Abstract
In the nucleus of the solitary tract (NTS), electrophysiological responses to taste stimuli representing four basic taste qualities (sweet, sour, salty, or bitter) can be often be discriminated by spike count, but, in units for which the number of spikes is variable across identical stimulus presentations, spike timing (i.e., temporal coding) can also support reliable discrimination. The present study examined the contribution of spike count and spike timing to the discrimination of stimuli that evoke the same taste quality but are of different chemical composition. Responses to between 3 and 21 repeated presentations of two pairs of quality-matched tastants were recorded from 38 single cells in the NTS of urethane-anesthetized rats. Temporal coding was assessed in 24 cells, most of which were tested with salty and sour tastants, using an information-theoretic approach (Victor & Purpura, 1996; 1997). Within a given cell, responses to tastants of similar quality were generally closer in magnitude than responses to dissimilar tastants; however, tastants of similar quality often reversed their order of effectiveness across replicate sets of trials. Typically, discrimination between tastants of dissimilar qualities could be made by spike count. Responses to tastants of similar quality typically evoked more similar response magnitudes but were more frequently, and to a proportionally greater degree, distinguishable based upon temporal information. Results showed that nearly every taste-responsive NTS cell has the capacity to generate temporal features in evoked spike trains that can be used to distinguish between stimuli of different qualities and chemical compositions.
Keywords: temporal coding, Nucleus of the solitary tract, taste, gustation
Introduction
The idea that the temporal characteristics of a taste response may convey information has been confirmed by several studies in recent years. For example, the presence of information conveyed by the temporal characteristics of taste-evoked spike trains has been described in both the brainstem (Di Lorenzo and Victor 2003, 2006) and gustatory cortex (Katz et al. 2001, 2002). In the nucleus of the solitary tract (NTS, the first synaptic relay in the central gustatory pathway), spike timing within the first 2 sec of a taste response was found to convey a significant amount of information about taste quality in about half of the cells tested (53%, 10 of 19; Di Lorenzo and Victor 2003). Stimuli that were used in that study each represented a separate taste quality and were thus at the limits of the perceptual domain of the system. That is, these stimuli were very distinct from one another. While taste stimuli are more diverse in the natural world, they can nevertheless be categorized into groups arranged according to taste qualities (Nowlis et al. 1980). Within these categories, taste stimuli of the same taste quality are more similar to each other than they are to taste stimuli of different qualities. Although we have demonstrated that temporal coding can be used to signal differences among tastants of different qualities, the extent to which temporal coding can also be used to signal both the similarity of tastants of the same taste quality (but different chemical composition, e.g. NaCl and LiCl, both salty) as well as the differences between them is as yet unclear.
In studies of neural coding in the gustatory system spatial theories such as labeled line (e.g. Frank 1973, 2000; Lundy and Contreras 1999; Scott and Giza 1990) and across neuron pattern (ANP; e.g. Doetsch and Erickson 1970; Ganchrow and Erickson 1970; Yamamoto and Yuyama 1987) theories have dominated the literature. These theories are based on the relative “response magnitudes” to a variety of tastants that represent the basic taste qualities (sweet, sour, salty, bitter and umami). This measure is the number of spikes evoked by a given tastant over an interval of time that includes the stimulus presentation (see Di Lorenzo and Lemon 2001 for a discussion). The labeled line theory assumes that each taste-responsive cell encodes stimuli of a single taste quality, typically defined by its “best” or most effective stimulus (reviewed by Spector and Travers 2005). In the ANP theory, the pattern of response magnitudes across the entire population is thought to convey the identity of a stimulus. Responses across cells to tastants that are of similar quality are more highly correlated than responses to dissimilar tastants. Both theories assume that taste-evoked spikes are integrated over the response interval and that the temporal arrangement of spikes within that interval is irrelevant; however, these assumptions may not be true (Di Lorenzo and Victor 2003, 2007; Hallock and Di Lorenzo 2006; Katz et al. 2002).
The use of the response magnitude as a starting point for spatial theories of taste coding is based on the premise that this measure is a reliable index of a cell’s sensitivity to a taste stimulus. Studies directed at examining response variability, however, have challenged this expectation. For example, Ogawa et al. 1973, 1974) have shown that responses in some chorda tympani fibers (CT, a branch of the VIIth nerve innervating taste buds on the rostral 2/3 of the tongue) can vary widely when taste stimulus presentations are repeated. In the NTS of the rat, taste responses in some cells can vary with repetition to such an extent that the best stimulus and the breadth of tuning can change (Di Lorenzo and Victor 2003). Although there are several variables that have been shown to change the best stimulus of taste-responsive NTS cells (Chang and Scott 1984; Di Lorenzo et al. 2003; Di Lorenzo and Monroe 1995; Jacobs et al. 1988; Smith and Li, 2000), variability on a trial-by-trial basis begs the question of how these cells convey an unambiguous message about a taste stimulus on the tongue.
The purpose of the present study was to study the neural coding of tastants of similar quality but different chemical composition in taste-responsive NTS cells in the rat. We first examined average firing rate and how it varied across repeated stimulus presentations. Our hypothesis was that responses to stimuli of similar quality would evoke responses whose firing rates were more tightly clustered than the distribution of firing rates elicited by stimuli of different taste qualities. In particular, we hypothesized that firing rate could be used to discriminate among different taste qualities (e.g., salty and bitter), but not between stimuli of similar taste qualities (e.g., NaCl and LiCl, both salty). The second set of analyses was aimed at quantifying the extent to which temporal coding could be used to distinguish between tastants of similar qualities as well as to differentiate among tastants of different taste qualities. Based on our previous studies (Di Lorenzo and Victor 2003, 2007), our hypothesis was that temporal coding would be used to discriminate among taste stimuli when differences in the number of spikes evoked by each of the tastants was insufficient to identify the tastant. Further, based also on these studies, we predicted that cells with the most variable responses would be more likely to exhibit temporal coding. Since past work has shown that temporal coding was most prominent in the initial portion of the response, we focused our analyses on the first 2 sec of the taste response.
Materials and Methods
Subjects
Thirty-eight male Sprague-Dawley rats (350 g – 450 g) were used in this study. Rats were pair housed with a 12 hr light-dark schedule (lights on at 7:00 a.m.) and given ad libitum access to fresh chow and water. A tasteless plastic tube was placed in the cage for environmental stimulation. Care was maintained as per the requirements of the Institutional Care and Use Committee of Binghamton University.
Surgery
Prior to surgery, rats were anesthetized with urethane (1.4 g/kg i.p. in 2 doses, 20 min apart). Animals received an injection of pentobarbital (Nembutal, 25 mg/kg i.p.) 20 minutes after the second dose of urethane. Supplementary injections of urethane were administered as needed to maintain anesthesia. Glycopyrrolate, a peripheral anticholinergic agent, was administered to facilitate breathing when necessary (Robinul diluted to 10% in isotonic saline, 0.0004 g/kg s.c.). Temperature was maintained between 35 –37° F with an anal thermistor probe connected to a heating pad (FHC, Inc.).
Animals were tracheotomized and placed in ear bars, and the head was held in place 5mm below the interaural line. The skin and fascia were removed to expose the skull, and a nontraumatic head holder was attached to the skull using stainless steel screws and dental cement. The occipital bone and underlying meninges were removed and the posterior cerebellum was gently aspirated to reveal the floor of the fourth ventricle.
Stimuli
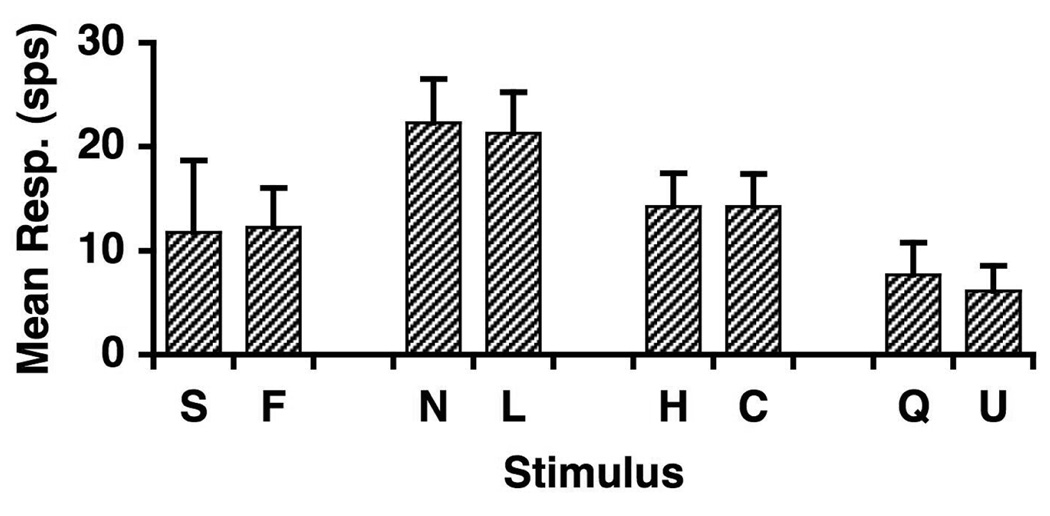
Four pairs of taste stimuli were used in this experiment, each stimulus of a pair predominantly evoking the same taste quality. Stimuli were reagent grade chemicals dissolved in distilled water, and present at room temperature. Pairs of stimuli were as follows: 0.1 M NaCl and 0.1 M LiCl (salty), 0.01 M HCl and 0.01 M citric acid (sour), 0.01 M quinine HCl and 1.0 M urea (bitter), and 0.5 M sucrose and 0.3 M fructose (sweet). The concentrations of the stimuli were both exemplars of a given taste quality evoked similar average response magnitudes across cells. Figure 1 shows the average response magnitudes evoked by each stimulus used in the present study.
Figure 1.
Mean ± SEM of all responses to each tastants tested. Abbreviations: S, sucrose; F, fructose; N, NaCl; L, LiCl; H, HCl; C, citric acid; Q, quinine; U, urea.
Stimulus delivery
Taste stimuli were bathed over the tongue through a specially designed stimulus delivery system. A mouthpiece consisting of six stainless steel tubes (1 mm dia.) with holes along the entire length top and bottom was positioned in the mouth so that the holes were facing the tongue surface and palate. These tubes were connected to pressurized (compressed air, ~ 10 lbs/inch2) reservoirs of tastants with polyethylene tubing through an array of computer-controlled solenoid valves. Each tube was associated with its own fluid reservoir, i.e. taste stimulus or distilled water. When a solenoid valve was actuated, fluid was sprayed over the entire mouth, including the nasoincisor ducts. Fast green dye was delivered to one animal, and its tongue was removed and inspected with an operating microscope in order to ensure that fluid was delivered to the fungiform, foliate, and circumvallate papillae. When the solenoid closed, flow of stimulus stopped without dripping due to the surface tension of the liquid in the tubes. Standard flow rate through this system was 5 ml/s, regulated by a pinch valve positioned on the tube leading from the reservoir to the mouth. Flow rates for all stimuli were calibrated daily prior to each experiment.
After surgery, the ear bars were removed, the animal's mouth opened, and a small weight was attached to the tip of the tongue so that the tongue was extended. The mouthpiece, as described above, was then inserted into the animal's mouth.
Recording and Testing
An etched tungsten microelectrode (18–20 MΩ, 1V @ 1 kHz, FHC Inc.) was slowly lowered into the brainstem above the rostral portion of the NTS, at 2.7mm anterior and 1.8mm lateral to the obex. The signal was amplified (Model P511, Grass Technologies) and monitored with a speaker and an oscilloscope. To locate the taste-responsive portion of the NTS, a solution of 0.1 M NaCl was periodically bathed over the tongue, followed by a distilled water rinse.
Single taste-responsive cells were isolated based on the consistencies of their waveform (see below). A cell's response profile was recorded as its response to presentations of exemplars of the four basic taste qualities. Stimuli were presented in the following order: NaCl, HCl, sucrose, and quinine. Each taste trial consisted of a 10 sec distilled water pre-rinse, 5 sec stimulus presentation, 5 sec pause, and a 20 sec distilled water rinse. Each stimulus delivery occurred at least 2 min after the onset of delivery of the previous stimulus.
Two of the four tastants that were tested initially were selected for further study, based upon the responsiveness of the cell. The tastants selected were usually, but not always, the stimuli that evoked the two most vigorous responses during the initial response profile. In some cases, selection of a “third-best” stimulus for further study rather than a “second-best” stimulus was made to ensure a representative sample of responsivity in our dataset. Given the variability in response magnitudes with repeated stimulus presentations that we have observed (Di Lorenzo and Victor 2003, 2007), the distinction between second-best and third-best was not always obvious and may not be meaningful. For all cells, two tastants were selected from among stimuli representing one of four taste qualities, along with two paired tastants evoking similar qualities to those selected. For example, if NaCl were selected, LiCl, another salty stimulus, would also be tested. Likewise, if HCl were selected, citric acid, another sour tastant, would also be tested. Tastants of similar quality were never presented consecutively.
Histology
At the end of the recording session, a lesion was made by passing DC current (1 mA cathodal, 5 sec) through the recording electrode. Brains were removed and placed in formalin for a minimum of 2 weeks. To verify the location of the lesion in the rostral NTS, the brainstems were cut into 40 µm sections and stained with cresyl violet.
Data Analysis
Electrophysiological responses were digitized with an analogue to a digital interface (Model1401, Cambridge Electronic Designs) and recorded on a computer. Waveforms associated with single cells were identified with template matching and principal component analysis using Spike2 software (Cambridge Electronic Designs). The precise timing of each spike (1 ms resolution) was recorded with respect to the onset of each stimulus delivery (as defined by the time of solenoid activation). A taste response was defined as a cell's average firing rate over the 2 sec of stimulus delivery minus its average firing rate during the 5 sec of water delivery immediately preceding stimulus onset. A response was considered significant when the firing rate during the first 2 sec of stimulus presentation was at least 50% greater than the firing rate during the preceding 5 sec of pre-rinse water presentation. Responses were expressed in spikes/sec (sps).
Variability in a cell's responses to a given tastant was assessed by calculating the coefficient of variation (CV, SD/Mean). Pearson's r was calculated for each cell's responses to all pairs of tastants, similar and dissimilar.
Analysis of temporal patterns of response
To characterize the contribution of the temporal structure of a response to the neural code for taste, spike trains were analyzed by the metric space method of Victor and Purpura (1996, 1997). These analytical methods provide a rigorous way to determine whether the statistics of the precise times of individual spikes, or of the pattern of interspike intervals, have the potential to carry information concerning taste quality. This analysis derives a family of metrics which measure “distance” (i.e., dissimilarity) between spike trains. Each of these metrics represents the “cost” of transforming one spike train into another by changing a different aspect of the spike trains that were compared. These included the number of spikes and the precise timing of spikes. The simplest of this family of metrics represents the difference in the number of spikes contained in two spike trains associated with two responses. To calculate cost in this case, each spike that is either deleted or added incurred a cost of “1”, so that this metric, called Dcount , is simply the arithmetic difference between the number of spikes contained in each response.
To measure the difference between two spike trains in terms of the arrangement of spikes in time requires a definition of how close in time two spikes need to occur to be considered “equivalent”. In the family of metrics described by Victor and Purpura (1996, 1997), the similarity of the timing of spikes, or the sequence of interspike intervals, in two responses is calculated at a variety of levels of precision, measured by a parameter called “q.” The cost of adding or deleting a spike is set at “1” as in Dcount and, in addition, the cost of moving a spike (or interspike interval) by an amount of time t is set at qt where q is in units of 1/sec. The resulting metric for spike timing is called Dspike[q].
Information (H) is calculated from these distances by determining the extent to which responses to each stimulus form distinct clusters (see Victor and Purpura, 1996, 1997 for details). Information is determined independently for a range (0 to 500) of values of q. The value of q at which information is maximized is denoted qmax, and the maximum value of H is denoted Hmax. In the present experiment, the data were analyzed as though each stimulus evoked a separate taste quality, even though the dataset contained responses from two sets of tastants of similar quality. Thus, the maximum possible value of H (in bits) for discrimination of any pair of tastants was 1 (log2 2 = 1). The relative contributions of spike count and spike timing to the information conveyed by taste responses were thus quantified by Hcount (obtained from Dcount , i.e., q = 0) and Hmax (obtained from Dspike[q] at q = qmax).
For some cells, the amount of information conveyed by spike count (Hcount, at q = 0) was at least as large as the amount of information conveyed by the spike timing, (Dspike[q]) at all other values of q (i.e. Hcount ≥ Hmax). For these cells, spike count was said to provide all the information available to distinguish between these stimuli. Alternatively, the rate envelope and/or spike timing pattern contributed to information when Hmax > Hcount. We used Hres, the amount of information conveyed by responses with randomized spike timing but the same rate envelope as the real responses, to distinguish between information contained in the rate envelope and information present in the precise timing of spikes; if Hmax > Hres +2SD and Hmax > Hcount, spike timing significantly contributed information to the taste responses above that contributed by spike count or the rate envelope. The rate envelope was said to provide information for a discrimination when Hmax ≤ Hres +2SD and Hmax > Hcount. The value of q at which Hmax was observed indicated the temporal precision with which spike timing was most significant. An additional control analysis was conducted in which the labels of each response were randomized and the information recalculated, called Hshuff. In the present dataset, these values were uniformly smaller that the information conveyed by the actual responses.
Importantly, two additional analyses served as controls for the possibility of spurious results. These are detailed in Victor and Purpura (1996, 1997). Briefly, in the first of these, called “shuffled,” values of H calculated as described above were compared with values H0 obtained from 10 to 40 surrogate datasets in which the tastants associated with each response were randomly scrambled. This control is necessary since estimates of H have an upward bias, which is conservatively estimated by H0. Only values of H that exceed the range (mean ± 2 SD) of values of H0 were considered to represent better-than-chance classification. In a second analysis, known as “exchange resampling” (Victor and Purpura 1996), surrogate data sets were created by resampling the original data so that responses to each tastant matched the post-stimulus time histograms of the observed responses, and individual responses also had the same number of spikes, but spike train patterns were destroyed. We then compared values of H obtained from the real data with values Hres obtained from the same analysis on 10 to 40 of these resampled datasets. If H was above the range (mean ± 2 SD) of values of Hres, we concluded that the observed temporal coding is not merely due to the average temporal profile of the response to each tastant (with the overall variability in spike count taken into consideration), and that the arrangement of spikes in time in individual trials must play a role in conveying information.
Results
General response characteristics
Responses to between three and 21 presentations (median = 11) of each of four taste stimuli (two exemplars of each of two taste qualities) were recorded from 38 cells. Across all cells the average spontaneous rate was 3.2 ± 0.7 sps. Among the 35 cells that had the initial response profiles available, 20 were identified as NaCl best, 5 as HCl best, 4 as sucrose best and 6 as quinine best. Complete response profiles are not available for 3 cells because of experimenter error. One cell responded equally well to NaCl and sucrose in the initial response profile; this cell was classified as NaCl best because it responded better to salts than sugars on all subsequent trials.
Recording sites were determined for 20 cells. Seven of these (35%) were located within the intermediate subdivision of the NTS, 7 (35%) within the lateral subdivision, 2 (10%) within the medial subdivision, 3 (15%) within the dorsomedial subdivision and 1 (5%) in the dorsolateral subdivision.
Response magnitude varied across trials
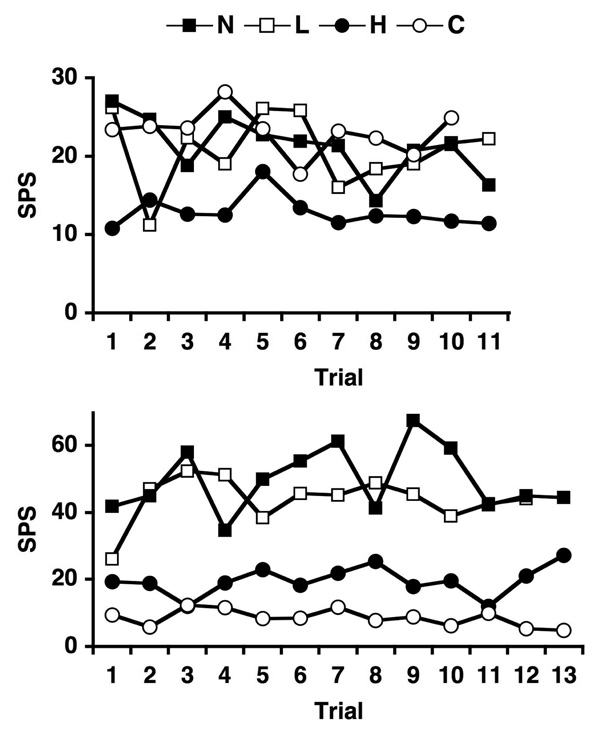
Several observations attest to the variability of response magnitude with stimulus repetition. For example, although most cells showed repeated significant responses throughout the recording session to the stimuli to which they responded initially, 16 cells (of 38; 42%) failed to respond to a given stimulus on at least one trial (Table 1). In addition, across repeated blocks of four trials (consisting of two pairs of tastants of similar qualities) both the order of effectiveness of stimuli of the same quality and that of different qualities changed in the majority of cells. These changes were found more frequently for responses to tastants of the same quality (33 cells with a median of 4 changes in order of effectiveness) compared with responses to tastants of different qualities (15 cells with a median of 2 changes in order of effectiveness). Figure 2 shows examples of both effects in two cells.
Table 1.
Response variability and frequency of significant responses in NTS cells. “CV” is the coefficient of variation (standard deviation / mean response across trials) averaged across tastants for each cell. "Blocks of Trials" is the number of times all four stimuli were presented, i.e. the minimum number of times any single stimulus was presented. "No. of Significant Trials" is the number of times the cell produced a significant response to the presentation of a given stimulus. In some cases there were more significant responses to a stimulus than the number of complete blocks of trials; this occurred when some stimuli were presented more often than others.
| No. of Significant Trials | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell | CV | Trials | NaCl | LiCl | HCl | Citric Acid | Quinine | Urea | Sucrose | Fructose |
| 1 | 0.40 | 21 | 10 | 6 | 16 | 19 | ||||
| 2 | 0.80 | 14 | 14 | 5 | 4 | 3 | ||||
| 3 | 0.49 | 16 | 6 | 3 | 7 | 0 | ||||
| 4 | 0.31 | 12 | 8 | 11 | 1 | 0 | ||||
| 5 | 0.36 | 11 | 5 | 4 | 2 | 2 | ||||
| 6 | 0.46 | 16 | 9 | 7 | 6 | 13 | ||||
| 7 | 0.35 | 13 | 12 | 11 | 10 | 8 | ||||
| 8 | 0.53 | 11 | 12 | 11 | 11 | 11 | ||||
| 9 | 0.56 | 12 | 10 | 9 | 10 | 12 | ||||
| 10 | 0.62 | 15 | 4 | 9 | 7 | 11 | ||||
| 11 | 0.49 | 6 | 10 | 10 | 10 | 10 | ||||
| 12 | 0.28 | 17 | 17 | 17 | 15 | 16 | ||||
| 13 | 0.24 | 11 | 11 | 11 | 12 | |||||
| 14 | 0.30 | 13 | 14 | 12 | 13 | 12 | ||||
| 15 | 0.21 | 10 | 11 | 11 | 11 | 10 | ||||
| 16 | 0.17 | 10 | 11 | 11 | 11 | 11 | ||||
| 17 | 0.14 | 14 | 14 | 15 | 14 | 15 | ||||
| 18 | 0.15 | 10 | 9 | 9 | 10 | 10 | ||||
| 19 | 0.17 | 14 | 13 | 12 | 14 | 14 | ||||
| 20 | 0.20 | 12 | 12 | 12 | 12 | 12 | ||||
| 21 | 0.21 | 12 | 12 | 12 | 11 | 12 | ||||
| 22 | 0.59 | 10 | 9 | 9 | 7 | 7 | ||||
| 23 | 0.11 | 11 | 10 | 10 | 9 | 10 | ||||
| 24 | 0.41 | 10 | 11 | 11 | 10 | 5 | ||||
| 25 | 0.23 | 11 | 11 | 11 | 10 | 10 | ||||
| 26 | 0.26 | 13 | 14 | 13 | 13 | 13 | ||||
| 27 | 0.30 | 8 | 8 | 8 | 3 | 1 | ||||
| 28 | 0.20 | 8 | 8 | 7 | 8 | 7 | ||||
| 29 | 0.08 | 3 | 3 | 3 | 3 | 2 | ||||
| 30 | 0.13 | 4 | 4 | 4 | 3 | 3 | ||||
| 31 | 0.29 | 5 | 5 | 5 | 3 | 4 | ||||
| 32 | 0.32 | 3 | 3 | 3 | 3 | 3 | ||||
| 33 | 0.66 | 9 | 9 | 2 | 6 | 3 | ||||
| 34 | 0.62 | 10 | 9 | 9 | 0 | 3 | 0 | |||
| 35 | 0.18 | 5 | 2 | 5 | 5 | |||||
| 36 | 0.19 | 6 | 6 | 6 | 4 | 2 | ||||
| 37 | 0.18 | 7 | 7 | 7 | 7 | 7 | ||||
| 38 | 0.25 | 8 | 5 | 1 | 2 | 1 | ||||
Figure 2.
Response rate (sps) across trials for salty and sour tastants in two cells. Abbreviations are as follows: N, NaCl; L, LiCl; H, HCl; C, citric acid. Top graph shows a cell with responses to different qualities (salty and sour) that reverse their order of effectiveness on different blocks of trials. Bottom shows a cell with responses to the same taste quality (salty) that reverse their order of effectiveness on different blocks of trials.
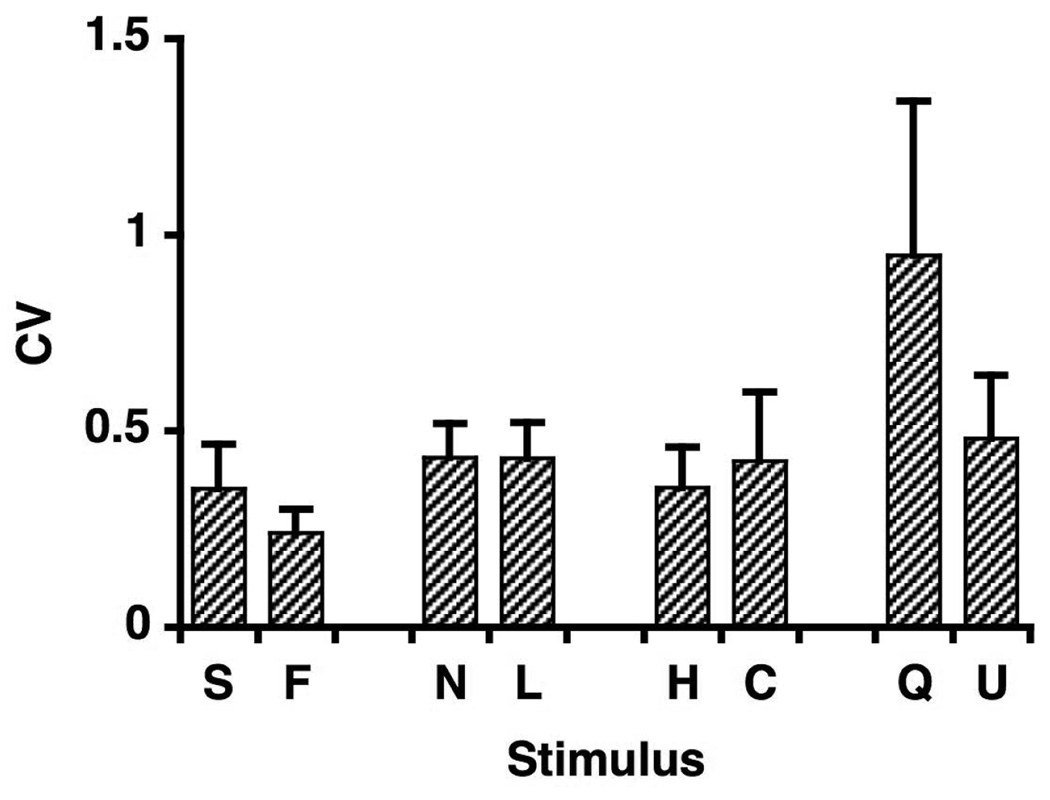
The coefficient of variation (CV; SD/mean), a measure of variability, was used to evaluate the stability of each cell’s response to each stimulus across trials. The average CV of all 2 sec responses, whether significant or not, to all tastants was 0.43 ± 0.05 SEM. Figure 3 shows the mean CV ± SEM for each stimulus. (Only stimuli that evoked at least 3 significant responses were included.) There were no significant differences in CVs across stimuli (one-way ANOVA, F7,132 = 0.91, p < 0.50), suggesting that responses to all stimuli were equally variable across repetitions. Even when responses to stimuli of the same quality were pooled, there were no significant differences in CV according to taste quality (one-way ANOVA, F3,136 = 1.51, p < 0.22). The fact that the CV did not differ across tastants reflects, in part, the strong correlation of the mean response to a given stimulus within a cell with the standard deviation of responses across trials (r = 0.69, p < 0.01). This correlation was equally strong when responses to individual taste stimuli were examined, with the exception of the sweet tastants where the sample was small (n = 5). These results suggest that stronger responses showed larger standard deviations; however, the significant negative correlation between the mean response and the CV (r = −0.33, p < 0.001) suggests that stronger responses were less variable than weaker ones.
Figure 3.
Graph of the mean ± SEM coefficient of variation (CV, standard deviation divided by the mean) across cells for each stimulus. Only those cells that showed at least three significant responses were included. Abbreviations and numbers of cells included were as follows: S, sucrose, n = 5; F, fructose, n = 5; N, NaCl, n = 35; L, LiCl, n = 33; H, HCl, n = 25; C, citric acid, n = 21; Q, quinine, n = 8; U, urea, n = 5.
Although the mean response magnitudes across cells for NaCl, LiCl, HCl and citric acid were all quite similar (see Figure 1), it was still possible that response magnitudes for these tastants might differ considerably within a particular cell. To assess this possibility, the mean of the absolute value of the difference between each pair of tastants across blocks of trials was calculated for each cell. These values were then averaged across cells. Because there were relatively few cells tested with sweet and bitter tastants, only those cells that were tested with both salty and sour tastants were used (n = 24 cells). The results, shown in Table 2, show that tastants of similar qualities evoke more similar response magnitudes within cells than tastants of dissimilar qualities. A one-way ANOVA of these differences revealed a main effect of paired tastant (F5,113 = 2.424, p < 0.04), but Newman-Keuls pairwise tests showed no significant differences. However, when comparisons across tastants of similar qualities (NaCl-LiCl and HCl-citric acid) were pooled and compared with all other comparisons as a group, differences between tastants of similar and dissimilar tastants were evident (Student’s t test, p < 0.001).
Table 2.
Differences (sps) between responses to salty and sour tastants within cells.
| Mean ± SEM | Median | Minimum | Maximum | |
|---|---|---|---|---|
| Similar taste quality | ||||
| NaCl vs. LiCl | 5.5 ± 1.0 | 3.6 | 1.2 | 20.9 |
| HCl vs. Citric Acid | 7.4 ± 1.4 | 6.8 | 0.7 | 23.5 |
| Dissimilar taste quality | ||||
| NaCl vs. HCl | 17.1 ± 4.5 | 10.0 | 0.9 | 78.0 |
| NaCl vs. Citric Acid | 16.9 ± 4.8 | 8.4 | 0.9 | 80.0 |
| LiCl vs. HCl | 16.0 ± 4.1 | 8.5 | 0.6 | 64.3 |
| LiCl vs. Citric Acid | 16.2 ± 4.0 | 11.9 | 1.0 | 59.6 |
We next examined correlations between mean firing rates elicited by different tastants. To do this, we calculated Pearson’s correlation coefficients. Table 4 shows the results of these analyses. Table 3A shows that responses to both similar and dissimilar stimuli did not covary over time; within-cell correlations therefore provide no indication that as a group they detect similarity between the two salty and the two sour tastants, as might be indicated by larger interstimulus correlations between similar-tasting stimuli. It should be noted, however, that the interval between stimulus presentations was two minutes for dissimilar stimuli, and four minutes for similar stimuli; fluctuations over shorter periods of time could not be detected with our experimental design. Median correlations across tastants were also quite low. Across cells, correlations between average responses (across trials) to similar tastants were greater than correlations between dissimilar tastants, though all interstimulus correlations were high, as seen in Table 3B.
Table 4.
Information conveyed by spike count and spike timing for comparisons of salty and sour tastants. Numbers are mean ± SEM.
| Hcount | Hmax | Hmax-Hcount | |
|---|---|---|---|
| Similar taste quality | |||
| NaCl vs. LiCl | 0.087 ± 0.041 | 0.370 ± 0.059 | 0.284 ± 0.054 |
| HCl vs. Citric Acid | 0.302 ± 0.063 | 0.583 ±0.085 | 0.281 ± 0.060 |
| Dissimilar taste quality | |||
| NaCl vs. HCl | 0.540 ± 0.098 | 0.718 ± 0.078 | 0.178 ± 0.067 |
| NaCl vs. Citric Acid | 0.425 ± 0.091 | 0.737 ± 0.074 | 0.312 ± 0.076 |
| LiCl vs. HCl | 0.533 ± 0.079 | 0.712 ± 0.059 | 0.178 ± 0.040 |
| LiCl vs. Citric Acid | 0.506 ± 0.102 | 0.723 ± 0.075 | 0.217 ± 0.075 |
Table 3.
Interstimulus correlations
| A. Interstimulus correlations within cells for salty and sour tastants (n = 23 cells) across blocks of trials. | ||||
|---|---|---|---|---|
| Range | ||||
| Mean ± SEM | Median | Minimum | Maximum | |
| Similar taste quality | ||||
| NaCl vs. LiCl | 0.18 ± 0.07 | 0.16 | −0.36 | 0.86 |
| HCl vs. Citric Acid | 0.06 ± 0.08 | 0.11 | −.72 | 0.67 |
| Dissimilar taste quality | ||||
| NaCl vs. HCl | 0.17 ± 0.07 | 0.19 | −0.33 | 0.95 |
| NaCl vs. Citric Acid | 0.19 ± 0.17 | 0.18 | −0.47 | 0.74 |
| LiCl vs. HCl | 0.07 ± 0.07 | 0 | −0.52 | 0.66 |
| LiCl vs. Citric Acid | 0.12 ± 0.06 | 0.15 | −0.34 | 0.60 |
| B. Interstimulus correlations across cells (n = 23 cells) for salty and sour tastants. | ||||
|---|---|---|---|---|
| NaCl | LiC | HCl | Citric Acid | |
| NaCl | 1.0 | |||
| LiCl | 0.98 | 1.0 | ||
| HCl | 0.80 | 0.84 | 1.0 | |
| Citric Acid | 0.65 | 0.68 | 0.90 | 1.0 |
Collectively, results of analyses of interstimulus correlations show that neither individual cells nor the across neuron patterns of response are competent to both distinguish among tastants as well as recognize the similarity of tastants of like taste quality. These results are based on the assumption that the response magnitude, i.e. spike count or firing rate, in the initial 2 sec of response can convey the critical information necessary for this task. It is possible, perhaps probable, that only certain cells use this coding mechanism and that cells may also use other coding mechanisms, e.g. temporal coding, to accomplish this discrimination. We therefore analyzed the contribution of the temporal characteristics of taste responses to the detection of differences among taste stimuli.
Temporal coding of tastants of similar and dissimilar quality
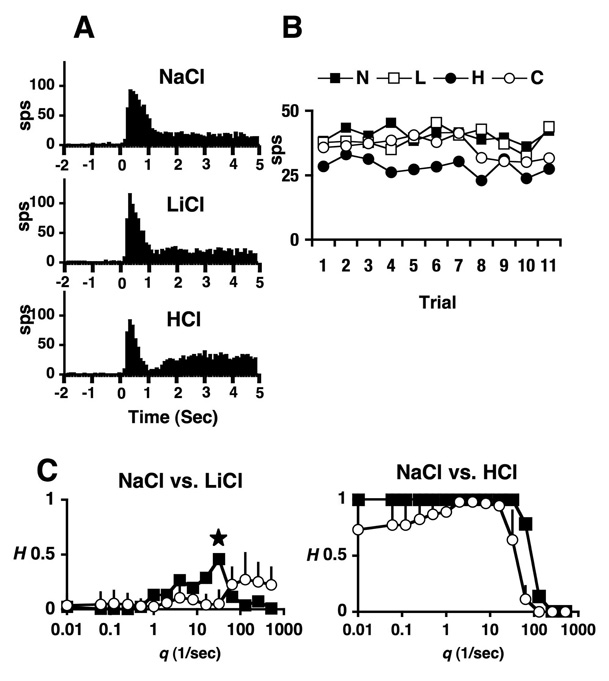
To analyze the contribution of temporal coding to the neural code for tastants of similar and dissimilar qualities, we utilized metric space analyses, as described in the Materials and Methods section above. These procedures allowed the quantification of the amount of information that was contributed by spike count alone, by the rate envelope (time course of the response), or by spike timing. Naturally, all three of these mechanisms might, and usually were, observed in any given comparison of responses to tastants. In addition, it is important to note that the maximum information that can be conveyed in any pairwise discrimination is 1 bit. In the present data only a few cells that used spike count alone achieved that value, though some cells for which spike timing and/or rate envelope contributed information came close. Of 40 stimulus-stimulus comparisons where Hmax = 1.0, 32 (80%) were between stimuli of different taste qualities and 30 (75%) also showed Hcount = 1. Figure 4–Figure 5 show examples of the results of analyses of temporal coding in two cells.
Figure 4.
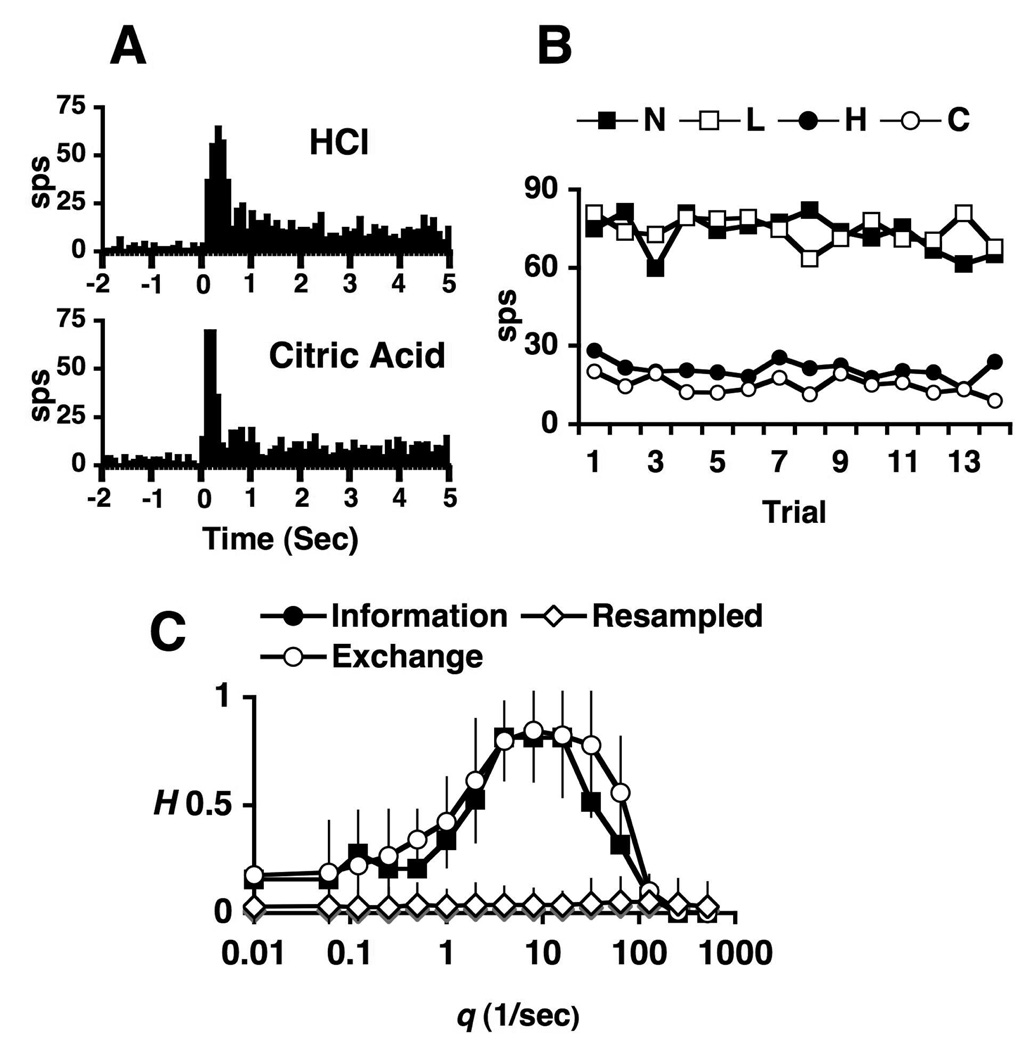
A. Peristimulus-time histograms (PSTHs) of responses to NaCl, LiCl and HCl. B. Responses (sps) to NaCl (N), LiCl (L), HCl (H) and citric acid (C) across trials. C. Metric space analysis of information contributed by responses (filled squares) at various levels of temporal precision, q(1/sec). Results of exchange (open squares) control analysis are also shown. Left graph shows that spike timing contributes a significant amount of information to the discrimination of NaCl vs. LiCl at q = 32, as indicated by a star. Right graph shows that spike count provides perfect discrimination of NaCl vs. HCl, as indicated by H = 1.0 at q = 0. See text for details.
Figure 5.
Example of a cell for which the rate envelope primarily accounts for the distinction between responses to HCl and citric acid. A. PSTHs of responses to HCl and citric acid. B. Responses (sps) to NaCl (N), LiCl (L), HCl (H) and citric acid (C) across trials. C. Metric space analysis of information contributed by spike timing of the responses (filled squares) at various levels of temporal precision, q(1/sec). Results of exchange (open circles) and resampling (open diamonds) control analyses are also shown. Spike timing provides no more information than the exchange analyses. See text for details.
Twenty-five cells had sufficient numbers of trials of each of four tastants to permit the analyses of temporal coding. Twenty-four cells were tested with salty (NaCl and LiCl) tastants, 19 cells were tested with sour (HCl and citric acid) tastants, four cells were tested with salty and bitter (quinine and urea) tastants and two cells were tested with sour and sweet (sucrose and fructose) tastants. The proportion of cells tested with each pair of taste qualities reflects the relative incidence of responsiveness to these stimuli among NTS cells. Thus, since almost all cells responded well to NaCl and HCl, salty and sour tastants were most often tested and most of our conclusions are based on data obtained from cells that responded well to these stimuli.
Analyses of information contributed by temporal coding showed that comparisons of tastants of similar qualities were encoded differently than those for tastants of dissimilar qualities. As shown in Table 4, the amount of information contributed by spike count alone was significantly less for tastants of similar quality than that for tastants of dissimilar quality (Student’s t test, p < 0.001). This was not surprising given the observation that the average difference between response magnitudes was higher for pairs of tastants of dissimilar qualities than for pairs of tastants of similar qualities (see Table 3). Moreover, the larger the mean absolute difference in response magnitude, the larger the amount of information conveyed by spike count (r = 0.49, p < 0.01).
The amount of information contributed by the temporal characteristics of the response in addition to that contributed by spike count was about the same for all pairwise comparisons. Given the fact that the total amount of information contributed by the temporal characteristics of a response was significantly larger for tastants of dissimilar quality than for those of similar quality (Student’s t test, p < 0.001), the temporal characteristics of responses contributed proportionately more information than spike count for distinguishing among tastants of similar quality than for tastants of dissimilar quality. The amount of information provided by the spike timing was therefore proportionally greater for distinctions between tastants of similar quality.
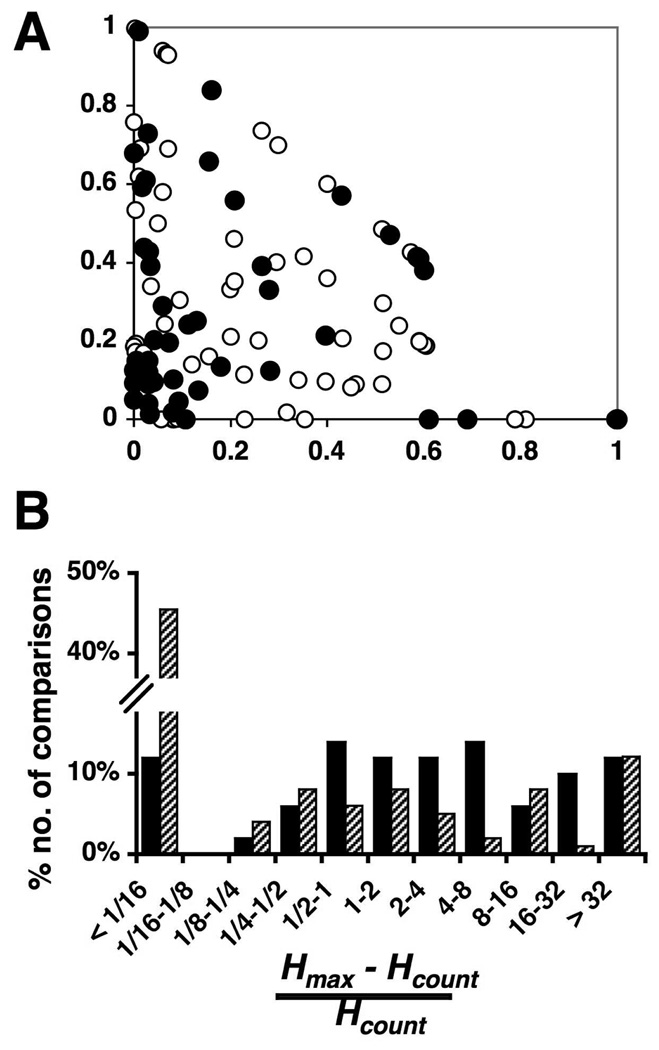
It is important to distinguish between classifying discriminations as easy vs. difficult, and classifying discriminations on the basis of whether the tastants are of similar vs. dissimilar qualities. We are operationally defining an “easy” discrimination as one that can be made by spike count, and a “difficult” discrimination as one that cannot be made by spike count). As shown in Table 2, on average, the response magnitudes evoked by two tastants of similar quality are similar. For cells where this applies, distinction between these two stimuli would qualify as difficult. However, in some cells, two tastants of different qualities evoke similar response magnitudes. This condition would also qualify as a difficult distinction. So, the question that arises is whether temporal coding is used most often for distinguishing between tastants of similar quality, or for difficult discriminations regardless of taste quality. To answer this question, in Figure 6, we plotted the information conveyed by spike count (Hcount) vs. the additional information contributed by temporal characteristics of the response (Hmax –Hcount) for tastants of similar (filled circles) and dissimilar qualities (open circles). This figure shows that there was a range of coding strategies (the scatter across the entire triangle), with temporal coding contributing more when Hcount was low, i.e., a difficult discrimination. This happens whether the comparison is between tastants of similar or dissimilar quality.
Figure 6.
Information conveyed by Hcount relative to the contribution of the temporal characteristics of a response to the total amount of information conveyed by taste responses. A. Graph of Hcount plotted against Hmax – Hcount for all stimulus-stimulus comparisons for all NTS cells that were analyzed for temporal coding (n = 25). Filled circles indicate comparisons of tastants of similar quality and open circles indicate comparisons of tastants of dissimilar quality. B. Histogram of the percent of the total number of stimulus-stimulus comparisons that showed various ratios of the contribution of temporal coding (Hmax –Hcount) in relation to the contribution of spike count (Hcount). Solid bars indicate comparisons of tastants of similar quality and hashed bars indicate comparisons of tastants of dissimilar quality. See text for details.
The proportional contribution of temporal coding is shown in Figure 6B. This value was calculated as follows:
Values of this ratio that are less than 1.0 (left side of graph, corresponding to the lower left triangle of Figure 6A) indicate that spike count conveys proportionately more information than the temporal characteristics of the response. Conversely, values of this ratio that are greater than 1.0 (right side of graph, corresponding to the upper right triangle in Figure 6A) indicate that the temporal characteristics of the response contribute proportionately more information than the spike count alone. This figure reveals differences between comparisons of tastants of similar and dissimilar quality. For comparisons of tastants of dissimilar qualities (hashed), the percentage of cells that convey proportionately more information by spike count (left side of graph) was quite high compared to the corresponding percentage of cells that convey proportionately more information by spike timing. This was due primarily to the large number (43 of 99 comparisons, 43%) of comparisons for which spike count provided the maximum amount of information conveyed by the cell for that comparison. However, for comparisons of tastants of similar quality (solid), ratios > 1.0 were more common than ratios < 1.0 suggesting that spike timing conveyed proportionately more information in these comparisons than spike count. In fact, 66% of all comparisons of tastants of similar qualities had ratios > 1.0, compared with only 36% of comparisons of tastants of dissimilar qualities. Thus, when equated for “difficulty” of discrimination (i.e., similar values of Hcount), discriminations between tastants of similar quality rely more heavily on spike timing than discriminations between tastants of dissimilar quality.
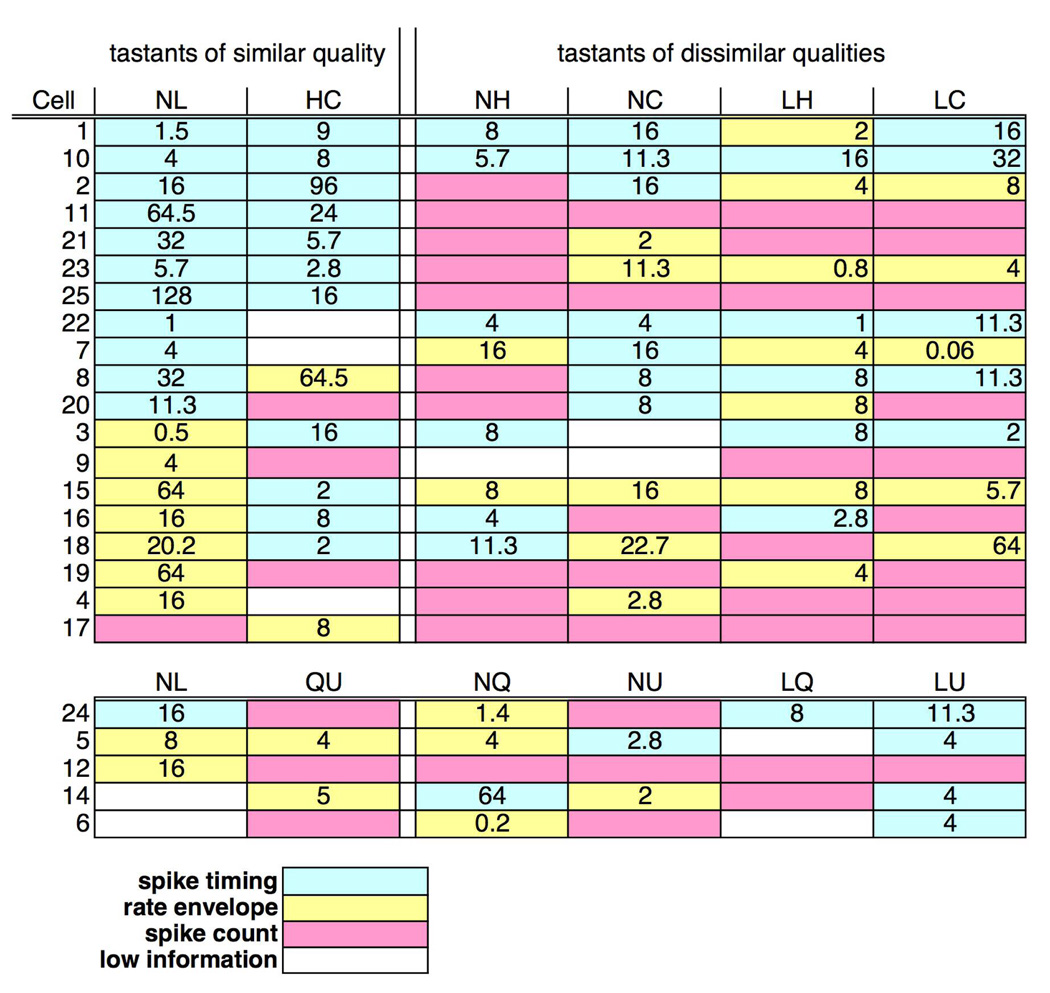
Data presented in Figure 7 further support the idea that temporal coding is evident more frequently for comparisons of tastants of similar quality than dissimilar quality. It can be seen, for example, that the comparison between the two salty and the two sour tastants showed the largest proportion of cells with a significant contribution of spike timing (NaCl vs. LiCl, 12 of 24 cells, 50%; HCl vs. citric acid, 11 of 19 cells, 58%) relative to all other pairwise comparisons (≤ 32% of the cells). In contrast, fewer cells used spike count to differentiate NaCl vs. LiCl (1 of 24 cells, 4%) and HCl vs. citric acid (3 of 19 cells, 16%) than were used with the other pairwise comparisons (range of 5 to 10 cells of 19, 26 to 53%). If the number of cells with responses showing a significant contribution of spike timing and the rate envelope were combined, it was evident that the temporal characteristics of response were more widely available to convey information about NaCl vs. LiCl (21 of 24 cells, 88%) and HCl vs. citric acid (13 of 19 cells, 68%) than the other comparisons (range of 8 to 10 cells of 19, 42 to 53%).
Figure 7.
Incidence of temporal coding by spike timing (blue squares), rate envelope (yellow squares) and spike count (pink squares). Empty squares indicate that the total information contributed by the response was low; i.e. Hmax < 0.1. Numbers indicate the value of q at Hmax for cells that show a significant contribution of spike timing. Upper set of rows: cells tested with pairs of salty and sour stimuli. Lower set of rows: cells tested with pairs of salty and bitter stimuli.
Several additional results are worth noting. First, spike timing contributed information to at least one, but not necessarily to every (see Figure 6), pairwise comparison in almost all NTS cells (15 of 19, 79%). Second, the level of precision at which spike timing was significant was generally between ~70–125 msec (median q for all pairwise comparisons ranged between 8 and 13.7). Third, as seen in Figure 3, four out of five cells tested with salty and bitter tastants used spike timing; however, none of these cells used spike timing to differentiate between quinine and urea.
Discussion
Electrophysiological responses to taste stimuli of similar and dissimilar qualities were recorded from 38 single cells in the NTS of anesthetized rats. Results showed that the magnitudes of response to all taste stimuli tested varied widely with repetition. Responses to tastants of similar quality were generally closer in magnitude than responses to dissimilar tastants; however, within a cell, tastants of similar quality often reversed their order of effectiveness across replicate sets of trials. Strong responses were less variable and thus more consistent than weak responses. Spike timing or the rate envelope (time course) of the response contributed a significant amount of information for interstimulus discrimination in 79% (15 of 19) of the cells tested for at least one pairwise comparison of taste stimuli. Temporal coding was evident in more cells and contributed proportionately more information than spike count alone for discrimination of tastants of similar quality compared with tastants of dissimilar quality. Since the average difference in response magnitude was smaller for tastants of similar quality compared with that for tastants of dissimilar quality, these results suggest that temporal coding may be used when differences in firing rate between two stimuli are small. Overall, these results demonstrate that temporal coding can contribute a significant amount of information to the discrimination of taste stimuli, even those that evoke a similar taste quality.
Several observations suggested that response magnitude was only a moderately reliable measure of a cell’s sensitivity to a given tastant. For example, variability in response magnitude with repetition was evidenced by the failure to respond to a normally effective tastant (a tastant that produced a response) and by changes in the order of effectiveness of tastants across blocks of trials. Reversal of the more effective stimulus of a pair of tastants across blocks of trials was observed more frequently when both tastants were of similar quality, and therefore of similar response magnitude (see Table 3). If this variability were due to some underlying generating factor on a long time scale (i.e. on a scale of minutes), then responses to tastants of similar quality might be predicted to vary in parallel, resulting in a high correlation of responses across trials. Contrary to this expectation, interstimulus correlations among salty and sour tastants were quite low (< 0.20) for both tastants of similar and dissimilar quality. Of course, there may be some underlying factor that modulates responses on a shorter time scale, say tens of seconds and that might account for the poor interstimulus correlations of responses to tastants of similar quality. Even so, our data are consistent with the suggestion that the response of an individual cell could potentially distinguish between any two tastants regardless of quality (due to the dissimilarity of their across neuron patterns; ANPs) but could not necessarily group tastants of similar quality. Similarly low interstimulus correlations for both similar and dissimilar pairs of stimuli imply that trial-to-trial variability is not quality-specific. In contrast, interstimulus correlations across cells were lower for comparisons of salty vs. sour tastants compared with those for salty vs. salty and sour vs. sour tastants. Unlike individual cells, the entire population of taste-responsive NTS cells may provide more reliable information about the similarity or dissimilarity of stimuli. In other words, the ANPs of responses to tastants of the same quality are more similar than ANPs of responses to tastants of dissimilar quality. In the ANP theory of taste coding, these differences form the basis for both discrimination of tastants of different qualities and the grouping tastants of similar taste qualities (Doetsch and Erickson 1970; Ganchrow and Erickson 1970; Yamamoto and Yuyama 1987).
In previous work using four tastants, each of which represented a different quality, the amount of variability (as measured by the mean CV of responses within a cell) was a strong predictor of the proportional contribution of temporal coding (r = 0.85; Di Lorenzo and Victor 2003). No such relationship was apparent in the present study for any pairwise comparison. At least two differences between the previous and present studies might account for this difference. First, the fact that we selected cells to a great extent on the basis of their responsiveness to our test stimuli (usually, but not always, avoiding weaker responses) may have produced a sample of cells with less variable responses than a more random sample might have. The observation that more robust responses are less variable than weaker ones is consistent with this possibility. Second, the use of stimuli representing only two taste qualities may also have impacted the CV. Previous results showed that the CVs of responses to NaCl, HCl, sucrose and quinine were not significantly different from each other, though the CVs for sucrose and quinine were larger than those for NaCl and HCl. In the present study, we also found no significant differences in variability across tastants, even when tastants were grouped according to quality. However, since most of our analyses were based on responses to salty and sour tastants, it may be that the CVs produced by these stimuli would underestimate the CV of the cell had it been tested with an array of tastants more representative of the entire perceptual domain.
Although response variability was not a good predictor of the relative contribution of temporal coding, we did find that the most difficult distinctions, i.e. between tastants of similar quality and/or similar response magnitude, showed evidence of temporal coding more frequently and to a proportionately greater degree. Conversely, comparisons of tastants of dissimilar qualities were found to rely more on spike count than the temporal characteristics of the response. Perfect discrimination between any two tastants, even two tastants of the same quality, requires 1 bit of information. Accordingly, distinguishing between two tastants of the same quality would require that same amount of information as distinguishing between two tastants of different qualities, even though it is intuitively more difficult to distinguish between two similar tastants than two dissimilar tastants. In the present study, the only pairings for which taste responses conveyed 1 bit of information were those in which spike count sufficed (see Figure 6), and these were mostly pairings of tastants of dissimilar qualities. Conversely, in the more difficult distinctions (including all of those between two tastants of similar quality), the information is less than 1 bit – indicating that distinction based on spike count and timing would be less than perfect. In particular, Hmax was significantly lower for comparisons between the two salty and the two sour tastants than for comparisons between a salty and sour tastant. In addition, within a given cell, tastants of similar qualities often evoked similar response magnitudes (at the concentrations employed here) but different response magnitudes for tastants of dissimilar qualities (see Table 2). Thus, it might be predicted that the spike count alone, Hcount, would be more informative for comparisons of tastants of dissimilar qualities. Present data also confirm this prediction. However, although the contribution of temporal coding was more frequent when spike counts were similar, the amount of information contributed by temporal coding could not be predicted by the average difference in response magnitude between any given pair of tastants. This result contradicts the strong form of our hypothesis that temporal coding is always used to discriminate among taste stimuli when differences in the number of spikes evoked by each of the tastants was insufficient to identify the tastant: there were many instances in which neither spike count nor temporal coding allowed discrimination.
The observation that temporal coding was evident for at least one pairwise comparison in nearly every cell was a surprise, given previous findings suggesting that only a subset of cells evidence temporal coding (Di Lorenzo and Victor 2003, 2007). Early studies of temporal coding in the CT nerve also found that only a subset of incoming fibers to the NTS convey information through the temporal characteristics of a response (Bradley et al. 1983; Mistretta 1972; Nagai and Ueda 1981). Other investigations in the central nervous system (Di Lorenzo and Schwartzbaum 1982; Katz et al. 2001; Nuding et al. 1991) have also implied that temporal coding is present in the responses of only a subset of cells. The use of taste stimuli that were of the same taste quality in the present study revealed that a larger proportion of NTS cells may convey information using the temporal characteristics of their responses than was thought previously (Di Lorenzo and Victor 2003, 2007). This suggests that most NTS cells may use temporal coding depending on the task required, e.g. distinguishing among very similar-tasting stimuli.
The criteria that we used for determination of which responses displayed temporal coding were conservative, in the following sense. In our analyses, if spike count provided enough information to support perfect discrimination, then we ignored the possibility that temporal coding (by envelope or pattern) might also be used. Consequently, when both "temporal codes" and "count codes" led to perfect discrimination, the cell (or discrimination) was classified as using a count code. In these discriminations, performance based on spike count would be less than perfect had one made the task more challenging, e.g. by using a shorter presentation time or lower concentration -- and this might have unmasked a contribution of temporal coding. Thus, our analysis likely underestimates the number of cells that make use of temporal coding.
It is important to keep in mind that we investigated only pairwise discrimination of tastants; in nature, the gustatory system is faced with the problem of identifying as taste stimulus from a wider array of qualities. Taken together with previous observations (Di Lorenzo and Victor 2003), our results suggest that, for any given cell, the spike count can suffice for an “approximate” classification of a taste stimulus, but fine discriminations may rely on the temporal features of the response. For cells that respond equally well to tastants of different taste qualities, these results predict that temporal coding will take on a more prominent role. These results are in agreement with similar results of a study of contrast discrimination in the visual cortex (Reich et al. 2001). Specifically, Reich et al. (2001) found that spike count works well in the low and intermediate range of contrasts, but in the high contrast range, where firing rates saturate, temporal coding plays an increasing role. Also in agreement with present results are data from the olfactory system of the honeybee showing that disruption of synchronized activity in the antennal lobe of the brain adversely affects discrimination of similar, but not dissimilar odorants (Stopfer 1997). Considered collectively, these data along with our own support the idea that temporal coding is most prominent when stimuli are difficult to discriminate from one another.
Implications and predictions
The present results are not inconsistent with either the labeled line or ANP theories of taste coding but rather add a dimension of complexity to both. For example, the reliance of the labeled line theory on the response characteristics of individual cells is fully consistent with the use of temporal coding to distinguish among tastants. However, the (now twice replicated; Di Lorenzo and Victor, 2003, 2007) observation that response magnitudes are often highly variable across repeated trials suggests that discovering the “true” sensitivity profile of a cell may require multiple stimulus trials. It further suggests that, even if statistical differences in response magnitude can be identified, temporal patterns may be more reliable for discriminations based on single-trial responses. Moreover, there may be some cells that do not show straightforward preferences for tastants of a single quality. What role these cells play in taste coding is not explained by a labeled line conceptualization. Similarly, the ANP theory is also consistent with the use of temporal coding since it is possible to argue, as Katz has done (Katz et al. 2002), that temporal patterns of response are dynamic and evolve over the time course of the response.
Data from the present study also lead to some predictions about taste-related behavior. For example, results of the temporal coding analyses suggest that the behavioral discrimination of NaCl and LiCl (each at 0.1 M) would be possible, albeit difficult, using gustatory cues. Although it is possible to train rats to discriminate NaCl from LiCl at or near the concentrations used in the present study (Balgura and Smith 1970; Kieffer 1978; Ossenkopp et al. 1997; Nachman 1963; Strom 1970), many investigators believe this discrimination to be based on postingestional associations rather than taste per se. Contrary to this belief, Kiefer (1978) has shown that rats can use taste cues alone to make behavioral distinctions between NaCl and LiCl at concentrations above ~0.01 M. To our knowledge, similar data related to behavioral discrimination of HCl and citric acid are not available. However, present data suggest that these two tastants would be more easily discriminable than the two salts at the concentrations used here. This prediction is based on the observations that the average absolute difference in magnitudes of response to HCl and citric acid is larger than that between NaCl and LiCl, and that spike count accounts for all information available for the discrimination between HCl and citric acid in proportionately more cells than for the discrimination between NaCl and LiCl.
Conclusions
For any theory of neural coding in a perceptual system, the questions of what drives the respective neural elements to respond as they do and what targets of these elements read their signals are important ones to answer. In the case of the rat NTS, recent studies have shown that the incoming message to these cells originates from receptors that are specific for the various taste qualities (Zhao et al. 2003; Zhang et al. 2003; Chandreshakar et al. 2000; Huang et al. 2006). However, incoming fibers are known to diverge to synapse with many NTS cells (Whitehead and Frank 1983). Conversely, any given NTS cell receives input from fibers that may have a variety of taste sensitivities. As a result, NTS cells are more broadly tuned than CT fibers (Doetsch and Erickson 1970). Temporal characteristics of responses may represent a potential mechanism for producing more reliable outputs and expanding the communication capacity of individual cells. Regarding the targets of NTS cells, located mainly in the parabrachial nucleus of the pons and the reticular formation (Halsell et al. 1996), whether and how these cells can interpret temporal patterns of input is as yet unknown.
Acknowledgements
Supported by NIDCD grant DC06914 to PMD and NIMH 1-R01-MH68012 (PI: Dan Gardner) to JDV.
References
- Balagura S, Smith DF. Role of LiCl and environmental stimuli on generalized learned aversion to NaCl in the rat. Am J Physiol. 1970;219:1231–1234. doi: 10.1152/ajplegacy.1970.219.5.1231. [DOI] [PubMed] [Google Scholar]
- Bradley RM, Stedman HM, Mistretta CM. Superior laryngeal nerve response patterns to chemical stimulation of sheep epiglottis. Brain Res. 1983;276:81–93. doi: 10.1016/0006-8993(83)90550-4. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Chang FC, Scott TR. Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. J Neurosci. 1984;4(7):1850–1862. doi: 10.1523/JNEUROSCI.04-07-01850.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM. The neural code for taste in the brain stem: Response profiles. Physiology & Behavior. 2000;69(1–2):87–96. doi: 10.1016/s0031-9384(00)00191-8. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Lemon CH. Methodological considerations for electrophysiological recording and analysis of taste-responsive cells in the brain stem of the rat. In: Simon SA, Nicolelis MAL, editors. Methods in Chemosensory Research. New York: CRC Press; 2001. pp. 293–324. [Google Scholar]
- Di Lorenzo PM, Lemon CH, Reich CG. Dynamic coding of taste stimuli in the brain stem: effects of brief pulses of taste stimuli on subsequent taste responses. J Neurosci. 2003;23:8893–8902. doi: 10.1523/JNEUROSCI.23-26-08893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. Corticofugal influence on taste responses in the nucleus of the solitary tract in the rat. J Neurophysiol. 1995;74(1):258–272. doi: 10.1152/jn.1995.74.1.258. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Schwartzbaum JS. Coding of gustatory information in the pontine parabrachial nuclei of the rabbit: Temporal patterns of neural response. Brain Res. 1982;251:245–257. doi: 10.1016/0006-8993(82)90742-9. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Victor JD. Taste response variability and temporal coding in the nucleus of the solitary tract of the rat. J Neurophysiol. 2003;90:1418–1431. doi: 10.1152/jn.00177.2003. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Victor JD. Neural coding mechanisms for flow rate in taste-responsive cells in the nucleus of the solitary tract of the rat. J Neurophysiol. 2007;97(2):1857–1861. doi: 10.1152/jn.00910.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch GS, Erickson RP. Synaptic processing of taste-quality information in the nucleus tractus solitarius of the rat. J Neurophysiol. 1970;33:490–507. doi: 10.1152/jn.1970.33.4.490. [DOI] [PubMed] [Google Scholar]
- Frank M. An analysis of hamster afferent taste nerve response functions. J Gen Physiol. 1973;61:588–618. doi: 10.1085/jgp.61.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME. Neuron types, receptors, behavior, and taste quality. Physiol Behav. 2000;69(1–2):53–62. doi: 10.1016/s0031-9384(00)00188-8. [DOI] [PubMed] [Google Scholar]
- Ganchrow JR, Erickson RP. Neural correlates of gustatory intensity and quality. J Neurophysiol. 1970;33(6):768–783. doi: 10.1152/jn.1970.33.6.768. [DOI] [PubMed] [Google Scholar]
- Hallock RM, Di Lorenzo PM. Temporal coding in the gustatory system. Neurosci Biobehavi Rev. 2006;30(8):1145–1160. doi: 10.1016/j.neubiorev.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neurosci. 1996;72(1):185–197. doi: 10.1016/0306-4522(95)00528-5. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442(7105):934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Mark GP, Scott TR. Taste responses in the nucleus tractus solitarius of sodium-deprived rats. J Physiol. 1988;406:393–410. doi: 10.1113/jphysiol.1988.sp017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DB, Nicolelis MA, Simon SA. Gustatory processing is dynamic and distributed. Curr Opin Neurobiol. 2002;12(4):448–454. doi: 10.1016/s0959-4388(02)00341-0. [DOI] [PubMed] [Google Scholar]
- Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;21(12):4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SW. Two-bottle discrimination of equimolar NaCl and LiCl solutions by rats. Physiol Psychol. 1978;6(2):191–198. [Google Scholar]
- Lundy RF, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol. 1999;82(6):2970–2988. doi: 10.1152/jn.1999.82.6.2970. [DOI] [PubMed] [Google Scholar]
- Mistretta CM. A quantitative analysis of rat chorda tympani fiber discharge patterns. In: Schneider D, editor. Olfaction and Taste IV. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 1972. pp. 294–300. [Google Scholar]
- Nachman M. Learned aversion to the taste of lithium chloride and generalization to other salts. J. Comp. Physiol Psychol. 1963;56:343–349. doi: 10.1037/h0046484. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ueda K. Stochastic properties of gustatory impulse discharges in rat chorda tympani fibers. J Neurophysiol. 1981;45:574–592. doi: 10.1152/jn.1981.45.3.574. [DOI] [PubMed] [Google Scholar]
- Nuding SC, McPheeters M, Frank ME. Interspike interval patterns of taste neurons in the hamster solitary nucleus. Chem Senses. 1991;16(5):429–446. [Google Scholar]
- Ogawa H, Sato M, Yamashita S. Variability in impulse discharges in rat chorda tympani fibers in response to repeated gustatory stimulation. Physiol Behav. 1973;11:469–479. doi: 10.1016/0031-9384(73)90033-4. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Yamashita S, Sato M. Variation in gustatory nerve fiber discharge pattern with change in stimulus concentration and quality. J Neurophysiol. 1974;37:443–447. doi: 10.1152/jn.1974.37.3.443. [DOI] [PubMed] [Google Scholar]
- Ossenkopp K-P, Ladowsky RL, Eckel LA. Forced-choice discrimination of equimolar NaCl and LiCl solutions in rats: effects of ablating the chemosensitive area postrema on acquisition and retention. Behav Brain Res. 1997;87:15–24. doi: 10.1016/s0166-4328(97)02279-1. [DOI] [PubMed] [Google Scholar]
- Reich DS, Mechler F, Victor JD. Temporal coding of contrast in primary visual cortex: when, what, and why? J Neurophysiol. 2001;85:1039–1050. doi: 10.1152/jn.2001.85.3.1039. [DOI] [PubMed] [Google Scholar]
- Scott TR, Giza BK. Coding channels in the taste system of the rat. Science. 1990;249:1585–1587. doi: 10.1126/science.2171145. [DOI] [PubMed] [Google Scholar]
- Smith DV, Li CS. GABA-mediated corticofugal inhibition of taste responsive neurons in the nucleus of the solitary tract. Brain Res. 2000;858(2):408–415. doi: 10.1016/s0006-8993(99)02484-1. [DOI] [PubMed] [Google Scholar]
- Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 2005;4(3):143–191. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagaven S, Smith BH, Laurant G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Strom C, Lingenfelter A, Brody JF. Discrimination of lithium and sodium chloride solutions by rats. Psychon Sci. 1970;8:290–291. [Google Scholar]
- Victor JD, Purpura KP. Nature and precision of temporal coding in visual cortex: a metric- space analysis. J Neurophysiol. 1996;76:1310–1326. doi: 10.1152/jn.1996.76.2.1310. [DOI] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. Metric-space analysis of spike trains: theory, algorithms and application. Network. 1997;8:127–164. [Google Scholar]
- Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol. 1983;220(4):378–395. doi: 10.1002/cne.902200403. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N. On a neural mechanism for cortical processing of taste quality in the rat. Brain Res. 1987;400(2):312–320. doi: 10.1016/0006-8993(87)90630-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umani tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112(3):293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]