Abstract
Objectives
Organ failure worsens outcome in sepsis. The Sequential Organ Failure Assessment (SOFA) score numerically quantifies the number and severity of failed organs. We examined the utility of the SOFA score for assessing outcome of patients with severe sepsis with evidence of hypoperfusion at the time of emergency department (ED) presentation.
Design
Prospective observational study.
Setting
Urban, tertiary ED with an annual census of >110,000.
Patients
ED patients with severe sepsis with evidence of hypoperfusion. Inclusion criteria: suspected infection, two or more criteria of systemic inflammation, and either systolic blood pressure <90 mm Hg after a fluid bolus or lactate >4 mmol/L. Exclusion criteria age <18 years or need for immediate surgery.
Interventions
SOFA scores were calculated at ED recognition (T0) and 72 hours after intensive care unit admission (T72). The primary outcome was in-hospital mortality. The area under the receiver operating characteristic curve was used to evaluate the predictive ability of SOFA scores at each time point. The relationship between Δ SOFA (change in SOFA from T0 to T72) was examined for linearity.
Results
A total of 248 subjects aged 57 ± 16 years, 48% men, were enrolled over 2 years. All patients were treated with a standardized quantitative resuscitation protocol; the in-hospital mortality rate was 21%. The mean SOFA score at T0 was 7.1 ± 3.6 points and at T72 was 7.4 ± 4.9 points. The area under the receiver operating characteristic curve of SOFA for predicting in-hospital mortality at T0 was 0.75 (95% confidence interval 0.68 - 0.83) and at T72 was 0.84 (95% confidence interval 0.77-0.90). The Δ SOFA was found to have a positive relationship with in-hospital mortality.
Conclusions
The SOFA score provides potentially valuable prognostic information on in-hospital survival when applied to patients with severe sepsis with evidence of hypoperfusion at the time of ED presentation.
Keywords: sepsis, severe sepsis, scoring system, Sequential Organ Failure Assessment, mortality
Recent estimates indicate that the rate of severe sepsis hospitalizations doubled during the past decade, and the age-adjusted population-based mortality is increasing, resulting in at least 215,000 deaths in the United States yearly (1, 2). It is estimated that one half of sepsis hospitalizations originate in the emergency department (ED), underscoring the importance of developing and evaluating accurate and reliable methods for assessing illness severity and prognosis to allow for proper allocation of limited hospital resources (3-5) and inclusion in early interventions (6, 7).
There are several outcome prediction models that are currently available for use in clinical practice. Among them are the Acute Physiology and Chronic Health Evaluation IV Score (8), the Simplified Acute Physiology Score III (9), the Logistic Organ Dysfunction Score (10), and the Mortality Probability Model III (11), which were derived and validated on large groups of intensive care unit (ICU) patients and require historical data or data after ICU admission for calculation. Previous investigations have shown that most of these scores possess inadequate predictive abilities when adapted to ED populations (12). The one ED-based scoring system, the Mortality in Emergency Department Sepsis score, was derived and validated on large groups of ED patients with suspected infection (13). Although the Mortality in Emergency Department Sepsis rule has performed reasonably well in the general population of ED patients with suspected infection (14), it has been reported to be less accurate in patients who are more severely ill, where prediction is perhaps more important (15). Accordingly, the validation of a simple scoring system that would remain accurate when applied to patients with severe sepsis at the time of ED presentation is of high priority for both bedside clinical care and clinical research trials.
Previous investigators have identified a link between the number of dysfunctional organs and both short-term and long-term mortality among ED patients with infection (16). The Sequential Organ Failure Assessment (SOFA) score is a simple and objective score that allows for calculation of both the number and the severity of organ dysfunction in six organ systems (respiratory, coagulatory, liver, cardiovascular, renal, and neurologic) (Table 1), and the score can measure individual or aggregate organ dysfunction (17). The aims of this study were to determine whether 1) the SOFA score, when applied to a cohort of ED patients with severe sepsis with evidence of hypoperfusion, would perform with good accuracy for predicting hospital mortality, and 2) the Δ SOFA, defined as the change in SOFA score for a predefined time interval, is positively associated with changes in mortality.
Table 1.
The Sequential Organ Failure Assessment (SOFA) score
| SOFA score | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Respirationa | ||||
| PaO2/FIO2 (mm Hg) | <400 | <300 | <220 | <100 |
| SaO2/FIO2 | 221-301 | 142-220 | 67-141 | <67 |
| Coagulation | ||||
| Platelets ×103/mm3 | <150 | <100 | <50 | <20 |
| Liver | ||||
| Bilirubin (mg/dL) | 1.2-1.9 | 2.0-5.9 | 6.0-11.9 | >12.0 |
| Cardiovascularb | ||||
| Hypotension | MAP <70 | Dopamine ≤5 or dobutamine (any) | Dopamine >5 or norepinephrine ≤0.1 | Dopamine >15 or norepinephrine >0.1 |
| CNS | ||||
| Glasgow Coma Score | 13-14 | 10-12 | 6-9 | <6 |
| Renal | ||||
| Creatinine (mg/dL) or urine output (mL/d) | 1.2-1.9 | 2.0-3.4 | 3.5-4.9 or <500 | >5.0 or <200 |
MAP, mean arterial pressure; CNS, central nervous system; SaO2, peripheral arterial oxygen saturation.
PaO2/FIO2 ratio was used preferentially. If not available, the SaO2/FIO2 ratio was used
vasoactive mediations administered for at least 1 hr (dopamine and norepinephrine μmg/kg/min).
MATERIALS AND METHODS
This study was a preplanned secondary analysis of a prospective registry of consecutive ED patients with severe sepsis with evidence of hypoperfusion treated with an institutional quantitative resuscitation protocol that is initiated in the ED at the time of recognition of sepsis (18). This study protocol was reviewed and approved by the institutional review board for the conduct of human research before enrollment of patients.
Subjects were enrolled from November 2005 through October 2007 in the ED at Carolinas Medical Center, an 800-bed teaching hospital with 120,000 ED patient visits per year. Explicit criteria for enrollment included 1) age >17 years; 2) suspected or confirmed infection; 3) two or more systemic inflammatory response syndrome criteria (19): heart rate >90 beats per minute, respiratory rate >20 breaths per minute, temperature >38°C or <36°C, white blood cell count >12,000 or <4000 cells/mm3 or >10% bands; and 4) systolic blood pressure <90 mm Hg or mean arterial pressure <65 mm Hg after a isotonic fluid bolus and anticipated need for ICU care, or a serum lactate concentration ≥4.0 mmol/L and anticipated need for ICU care. Exclusion criteria included 1) age <18 years; 2) need for immediate surgery; and 3) absolute contraindication for a chest central venous catheter.
Eligible subjects were identified by board-certified emergency physicians and were treated in the ED and medical ICU with an institution-approved quantitative resuscitation protocol that was previously described (20). All data elements required for calculation of the SOFA score at the time of ED recognition and resuscitation (T0) and 72 hours after ICU admission (T72), as well as hospital outcomes, were prospectively collected on standardized forms and entered into a database for later analysis. For T0 scores, only data available in the ED were used for calculation; and for T72 scores, data available within 12 hours of the 72-hour time point were used for calculation. To our knowledge, no physician in the ED had any independent knowledge of the SOFA score. For purposes of this study, we made one modification in the calculation of the respiratory component of the SOFA score (Table 1). We preferentially used the PaO2 to FIO2 ratio (PaO2/FIO2) when arterial blood gases were obtained. In cases where the PaO2 was not available, we used the peripheral arterial oxygen saturation (SaO2) to FIO2 ratio (SaO2/FIO2). This substitution has been previously validated with high correlation (21). The definitions of SOFA score variables were otherwise identical to those reported in the original publication by Vincent et al (17).
Data Analysis
We defined the primary dependent variable for statistical analysis as in-hospital mortality. The predictive ability of both the T0 and T72 SOFA scores was evaluated using receiver operating characteristic (ROC) curve analysis generated on standard statistical software (StatsDirect v 2.7.2). The area under the curve (AUC) was used to compare the discriminatory power of the scoring system or other clinical variables of interest, with an AUC 1.0 considered perfect discrimination and 0.5 considered equal to chance (22). Odds ratios were calculated to determine independent predictors of in-hospital mortality by using logistic regression with bootstrap correction for 95% confidence intervals (CI) (23). Common ED variables known to predict in-hospital mortality in critically ill patients were entered into the regression analysis (24, 25). The model was limited to contain no more than one dependent variable for every eight outcomes. Because of colinearity with the SOFA score, we did not input any physiologic data contained in the SOFA score into the model as a stand-alone variable.
Continuous data are presented as means and SD and were compared using unpaired Student's t tests or Mann-Whitney U test, as appropriate. Categorical data are presented as percentages and were compared using chi-square test. We calculated the Δ SOFA, defined as the change in SOFA score over 72 hours (T0 SOFA - T72 SOFA) and examined the relationship between the Δ SOFA and in-hospital mortality graphically and using the Armitage chi-square test for linear trend (26). For all statistical testing, p < 0.05 was considered significant.
Given that this study examined both absolute values and changes in SOFA score at two time points (T0 and T72), there was the potential for subjects to die, be transferred, or be discharged from the hospital before the 72-hour time point. To account for these potential dropouts, we followed the last observation carried forward principle. Thus, for subjects who were not available for calculation of 72-hour SOFA (due to death, transfer, or discharge), we used the available data that were most temporally related to the 72-hour time point. To determine the impact of this strategy on our results, we performed a sensitivity analysis in which we recalculated the ROC and relationship between the Δ SOFA and in-hospital mortality using only subjects with complete data at 72 hours.
RESULTS
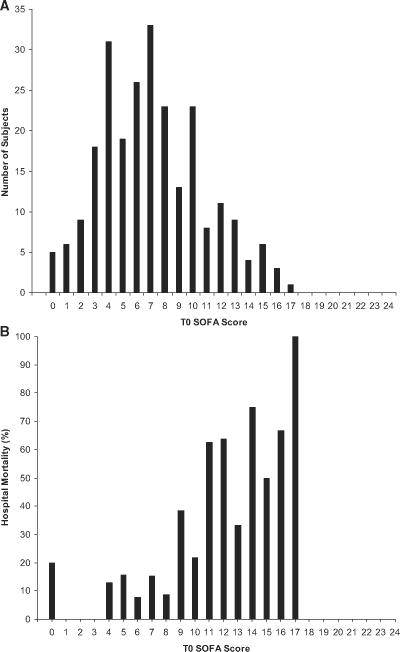
A total of 248 patients were included with an overall in-hospital mortality rate of 21% (51/248). Table 2 summarizes the demographics and initial clinical characteristics of the entire study population. The average SOFA score at T0 was 7.1 ± 3.6 points and at T72 was 7.4 ± 4.9 points in the entire population. Nonsurvivors had significantly higher mean (9.8 points) T0 and mean (11.8 points) T72 SOFA scores when compared with survivors' T0 and T72 (6.5 and 6.2) mean scores, respectively (Mann-Whitney U test, p < 0.001). The distribution of T0 SOFA scores and their respective associated in-hospital mortality rates are shown in Figure 1. As can be seen, the distribution of scores were slightly skewed, because very high values (>17) were not present in the sample. The in-hospital mortality associated with each individual T0 score, in general, showed an increase as the total score increased.
Table 2.
Initial patient demographic and clinical characteristics
| Variable | All (n = 248) | Survivor (n = 197) | Nonsurvivor (n = 51) | Pa |
|---|---|---|---|---|
| Age (yrs) | 57 ± 17.4 | 56 ± 17.4 | 62 ± 16.4 | 0.008 |
| Race (%) | 0.53 | |||
| White | 49 | 54 | 49 | |
| Black | 38 | 39 | 49 | |
| Gender (%) | 0.32 | |||
| male | 48 | 51 | 41 | |
| Female | 52 | 49 | 59 | |
| T0 SOFA score | ||||
| Respiratory | 1.5 ± 1.3 | 1.3 ± 1.2 | 2.2 ± 1.5 | |
| Coagulation | 0.4 ± 0.8 | 0.4 ± 0.8 | 0.5 ± 1.0 | |
| Liver | 0.5 ± 0.8 | 0.4 ± 0.7 | 0.6 ± 1.0 | |
| Cardiovascular | 2.4 ± 1.3 | 2.2 ± 1.3 | 3.0 ± 1.3 | |
| CNS | 0.9 ± 1.3 | 0.7 ± 1.1 | 1.5 ± 1.6 | |
| Renal | 1.5 ± 1.3 | 1.4 ± 1.2 | 2.0 ± 1.5 | |
| Total | 7.1 ± 3.6 | 6.5 ± 3.3 | 9.8 ± 3.5 | <0.001 |
| T72 SOFA score | ||||
| Respiratory | 1.5 ± 1.5 | 1.2 ± 1.3 | 2.7 ± 1.3 | |
| Coagulation | 0.6 ± 0.9 | 0.5 ± 0.9 | 0.8 ± 1.1 | |
| Liver | 0.5 ± 0.8 | 0.4 ± 0.7 | 0.7 ± 1.0 | |
| Cardiovascular | 2.3 ± 1.4 | 2.1 ± 1.4 | 3.3 ± 1.2 | |
| CNS | 1.2 ± 1.4 | 0.9 ± 1.2 | 2.5 ± 1.5 | |
| Renal | 1.3 ± 1.3 | 1.1 ± 1.2 | 1.9 ± 1.5 | |
| Total | 7.4 ± 4.9 | 6.2 ± 3.8 | 11.8 ± 4.1 | <0.001 |
| Initial suspected infection site (%)a | ||||
| Pneumonia | 41 | 40 | 45 | |
| Urinary | 29 | 31 | 22 | |
| Intra-abdominal | 21 | 22 | 20 | |
| Skin/soft tissue | 15 | 15 | 12 | |
| Blood | 11 | 11 | 10 | |
| Other | 16 | 17 | 12 | |
| Lowest SBP (mm Hg) | 72 ± 17.2 | 73 ± 17.8 | 68 ± 14.1 | 0.03 |
| Highest RR (beats/min) | 119 ± 25.4 | 119 ± 26.1 | 120 ± 23 | 0.81 |
| Highest RR (breaths/min) | 30 ± 11.7 | 30 ± 11.4 | 30 ± 13.1 | 0.81 |
| Temperature (F) | ||||
| Minimum | 97 ± 9.3 | 97 ± 10.2 | 96 ± 3.9 | 0.61 |
| Maximum | 101 ± 21.3 | 101 ± 23.7 | 99 ± 3.5 | 0.29 |
| CVP (mm Hg) | ||||
| Minimum | 9 ± 14.4 | 10 ± 14.4 | 8 ± 14.6 | 0.55 |
| Maximum | 13 ± 6.1 | 14 ± 6.2 | 12 ± 5.7 | 0.24 |
| Scvo2 (%) | ||||
| Minimum | 67 ± 15.8 | 69 ± 13.1 | 65 ± 14.3 | 0.19 |
| Maximum | 77 ± 16.5 | 79 ± 12.0 | 77 ± 17.9 | 0.61 |
| Lowest SaO2 (%) | 91 ± 10.3 | 91 ± 11.1 | 93 ± 6.2 | 0.04 |
| Lowest GCS | 13 ± 6.1 | 13 ± 3.2 | 11 ± 4.8 | 0.002 |
| WBC (cell/mm3) | 16 ± 10 | 13 ± 9.5 | 17 ± 11.7 | 0.54 |
| Lactate (mmol/L) | 5 ± 11.1 | 3 ± 3.0 | 6 ± 4.5 | 0.003 |
T0, time zero; T72, time 72 hr after admission; SOFA, sequential organ failure assessment; CNS, central nervous system; SBP, systolic blood pressure; RR, respiratory rate; CVP, central venous pressure; Scvo2, central venous oxygen saturation; SaO2, peripheral arterial oxygen saturation; GCS, Glasgow Coma Score; WBC, white blood cell count.
p values calculated using unpaired t tests, Mann-Whitney U tests, or chi-square tests where appropriate
totals >100% due to more than one potential site of infection. All values presented as means ± SD or percentages.
Figure 1.
Analysis of time zero (T0) Sequential Organ Failure Assessment (SOFA) score. A, Number of all subjects categorized by their calculated total T0 SOFA score; B, occurrence of in-hospital mortality according to T0 SOFA score.
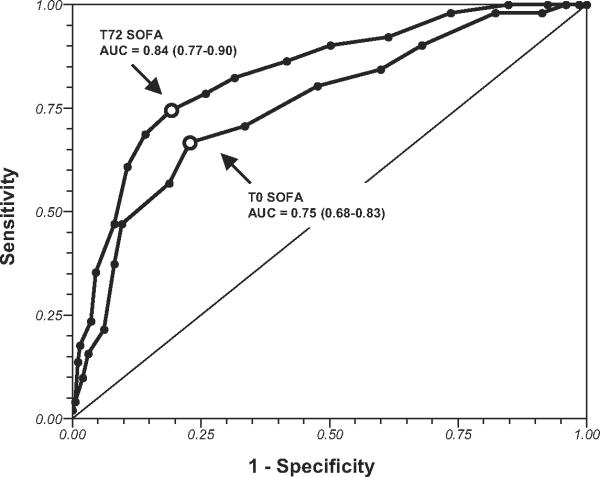
Figure 2 graphically depicts the ability of T0 and T72 SOFA scores for predicting mortality using ROC curve analysis. The T0 SOFA score demonstrated AUC, suggesting fair accuracy for mortality prediction (AUC, 0.75; 95% CI 0.68-0.83). The T72 SOFA score demonstrated AUC, suggesting good accuracy for mortality prediction (AUC, 0.84; 95% CI 0.77-0.90).
Figure 2.
Receiver operating characteristic curves of time zero (T0) and T72 (72 hours after ICU admission) Sequential Organ Failure Assessment (SOFA) scores for predicting hospital mortality. Area under the curve (AUC) followed by 95% confidence intervals are shown.
To compare the predictive ability of T0 SOFA with those of other clinical variables commonly available in the ED, ROC curves were constructed to determine the ability of these variables to predict mortality. The T0 SOFA had a higher AUC (0.75) than did any of the other variables investigated, including lowest ED systolic blood pressure (AUC, 0.62), highest ED temperature (AUC, 0.60), highest ED respiratory rate (AUC, 0.50), highest ED heart rate (AUC, 0.50), lowest ED Glasgow Coma score (AUC, 0.62), ED white blood cell count (AUC, 0.52), highest ED lactate (AUC, 0.65), and age (AUC, 0.61).
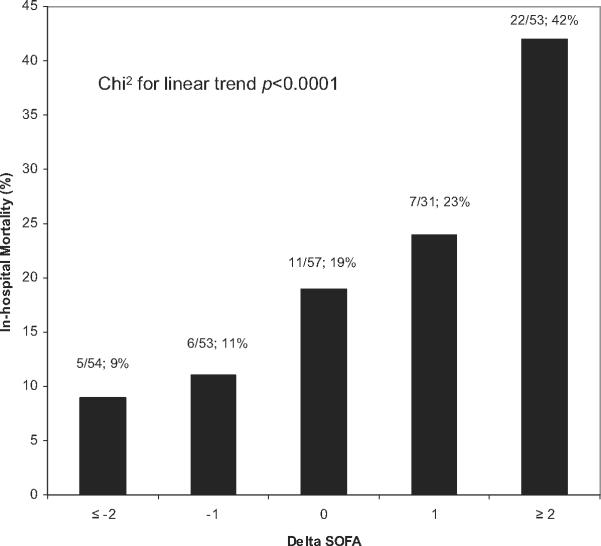
Figure 3 shows the relationship between Δ SOFA and in-hospital mortality. A positive relationship was found between the Δ SOFA over 72 hours and in-hospital mortality (chi-square for linear trend, p < 0.001). Subjects with a Δ SOFA of ≥2 points had a two-fold higher mortality rate (42%) than did the entire cohort (21%). Subjects with a Δ SOFA of ≤-2 points had a mortality rate of less than one-half (9%) of the entire cohort. Any increase in Δ SOFA (score worsened over 72 hours) was associated with a 35% in-hospital mortality rate, and any decrease in Δ SOFA (score improved over 72 hours) was associated with 10% in-hospital mortality.
Figure 3.
The relationship between delta Sequential Organ Failure Assessment (Δ SOFA) score and in-hospital mortality. Δ SOFA calculated by subtracting time 72 SOFA score from time zero SOFA score.
Recognizing the concern of the potential for confounding variables to affect the prognostic performance of SOFA, we constructed a logistic regression model and calculated the bias-adjusted odds ratio using the confounders of age, active malignancy, human immunodeficiency virus/acquired immunodeficiency syndrome, congestive heart failure, and highest lactate. This model found T0 SOFA to be a statistically significant predictor of in-hospital mortality, and it had the highest bias-adjusted odds ratio (1.3, 95% CI 1.14-1.41).
Among the initial cohort of 248 subjects, 19 of 51 nonsurvivors (37%) died in the first 72 hours of hospitalization, and 11 of 197 survivors (6%) were transferred or discharged from the hospital in the first 72 hours. We conducted a sensitivity analysis using only subjects who had complete data at 72 hours (n = 218). The T72 SOFA score AUC slightly declined, and the CIs slightly widened (AUC, 0.79, 95% CI 0.69-0.86). The strong linear relationship between the Δ SOFA and the in-hospital mortality was unchanged (chi-square for linear trend, p < 0.001).
DISCUSSION
The results of this study suggest that the SOFA score functions with fair to good accuracy for predicting in-hospital mortality when applied to patients with severe sepsis with evidence of hypoperfusion at the time of ED presentation. Furthermore, we found that the Δ SOFA over 72 hours has a statistically significant positive relationship to in-hospital mortality. These data suggest that use of the SOFA score is an acceptable method for risk stratification and prognosis of patients with severe sepsis with evidence of hypoperfusion at the time of ED presentation. Using absolute values or changes over time, the SOFA score appears to be a potentially useful tool for either the clinician during bedside assessment or for purposes of clinical research trials of sepsis.
The SOFA score was created by the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine, with the intent of creating an objective tool to describe individual and aggregate organ failure (17). The usefulness of the score has been previously validated in large cohorts of critically ill patients (27, 28). The SOFA score has several desirable characteristics for application in the ED, because it is easy to calculate at the bedside and includes clinical and laboratory data that are routinely available in the ED. We are aware of no previous study that has demonstrated the utility of applying the SOFA score in the ED at the time of recognition and resuscitation of patients with severe sepsis with evidence of hypoperfusion.
The adaptation of ICU-based scoring systems to application in the ED has been studied in previous investigations (12, 29). These studies have found the predictive abilities of these scoring systems to be modest at best, and given that these scores are often complex and require special software to calculate, the utility of applying them in real time in the ED is limited. The SOFA score is more practical for use in the ED, given that it is easy to calculate at the bedside, includes only vital sign and laboratory data that are routinely available, and does not require a definitive final diagnosis of the acute process. These facts, in addition to the equivalent performance of the SOFA score observed in this study, suggest that it may be preferred more than other scores for risk stratification and prognosis.
We made one adaptation to the SOFA score, as originally reported by substituting the SaO2/FIO2 ratio for the PaO2/FIO2 ratio (21). The ability to make this substitution makes the SOFA score more desirable and generalizable to the ED setting where it may not necessarily be routine to obtain arterial blood gases, particularly in patients who are not receiving mechanical ventilation. However, it remains possible that if we had used the PaO2/FIO2 ratio in all subjects, our results may have been altered.
Previous investigations have reported the usefulness of assessing the change in SOFA during ICU care to assess outcome (28, 30). To our knowledge, this is the first report examining the Δ SOFA incorporating calculations from the time of ED presentation and identification through the early phases of ICU care. We found that the Δ SOFA in this study was associated with positive, direct, and statistically significant changes in the in-hospital mortality. The importance of this point is the potential utility of such a measurement to be used as a method for evaluating clinical treatment progress and as a patient-oriented outcome in clinical research trials incorporating early sepsis interventions.
This report has several limitations to be considered. First, we made an adaptation to the respiratory component of the SOFA score, as previously described. It is possible that our results would have been different if this adaptation had not been made. Second, all scores were calculated post hoc and not applied in real time. If the scores had been calculated and applied prospectively, they might have performed with different accuracy because of their potential impact on disposition decisions. Third, the relatively small size of the sample studied might have resulted in a less precise estimation of the accuracy of the SOFA score. We followed the principle of last observation carried forward to account for subjects who were not available at 72 hours for calculation of the SOFA score. Although this is an accepted practice in many clinical trials, we performed a sensitivity analysis to determine the impact of this strategy on our results. This sensitivity analysis revealed minimal effect on the T72 SOFA ROC curve results and no effect of the positive trend analysis for relationship between Δ SOFA and mortality. Additionally, we studied only a subset of severe sepsis patients, those with cardiovascular or metabolic evidence of hypoperfusion. Therefore, our results may not be generalizable to severe sepsis patients with other criteria for organ dysfunction. Finally, the 72-hour time point to evaluate SOFA may not have been the optimal time point, and other time points (e.g., 6 or 24 hours) may have yielded different results.
CONCLUSIONS
The SOFA score demonstrated fair to good accuracy for predicting in-hospital mortality when applied to patients with severe sepsis with evidence of hypoperfusion at the time of ED presentation. The Δ SOFA over 72 hours has a significant positive relationship to in-hospital mortality. These data suggest that use of the SOFA score is an acceptable method for risk stratification and prognosis of ED patients with severe sepsis with evidence of hypoperfusion and that the Δ SOFA score may be a useful measurement to follow in clinical and research settings.
Acknowledgments
Supported, in part, by grant K23GM076652-01A1, from the National Institute of General Medical Sciences/National Institutes of Health to Dr. Jones and K23GM083211 to Dr. Trzeciak. Dr. Jones has received a grant from Critical Biologics Corporation. Dr. Kline has stock ownership in CP Diagnostics. Dr. Kline has received patents from US Patent # 7,083,574. Dr. Trzeciak has received grant support from Novo Nor-disk, Eli Lilly, Biosite.
Footnotes
See also p. 1807.
REFERENCES
- 1.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Strehlow MC, Emond SD, Shapiro NI, et al. National study of emergency department visits for sepsis, 1992 to 2001. Ann Emerg Med. 2006;48:326–331. doi: 10.1016/j.annemergmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Fromm RJ, Gibbs LR, McCallum WG, et al. Critical care in the emergency department: A time-based study. Crit Care Med. 1993;21:970–976. doi: 10.1097/00003246-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Wang HE, Shapiro NI, Angus DC, et al. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 6.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1677. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman JE, Kramer A. Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 9.Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2. Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Gall JR, Klar J, Lemeshow S, et al. The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA. 1996;276:802–810. doi: 10.1001/jama.276.10.802. [DOI] [PubMed] [Google Scholar]
- 11.Higgins TL, Teres D, Copes WS, et al. Assessing contemporary intensive care unit outcome: An updated Mortality Probability Admission Model (MPMO-III) Crit Care Med. 2007;35:827–835. doi: 10.1097/01.CCM.0000257337.63529.9F. [DOI] [PubMed] [Google Scholar]
- 12.Jones AE, Fitch MT, Kline JA. Operational performance of validated physiologic scoring systems for predicting in-hospital mortality among critically ill emergency department patients. Crit Care Med. 2005;33:974–978. doi: 10.1097/01.ccm.0000162495.03291.c2. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro NI, Wolfe RE, Moore RB, et al. Mortality in Emergency Department Sepsis (MEDS) score: A prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31:670–675. doi: 10.1097/01.CCM.0000054867.01688.D1. [DOI] [PubMed] [Google Scholar]
- 14.Sankoff JD, Goyal M, Gaieski DF, et al. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS) Crit Care Med. 2008;36:421–426. doi: 10.1097/01.CCM.0B013E3181611F6A0. [DOI] [PubMed] [Google Scholar]
- 15.Jones AE, Saak K, Kline JA. Performance of the mortality in ED sepsis score for predicting hospital mortality among patients with severe sepsis and septic shock. Am J Emerg Med. 2008;26:689–692. doi: 10.1016/j.ajem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro N, Howell MD, Bates DW, et al. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006;48:583–590. doi: 10.1016/j.annemergmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Jones AE, Brown MD, Trzeciak S, et al. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: A meta-analysis. Crit Care Med. 2008;36:2734–2739. doi: 10.1097/CCM.0b013e318186f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 20.Jones AE, Focht A, Horton JM, et al. Prospective external validation of the clinical effectiveness of an emergency department-based early goal directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandharipande PP, Sanders N, Jacques P, et al. Calculating SOFA scores when arterial blood gasses are not available: Validating SpO2/FIO2 ratios for imputing PaO2/FIO2 ratios in the SOFA scores. Crit Care Med. 2006;34:A1. [Google Scholar]
- 22.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 23.Efron B, Tibshirani R. Improvements on cross-validation: The .632+ bootstrap method. J Am Stat Assoc. 1997;92:548–560. [Google Scholar]
- 24.Jones AE, Stiell IG, Nesbitt LP, et al. Non-traumatic out-of-hospital hypotension predicts inhospital mortality. Ann Emerg Med. 2004;43:106–113. doi: 10.1016/j.annemergmed.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Jones AE, Yiannibas V, Johnson CL, et al. Emergency department hypotension predicts sudden unexpected in-hospital mortality: A prospective cohort study. Chest. 2006;130:941–946. doi: 10.1378/chest.130.4.941. [DOI] [PubMed] [Google Scholar]
- 26.Armitage P, Berry G. Statistical Methods in Medical Research. Third Edition Blackwell Scientific; Oxford: 1994. [Google Scholar]
- 27.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 28.Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999;25:686–696. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen HB, Banta J, Cho T, et al. Mortality predictions using current physiologic scoring systems in patients meeting criteria for early goal-directed therapy and the severe sepsis resuscitation bundle. Shock. 2008;30:23–28. doi: 10.1097/SHK.0b013e3181673826. [DOI] [PubMed] [Google Scholar]
- 30.Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med. 2005;33:2194–2201. doi: 10.1097/01.ccm.0000182798.39709.84. [DOI] [PubMed] [Google Scholar]