar]
- 32.The North American Study Group on Interferon β-1b in Secondary Progressive MS. Interferon beta-1b in secondary progressive MS. Neurology. 2004;63:1788–1795. doi: 10.1212/01.wnl.0000146958.77317.3e. [DOI] [PubMed] [Google Scholar]
- 33.Grant AM, Altman DG, Babiker AB, et al. Damocles Study Group. Issues in data monitoring and interim analyses of trials. Health Technology Assessment (Winchester, England) 2005;9:24. doi: 10.3310/hta9070. [DOI] [PubMed] [Google Scholar]
- 34.Lilford RJ, Braunholtz D, Edwards S, et al. Monitoring clinical trials: interim data should be publicly available. British Medical Journal. 2001;323:441–442. doi: 10.1136/bmj.323.7310.441. [DOI] [PMC free article] [PubMed] [Google Scholar]