Abstract
Purpose
The objective of this study was to determine the phenotypic characterization of ligamentum flavum cells from patients with ossification of the ligamentum flavum (OLF).
Materials and Methods
Ligamentum flavum tissues were harvested from OLF and non-OLF patients during surgery. OLF and non-OLF cells were isolated from explant cultures. Cultured cells were analyzed using immunofluorescence staining and reverse transcription-polymerase chain reaction.
Results
OLF cells exhibited various appearances compared with the typical fibroblast-like morphology of non-OLF cells. Expressions of collagen type I and collagen type III were observed in OLF and non-OLF cells. OLF cells uniquely expressed osteocalcin, which is a marker for osteoblasts, and collagen type II which is a marker for chondrocytes, whereas they were negative in non-OLF cells.
Conclusion
These findings indicate that OLF cells have phenotypic characterization of osteoblasts and chondrocytes which could play a role in the pathophysiology of OLF.
Keywords: Cell phenotype, ossification, ligamentum flavum
INTRODUCTION
The ligamentum flavum is a spinal ligament connecting the laminae of the adjacent vertebrae from the axis to the lumbosacral interval, and forms the posterior wall of the spinal canal. Ossification of the ligamentum flavum (OLF) is characterized by ectopic bone formation within the ligament flavum which is normally composed of elastic and connective tissue fibres.1 Enlarged ossified tissue can compress the spinal cord and its roots, resulting in various degrees of neurological symptoms, ranging from discomfort to severe myelopathy.2 The lower thoracic spine is the most frequently affected area, and OLF has been increasingly recognized as an important cause of thoracic myeloradiculopathy.2-5 Treatment of symptomatic OLF has most often been reported as posterior decompressive laminectomy with removal of ossified ligaments, but surgical decompression does not always produce a satisfactory result due to recurrent ossification at another or the same spinal level.6 Up to date, no therapeutic agent is known to prevent the development of OLF.
Etiological studies have shown possible links between OLF and genetic factors.7,8 In addition, several factors, including mechanical stress,9,10 metabolic abnormalities,11,12 regional cytokines or growth factors,13-16 have also been reported to contribute to the development and progression of ossification. However, the exact pathomechanism of OLF is still largely unknown, although the involvement of various genetic and environmental factors has been acknowledged.
Histopathological data indicated that the process of OLF was a form of endochondral ossification,17 but little is known about the nature of cultured cells derived from the ossified ligamentum flavum. In the present study, we investigated phenotypic characterization of cultured ligamentum flavum cells isolated from OLF patients, as compared to that of ligamentum flavum cells from non-OLF patients. Immunofluorescence and reverse transcription-polymerase chain reaction (RT-PCR) analyses were performed to examine the expressions of osteocalcin, collagen type I, type II, and type III.
MATERIALS AND METHODS
Specimens
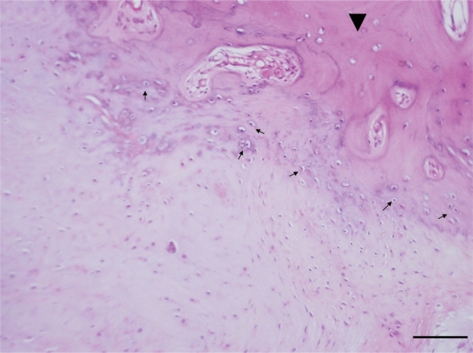
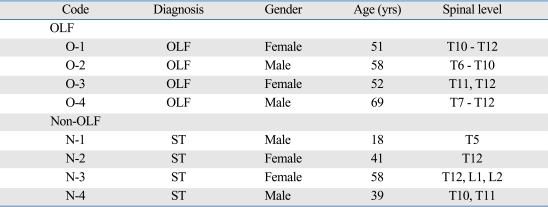
Ligamentum flavum tissues were harvested aseptically during surgery, which was performed to decompress the spinal cord for myeloradiculopathy, from four thoracic OLF patients who were documented by magnetic resonance imaging and computed tomography. As a control, the ligamentum flavum was obtained from four non-OLF patients during surgery for thoracic spine trauma. OLF or non-OLF was preoperatively diagnosed by X-ray, computerized tomography, and magnetic resonance imaging, and further confirmed by histological examination. Fig. 1 shows histological characterization of the ossified ligamentum flavum. Details of these patients are shown in Table 1. Informed consent was obtained from each patient, and this study was approved by the Ethics Committee of the Southern Medical University.
Fig. 1.
Histological characterization of the OLF. Ossification (arrowhead) and chondroid cells (arrow) were observed in the ossified ligamentum flavum (hematoxylin and eosin stain, bar = 100 µm).
Table 1.
Clinical Details of Individual Patients
OLF, ossification of the ligamentum flavum; ST, spinal trauma; T, thoracic vertebrae; L, lumbar vertebrae.
Cell culture experiments
Cells were isolated from the ligamentum flavum tissues as described previously18 with some modifications. Briefly, the specimens were washed with phosphate-buffered saline (PBS), after which any surrounding tissues attached to the specimen were carefully removed, including bone, fat, and connective tissue. The collected ligaments were minced into pieces of approximately 0.5 mm3 and washed twice with PBS. The minced tissue was digested at 37℃ for 60 min with 0.2% collagenase type I (Sigma-Aldrich Corp., St. Louis, MO, USA) in Dulbecco's Modified Eagle's medium (DMEM, Gibco BRL, Melbourne, Victoria, Australia) containing no serum. Collagenase-treated ligament chips were washed with DMEM and then placed in 6-well plates in DMEM supplemented with 10% fetal bovine serum (Gibco BRL.), 100 U/mL penicillin and 100 pg/mL streptomycin, and incubated in a humidified atmosphere of 95% air and 5% CO2 at 37℃. The medium was changed at two-day intervals, and the explants were daily checked for cell outgrowth using an inverted light microscope. The outgrown cells were harvested before confluence and subcultured after trypsinization with 0.2% trypsin/ 0.02% ethylenediamine tetraacetic acid (EDTA). Cells at the third passage were used for experiments. Cell lines obtained from OLF and non-OLF patients were named as OLF cells and non-OLF cells, respectively.
Immunofluorescence microscopy
For immunofluorescence analysis, monolayers of cells were grown on glass coverslips inserted in 6-well plates. After rinsing with PBS, the cells were fixed in 4% paraformaldehyde in PBS for 10 min and permeabilized with PBS containing 0.5% TritonX-100 for 5 min at room temperature. Blocking was performed with 5% bovine serum albumin (BSA) in PBS for 30 min, and the cells were subsequently incubated overnight at 4℃ with primary antibodies. The antibodies were used at the following dilutions: goat polyclonal anti-collagen type I antibody 1 : 50 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and goat polyclonal anti-Collagen type III antibody 1 : 50 (Santa Cruz Biotech-nology.); goat polyclonal anti-Osteocalcin antibody 1 : 25 (Santa Cruz Biotechnology.); mouse monoclonal anti-Collagen type II antibody 1 : 25 (Thermo Fisher Scientific Inc, Fremont, CA, USA). Following three washes in PBS (5 mins each), the cells were incubated for 60 min at room temperature with a 1 : 100 dilution of the secondary anti-bodies: fluorescein isothiocyanate (FITC)-conjugated rabbit anti-goat IgG (ZSGB-BIO Inc., Beijing, China) and FITC-conjugated goat anti-mouse IgG (ZSGB-BIO Inc.) respec-tively. After three additional washes in PBS, the cell nuclei were counterstained with 10 µg/mL propidium iodide (PI) (Sigma-Aldrich Corp.) for 10 min at room temperature and rinsed again in PBS. Finally, the coverslips were mounted onto microscopic slides using 50% glycerol in PBS. Negative controls were performed in parallel, except that cells were treated with 5% BSA in PBS without primary antibody. Images were obtained and analyzed using the LSM-510 laser confocal microscope system (Carl Zeiss, Göttingen, Germany).
RNA extraction and RT-PCR
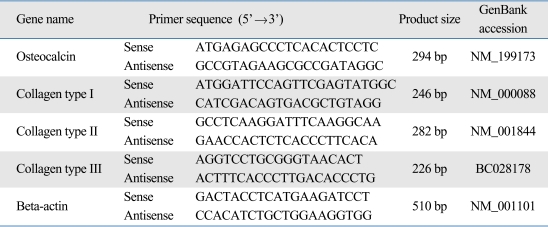
Total RNA was extracted from cultured cells using a TRIZOL® Reagent kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. First-strand cDNA was synthesized by reverse transcription of 5 µg of total RNA using SuperScript™ III First-Strand Synthesis System (Invitrogen.). PCR amplification was performed with GoTaq® Green Master Mix (Promega, Madison, WI, USA) using the human specific primers, which are shown in Table 2. The house-keeping gene beta-actin was used as an internal control. Cycling conditions for PCR were as follows: for osteocalcin, collagen type I, collagen type II and collagen type III, 35 cycles of 45 s at 94℃, 45 s at 60℃, 1 min at 72℃, and 5 min at 72℃; for beta-actin, 35 cycles of 30 s at 94℃, 30 s at 55℃, 30s at 72℃, and 5 min at 72℃. PCR products were subjected to 2% agarose gel electrophoresis and visualized by ethidium bromide staining under ultraviolet light exposure.
Table 2.
Primer Sequences Used in RT-PCR
RT-PCR, reverse transcription-polymerase chain reaction.
RESULTS
In the present study, ligamentum flavum cells were successfully isolated using an explant method. Within 14 days after explantation, outgrowth of cells from ligament tissue was observed and became monolayer. Morphologically, OLF cell lines varied widely in appearance, ranging from thin, spindle-shaped cells to polygonal cells or oval-shaped cells at confluence. Non-OLF cell lines exhibited features of fibroblast-like cells with spindle shape.
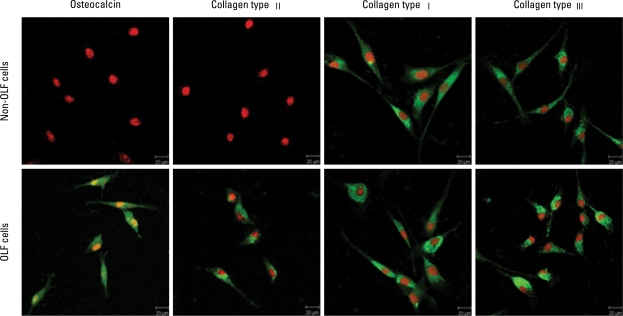
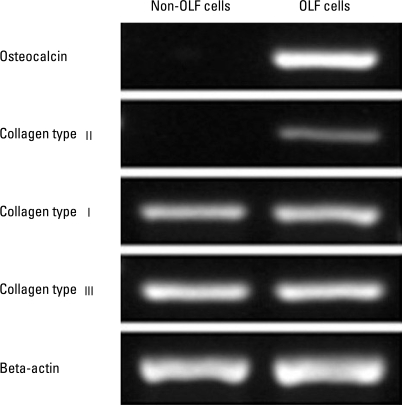
As demonstrated by immunofluorescence, osteocaclin (a specific marker of osteoblasts19), and collagen type II (a specific marker of chondrocytes20) were expressed in OLF cells, but not in non-OLF cells; the expression of collagen type I and collagen type III was observed in OLF and non-OLF cells. The data with four different OLF cell lines and non-OLF cell lines were reproducible (Fig. 2). Moreover, phenotype difference between OLF and non-OLF cells was further confirmed using RT-PCR analysis for genes of oastocalcin, collagen type I, collagen type II and collagen type III (Fig. 3).
Fig. 2.
Immunofluorescence analysis of OLF cells and non-OLF cells. Immunofluorescence was visualized using FITC (green) and the nuclei by PI (red). OLF cells expressed osteocalcin, collagen type I, II and III; non-OLF cells expressed collagen type I and III, but not osteocalcin and collagen type II (bar = 20 µm).
Fig. 3.
Gene expression analysis for osteocalcin, collagen type I, collagen type II and collagen type III in OLF cells and non-OLF cells by RT-PCR. Data shown are agarose gel electrophoresis of amplification products.
DISCUSSION
OLF has been shown to be predominant in Asian populations, particularly in Japanese patients.2,4,6,14,17 However, over the past decade, the number of OLF case reports has been increasing also in non-Asian patients.1,3,5,21 Now, OLF is recognized as an important disease occurring not only in Asians, but also in peoples in Europe and the United States.
OLF has been described as an isolated clinical entity, although it can also be found in association with ossification of other spinal ligaments such as posterior and anterior longitudinal ligaments and the supraspinous ligaments. The pathogenesis of OLF has not been studied as vigorously as that of ossification of posterior longitudinal ligament (OPLL),22 and the precise pathogenesis of OLF has not been conclusively established.
The phenotypic characterization of OLF cells has not been studied in detail so far. The present study found that OLF cells had various appearances compared to the typical fibroblast-like morphology of non-OLF cells, and we demonstrated the presence of osteoblast phenotype and chondrocyte phenotypes in the OLF cells using immunofluorescence and RT-PCR analyses.
OLF is definitely a lesion of ectopic bone formation developed within the ligamentum flavum, and the developmental mode of OLF is mainly endochondral ossification.17 Histological studies revealed fibroblastic proliferation as endochondral ossification process, followed by chondroblasts, which are eventually transformed into osteoblasts with blood vessels and Haversian canals.23 Collagen type II is recognized as a specific marker for chondrocytes,20 and osteocalcin is a typical marker of osteoblasts and a good indication for late stages of osteoblastic differentiation.19 The expression of osteocaclin and collagen type II was observed in cultured OLF cells, indicating that OLF cells have osteoblast phenotype and chondrocyte phenotype. Hence, differentiation of ligament cells toward chondrocyte phenotype and osteoblast phenotype could play a pivotal role in initiation and development of OLF.
Collagen type I and type III are the important extracellular matrix proteins of ligamentum flavum cells.24 The present data showed that they were expressed in cultured OLF cells and non-OLF cells. Howerver, non-OLF cells were negative in collagen type II and osteocaclin, and showed fibroblast-like characteristics.
The result in the present culture model indirectly agrees with previous histological and immunohistochemical findings: collagen type II-positive chondrocyte-like cells and alkaline phosphatase-positive osteoblast-like cells were observed within and around the ossification fronts.14,16 A number of studies have also documented that osteogenesis cytokines, such as bone morphogenetic proteins (BMPs), and transforming growth factor (TGF)-β, are localized around the ossification front.13,14,16 These causative factors could play a role in the initiation of cellular events by which ligament flavum cells differentiate into chondrocytes and osteoblasts.
In conclusion, the present study reveals that cultured cells from the ossified ligamentum flavum have phenotypic characteristics for osteoblasts and chondrocytes which could have a role in the pathophysiology of OLF. The findings will be helpful in better understanding the nature of OLF. Future studies will need to investigate the causal mechanisms underlying cell differentiation in the ligament.
References
- 1.al-Orainy IA, Kolawole T. Ossification of the ligament flavum. Eur J Radiol. 1998;29:76–82. doi: 10.1016/s0720-048x(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 2.Kuh SU, Kim YS, Cho YE, Jin BH, Kim KS, Yoon YS, et al. Contributing factors affecting the prognosis surgical outcome for thoracic OLF. Eur Spine J. 2006;15:485–491. doi: 10.1007/s00586-005-0903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascal-Moussellard H, Cabre P, Smadja D, Catonné Y. Symptomatic ossification of the ligamentum flavum: a clinical series from the French Antilles. Spine. 2005;30:E400–E405. doi: 10.1097/01.brs.0000169450.49181.91. [DOI] [PubMed] [Google Scholar]
- 4.Aizawa T, Sato T, Sasaki H, Kusakabe T, Morozumi N, Kokubun S. Thoracic myelopathy caused by ossification of the ligamentum flavum: clinical features and surgical results in the Japanese population. J Neurosurg Spine. 2006;5:514–519. doi: 10.3171/spi.2006.5.6.514. [DOI] [PubMed] [Google Scholar]
- 5.Tokala DP, Lam KS, Prince HG. Ossification of the proximal thoracic ligamenta flava causing acute myelopathy in a Caucasian: case report and literature review. Spinal Cord. 2007;45:310–313. doi: 10.1038/sj.sc.3101953. [DOI] [PubMed] [Google Scholar]
- 6.Nishiura I, Isozumi T, Nishihara K, Handa H, Koyama T. Surgical approach to ossification of the thoracic yellow ligament. Surg Neurol. 1999;51:368–372. doi: 10.1016/s0090-3019(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 7.Shiigi E, Sugiyama T, Tanaka H, Murata H, Shirakura Y, Kawai S. Possible involvement of vitamin D receptor gene polymorphism in male patients with ossification of spinal ligaments. J Bone Miner Metab. 2001;19:308–311. doi: 10.1007/s007740170015. [DOI] [PubMed] [Google Scholar]
- 8.Kong Q, Ma X, Li F, Guo Z, Qi Q, Li W, et al. COL6A1 polymorphisms associated with ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament. Spine. 2007;32:2834–2838. doi: 10.1097/BRS.0b013e31815b761c. [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto N, Maeda T, Miura H, Jingushi S, Hosokawa A, Harimaya K, et al. Repetitive tensile stress to rat caudal vertebrae inducing cartilage formation in the spinal ligaments: a possible role of mechanical stress in the development of ossification of the spinal ligaments. J Neurosurg Spine. 2006;5:234–242. doi: 10.3171/spi.2006.5.3.234. [DOI] [PubMed] [Google Scholar]
- 10.Maigne JY, Ayral X, Guérin-Surville H. Frequency and size of ossifications in the caudal attachments of the ligamentum flavum of the thoracic spine. Role of rotatory strains in their development. An anatomic study of 121 spines. Surg Radiol Anat. 1992;14:119–124. doi: 10.1007/BF01794886. [DOI] [PubMed] [Google Scholar]
- 11.Shirakura Y, Sugiyama T, Tanaka H, Taguchi T, Kawai S. Hyperleptinemia in female patients with ossification of spinal ligaments. Biochem Biophys Res Commun. 2000;267:752–755. doi: 10.1006/bbrc.1999.2027. [DOI] [PubMed] [Google Scholar]
- 12.Terakado A, Tagawa M, Goto S, Yamazaki M, Moriya H, Fujimura S. Elevation of alkaline phosphatase activity induced by parathyroid hormone in osteoblast-like cells from the spinal hyperostotic mouse TWY (twy/twy) Calcif Tissue Int. 1995;56:135–139. doi: 10.1007/BF00296345. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Ishidou Y, Yonemori K, Nagamine T, Origuchi N, Maeda S, et al. Expression and localization of bone morphogenetic proteins (BMPs) and BMP receptors in ossification of the ligamentum flavum. Bone. 1997;21:23–30. doi: 10.1016/s8756-3282(97)00080-x. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto R, Ikata T, Murase M, Hasegawa T, Fukushima T, Hizawa K. Comparative study between magnetic resonance imaging and histopathologic findings in ossification or calcification of ligaments. Spine. 1991;16:1253–1261. doi: 10.1097/00007632-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, Nagai E, Murata H, Tsubone T, Shirakura Y, Sugiyama T, et al. Involvement of bone morphogenic protein-2 (BMP-2) in the pathological ossification process of the spinal ligament. Rheumatology (Oxford) 2001;40:1163–1168. doi: 10.1093/rheumatology/40.10.1163. [DOI] [PubMed] [Google Scholar]
- 16.Yayama T, Uchida K, Kobayashi S, Kokubo Y, Sato R, Nakajima H, et al. Thoracic ossification of the human ligamentum flavum: histopathological and immunohistochemical findings around the ossified lesion. J Neurosurg Spine. 2007;7:184–193. doi: 10.3171/SPI-07/08/184. [DOI] [PubMed] [Google Scholar]
- 17.Okada K, Oka S, Tohge K, Ono K, Yonenobu K, Hosoya T. Thoracic myelopathy caused by ossification of the ligamentum flavum. Clinicopathologic study and surgical treatment. Spine. 1991;16:280–287. doi: 10.1097/00007632-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Specchia N, Pagnotta A, Gigante A, Logroscino G, Toesca A. Characterization of cultured human ligamentum flavum cells in lumbar spine stenosis. J Orthop Res. 2001;19:294–300. doi: 10.1016/S0736-0266(00)00026-7. [DOI] [PubMed] [Google Scholar]
- 19.zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003;71:18–27. doi: 10.1046/j.1432-0436.2003.700602.x. [DOI] [PubMed] [Google Scholar]
- 20.Huh YH, Ryu JH, Chun JS. Regulation of type II collagen expression by histone deacetylase in articular chondrocytes. J Biol Chem. 2007;282:17123–17131. doi: 10.1074/jbc.M700599200. [DOI] [PubMed] [Google Scholar]
- 21.Pantazis G, Tsitsopoulos P, Bibis A, Mihas C, Chatzistamou I, Kouzelis C. Symptomatic ossification of the ligamentum flavum at the lumbar spine: a retrospective study. Spine. 2008;33:306–311. doi: 10.1097/BRS.0b013e3181624535. [DOI] [PubMed] [Google Scholar]
- 22.Inamasu J, Guiot BH, Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58:1027–1039. doi: 10.1227/01.NEU.0000215867.87770.73. [DOI] [PubMed] [Google Scholar]
- 23.Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37:129–136. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 24.Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta1. J Orthop Res. 2002;20:1380–1386. doi: 10.1016/S0736-0266(02)00046-3. [DOI] [PubMed] [Google Scholar]