Abstract
Introduction
Theoretically, the physiological response to stress should inhibit the sexual response. This has been demonstrated experimentally in animal models, and correlationally in studies of human reproduction. It is reasonable to expect, then, that the stress response would be blunted during sexual arousal, and several researchers have found a pattern of decreasing cortisol during sexual arousal.
Aim
In the present study, we explored individual differences in women’s cortisol response to sexual arousal in a laboratory setting. We also examined how cortisol response in the laboratory related to a validated measure of sexual arousal functioning in real life.
Main Outcome Measures
Cortisol levels were measured in saliva via enzyme immunoassay. Subjective arousal was measured by a self-report questionnaire, and genital arousal was measured by a vaginal photoplethysmograph.
Methods
Subjective and physiological responses to an erotic film were assessed in 30 women. Saliva samples were taken at baseline and following the film.
Results
The majority of women (N = 20) showed a decrease in cortisol; nine women showed an increase in response to an erotic film. The women who showed an increase in cortisol had lower scores on the Arousal, Desire, and Satisfaction domains of the Female Sexual Function Index. Genital arousal in the laboratory was not related to cortisol change.
Conclusions
Women who show an increase in cortisol in response to sexual stimuli in the laboratory have lower levels of functioning in certain areas of their sexual life compared with women who show a decrease in cortisol. Stress related to sexual performance may interfere with sexual arousal.
Keywords: Cortisol, Sexual Arousal, Human Females, Stress
Introduction
In response to a stressor, most organisms have an automatic reaction that engages the mechanisms necessary for mobilization. This response, automatically activated as a defense against any threat, is designed to provide the energy resources necessary for survival and to shut down all unnecessary functions, such as digestive and reproductive functions. Consequently, in order for an organism to engage in sexual activity, the stress response would need to be inactive.
Cortisol release from the adrenal cortex is a key component of the stress response. Although there are a series of autonomic and endocrine responses that occur when an organism is faced with a stressor, cortisol has become commonly known as “the stress hormone.” Cortisol’s role in the endocrine system is metabolic, and it is released both after eating and in response to stressful situations. As part of the stress response, cortisol acts on various metabolic pathways to provide energy where it is needed in the body during a stressful fight or flight situation. Although increased cortisol release is not the only marker of the stress response, measuring cortisol response is a simple way to make a reasonable judgment about whether or not an organism is experiencing a stress response. This is particularly useful in sexual arousal studies because cortisol is only active in specific instances, whereas, for example, the sympathetic nervous system is activated in a variety of situations including both sexual arousal and during stress.
Three studies examining cortisol response during sexual arousal and orgasm provided evidence that the stress response is inactive during the sexual response in women [1-3]. Heiman et al. examined women’s endocrine responses to erotic stimuli by having an experimental group watch two 18-minute erotic films separated by 80 minutes, and a control group to watch a non-sexual documentary for 18 minutes followed by an erotic film 80 minutes later [3]. Blood was sampled continuously throughout the films. Both groups showed a nonsignificant decline in cortisol over the course of the study. Exton et al. noted similar results in 10 women who watched a film series consisting of a 20-minute documentary, a 20-minute erotic film, and a second 20-minute documentary [1]. Ten minutes into the erotic film, participants were instructed to masturbate until orgasm. Continuous blood samples revealed a significant decline in cortisol across the 60 minutes. As a control condition, the same women watched a 60-minute documentary film on a different day. Cortisol response during the control condition paralleled that of the experimental condition, suggesting that cortisol is not affected by sexual stimuli. Using a similar paradigm, but measuring only arousal, Exton and colleagues showed a significant decline in cortisol from the beginning of the study throughout the entire 60 minutes in both the experimental and control conditions [2]. Together, the findings from these studies suggest cortisol either decreases or does not change in response to sexual arousal or orgasm.
In a recent study, cortisol responses during sexual arousal showed a divergent pattern among women with and without a history of childhood sexual abuse (CSA; Rellini et al., unpublished manuscript) Although the majority of women in both groups responded to an erotic film with decreased cortisol, a substantially greater percentage of women with a history of childhood sexual abuse vs. non-abused women showed an increase in cortisol in response to an erotic film. Previously, Rellini and Meston proposed that abnormal cortisol patterns in women with a history of CSA could, in part, account for the high incidence of sexual arousal disorder among women with a history of childhood sexual abuse [4]. In the former study, (Rellini et al., unpublished manuscript) a small percentage of non-sexually abused women also showed an increase in cortisol in response to erotic stimuli, which was an unexpected finding given the assumption that the stress response should be inactive during sexual arousal in non-sexually abused women. We decided to follow up on this novel finding with further investigation.
The present study was designed to examine whether differences in sexual functioning (as measured by a questionnaire) could account for differences between non-sexually abused women who respond with an increase vs. a decrease in cortisol during a sexual scenario. It may be expected that women with a history of sexual arousal difficulties would experience higher levels of anxiety during a laboratory assessment of sexual arousal than would women with no sexual arousal concerns, which could lead to an increase in cortisol. Negative affect and expectations about sexual situations can reduce genital sexual arousal in men [5]. This would be consistent with recent findings that women with high levels of state anxiety showed significantly lower levels of genital arousal to an erotic film than women with moderate levels of state anxiety did [6]. Women with high levels of chronic stress and those exposed to acute stress also show lower levels of genital arousal [7].
Aims
The present study had two goals: (i) to further examine whether differences in the direction of cortisol responses to laboratory-induced sexual arousal exist in women; and if so, (ii) whether the direction of cortisol response is related to real-life sexual functioning. Specifically, we hypothesized that if differences in the direction of cortisol responses to a laboratory sexual situation exist between women, it will be in a direction such that increased cortisol is associated with decreased sexual arousal functioning.
Methods
Participants
The participants were 30 women aged 21-51 years. They were drawn from a community sample and were recruited via newspaper advertisements for women interested in participating in a study on sexuality. See Table 1 for demographic information. All participants had engaged in sexual activity within 4 weeks before beginning the study. Six (20.0%) of the women were taking hormonal contraceptives, and the remainder were freely cycling. All participants were screened via a telephone interview prior to scheduling their appointment. The interview began by informing the women that they would be viewing a sexually explicit film while having their genital arousal measured. If they were still interested in participating, the interview continued. Women were excluded from participating in the study if they had a history of sexual trauma or Axis I disorders, or were currently diagnosed with an Axis I disorder, if they were peri or postmenopausal, if they were taking medications known to affect vascular or sexual functioning, or if they had a medical condition that could feasibly affect sexual arousal or cortisol response. Women on hormonal contraceptives were included in the study. Although cortisol response has been shown to fluctuate with the menstrual cycle, women on oral contraceptives and women in their follicular phase do not differ in their salivary cortisol responses to acute stressors [8]. Women were informed that they would be viewing a sexually explicit film while having their genital arousal measured.
Table 1.
Participants’ demographic information
| Participants (N = 16) |
||
|---|---|---|
| M | SD | |
| Age | 28.5 | 6.03 |
| N | % | |
| Education | ||
| High school/G.E.D. | 4 | 13.3 |
| Some college/4-year degree | 23 | 76.7 |
| Advanced degree | 3 | 10.0 |
| Relationship status | ||
| Single, not dating | 2 | 6.7 |
| Single, dating | 11 | 36.7 |
| In a committed relationship | 13 | 43.3 |
| Married | 4 | 13.3 |
| Ethnicity | ||
| European American/White | 17 | 56.7 |
| Hispanic/Latina | 4 | 13.3 |
| African American/Black | 3 | 10.0 |
| Asian American | 3 | 10.0 |
| Mixed | 2 | 6.7 |
| Pacific Islander | 1 | 3.3 |
| Hormonal contraception | ||
| Currently using | 6 | 20.0 |
Materials and Apparatus
Film Scale
The film scale is a 41-item questionnaire that can be subdivided into four subscales: subjective experience of physiological sexual arousal, subjective experience of mental sexual arousal, negative affect, and positive affect [9]. Participants are given a word or phrase that describes how they feel and are asked to rate the item on a 7-point Likert scale indicating not at all to intensely. Examples of the words and phrases used are genital pulsing (physiological arousal), sexually turned on (mental arousal), relaxed (positive affect), and nervous (negative affect). The film scale is designed to be administered before and after a film, and the difference from pre- to post-film is assessed.
Female Sexual Function Index (FSFI)
To assess levels of sexual functioning, participants completed the FSFI, a questionnaire composed of 19 items divided into six domains: desire (2 items), arousal (4 items), lubrication (4 items), orgasm (3 items), satisfaction (3 items), and pain (3 items) [10]. The FSFI has been shown to reliably discriminate between women with DSM-IV-TR [11] diagnosed Female Sexual Arousal Disorder and control patients [10], and women with DSM-IV-TR diagnosed Hypoactive Sexual Desire Disorder or Female Orgasmic Disorder and healthy controls [12] on each of the six domains and the full-scale score.
Vaginal Photoplethysmograph
A vaginal photoplethysmograph was used to assess vaginal response to the sexual films. A data acquisition unit Model MP100WS (BIOPAC Systems, Inc., Santa Barbara, CA) and a software program, ACQKnowledge version 3.7.3 (BIOPAC Systems, Inc.), were used for the transformation of analog/digital data. The vaginal pulse amplitude (VPA) signal was sampled 80 times per second and the amplitude of each pulse wave was recorded in millivolts (mV).
Cortisol
Saliva samples were collected using Sarstedt salivettes with cotton swabs (Sarstedt, Newton, NC, USA). Participants were instructed to place the cotton swab into their mouth for 3 minutes to collect saliva. Samples were frozen until they were assayed for cortisol. Prior to assay, the salivettes were thawed and centrifuged for 10 minutes at 3,500 rpm. Assays were then run using a Salimetrics Cortisol EIA kit (Salimetrics, State College, PA, USA). Intra-assay covariance ranged from 1-10% and inter-assay covariance was 10.3%.
Since various daily life experiences can alter cortisol secretion, information on participant stress was collected using the Daily Inventory of Stressful Events, which measures anxiety and depression experienced in the previous 24 hours [13]. Levels of stress during the previous month and levels of stress during the previous 24 hours were also reported using 10 items from Kessler’s screening scale for psychological distress [14]. The McArthur Research Network’s guidelines for salivary cortisol measurement were followed [15]. Participants were asked: (i) “How much did you feel happy, excited, or content when you woke up?” and (ii) “How much did you feel worried, anxious, or fearful when you woke up?” These items were ranked on a 5-point Likert scale. No participants indicated an abnormal amount of stress or anxiety in these measures. Additional information was collected on cigarette smoking, alcohol consumption, drugs taken, vigorous exercise, time of awakening, and the most stressful event of the day over a 24-hour reference period.
Film Sequence
Participants viewed a 15-minute film sequence that consisted of 1 minute with the word “Relax” on a black screen, 4 minutes of a neutral film (a travel documentary), and 10 minutes of a sexual film. The sexual film was drawn from the Sexual Psychophysiology Laboratory film library, which includes sexual films directed and produced by women. Women show higher levels of positive affect and lower levels of negative affect to sexual films created by women compared with those created by men [16]. All films in this library have been standardized in terms of length of different types of sexual scenes (i.e., foreplay, oral sex, and vaginal intercourse), and scenes depicting violence have been edited out of the film.
Procedure
Prior to the laboratory visit, participants were instructed to avoid caffeine consumption, chocolate, and exercise for 12 hours prior to their visit since these factors are believed to impact cortisol measures [14]. Participants were also asked not to brush their teeth, eat, smoke cigarettes, or chew gum for 2 hours prior to their study visit. Cortisol data from women who did not comply with these requests was not included in the analyses (N = 1). Participants were scheduled for testing between 2:00 pm and 6:00 pm to control for daily fluctuations in cortisol. Because there is some evidence that cortisol reactivity changes over the menstrual cycle [8], menstrual cycle phase was controlled for by scheduling all participants between days 5 and 10 of their menstrual cycle.
After the participants reviewed and signed a consent form, a female experimenter explained how to insert and remove the vaginal photoplethysmograph. Participants were then left alone in a private room to fill out a demographics questionnaire and the film scale. Once participants had been in the lab for 30 minutes, they were asked to provide the baseline saliva sample (baseline). They were then instructed to insert the vaginal photoplethysmograph and sit comfortably for 10 minutes before the film sequence began. At the end of the sexual film, the participants completed the film scale a second time. Twenty-five minutes after the end of the film, participants provided the second saliva sample (post-film sample). Because cortisol takes approximately 30 minutes to peak in saliva, these sampling times corresponded to baseline and mid-erotic film cortisol levels [17]. Participants were paid US $50 for their participation in the study. All procedures for this study were approved by The University of Texas at Austin Institutional Review Board.
Data Analysis
All data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Inspection of individual cortisol results revealed that there were two distinct groups of responders: nine women showed increased cortisol in response to the erotic film (Increasers), and 20 women showed decrease in cortisol in response to the erotic film (Decreasers). All data were analyzed with group membership as the between-subjects variable. To examine whether oral contraceptive use influenced cortisol responses, we conducted t-tests between the women who were, and were not, using oral contraceptives. There were no significant differences between (i) oral contraceptive users and non-users at baseline, t(27) = -0.1, P = 0.92; (ii) post-film, t(27) = 1.49, P = 0.16; or (iii) in the percent change over baseline, t(27) = -1.07, P = 0.3.
VPA data were analyzed by calculating the total change in amplitude for each pulse wave. This was done by finding the peak and nadir for each pulse wave and computing the difference between the two. These total amplitude values were then averaged every 10 seconds, and the 10-second epochs were averaged over the neutral film and the erotic film. In order to compare across groups for physical genital arousal, we calculated the percent change over baseline for each participant’s VPA data.
Results
In line with previous studies, we found an overall decline in cortisol over the course of the study. A repeated measures anova with time as the within-subjects variable, and group (Increasers, Decreasers) as the between-subjects variable found a significant main effect of time: cortisol decreased from 0.115 (μg/dL), standard deviation (SD) = 0.013 to 0.1 (μg/dL), SD = 0.009, F(1,27) = 4.59, P = 0.04. The interaction between group and time was also significant, F(1,27) = 23.38, P < 0.001. Post-hoc repeated measures anovas revealed that both the Increasers’, F(1,8) = 8.61, P = 0.02, and Decreasers’, F(1,19) = 30.37, P < 0.001, cortisol levels changed significantly over time (Figure 1). An independent samples t-test showed that there was no significant difference between the two groups at baseline, t(27) = 1.17, P = 0.25.
Figure 1.
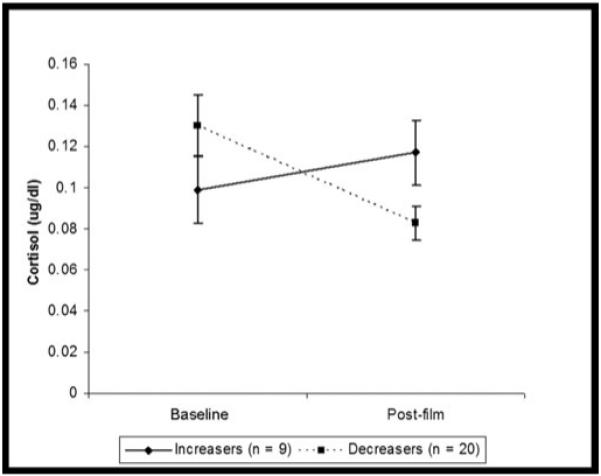
Cortisol changes by group.
As predicted, women who showed an increase in cortisol in response to the film had lower scores on the arousal subscale of the FSFI compared with Decreasers, t(27) = 2.95, P = 0.007. In addition, Increasers also had lower scores than Decreasers on the desire, t(27) = 3.02, P = 0.03, and satisfaction, t(27) = 2.48, P = 0.03, domains. Lower scores on the FSFI indicate a lower level of sexual functioning. Figure 2 shows that the Increasers had lower scores on all domains of the FSFI, although some were not significantly different. On the full scale, the Increasers scored a mean of 26.76 (SD = 3.22) and Decreasers scored 30.91 (SD = 4.83), t(27) = 2.34, P = 0.03.
Figure 2.
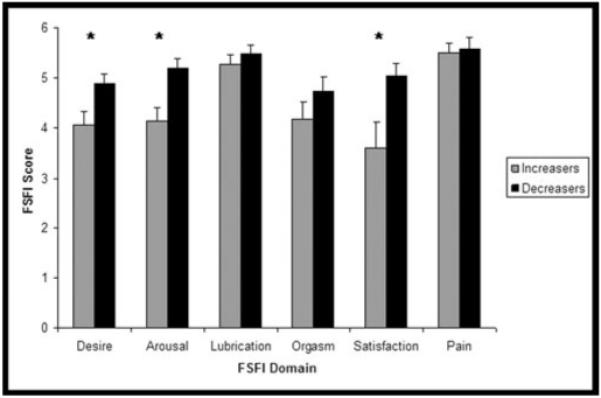
Female Sexual Function Index (FSFI) scores of Increasers and Decreasers. *P < 0.05.
In response to the erotic film, overall the participants showed an increase in both subjective mental sexual arousal, F(1,27) = 8.42, P = 0.007, and subjective physiological sexual arousal, F(1,24) = 11.72, P = 0.003. There were no differences between groups on any of the four subscales of the film scale at either the pre-film or post-film time points. Examination of individual items related to stress revealed a pre-film difference on relaxed that disappeared post-film, and no difference before or after the film on the anxiety item. Increasers reported being less relaxed t(26) = 3.24, P = 0.003 before the film, but not after, t(26) = 0.43 P = 0.67. We also examined the relationship between the arousal subscales of the film scale and FSFI arousal domain. Correlations between the laboratory measure of subjective mental arousal and FSFI Arousal revealed a significant relationship for Increasers, r(8) = 0.77, P = 0.02, but not Decreasers, r(18) = 0.08, P = 0.74. The relationship between subjective reports of physiological sexual arousal and FSFI arousal was not significant for either group. Increasers had a mean increase in VPA of 36% (SD = 45.4) over baseline, while Decreasers had a mean increase of 43% (SD = 48.1). The two groups were not significantly different from one another, t(27) = 0.37, P = 0.71.
Discussion
With one exception, Rellini et al.’s (unpublished manuscript) past research has found that cortisol decreases or stays the same in response to sexual stimuli [1-3]. Consistent with the former study, the present study found that some women respond to sexual situations with an increase in cortisol. Novel to this study was the finding that these women who showed increased cortisol responses (Increasers) had lower scores on a validated measure of sexual function than women who responded to sexual stimuli with a decrease in cortisol (Decreasers) did. Specifically, Increasers had lower scores on the Arousal, Desire, and Satisfaction domains of the FSFI than did women who showed the more commonly reported decrease in cortisol during sexual arousal. It should be noted that the participants in our study were a nonclinical sample, and neither group fell below the FSFI cutoff score for sexual dysfunction [18]. We interpreted the difference as an indication that the Increasers may have more sexual concerns than the Decreasers.
One possible explanation for the increase in cortisol among women with lower scores on a measure of sexual functioning is that a laboratory test of sexual responding, such as that conducted in this investigation, is more anxiety-eliciting for women who may have occasionally experienced problems with arousal. That is, the performance demands of the laboratory situation may have elevated cortisol levels for women who were not entirely confident in their ability to become aroused. Consistent with this hypothesis, when measured at the beginning of the laboratory sessions, Increasers reported being significantly less relaxed than did Decreasers. Inconsistent with this hypothesis is the finding that post-film ratings of feeling relaxed did not differ significantly between the Increasers and Decreasers. Although highly speculative, it is possible that post-film, once the women realized they were becoming psychologically and genitally sexually aroused to the erotic film, their negative cognitions and anxiety about being in a sexual situation diminished.
As previously noted, the present study was not conducted on a clinical sample. Future studies are needed to examine whether a clinical sample of women with female sexual arousal disorder and increased cortisol responses to a laboratory test of sexual response would show a similar pattern of genital response in the laboratory as the women noted here. Having an increased cortisol response to sexual stimuli could potentially impair the endocrine balance needed for successful sexual responding. At least two studies have shown that in men, cortisol release triggered by acute stressors was associated with a decrease in testosterone [19,20], but to our knowledge, the effects of cortisol on androgens in premenopausal women have not yet been investigated.
We found that there were no significant differences in genital arousal between women who showed an increase in cortisol, and those who showed a decrease. Possibly, increased levels of cortisol do not have a negative effect on sexual response in women. We based our assumption that cortisol release would negatively affect sexual response, on findings from the animal literature and on human studies that assessed laboratory measures of sexual arousal. Also, several questionnaire studies have indicated that stress can interfere with sexual desire and arousal [21,22]. To our knowledge, no study has examined the cortisol response during real-life sexual activity. Physical exercise has been shown to increase cortisol [23], and it is possible that the physical exertion associated with sexual activity could increase cortisol levels.
The finding that Increasers, but not Decreasers, showed a positive correlation between their scores on the FSFI Arousal domain and rating of arousal to the erotic film, warrants mention. Because the FSFI is a measure of a women’s sexual functioning in their daily life, this could indicate that for Increasers, who have lower scores on FSFI Arousal (indicating they were not always able to become aroused in sexual situations) their own sexual problems are made salient in a laboratory test where they are expected to become sexually aroused.
Conclusions
We have demonstrated that some women respond to sexual stimuli with an increase in cortisol, which is opposite to that seen in previous studies of women who do not have a history of sexual trauma. Compared with women who showed a decline in cortisol in response to the sexual stimuli, women who showed an increase in cortisol had lower scores on a standardized measure of sexual functioning in the domains of sexual arousal, desire, and satisfaction. Given that feedback of an increased cortisol response could trigger disruptions in other hormonal mechanisms, if replicated, our findings have important implications for both reproductive health and sexual functioning in women.
Acknowledgment
The authors would like to thank Yvon Delville for his assistance with cortisol assays, and to Emily Fogle for her assistance with data collection. This publication was made possible by Grant Number 1 RO1 HD051676-01 A1 from the National Institute of Child Health and Human Development to Cindy M. Meston, and by Grant Number F31 MH68165 from the National Institute of Mental Health to Alessandra H. Rellini. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institute of Mental Health.
References
- 1.Exton MS, Bindert A, Kruger T, Scheller F, Hartmann U, Schedlowki M. Cardiovascular and endocrine alterations after masturbation-induced orgasm in women. Psychosom Med. 1999;61:280–9. doi: 10.1097/00006842-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Exton NG, Truong TC, Exton MS, Wingenfeld SA, Leygraf N, Saller B, Hartmann U, Schedlowski M. Neuroendocrine response to film-induced sexual arousal in men and women. Psychoneuroendocrinology. 2000;25:187–99. doi: 10.1016/s0306-4530(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 3.Heiman JR, Rowland DL, Hatch JP, Gladue BA. Psychophysiological and endocrine responses to sexual arousal in women. Arch Sex Behav. 1991;20:171–86. doi: 10.1007/BF01541942. [DOI] [PubMed] [Google Scholar]
- 4.Rellini AH, Meston CM. Psychophysiological sexual arousal in women with a history of childhood sexual abuse. J Sex Marital Ther. 2006;32:5–22. doi: 10.1080/00926230500229145. [DOI] [PubMed] [Google Scholar]
- 5.Barlow DH. Causes of sexual dysfunction: The role of anxiety and cognitive interference. J Consult Clin Psychol. 1986;54:140–8. doi: 10.1037//0022-006x.54.2.140. [DOI] [PubMed] [Google Scholar]
- 6.Bradford A, Meston CM. The impact of anxiety on sexual arousal in women. Behav Res Ther. 2006;44:1067–77. doi: 10.1016/j.brat.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ter Kuile MM, Vigeveno D, Laan E. Preliminary evidence that acute and chronic daily psychological stress affect sexual arousal in sexually functional women. Behav Res Ther. 2007;45:2078–89. doi: 10.1016/j.brat.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Kirshbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–62. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Heiman JR, Rowland DL. Affective and physiological sexual response patterns: The effects of instructions on sexually functional and dysfunctional men. J Psychosom Res. 1983;27:105–16. doi: 10.1016/0022-3999(83)90086-7. [DOI] [PubMed] [Google Scholar]
- 10.Rosen R, Brown C, Heiman J, Leiblum S, Meston CM, Shabsigh R, Ferguson D, D’Agostino R., Jr The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edition. Author; Washington, DC: 2006. text revision. [Google Scholar]
- 12.Meston CM. Validation of the Female Sexual Function Index (FSFI) in women with female orgasmic disorder and in women with hypoactive sexual desire disorder. J Sex Marital Ther. 2003;29:39–46. doi: 10.1080/713847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida DM, Wethington E, Kessler RC. The Daily Inventory of Stressful Experiences (DISE): An interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, Andrews G, Colpe L, Hiripi E, Mroczek DK, Normand SL, Walters EE, Zaslavsky AM. Short screening scales to monitor population prevalence and trends in nonspecific psychological distress. Psychol Med. 2002;32:959–76. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 15.Stewart J, John D, Catherine T. Salivary cortisol measurement. Mac-Arthur Research Network on Socioeconomic Status and Health; 2002. [accessed November 21, 2006]. Summary of findings by the. Available at: http://www.macses.ucsf.edu/Research/Allostatic/notebook/salivarycort.html. [Google Scholar]
- 16.Laan E, Everaerd W, van Bellen G, Hanewald G. Women’s sexual and emotional responses to male- and female-produced erotica. Arch Sex Behav. 1994;23:153–69. doi: 10.1007/BF01542096. [DOI] [PubMed] [Google Scholar]
- 17.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology. 1989;22:150–69. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 18.Wiegel M, Meston CM, Rosen RC. The Female Sexual Function Index (FSFI): Cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 19.Chatterton RT, Jr, Vogelsong KM, Lu Y, Hudgens GA. Hormonal responses to psychological stress in men preparing for skydiving. J Clin Endocrinol Metab. 1997;82:2503–9. doi: 10.1210/jcem.82.8.4133. [DOI] [PubMed] [Google Scholar]
- 20.Cummings DC, Quigley ME, Yen SS. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab. 1983;57:671–3. doi: 10.1210/jcem-57-3-671. [DOI] [PubMed] [Google Scholar]
- 21.Colson MH, Lemaire A, Pinton P, Hamidi K, Klein P. Sexual behaviors and mental perception, satisfaction and expectations of sex life in men and women in France. J Sex Med. 2006;3:121–31. doi: 10.1111/j.1743-6109.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 22.Morokoff PJ, Gillilland R. Stress, sexual functioning, and marital satisfaction. J Sex Res. 30:43–53. [Google Scholar]
- 23.Cummings DC, Rebar RW. Exercise and reproductive function in women. Am J Int Med. 1983;4:113–25. [PubMed] [Google Scholar]


