Abstract
One hypothesis that couples infection with autoimmune disease is molecular mimicry. Molecular mimicry is characterized by an immune response to an environmental agent that cross-reacts with a host antigen, resulting in disease1,2. This hypothesis has been implicated in the pathogenesis of diabetes, lupus and multiple sclerosis (MS)1–4. There is limited direct evidence linking causative agents with pathogenic immune reactions in these diseases. Our study establishes a clear link between viral infection, autoimmunity and neurological disease in humans. As a model for molecular mimicry, we studied patients with human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP), a disease that can be indistinguishable from MS (refs. 5–7). HAM/TSP patients develop antibodies to neurons8. We hypothesized these antibodies would identify a central nervous system (CNS) autoantigen. Immunoglobulin G isolated from HAM/TSP patients identified heterogeneous nuclear ribonuclear protein-A1 (hnRNP-A1) as the autoantigen. Antibodies to hnRNP-A1 cross-reacted with HTLV-1-tax, the immune response to which is associated with HAM/TSP (refs. 5,9). Immunoglobulin G specifically stained human Betz cells, whose axons are preferentially damaged7. Infusion of autoantibodies in brain sections inhibited neuronal firing, indicative of their pathogenic nature. These data demonstrate the importance of molecular mimicry between an infecting agent and hnRNP-A1 in autoimmune disease of the CNS.
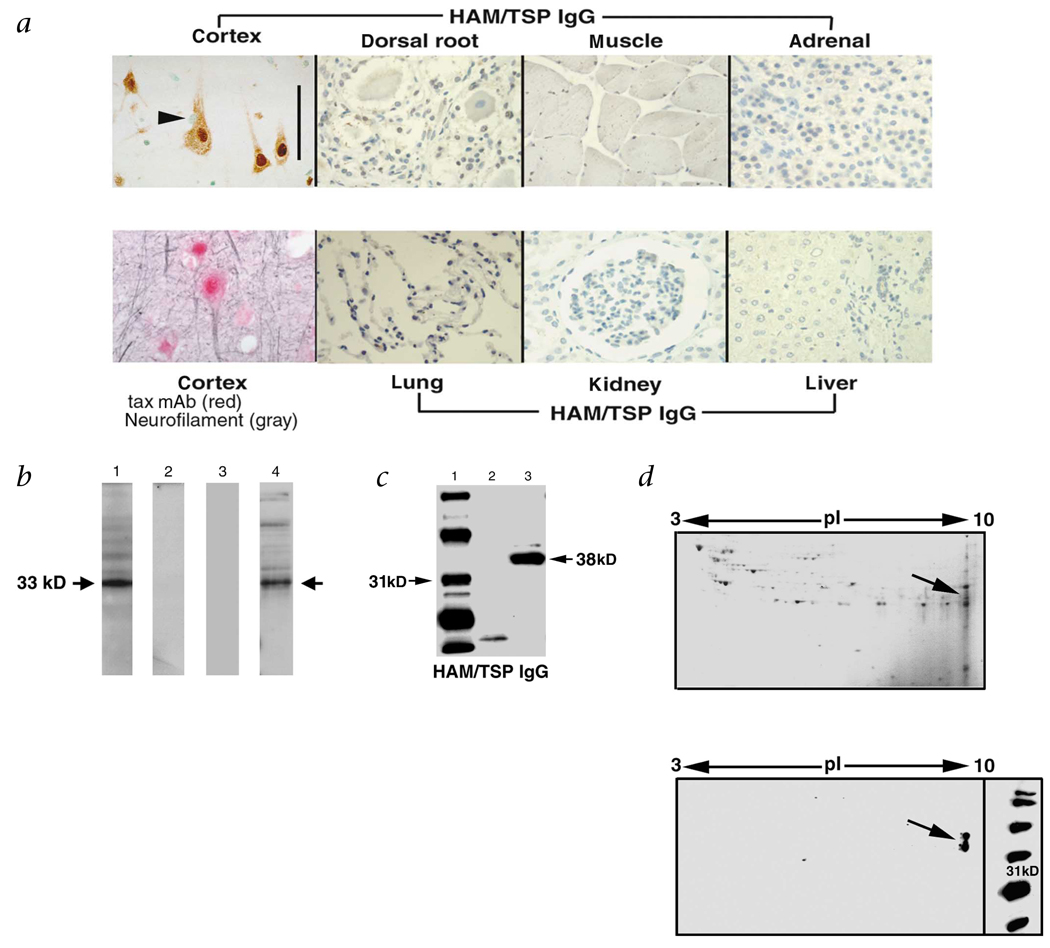
To test for molecular mimicry between an environmental agent and the central nervous system (CNS), we isolated immunoglobulin G (IgG) from the serum of patients with human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and tested it for reactivity with human tissues (Fig. 1a). There was intense staining of neurons in brain and no staining of glia, dorsal root ganglion (peripheral nervous system) or systemic organs. A monoclonal antibody to HTLV-1-tax (tax mAb) mimicked IgG staining of neurons. To identify the protein, cortical neurons were isolated, proteins extracted and subjected to SDS–PAGE and western-blot analysis. The IgG recognized a band of approximately 33 kD (Fig. 1b), whereas IgG isolated from controls did not. Importantly, the tax mAb that stained CNS neurons reacted with the antigen. All patients with HAM/TSP (13/13) developed antibodies recognizing the neuronal antigen8. Nine of ten HTLV-1-seropositive patients without neurological symptoms and 12 HTLV-1-seronegative controls showed no reactivity (P < 0.0001 versus HAM/TSP)8. Clinically, the HAM/TSP patients presented with progressive neurological disease in which corticospinal tract damage (weakness, spasticity and pathological reflexes) predominated6. Many of our patients were originally diagnosed with MS. In fact, one of our patients was diagnosed with MS for 20 years before HTLV-1 testing10.
Fig. 1.
Specificity of HAM/TSP IgG for CNS neurons and isolation of the neuronal antigen. a, Immunohistochemistry of human tissues. Biotinylated HAM/TSP IgG stained CNS neurons (cortex, brown reaction product) (scale bar, 100 µm). There was no staining of glia (arrowhead), dorsal root ganglion or systemic organs. A tax mAb mimicked HAM/TSP IgG staining indicated by the red stain. Neurofilament (gray) confirmed localization of tax mAb to neurons. b, Western blots of proteins derived from CNS neurons. HAM/TSP IgG showed an intense signal at 33 kD (lane 1). There was no reactivity using IgG isolated from HTLV-1 seronegative (lane 2) or HTLV-1 seropositive, neurologically asymptomatic individuals (lane 3). The tax mAb recognized the 33-kD neuronal antigen (lane 4). c, Purification and western blots of the neuronal protein following high salt extraction and centrifugation. Molecular weight markers (lane 1), precipitate (lane 2) and supernatant (lane 3). HAM/TSP IgG reacted with the supernatant (lane 3), but not the precipitate (lane 2). d, 2-D gel electrophoresis and western blot of the neuronal protein purified from the supernatant. Coomassie stain of gel (left). Western blot of the neuronal extract (right), HAM/TSP IgG reacted at 33–38 kD, pI = 9.3 (arrow).
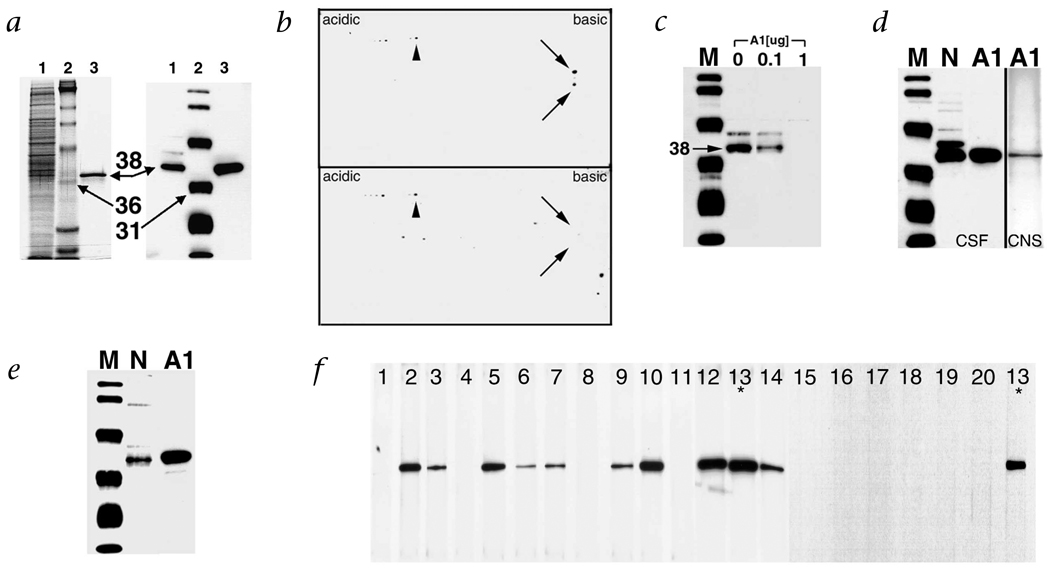
To establish a direct link between the immune response to HTLV-1 and the neurological disease, we purified the proteins recognized by the immune sera. Neuronal proteins were extracted under high salt conditions, separated by centrifugation and subjected to SDS–PAGE followed by western-blot analysis. Under these conditions, HAM/TSP IgG reacted with an antigen of 38 kD (Fig. 1c). Thus, depending on the extraction conditions, either a 33- or 38-kD autoantigen was recognized. Subsequently, the supernatant was subjected to two-dimensional (2-D) gel electrophoresis followed by western blot. HAM/TSP IgG was immunoreactive with proteins at a molecular weight of 33 and 38 kD and a pI of 9.3 (Fig. 1d). The proteins were dissected from 2-D gels, digested with trypsin and analyzed by matrix-assisted laser desorption ionization mass spectroscopy11. The molecular weights of the tryptic peptides aligned with hnRNP-A1 and A1B. Approximately 93% of the molecular weights matched these proteins with probability scores as high as 3.13 × 103 (data not shown). Heterogeneous nuclear ribonuclear protein-A1 (hnRNP-A1) and A1B are identical except for an additional 50 amino acids in hnRNP-A1. There were no matches with this unique sequence of hnRNP-A1. These data strongly suggest that HAM/TSP patients had autoimmunity to a common epitope in hnRNP-A1 and A1B. The calculated molecular weight and pI of these proteins approximate what we found by western blot (A1 = 38.8, A1B = 34.2, pI = 9.3). To prove that the immunoreactivity was specific for hnRNP-A1, we cloned and expressed recombinant hnRNP-A1 (rhnRNP-A1) from human brain. HAM/TSP IgG reacted with rhnRNP-A1 and the neuronal extract at an identical molecular weight (Fig. 2a). Pre-incubation of HAM/TSP IgG with rhnRNP-A1 abolished immunoreactivity of the western blots (Fig. 2b and c). This confirms that the protein dissected from the gel was the same protein identified by western blot and that the neuronal antigen recognized by HAM/TSP IgG is hnRNP-A1. IgG isolated from the cerebrospinal fluid (CSF) and brain of a HAM/TSP patient reacted with hnRNP-A1 (Fig. 2d). These data indicate that there is IgG specific for hnRNP-A1 intrathecally and within the brain parenchyma of HAM/TSP patients. Finally, the tax mAb that was immunoreactive with neurons reacted with hnRNP-A1 (Fig. 2e), suggesting molecular mimicry between the epitope recognized by the mAb and hnRNP-A1. The epitope specificity of the mAb includes the C-terminus of tax (tax346–353) (M.C.L., personal communication) which is coincident with the immunodominant epitope in HAM/TSP patients9. Database analysis showed no exact match between tax346–353 (KHFRETEV) and hnRNP-A1. Molecular mimicry due to immunologic cross-reactivity as shown by these and other data may have increased biological significance compared with mimics defined by primary sequences1–3. Importantly, all of the HAM/TSP patients and none of the HTLV-1-seronegative patients reacted with rhnRNP-A1 (Fig. 2f).
Fig. 2.
Immunoreactivity of HAM/TSP IgG with the neuronal extract and hnRNP-A1. a, Left panel, Coomassie staining; right panel, HAM/TSP IgG immunoreactivity by western blot. Neuronal extract (lane 1), molecular weight markers (lane 2) and rhnRNP-A1 (lane 3). rhnRNP-A1 is identified as a 38-kD band that is recognized by HAM/TSP IgG (right panel, third lane) and corresponds to an immunoreactive band in the neuronal extract (right panel, lane 1). b, Reactivity by western blot following 2-D gel electrophoresis of neuronal extract is adsorbed by pre-incubation with rhnRNP-A1. Immunoreactivity of HAM/TSP IgG with neuronal proteins (top panel) was adsorbed by pre-incubation with rhnRNP-A1 (bottom panel, arrows). There was immunoreactivity to other neuronal proteins that did not adsorb with rhnRNP-A1 (arrowheads). M, molecular weight markers; N, neurons; A1, rhnRNP A1. c, Pre-incubation of HAM/TSP IgG with 0, 0.1 and 1.0 µg rhnRNP-A1 followed by western blot of the neuronal extract demonstrated concentration-dependent adsorption of immunoreactivity. d, IgG purified from the CSF and CNS of a patient with HAM/TSP reacted with neurons and rhnRNP-A1. e, The tax mAb reacted with neurons and rhnRNP-A1. f, Screening of patient sera demonstrated 10/10 HAM/TSP patients (numbers 2, 3, 5–7, 9, 10 and 12–14) and 0/10 HTLV-1 seronegative controls (1, 4, 8, 11 and 15–20) reacted with rhnRNP-A1. *, patient 13 was used as a positive control for the assay that tested patients 15–20.
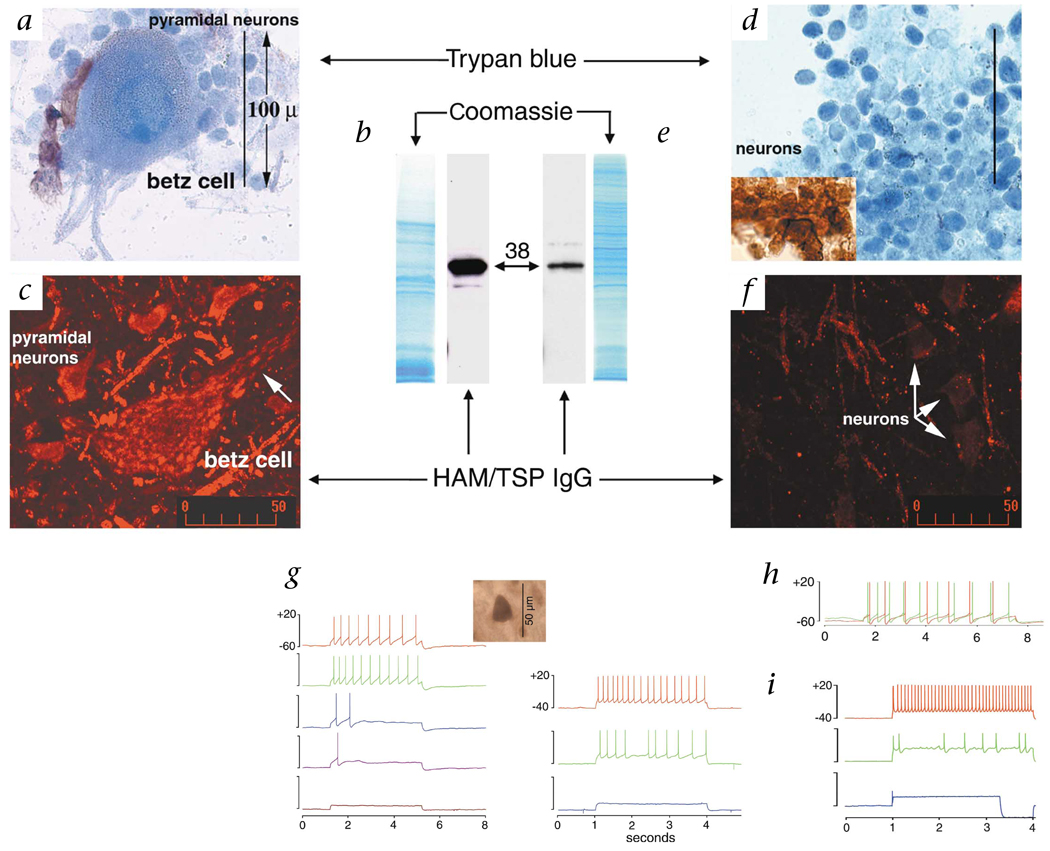
The hnRNPs are a group of approximately 30 nuclear riboproteins associated with systemic, but not neurological, autoimmune diseases. Autoantibodies against hnRNP-A1 and A2/B1 have been found in patients with lupus, mixed connective tissue disease and rheumatoid arthritis12. hnRNP-A1 is critical to transport of mRNA from the nucleus to the cytoplasm and cellular processes13–15. In the CNS, hnRNP-A1 shows greater expression in neurons compared with glia16. We hypothesized that hnRNP-A1 would be highly expressed in large cells of the CNS in which efficient transport of mRNA is critical, such as pyramidal neurons of the precentral gyrus, including Betz cells. These motor neurons control movement and their axons comprise most of the corticospinal tract. Betz cells are the largest neurons in the human CNS with cell bodies that are 60–120 µm in height and axons up to a meter long17. To determine if these cells preferentially bound HAM/TSP autoantibodies, we separated neurons of the precentral gyrus from other areas of brain (Fig. 3). Trypan blue staining confirmed the presence of pyramidal and Betz cells in the pre-central gyrus and cortical neurons in the parietal- occipital lobe (Fig. 3a and d). All cells stained with neurofilament, indicating there was no contamination by non-neuronal cells (Fig. 3d, inset). Western blots of pre-central gyrus neurons including Betz cells, in contrast to smaller cortical neurons, showed an intense signal with HAM/TSP IgG (Fig. 3b and e). This correlated with immunohistochemical staining (Fig. 3c and f). These data suggest that HAM/TSP IgG is specific for neurons of the corticospinal tract, the major target of neurological dysfunction in HAM/TSP.
Fig 3.
Target specificity and biologic activity of HAM/TSP IgG. a–f, CNS neurons were isolated from the precentral gyrus (a–c) and posterior parietal-occipital cortex (d–f). a and d, Trypan blue staining confirmed the presence of large pyramidal neurons and Betz cells (a) compared with typical cortical neurons (d). All cells stained with neurofilament (d, inset). b and e, Coomassie staining and western blots of neuronal proteins. There was an intense HAM/TSP IgG signal from pyramidal neurons derived from the pre-central gyrus that included Betz cells (b), compared with parietal-occipital cortex (e) . Coomassie staining shows comparable protein loading. c and f, Immunohistochemistry of the pre-central gyrus of human motor cortex and of the parietal-occipital cortex. c, A Betz cell is shown surrounded by pyramidal neurons. Staining is present throughout the cytoplasm and extends into neuronal processes (arrow). f, Staining is less intense in parietal-occipital cortex. g–i, Patch clamp recording of neurons in rat brain following extracellular infusion of antibodies (normal CSF - red). g, Left, Physiological concentrations of HAM/TSP IgG (5, 10, 15 and 20 µg/ml, respectively) decreased neuronal firing without affecting calcium (data not shown). HAM/TSP IgG localized to the cytoplasm of neurons (inset). Right, Experiments were replicated using the monospecific antibody to hnRNP-A1 (5 and 10 µg/ml, respectively). h, Application of normal IgG (10 µg/ml; green) showed no slowing in neuronal firing. i, The tax mAb decreased (10 µg/ml; green), then completely inhibited neuronal firing (20 µg/ml; blue).
We have determined that HAM/TSP patients develop antibodies to CNS neurons that cross-react with HTLV-1-tax. The neuronal autoantigen is hnRNP-A1. The distribution of hnRNP-A1 in the CNS corresponds to structures affected in HAM/TSP. These data establish a strong relationship between the autoimmune response and the neurological disease. To provide further proof that the autoimmune reaction contributes to the neurological disease, we used patch-clamp studies to test the ability of autoantibody to alter neuronal function. Here, we studied individual neurons in rat brain sections that maintain interactions with other neurons and non-neuronal cells18. Infusion into the extracellular space with HAM/TSP IgG (at concentrations of IgG in human CSF) completely inhibited neuronal firing (Fig. 3g). HAM/TSP IgG localized only to the neuronal cytoplasm, suggesting that IgG entered the cell, bound hnRNP-A1 and resulted in decreased neuronal firing. A monospecific antibody against hnRNP-A1 purified from HAM/TSP patients replicated this response. These data are consistent with HAM/TSP IgG being biologically active and pathogenic. Infusion of IgG from normal individuals showed no change in neuronal firing (Fig. 3h). Importantly, inhibition was reproduced by the infusion of tax mAb (Fig. 3i), suggesting that biological activity is dependent upon an immunodominant cross-reactive epitope between HTLV-1-tax and hnRNP-A1.
How CNS damage develops in HAM/TSP is incompletely understood. It does not seem to result from direct infection with HTLV-1, but infection associated with an autoimmune mechanism. The immune response to tax differentiates patients with HAM/TSP from controls5,9. Specifically, HAM/TSP patients develop a CD8+ cytotoxic T-lymphocyte (CTL) response specific for the N terminus of HTLV-1-tax (tax11–19 associated with human leukocyte antigen-A2)5 and an antibody response to its C terminus (tax316–353)9. Viral load may be important in stimulating these responses since HAM/TSP patients have elevated viral loads of tax19. In addition to tax, antibodies from HTLV-1 infected patients react with several intracellular autoantigens20. These data suggest that a cross-reactive immune response between HTLV-1-tax and a CNS autoantigen may exist and contribute to the pathogenesis of HAM/TSP. Until now, a specific CNS autoantigen that may act as a target for cellular or antibody-mediated autoimmune responses had not been identified in HAM/TSP patients.
How antibodies enter the CNS and bind to intracellular antigens such as neuronal hnRNP-A1 in autoimmune diseases such as HAM/TSP and MS is not clear. Data indicate that IgG has a significant role in the pathogenesis of these diseases7,8,21. In HAM/TSP, patients are infected with HTLV-1, which is tropic for CD4+ T-lymphocytes7. Infection of CD4+ T-lymphocytes results in their activation and may allow them to cross the blood–brain barrier (BBB)22,23. Interaction of these cells with the CNS results in cytokine expression, adhesion molecule and receptor upregulation, and metalloproteinase secretion22,23. These events are associated with disruption of the BBB after which CTLs and antibodies against tax may enter the CNS (refs. 5–9). Tax-specific antibodies may hone to and enter corticospinal neurons that express high levels of hnRNP-A1. Antibodies can disrupt neuronal function, as suggested by this and other studies24.
Our data show that there is molecular mimicry between HTLV-1 and neuronal hnRNP-A1. This mimicry is likely to be significantly involved in the pathogenesis of HAM/TSP. The data establish a clear link between chronic viral infection, autoimmunity and neurological disease in humans. Recently, investigators have shown that hnRNP-A2, a ribonucleoprotein with significant homology to hnRNP-A1, has a critical role in the transport of myelin basic protein (MBP) mRNA to the processes of oligodendrocytes25. Many studies implicate molecular mimicry involving MBP in the pathogenesis of MS (refs. 1–4). Future studies may find that cellular and humoral autoimmunity to hnRNP-A complexes are involved in the pathogenesis of other immune-mediated diseases of the CNS such as MS.
Methods
IgG isolation and immunohistochemistry
IgG was isolated from serum or CSF by adsorption to protein A-Sepharose (Sigma) and biotinylated (biotin-N-hydroxysuccinimide, Vector, Burlingame, California). Autopsy tissues were obtained from HTLV-1 seronegative individuals. Immunohistochemistry7 was performed on formalin-fixed, paraffin-embedded 5-µm sections. IgG (1:50) was detected with avidin-biotin peroxidase/diamino-benzidine HCl or Texas Red Avidin-D (Vector). Fluorescence was imaged by confocal microscopy (Bio-Rad MRC-1024, Hercules, California). The tax mAb (1:50) was coupled with secondary antibody followed by Alkaline Phosphatase (Vector-Red). Neurofilament was detected with NCL-NF68 antibody (Novocastra, Newcastle-upontyne, UK) and secondary antibody coupled with SG substrate peroxidase or avidin-biotin-peroxidase/diamino-benzidine HCl (Vector). IgG was isolated from HAM/TSP brain21. A monospecific antibody to hnRNP-A1 was produced by purifying HAM/TSP IgG with rhnRNP-A1 bound to sepharose.
Neuronal preparations and western blots
Human cortex was dissected, suspended in Ficoll/sucrose buffer, filtered through nylon mesh and neurons isolated by centrifugation. Whole neurons were mixed with TRYPAN blue, adhered to slides and photographed. For initial SDS–PAGE/western blots, neurons were mixed in reducing buffer and boiled before loading onto 12% SDS–PAGE gels7. To purify the antigen, neurons were subjected to guanidinium hydrochloride (6 M, 125 mM Tris/HCl, pH 6.8) and centrifuged (10,000g). The supernatant was used for subsequent studies. After SDS–PAGE, proteins were transferred to polyvinylidene fluoride (PVDF) membranes for western blotting with biotinylated IgG (1:5,000) and detected with avidin-horseradish peroxidase followed by enhanced chemiluminescence (ECL, Amersham, Piscataway, New Jersey). The tax mAb (1:5,000) was detected with biotinylated-goat anti-mouse antibody and ECL. For adsorption studies, HAM/TSP IgG was incubated with 0, 0.1 or 1.0 µg rhnRNP-A1 prior to western blotting.
2-D gel electrophoresis and purification and mass spectroscopy of dissected proteins
Samples were solubilized in 0.5% (v/v) immobilized pH gradient (IPG) buffer (pH 3–10, Pharmacia) and bromophenol blue. A 17-cm IPG strip was used for isoelectric focusing of 30 µg samples. The first-dimension isoelectric focusing program was as follows: 0 V, 12 h; 500 V, 1 h; 1,000 V, 1 h; and 8,000 V, 4 h. After equilibration in buffer with DTT and iodoacetamide, the second-dimension was done at 40 mA. Gels were stained with Coomassie (Gel-Code Blue Reagent, Pierce, Rockford, Illinois) or electroblotted onto PVDF membranes for western blotting. Spots identified by western blotting were analyzed by in-gel digestion. Excised plugs were washed with NH4HCO3/acetonitrile and dried. Trypsin was added. Supernatant was removed, digestion buffer added, and incubation continued (37 °C, 12 h). The sample was centrifuged and transferred to a tube containing hydrophilic peptides. Peptides were extracted with 60% acetonitrile/0.01% trifluoracetic acid (TFA). Samples were dried, TFA and water were added. Peptides were eluted, purified with ZipTip C18-microcolumns (Millipore, Bedford, Massachusetts) and deposited directly on the MALDI (Matrix-Assisted Laser Desorption Ionization) target. MALDI-time-of-flight mass spectroscopy was used to obtain mass fingerprinting for proteins using a Voyager DE-ER instrument (Applied Biosystems, Framingham, Massachusetts). Spectra were acquired in the delayed extraction (DE), reflectron (R) mode. 100–200 scans were averaged to produce final spectra. The mass scale was calibrated using the masses of trypsin autodigestion products. Protein identification was performed by searching peptide masses in a comprehensive non-redundant protein sequence database (Protein Prospector)11. Primary sequences were compared using Pustell Protein Matrix (MacVector) and Pairwise-Blast, NCBI.
Cloning of human hnRNP-A1
Human brain mRNA was isolated using a commercial kit (Clontech, San Francisco, California). hnRNP-A1 cDNA was prepared by RT-PCR using primers representing the coding region (sense, 5′-GGATCCATGTCTAAGTCAGAGTCTCCTAAA-3′; antisense, 5′-AAGCTTTTAAAATCTTCTGCCACTGCCATA-3′). PCR product size was confirmed by agarose gel electrophoresis. PCR product was excised from the gel, purified, and subcloned into TOPO PCR 2.1 vector (Invitrogen, Carlsbad, California). Positive clones were selected by white/blue screening. Plasmids were purified (Wizard Plus SV Miniprep, Promega, Madison, Wisconsin), subjected to BamHI/HindIII double digestion and cloned into pQE 30 vector (Qiagen, Valencia, California) in which the gene product was expressed with 6 X His tag for purification. The recombinant hnRNP-A1 was expressed by adding IPTG (1 mM) in M15 (pRep4), an E. coli strain (Qiagen), and purified using Nichelating affinity chromatography.
Patch-clamp experiments
300-µm brain slices dissected from Sprague–Dawley rats were prepared in ice-cold artificial cerebrospinal fluid (ACSF). Slices were submerged in the recording chamber and perfused with ACSF, followed by the test antibody (in ACSF). Neurons were visualized with a Photometrics PXL charge-coupled camera and patched with a glass electrode filled with a physiologic intracellular solution. Neurons were held below −50 mV and constant depolarization with 1–10 Hz pacemaker firing (IgG) and below −40 mV and constant depolarization of 5–15 Hz (tax mAb and monospecific ab). Electrode resistance was between 5 and 8 MΩ. Recordings were made in a current clamp mode synchronously with calcium imaging and analyzed by Igor Pro 3.13 (ref. 18).
Acknowledgments
We thank K. Troughton, E. Umstot and J. Berk for technical assistance; C. Raine for autopsy material; S. Jacobson for some of the serum samples; and B. Langton for the HTLV-1-tax monoclonal antibody obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. This material is based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs. This study was funded by the VA Career Development Award and NIH RO1-NS-38876 (to M.C.L.), and NIH RR 10522 and NSF-DBI-9604633 (to D.M.D.).
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Oldstone M. Molecular mimicry and immune mediated disease. FASEB J. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert LJ, Inman RD. Molecular mimicry and autoimmunity. New Engl. J. Med. 1999;341:2068–2074. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- 3.Gran B, Hemmer B, Vergelli M, McFarland H, Martin R. Molecular mimicry and multiple sclerosis: Degenerate T-cell recognition and the induction of autoimmunity. Ann. Neurol. 1999;45:559–567. doi: 10.1002/1531-8249(199905)45:5<559::AID-ANA3>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujinami R, Oldstone M. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: A mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson S, Shida H, McFarlin D, Fauci A, Koenig S. Circulating CD8+ cytotoxic lymphocytes specific for HTLV-1 in patients with HTLV-1 associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 6.Levin M, et al. Immunopathogenesis of HTLV-1 associated neurologic disease based on a spinal cord biopsy from a patient with HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) New. Engl. J. Med. 1997;336:839–845. doi: 10.1056/NEJM199703203361205. [DOI] [PubMed] [Google Scholar]
- 7.Levin M, Jacobson S. HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP): A chronic progressive neurologic disease associated with immunlogically mediated damage to the central nervous system. J. Neurovirol. 1997;3:126–140. doi: 10.3109/13550289709015802. [DOI] [PubMed] [Google Scholar]
- 8.Levin M, et al. Neuronal molecular mimicry in immune mediated neurologic disease. Ann. Neurol. 1998;44:87–98. doi: 10.1002/ana.410440115. [DOI] [PubMed] [Google Scholar]
- 9.Lal R, Giam C, Coligan J, Rudolph D. Differential immune responsiveness to the immunodominant epitopes of regulatory proteins (tax and rex) in human T-cell lymphotropic virus type I associated myelopathy. J. Infect. Dis. 1994;169:496–503. doi: 10.1093/infdis/169.3.496. [DOI] [PubMed] [Google Scholar]
- 10.Kahn R, Bertorini T, Levin M. Neurologic complications of HTLV-1infection. The Neurologist. 2001;7:271–278. doi: 10.1097/00127893-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Beranova-Giorgianni S, Desiderio D. Mass spectrometry of the human pituitary proteome: Identification of selected proteins. Rapid Commun. Mass Spectrom. 2000;14:161–167. doi: 10.1002/(SICI)1097-0231(20000215)14:3<161::AID-RCM859>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Biamonti G, Ghigna C, Caporali R, Montecucco C. Heterogeneous nuclear ribonucleoproteins (hnRNPs): An emerging family of autoantigens in rheumatic diseases. Clin. Exp. Rheumatol. 1998;16:317–326. [PubMed] [Google Scholar]
- 13.Krecic AM, Swanson MS. hnRNP complexes: Composition, structure, and function. Curr. Opin. Cell Biol. 1999;11:363–371. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- 14.Nakielny S, Dreyfuss G. Nuclear export of proteins and RNAs. Curr. Opin. Cell Biol. 1997;9:420–429. doi: 10.1016/s0955-0674(97)80016-6. [DOI] [PubMed] [Google Scholar]
- 15.Shyu A-B, Wilkinson MF. The double lives of shuttling mRNA binding proteins. Cell. 2000;102:135–138. doi: 10.1016/s0092-8674(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 16.Kamma H, Portman DS, Dreyfuss G. Cell type-specific expression of hnRNP proteins. Exp. Cell Res. 1995;221:187–196. doi: 10.1006/excr.1995.1366. [DOI] [PubMed] [Google Scholar]
- 17.Lassek AM. The human pyramidal tract. IV. A study of the mature, myelinated fibers of the pyramid. J. Comp. Neurol. 1942;76:217–225. [Google Scholar]
- 18.Callaway J, Lasser-Ross N, Stuart N, Ross W. Dynamics of intracellular free calcium concentration in the presynaptic arbors of individual barnacle photoreceptors. J. Neurosci. 1993;13:1157–1166. doi: 10.1523/JNEUROSCI.13-03-01157.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagai M, et al. Analysis of HTLV-1 proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-1 carriers: High proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 20.Muller S, et al. IgG Autoantibody response in HTLV-1-infected patients. Clin. Immunol. Immunopathol. 1995;77:282–290. doi: 10.1006/clin.1995.1154. [DOI] [PubMed] [Google Scholar]
- 21.Warren K, Catz I, Steinman L. Fine specificity of the antibody response to myelin basic protein in the central nervous system in multiple sclerosis: The minimal B-cell epitope and a model of its features. Proc. Natl. Acad. Sci. USA. 1995;92:11061–11065. doi: 10.1073/pnas.92.24.11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero I, et al. Interactions between brain endothelial cells and human T-cell leukemia virus type 1 infected lymphocytes: Mechanisms of viral entry into the central nervous system. J. Virol. 2000;74:6021–6030. doi: 10.1128/jvi.74.13.6021-6030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giraudon P, et al. T lymphocytes activated by persistent viral infection differentially modify the expression of metalloproteinases and their endogenous inhibitors, TIMPs, in human astrocytes: Relevance of HTLV-I-induced neurologic disease. J. Immunol. 2000;164:2718–2727. doi: 10.4049/jimmunol.164.5.2718. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Elias RV, Cao W, Lerious V, McGinnis JF. Anti-recoverin antibodies cause the apoptotic death of mammalian photoreceptor cells in vitro. J. Neurosci. Res. 1999;57:706–718. [PubMed] [Google Scholar]
- 25.Munro TP, et al. Mutational analysis of a heterogeneous nuclear ribonucleoprotein A2 response element for RNA trafficking. J. Biol. Chem. 1999;274:34389–34395. doi: 10.1074/jbc.274.48.34389. [DOI] [PubMed] [Google Scholar]