Colorectal cancer (CRC) is one of the most common malignancies in the United States. In 2002, it is estimated that 148,300 new cases of CRC will be diagnosed, resulting in approximately 56,600 deaths.1 The latter renders CRC the second leading cause of deaths from cancer in the United States. An individual’s life-time risk of developing CRC is 6%, with greater than 90% of cases occurring after age 50.2 Together, these facts indicate that CRC is an important health concern.
It has been recognized for some time that cancer is the result of sequential mutations in key genes, such as oncogenes and tumor-suppressor genes. The existence of hereditary predisposition to certain cancers including CRC often paves the foundation for identifying genes whose mutations are involved in the formation of that particular cancer. Thus, the identification of the adenomatous polyposis coli (APC) gene as the gene mutated in the germline DNA of patients affected by familial adenomatous polyposis (FAP) was heralded as a major breakthrough in understanding the molecular pathogenesis of CRC.3,4 Indeed, since its identification in 1991, there have been over 2000 studies published on APC. These studies demonstrate that mutations in the APC gene are not only important for the pathogenesis of FAP, but are responsible for the majority (>80%) of sporadic cases of CRC.5 They also indicate that mutations in APC occur early in the tumorigenic process, netting it the name “gatekeeper.”6
APC turns out to be a remarkably fascinating gene. It encodes a large protein consisting of 2843 amino acids and has a predicted molecular weight of greater than 300,000 kilodaltons. It is present in a variety of epithelial tissues, usually in cells that are postmitotic.7 Immunocytochemical studies show that APC is often diffusely distributed in the cytoplasm, although it can sometimes be found in the apical or lateral regions of epithelial cells.7 Studies indicate that APC participates in a variety of cellular functions including proliferation, differentiation, apoptosis, adhesion, migration, and chromosomal segregation.8 To date, the question remains: Which of these functions is critically involved in the tumorigenic process when APC is mutated?
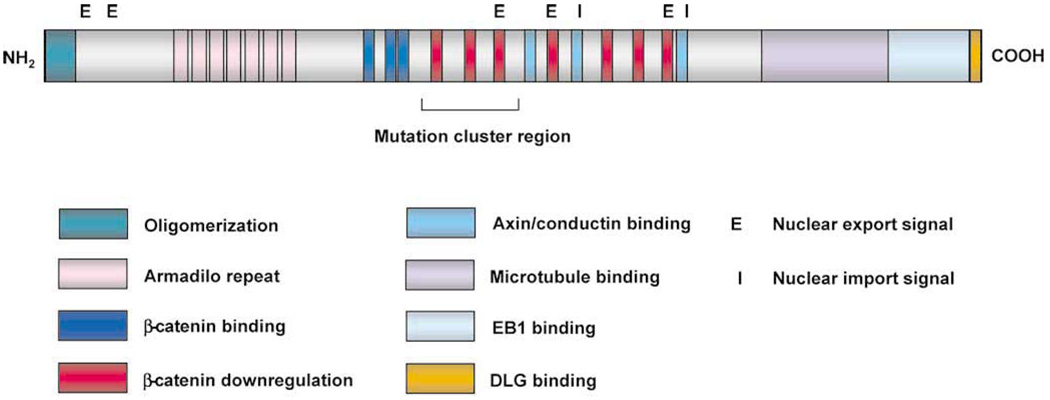
A glimpse to the answer may be provided by knowing which proteins interact with APC. Figure 1 shows the various domains within APC that interact with other proteins. The armadillo repeat in the N terminal portion, for example, binds to the B56 regulatory subunit of protein phosphatase 2A and APC-stimulated guanine nucleotide exchange factor.8 These 2 proteins may be involved in the Wnt signaling pathway, of which APC is a component.9,10 Another important domain includes three 15-amino acid repeats that bind β-catenin and seven 20-amino acid repeats that are required for the down-regulation of β-catenin.11,12 Sites in APC have also been identified that interact with axin and conductin,13,14 2 inhibitory proteins of the Wnt signaling pathway. The C terminal portion of the protein is involved in binding to microtubules and a tubulin-binding protein, EB1.15 Lastly, APC contains both nuclear export and import signals,16,17 suggesting that shuttling protein partners between the nucleus and cytoplasm may be a function of APC.
Figure 1.
The different domains of the APC protein.
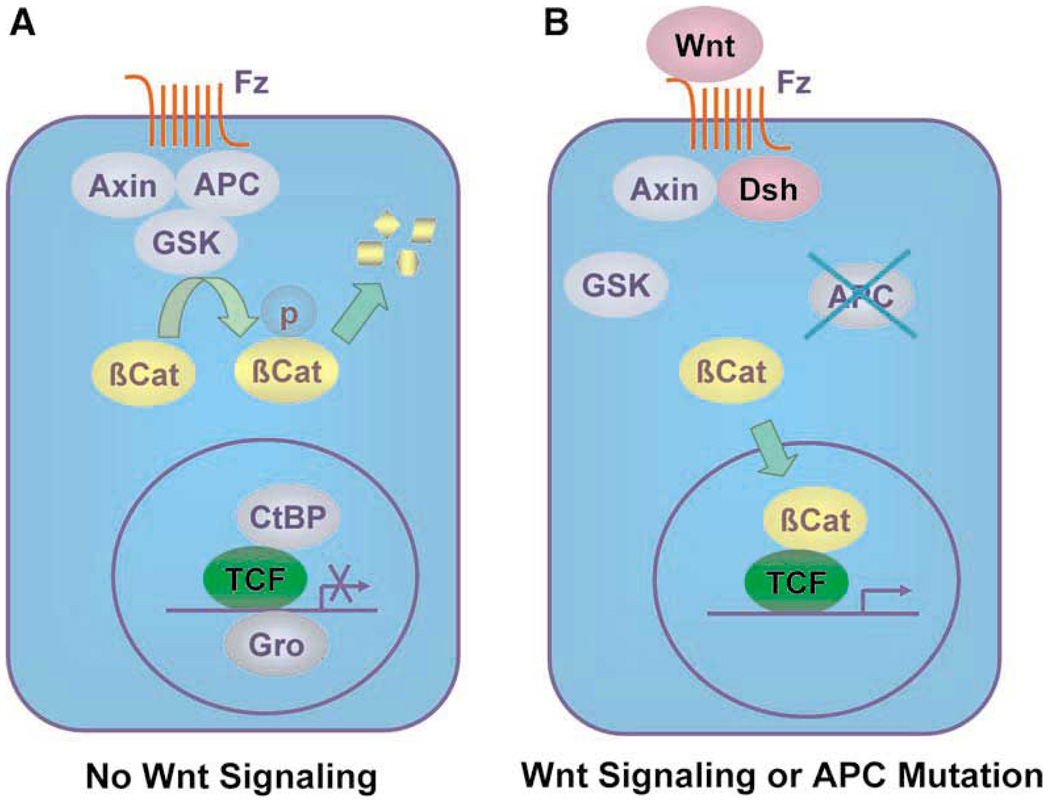
Mutational analysis of the APC gene indicates that the majority of germline mutations found in FAP patients are nonsense mutations, leading to the formation of a truncated protein.4 More than 60% of the APC mutations are found in the central region (between codons 1284–1580) of the protein, which is called the mutation cluster region (MCR).18 The fact that MCR coincides with the region in APC important for down-regulation of β-catenin suggests that this function is important for the pathogenesis of CRC. Subsequent studies demonstrate that APC and β-catenin are important parts of the Wnt signaling pathway. As seen in Figure 2A, cells are quiescent in the absence of Wnt signaling. This is caused by the interaction among axin/conductin, APC and glycogen synthase kinase 3β (GSK) targets β-catenin for degradation by the ubiquitination pathway.19 In the absence of free β-catenin, gene transcription directed by the T-cell factors (TCFs) is silenced by repressors such as CtBP and Gro. Upon binding of Wnt to its member receptor frizzled, a protein called “disheveled” is activated.20 This leads to the dissociation of the complex formed by axin/conductin, APC and GSK, resulting in the accumulation of free cytoplasmic β-catenin (Figure 2B). β-catenin is then translocated to the nucleus where it forms a complex with TCFs,21 resulting in the activation of gene transcription and subsequent cell proliferation. Mutations in APC have the same effect as Wnt signaling in destabilizing the axin/APC/GSK complex. Consequently, mutations in axin or conductin have been found in cancers.22,23 Alternatively, mutations resulting in the constitutive activation of β-catenin no longer subject to the inhibitory effect of axin/APC/GSK have also been identified in CRC in the absence of APC mutation.24
Figure 2.
The signaling pathways in (A) the absence and (B) presence of Wnt.
How does the β-catenin/TCF complexinduce cell proliferation? This, in large part, depends on the target genes regulated by this transcriptional complex. In this issue of Gastroenterology, Heinen et al.25 reports that a mechanism by which APC inhibits cell proliferation is to control entry into the S phase of the cell cycle by regulating β-catenin/TCF. This study stems from the previous observations that the oncogene c-MYC and the cell cycle regulator cyclin D1 are 2 direct targets of the β-catenin/TCF complex.26,27 Both c-MYC and cyclin D1 are involved in the transition between the G1-S checkpoint of the cell cycle and do so by influencing the activity of the retinoblastoma tumor-suppressor pRB.28 Using primarily transient transfection, Heinen et al.25 showed that colon cancer cells transfected with wild-type APC became arrested in the G1 phase of the cell cycle. This cell cycle arrest was abrogated by cotransfection with either a constitutively activated β-catenin or c-MYC and cyclin D1 combined. Moreover, they showed that APC inhibits pRB phosphorylation (thus inhibiting the release of E2F from pRB) and decreases levels of cyclin D1. They conclude that APC functions upstream of pRB and that both c-MYC and cyclin D1 are involved in APC-mediated regulation of the G1/S boundary of the cell cycle.
Is the cell cycle effect of APC reported by Heinen et al.25 sufficient in providing an explanation for the formation of CRC when APC is mutated? To address this question, one needs to consider what steps a cell must take in order for it to become cancerous. There is a minimum of 2 requirements: the cell must acquire a selective advantage to allow for its initial clonal expansion and genetic instability to allow for mutations in other genes responsible for the process of malignant transformation. The latter behavior constitutes a common phenotype in CRC called chromosomal instability (CIN). CIN tumors have a defect in chromosomal segregation, which is manifested by both qualitative and quantitative variations in chromosome numbers.29 Recent studies have shown that APC mutations can give rise to chromosomal instability30,31 and may elicit the CIN phenotype in synergy with other potent oncoproteins or tumor suppressors. This effect is likely dependent on the interaction between APC and EB1 and possibly between APC and microtubules. Combining the results of study by Heinen et al.25 and the CIN studies, APC seems to fulfill both requirements cited above for malignant transformation.
The importance of the cell cycle checkpoint function of APC during tumor formation can also be revealed by studying mouse models of CRC in which mice with various germline mutations in the APC gene have been generated. The classic mouse model is the APCMin mouse.32 APCMin carries a nonsense mutation in codon 850 of the APC gene and no longer contains the region required to regulate β-catenin. Heterozygous APC+/Min animals develop numerous adenomas in their intestinal tract. A second model has since been developed that involves a truncating mutation at codon 1638 of the APC gene (APC1638T).33 The APC1638T mutation truncates the C terminal portion of APC responsible for CIN-related functions but retains the β-catenin regulatory domain. Consequently, embryonic stem (ES) cells isolated from homozygous APC1638T animals are CIN.31 However, the corresponding mice are viable and tumorfree. These observations underscore the importance of selective advantage provided by the loss of β-catenin control in tumor formation and argues against the ability of chromosomal instability to initiate the transformation process.
The findings by Heinen et al.25 also carry potential therapeutic implications. If the checkpoint function of APC is involved in the initiation of CRC, therapies directed toward restoring the β-catenin down-regulatory activity of APC or inhibiting the overactive β-catenin may be used in the prevention of CRC, especially in high-risk individuals such as FAP patients. In contrast, one may wish to target the CIN-related function of APC in later stages of tumorigenesis. Additional studies will help differentiate the effectiveness of these 2 strategies.
Acknowledgments
Supported by grants from the National Institutes of Health (DK52230 and CA84197).
References
- 1.American Cancer Society. Cancer Facts & Figures 2002. http://www.cancer.org/eprise/main/docroot/stt/stt_0.
- 2.Burt RW. Colon cancer screening. Gastroenterology. 2000;119:837–853. doi: 10.1053/gast.2000.16508. [DOI] [PubMed] [Google Scholar]
- 3.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, McPherson J, Wasmuth J, LePaslier D, Abderrahim H, Cohen D, Leppert M, White R. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 4.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finniear R, Markham A, Groffen J, Boguski MS, Altschul SF, Horii A, Ando H, Miyoshi Y, Miki Y, Nishisho I, Nakamura Y. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 5.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 6.Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science. 1998;280:1036–1037. doi: 10.1126/science.280.5366.1036. [DOI] [PubMed] [Google Scholar]
- 7.Midgley CA, White S, Howitt R, Save V, Dunlop MG, Hall PA, Lane DP, Wyllie AH, Bubb VJ. APC expression in normal human tissues. J Pathol. 1997;181:426–433. doi: 10.1002/(SICI)1096-9896(199704)181:4<426::AID-PATH768>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.van Es JH, Giles RH, Clevers HC. The many faces of the tumor suppressor gene APC. Exp Cell Res. 2001;264:126–134. doi: 10.1006/excr.2000.5142. [DOI] [PubMed] [Google Scholar]
- 9.Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, Higuchi O, Akiyama T. Asef, a link between the tumor suppressor APC and G-protein signaling. Science. 2000;289:1194–1197. doi: 10.1126/science.289.5482.1194. [DOI] [PubMed] [Google Scholar]
- 11.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 12.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 13.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 14.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 15.Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- 16.Neufeld KL, Nix DA, Bogerd H, Kang Y, Beckerle MC, Cullen BR, White RL. Adenomatous polyposis coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm. Proc Natl Acad Sci U S A. 2000;97:12085–12090. doi: 10.1073/pnas.220401797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat Cell Biol. 2000;2:653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 19.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 21.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 22.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 23.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 25.Heinen CD, Heppner Goss K, Cornelius JR, Babcock GF, Knudsen ES, Kowalik T, Groden J. The APC tumor suppressor controls entryinto S-phase through its ability to regulate the cyclin D/Rb pathway. Gastroenterology. 2002;123:751–763. doi: 10.1053/gast.2002.35382. [DOI] [PubMed] [Google Scholar]
- 26.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 27.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 28.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 29.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 31.Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, van Es JH, Breukel C, Wiegant J, Giles RH, Clevers H. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 32.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 33.Smits R, Kielman MF, Breukel C, Zurcher C, Neufeld K, Jagmohan-Changur S, Hofland N, van Dijk J, White R, Edelmann W, Kucherlapati R, Khan PM, Fodde R. Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 1999;13:1309–1321. doi: 10.1101/gad.13.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]