Abstract
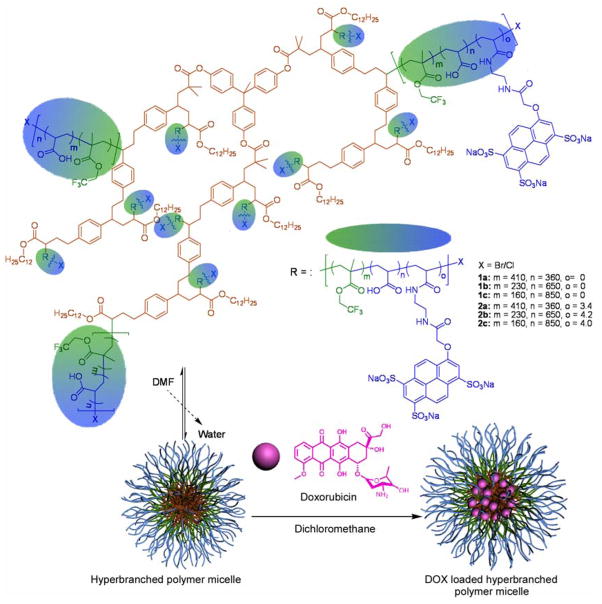
Micelles from amphiphilic star-block copolymers, having a hydrophobic hyperbranched core and amphiphilic fluoropolymer arms, were constructed as drug delivery-agent assemblies. A series of polymer structures was constructed from consecutive copolymerizations of 4-chloromethylstyrene with dodecyl acrylate and then 1,1,1-trifluoroethyl methacrylate with tert-butyl acrylate, followed by acidolysis to release the hydrophilic acrylic acid residues. These structures were labeled with cascade blue as a fluorescence reporter. The series of materials differed primarily in the ratio of 1,1,1-trifluoroethyl methacrylate to acrylic acid units, to give differences in fluorine loading and hydrophobicity/hydrophilicity balance. Doxorubicin (DOX) was used as a therapeutic to study the loading, release and cytotoxicity of these micellar constructs on an U87-MG-EGFRvIII-CBR cell line. The micelles, with TEM-measured diameters ranging from 5–9 nm and DLS-measured hydrodynamic diameters from 20–30 nm, had loading capacities of ca. 4 wt% of DOX. The DOX-loaded micelles exhibited potent cytotoxicity with cell viabilities of 60-25% at 1.0 μg/mL effective DOX concentrations, depending upon the polymer composition, as determined by MTT assays. These cell viability values are comparable to that of free DOX, suggesting an effective release of the cargo and delivery to the cell nuclei, which was further confirmed by fluorescence microscopy of the cells. 19F-NMR spectroscopy indicated a partial degradation of the surface-available trifluoroethyl ester linkages of the micelles, which may have accelerated the release of DOX. 19F-NMR spectroscopy was also employed to confirm and to quantify the cell uptake of the micelles. These dual fluorescent- and 19F-labeled and chemically-functional micelles may be used potentially in a variety of applications, such as cell labeling, imaging, and therapeutic delivery.
INTRODUCTION
Nanoscopic systems that can incorporate combined imaging and therapeutic capabilities are the focus of intensive research for applications in the growing field of nanomedicine.(1–7) Such systems offer the possibility to increase diagnostic accuracy and therapeutic effectiveness, while minimizing side effects from treatment. Nanoscopic polymeric assemblies from dendrimers,(8, 9) polymer micelles,(5, 10, 11) shell crosslinked knedel-like (SCKs) nanoparticles(12–14) and other polymer assemblies(15, 16) are promising candidates for such multifunctional therapeutic platforms, since these materials are conveniently synthesized, variably functionalized, and can be assembled to a range of sizes and compositions.
Such polymeric assemblies usually have an amphiphilic composition, in which the hydrophobic component serves as a reservoir for therapeutic cargo and/or imaging agents, and the hydrophilic component provides stability in solution and can be tailored for attaching imaging agents and/or targeting ligands. Therapeutic cargos, usually packed through hydrophobic-hydrophobic interactions, when not covalently attached, can be released through passive diffusion(12) or as a response to external stimuli such as pH,(8–10, 17) temperature,(17–19) and light.(19) In order to utilize polymer assemblies for systemic delivery of therapeutics, it is important to maximize the loading capacity to minimize injection volume for intravenous delivery, and also to achieve sustained and controlled release.
Our group has long interest in developing polymers for the construction of multi-functional nanoscopic assemblies for imaging and drug delivery purposes.(12, 14, 16, 20, 21) One such system is the 1,4,7,10-tetraazocyclododecane-N,N′N″N‴-tetraacetic acid (DOTA)-conjugated SCKs that can chelate 64Cu for labeling and in vivo PET imaging.(14, 22) Extension of the SCKs to serve as a therapeutic delivery system has also been reported.(12) Recently, we developed fluorinated hyperbranched star-like polymers that can serve as 19F magnetic resonance imaging (MRI) agents.(23) The multi-compartmental amphiphilicity of these structures gave rise to the hypothesis that they are capable of therapeutic agent packaging while also providing for detectable imaging or labeling for tracking of the nanoparticle locations. In this study, utilization of these materials as 19F and fluorescence dual-labeled imaging assemblies as well as nanocarriers for chemotherapeutic cargos was explored. 19F NMR/MRI techniques were successfully applied to track and quantify cell migration and uptake. Due to near-zero background, the technique is highly sensitive. In this study, 19F NMR was applied to quantify the number of micelles taken up by the cells and to determine the micelle stability. Doxorubicin (DOX) was selected the therapeutic agent and the loading, release, and cytotoxicity of the nanocarrier constructs was investigated in depth, and compared against the behaviors of the host nanomaterial and the small molecule drug, each individually and together. DOX has been studied extensively in micellar delivery strategies(5, 8, 24, 25) and is a common therapeutic for the treatment of a range of cancers.(26) It also exhibits antibiotic properties, and may have benefit toward other diseases that involve a need for reduction in cell growth, such as to interfere with restenosis by using drug eluting stents.(27) Most importantly, for these fundamental studies, DOX is a viable model drug, due to its inherent UV-vis/fluorescence characteristics. We found that these polymer micelle assemblies were capable of packaging DOX and that the DOX-micelle constructs exhibited cytotoxicity against U87-MG-EGFRvIII-CBR cancer cells that nearly matched that of the free DOX. The successful construction of dual-labeled micelles may find applications in the area of drug delivery, wherein monitoring their migration and uptake activity require careful monitoring.
EXPERIMENTAL PROCEDURES
Materials and methods
All chemicals were purchased from Sigma–Aldrich Chemical Company, unless otherwise noted. Cascade Blue® (CB) ethylenediamine tri-sodium salt and MTT assay kit were purchased from Invitrogen Co. The amphiphilic hyperbranched polymer materials, 1a–c, were prepared as previously reported.(23)
U87-MG-EGFRvIII-CBR cells (a human glioblastoma cell line modified to express the mutant EGFRvIII receptor and click beetle red luciferase) have been previously reported.(12) 1H-NMR spectra were recorded on a 500 MHz Varian Inova 500 spectrometer, with the solvent proton signal as standard. 19F-NMR spectra were recorded at 470 MHz on a Varian Inova 500 spectrometer equipped with a fluorine-observe 5 mm probe (Nalorac Co. CA) with trifluoroacetic acid (TFA) as an internal standard.
Dynamic light scattering (DLS) measurements were acquired using a Brookhaven Instruments (Worcestershire, U.K.) system, including a model BI-200SM goniometer, a model BI-9000AT digital correlator, a model EMI-9865 photomultiplier, and a model 95-2 Ar ion laser (Lexel, Corp.; Farmindale, NY) operated at 514.5 nm. Measurements were made at 25 ± 1 °C. Prior to analysis, solutions were filtered through a 0.22 μm Gelman Nylon Acrodisc® 13 membrane filter to remove dust particles. Scattered light was collected at a fixed angle of 90°. The digital correlator was operated with 522 ratio spaced channels, and initial delay of 5 μs, a final delay of 100 ms, and a duration of 10 minutes. A photomulitplier aperture of 400 μm was used, and the incident laser intensity was adjusted to obtain a photon counting of between, 200 and 300 kcps. The calculations of the particle size distributions and distribution averages were performed with the ISDA software package (Brookhaven Instruments Co.), which employed single-exponential fitting, cumulants analysis, non-negatively constrained least-squares (NNLS) and CONTIN particle size distribution analysis routines. The data is presented as the average values from four measurements.
Transmission electron microscopy (TEM) measurements were conducted on a Hitachi H600 microscope. Micrographs were collected at 100,000× magnification and calibrated using a 41 nm polyacrylamide bead from NIST. Carbon-coated copper grids were treated with oxygen plasma prior to deposition of the micellar samples. The samples were stained with 1% phosphotungstic acid (PTA) for 1 minute, and then the solution was wicked away and the samples were allowed to dry under ambient conditions. The number-average particle diameters (Dav) and standard deviations were generated from the analysis of a minimum of 100 particles from at least three different micrographs.
UV-vis spectroscopy was acquired on Varian Cary 1E UV-vis system (Varian Inc., Palo Alto, CA) using PMMA quvettes.
Synthesis
Preparation of cascade blue-conjugated micelles [2a–2c]
To three solutions of polymers 1a–c (20 mg each of 1a–1c, 0.20, 0.22 and 0.21 μmol for 1a–1c) in DMF (5 mL) was added HBTU (3.8 mg, 1 × 10−2 mmol) and HOBT (1.4 mg, 1 × 10−2 mmol). The solutions were allowed to stir under argon for 1 h. DIPEA (4 mg, 4 × 10−2 mmol) and Cascade Blue (0.63 mg, 1 × 10−3 mmol) were added. The reactions were stirred under argon overnight. DMF (15 mL) was added and the solutions were dialyzed (MWCO 30 kDa) against nanopure water for 84 h. Micellar solutions were thereby obtained and the amounts of Cascade Blue (CB) were determined by UV-Vis (400 nm, ε= 28000 M−1 cm−1 from manufacturer’s manual). A portion of the micelle solution was lyophilized to dryness for 1H and 19F NMR analyses.
2a: 3.4 CB/polymer (68%), micelle concentration, 0.33 mg mL−1. 1H-NMR (500 MHz, DMSO-d6): δ 0.8–2.3 (br, m, CH2 and CH of backbone), 4.3–4.7 (s, br, CH2CF3, PhCH2), 6.4–7.5 (m, br, ArH), 7.6–8.0 (m, br, ArH of CB), 8.2 (ArH of CB), 8.6 (s, ArH of CB), 9.0 (s, ArH of CB), 9.9 (s, ArH of CB), 12.0–12.5 (s, br, COOH) ppm. 19F-NMR (470 MHz, DMSO-d6): δ 2.7 (s) ppm. TEM: Dav: 9 ± 2 nm, DLS: Dh(n) 21 ± 2 nm, Dh(v): 29 ± 4 nm, Dh(i): 129 ± 20 nm.
2b: 4.2 CB/polymer (84%), micelle concentration 0.35 mg mL−1. 1H-NMR (500 MHz, DMSO-d6): δ 0.8–2.3 (br, m, CH2 and CH of backbone), 4.3–4.7 (s, br, CH2CF3, PhCH2), 6.4–7.5 (m, br, ArH), 7.6–8.0 (m, br, ArH of CB), 8.2 (ArH of CB), 8.6 (s, ArH of CB), 9.0 (s, ArH of CB), 9.9 (s, ArH of CB), 12.0–12.5 (s, br, COOH) ppm. 19F-NMR (470 MHz, DMSO-d6): δ 2.7 (s) ppm. TEM: Dav: 8 ± 2 nm; DLS: Dh(n) 26 ± 4 nm, Dh(v): 29 ± 6 nm, Dh(i): 135 ± 25 nm.
2c: 4.0 CB/polymer (80%), micelle concentration 0.32 mg mL−1. 1H-NMR (500 MHz, DMSO-d6): δ 0.8–2.3 (br, m, CH2 and CH of backbone), 4.3–4.7 (s, br, CH2CF3, PhCH2), 6.4–7.5 (m, br, ArH), 7.6–8.0 (m, br, ArH of CB), 8.2 (ArH of CB), 8.6 (s, ArH of CB), 9.0 (s, ArH of CB), 9.9 (s, ArH of CB), 12.0–12.5 (s, br, COOH) ppm. 19F-NMR (470 MHz, DMSO-d6): δ 2.7 (s) ppm. TEM: Dav: 5 ± 1 nm; DLS: Dh(n) 29 ± 3 nm, Dh(h): 33 ± 6 nm, Dh(n): 135 ± 20 nm.
General procedure for DOX loading
A solution of DOX (2.7 mg mL−1 in CH2Cl2 and 3 eq. of triethylamine, 30% w/w with respect to the micelle) was added to a vial containing a magnetic stir bar and the micelle (4 mL, polymer concentration ~ 0.30 mg mL−1). The solution were protected from light and stirred without a cap in a well-ventilated fume hood for 16 h. Insoluble DOX was removed by centrifugation (3000 rpm × 10 min) and the supernatants were transferred to a centrifugal filter device (Amicon Ultra 4, 30 kDa MWCO, Millipore Corp., Billerica MA, USA) and washed extensively with 5 mM PBS pH 7.4 buffer at 37 °C to remove all unincorporated DOX. After eight washing cycles, the filtrate was analyzed by UV-vis (488 nm) to confirm the successful removal of the free DOX. The DOX-loaded micelle solutions were then reconstituted to a final volume of 4 mL. The amount of incorporated DOX was determined by UV-vis (480 nm, ε = 12500 M−1cm−1 determined by a calibration curve in DMF/PBS 4:1) in a 4:1 v/v mixture of DMF and DOX-micelle solution.
General procedure for the release experiments
DOX-micelle solutions (2 mL) were transferred to a presoaked dialysis cassette (Slide-A-Lyzer, 10 kDa MWCO, Pierce Biotechnology, Rockford IL, USA). The cassettes were then stirred in a beaker containing 4 L of 5 mM PBS at pH 7.4 and 37 °C for a period of 48 h. Samples (~100 μL) were removed from the cassette at 0, 1, 2, 3, 4, 8, 16, 24, and 48 h and analyzed by UV-vis (488 nm, ε = 12500 M−1cm−1 determined by a calibration curve in PBS). All release experiments were conducted in triplicate.
Cell cytotoxicityassay on U87-MG-EGFRvIII-CBR cells
Cell cytotoxicity assay was conducted using MTT assay kit and followed the manufacturer’s protocol. Briefly,5000 cells/well of the U87-MG-EGFRvIII-CBR cells were plated in triplicate in 96-well plate 24 h before treatment. Cells were then washed twice with sterile PBS and exposed to different doses of either free DOX or DOX-micelles at 37 °C for 2 h in 10% FBS α-MEM. After 2 h of incubation, the cells were washed again twice with sterile PBS and re-fed with fresh media and further incubated at 37 °C for 72 h before the MTT assay was performed. To assess cell viability, 10 μL of MTT (5 mg ml−1) solution was added into each well and incubated at 37 °C for 4 h. The reaction was terminated with 10% SDS stop solution. Absorbance was read at 570 nm using μQuant plate reader (Bio-Tek Instruments Inc.) and viability was calculated as the percentage of control (cells receiving no treatment).
In vitro fluorescent microscopy study
87-MG-EGFRvIII-CBR cells were treated for 24 h with either 1 μg ml−1 of DOX or DOX-loaded micelles. Afterwards, cells were washed twice with PBS buffer and fixed with 4% paraformaldehyde. Fluorescence was observed using Zeiss fluorescence microscope equipped with a appropriate filter set (Axioskop plus, Carl Zeiss, Germany) and pictures were taken using 63× oil lenses.
Micelle stability studies by 19F-NMR
U87-MG-EGFRvIII-CBR cells (1 million) suspended in 200 μL PBS buffer were mixed with micelles in PBS buffer and this cell solution was then placed in a 5 mm NMR tube. TFA (0.050 mg/mL) was used as an internal reference. The pH was measured to be 7.4 before the kinetics studies. The degree of hydrolysis was calculated based on the relative integration value of the trifluoroethyl ester at 2.7 ppm and the integration value of trifluoroethanol at −1.2 ppm. In a control study, PBS buffer, instead of the cell solution was used and the protocol was identical. The parameters used were: d1= 10 s, sw = 19189.3 Hz, nt = 378, lb = 5 Hz, temp = 37 °C.
Cell uptake studies by 19F-NMR
U87-MG-EGFRvIII-CBR cells (4 million) were plated on a 6-well plate and then treated with 500 μL of micelle solution for at 37 °C 2 h. Afterwards, the cells were washed 3 times with PBS buffer and lifted by trypsin. Cells were washed again with PBS buffer and centrifuged 3 times (3000 rpm × 5 min). Cell pellet was collected and fixed with 4% paraformaldehyde and re-suspended into 250 μL PBS buffer. The cells were sonicated for 1 minute, 100 μL of D2O and 10 μL of 0.050 mg/mL TFA solution were added. The solution was transferred into a water compatible BMS-005TJ Advanced NMR microtube (Shigemi Inc., PA) and subjected to 19F-NMR spectroscopic analysis. In a control study, PBS buffer instead of micelle solution was used and the protocol was identical. The parameters used were: d1= 10 s, sw = 19189.3 Hz, nt = 8620, lb = 5 Hz.
RESULTS AND DISCUSSION
Synthesis and micellization of the cascade blue-conjugated fluoropolymers
The polymers 1a–1c(23) were functionalized with Cascade Blue (CB) ethylene diamine to give CB-functionalized polymers (Scheme 1). Coupling of CB ethylene diamine to the pre-established macromolecules, having a hyperbranched hydrophobic core and amphiphilic copolymer arms comprised of trifluoroethyl methacrylate and acrylic acid residues, was facilitated by the addition of standard peptide coupling reagents, N-hydroxybenzotriazole (HOBt) and O—benzotriazol-1-yl-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) in N,N-dimethylformamide (DMF), to give an amide linkage between the CB and the macromolecule acrylic acid sites. Initially, polymers with ca. two CB units per polymer chain were prepared, but the corresponding micelles failed to produce adequate fluorescence microscopy images with our instrumental resolutions. Therefore, the theoretical number of CBs per polymer was increased to ca. five during the coupling reactions, which afforded CB/polymer values of 3.3, 4.2, and 4.0 per polymer for samples 2a, 2b, and 2c, corresponding to conjugation efficiencies of 69%, 82% and 79%, respectively, as determined by UV-vis spectroscopy.
Scheme 1.
Chemical structures and compositions for micelles 1a–1c and 2a–2c, and schematic illustrations of the amphiphilic star-like structure, assembled as uni- or multi-molecular aggregates and the loading of DOX into those nanostructures.
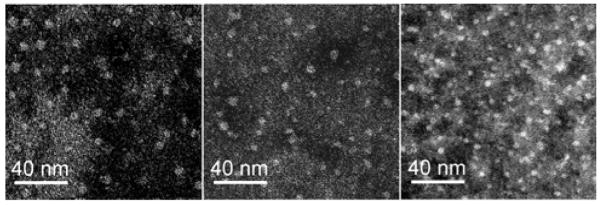
Recently, we reported that purification and micellization can be performed simultaneously,(28) thus, after the conjugations of CB, additional DMF was added to the reaction mixtures and the solutions were dialyzed subsequently against nanopure water for 84 h to induce self assembly into polymeric micelles while removing non-covalently-linked CB. The polymers readily formed micelles, as determined by transmission electron microscopy (TEM) and dynamic light scattering (DLS) studies (Figure 1 and Table 1). Compared to the original micelles of the precursors, 1a–1c(23), conjugation of CB did not change the TEM size of the micelles significantly (Table 1). The DLS data showed slight increases in number-average hydrodynamic diameter (Dh) values.
Figure 1.
TEM images of micelles 2a–2c (shown from left to right, respectively), on a carbon-coated copper grid with negative staining by 1% phosphotungstic acid.
Table 1.
Summary of characterization data and doxorubicin loading for micelles 2a–2c.
| Sample | TEM [nm] | Dh(n) [nm]a | Dh(v) [nm]a | Dh(i) [nm]a | DOX [μM]b | Loading capacity [wt %] | CB/polymerb |
|---|---|---|---|---|---|---|---|
| 2a | 9±2 | 21±2 | 29 ±4 | 129 ± 20 | 11 | 3.6 + 0.5 | 3.4 |
| 2b | 8±2 | 26 ±4 | 29 ±6 | 135 ±25 | 24 | 4.9 + 0.6 | 4.2 |
| 2c | 5±1 | 29 ±3 | 33 ±6 | 138 ±20 | 22 | 4.0 + 0.7 | 4.0 |
by DLS
by UV-vis
DOX loading and release profiles
DOX was incorporated into the micelles using a similar procedure as published previously(8) Dichloromethane was used as the organic solvent to solvate the DOX and to swell the core of the micelle, facilitating the loading of the therapeutic. The amount of DOX in the micelle solutions were determined by UV-vis measurements. The loading capacities for micelles 2a–2c were 3.6 ± 0.5, 4.9 ± 0.6 and 4.0 ± 0.7 wt% based on polymer mass, respectively (Table 1). During the preparation of the micelles, we noticed that the micelle solution 2a precipitated after ca. one week of storage, while micelles 2b and 2c were storage stable for months, indicating that a high fluorine content adversely affected the formation and stability of the micelles, which may be a reason for its low loading capacity. The balance of fluorine and carboxylic acid content also affected the DOX release profiles. The release of the DOX was assessed by monitoring the decrease of UV-vis absorbance over time.(8, 12) Samples were withdrawn from dialysis cassettes at progressive time intervals and analyzed by UV-vis spectroscopy. In this release model, DOX molecules are first released from the micelles, and then pass through the dialysis membrane. Since the rate-determining step is the rate that DOX is released from the micelles (Figure 2), the overall rate can be considered as the rate that DOX was released from the micelles. Using this release model, the DOX release behavior was similar for 2b and 2c, but 2a exhibited a faster release rate (Figure 2), possibly due the fact that 2a does not have a stable micelle structure. For 2b and 2c, a release of ca. 25% of the cargo was observed in 8 h, followed by a slow, sustained release over the course of 48 h. At the end of 48 h, 66%, 45% and 51% of DOX had released from micelles 2a, 2b and 2c, respectively. The release profiles were slower than those observed for highly branched dendritic constructs(8) loaded with DOX, but similar to release rates from shell crosslinked nanoparticles.(12)
Figure 2.
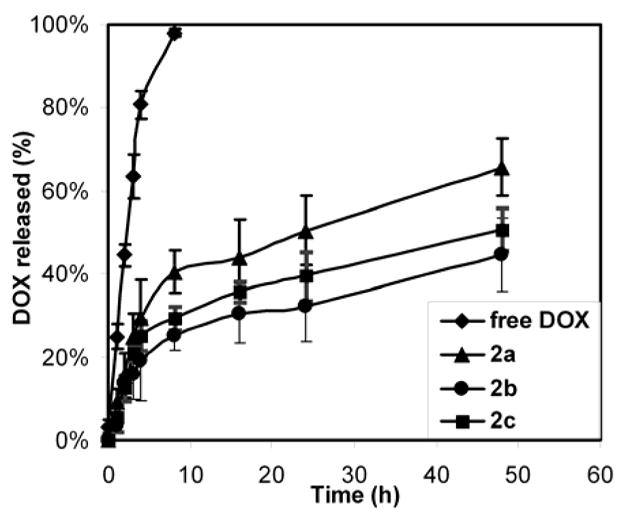
Release profiles of DOX from a dialysis cassette (control labeled as free DOX in the legend) and from dialysis cassettes containing micelles of 2a–2c over time in PBS buffer (5 mM, pH 7.4) at 37 °C.
Cell cytotoxicity studies
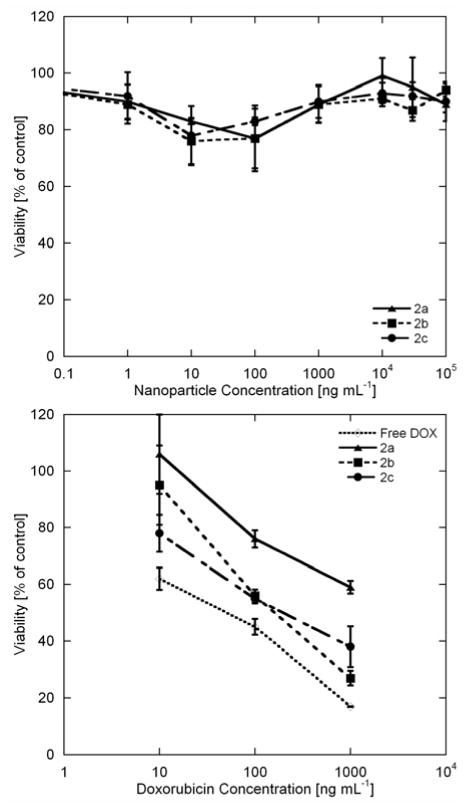
The cytotoxicities of 2a–2c were studied by incubating U87-MG-EGFRvIII-CBR cells with varying concentrations of the micelles. Cell viability was assessed using a Vibrant MTT cell proliferation assay kit. Cells were first incubated with DOX-loaded and non-loaded micelles for 2 h, subsequently, the cells were washed twice with PBS buffer and re-fed with fresh media. After 72 h of incubation, MTT assays were conducted. No significant toxicity was observed at concentrations up to 100,000 ng mL−1 for the non-loaded micelles (Figure 3, top). In comparison, the DOX-loaded micelles exhibited potent cytotoxic effects (Figure 3, bottom). At 1,000 ng mL−1, only 30% of the cells treated with DOX-loaded micelles survived (sample 2b), which is comparable to free DOX at the same dose concentration (20% survived, Figure 3, bottom), indicating that this nanocarrier can achieve excellent cytotoxic effect on this glioma cell line. Interestingly, compared to glassy amorphous SCK nanoparticles derived from poly(acrylic acid)-b-poly(styrene) (PAA-b-PS),(12) the micelles in this study have lower loading capacity of DOX, but showed significantly greater cytotoxicity. The improvement of therapeutic efficacy is possibly due to a higher extent of release of DOX in these systems (45–66% after 48 h, compared to the SCK system that retains more cargo, with only 30–55% release after 48 h), as this new system was designed to contain an integrated mixing between the hydrophobic pockets and hydrophilic domains, giving a more open, multi-compartment structure, in contrast to the core-shell structure of SCK nanoparticles.
Figure 3.
Cell viability studies usingMTT assay. U87-MG-EGFRvIII-CBR cells were incubated with 2a–2c without (top) and with DOX (bottom) loading at varied concentrations.
We also noticed that in the presence of cells, a partial degradation of the trifluoroethyl ester moiety occurred, which may further enhance the release of DOX. The substantial chemical shift difference between trifluoroethyl ester and trifluoroethanol, and the zero-background of 19F-NMR signal allows real-time monitoring of the hydrolysis of these fluorinated micelles, upon cellular incubation. As seen in Figure 4, the trifluoroethyl esters in the micelle exhibited a broad 19 F resonance at 2.7 ppm, and the degradation product, trifluoroethanol, gave a sharp triplet at −1.2 ppm with a 3JH-F = 8.5 Hz. While the micelle solutions were very stable in water and PBS (for months), as monitored by 19F-NMR spectroscopy, incubation with cells resulted in quick release of trifluoroethanol. Within 1 h, ca. 2% of trifluoroethanol was detected by 19F-NMR spectroscopy. The hydrolysis proceeded rapidly up to ca. 4 h, with a total of ca. 8% degradation. Afterwards, a sudden halt of degradation was observed, resulting in a non-linear degradation profile (see supporting information). A halt in degradation may be due to a loss of enzymatic activity. Alternatively, the hydrolysis may be limited to the surface-available trifluoroethyl ester groups, while others remain stable beneath the surface of the polymer or polymer micelle assembly. A thorough study of the hydrolysis of these micellar constructs using lipases and enzymatic inhibitors is currently underway. Nevertheless, the partial hydrolysis of trifluoroethyl ester during the cell incubation may result in a faster and more complete release of DOX in vitro, as compared to release data from the dialysis model. Although the hydrolysis product, trifluoroethanol, may be toxic to cells, we did not observe a significant negative effect on the viability of the cells incubated with the control micelle solutions (Figure 3, top).
Figure 4.
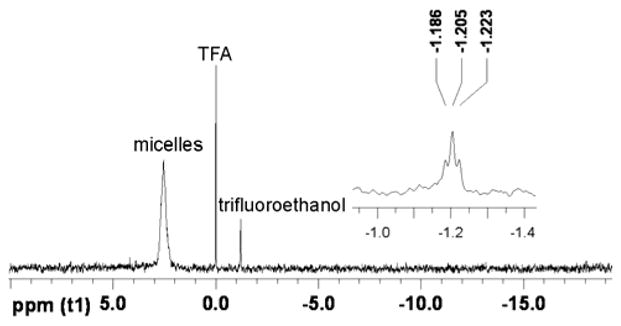
19F-NMR spectra demonstrating partial hydrolysis of the trifluoroethyl ester units of 2b (470 MHz, H2O/D2O, with TFA as an internal standard, 4 h after incubation of micelles with cells at 37 °C).
Fluorescence microscopy studies
The cellular uptake of the polymer micelles were also studied by fluorescence microscopy. We took advantage of the blue fluorescence from CB and red autofluorescence from DOX to study the cellular uptake of the polymeric micelles and localization of DOX within the cells delivered by these polymers. As shown in Figure 5, cells treated with all three control micelles (2a–2c) demonstrated significant amounts of blue fluorescence, mostly in the cytoplasm. This intracellular trafficking is in contrast with that of free CB dye as a small molecule, which binds strongly to the nucleus (data not shown), confirming that the fluorescence observed arises from polymer-dye conjugates. At this moment, the mechanism of cellular uptake of these micelles is not clear and remains to be investigated. However, the blue fluorescence clearly indicated that cell uptake indeed occurred.
Figure 5.
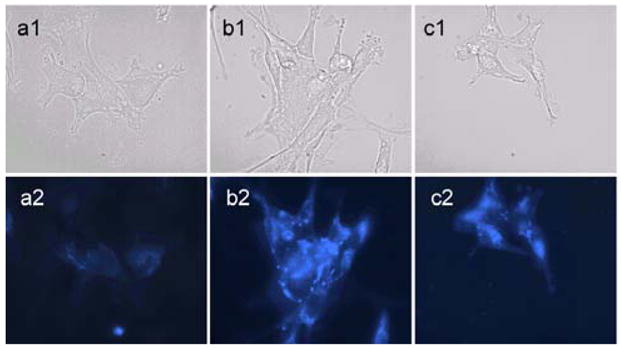
Cellular uptake of micelles by fluorescence microscopy study. U87-MG-EGFRvIII-CBR cells were incubated with micelle solutions of 2a, 2b and 2c, respectively. 1: phase contrast. 2: blue fluorescence. The cells were treated with mice lle (0.1 mg/mL) for 2 h at 37 °C, followed by repeated washings and then fixed with 4% paraformaldehyde.
Because micelles 2b showed the best cell viability reduction (Figure 3, bottom), we chose micelles 2b for further microscopy studies on DOX localization. As seen in Figure 6, within 2 h treatment, DOX molecules were already delivered inside the cells, although mostly present in the cytoplasm surrounding the nucleus (Figure 6, a2). In contrast, after 24 h treatment, DOX was located within the nucleus (Figure 6, b2); the nuclei are brighter, indicating more DOX molecules have been incorporated. These images indicated efficient deliveries of DOX to the nucleus, which may be responsible for cell viability reductions.
Figure 6.
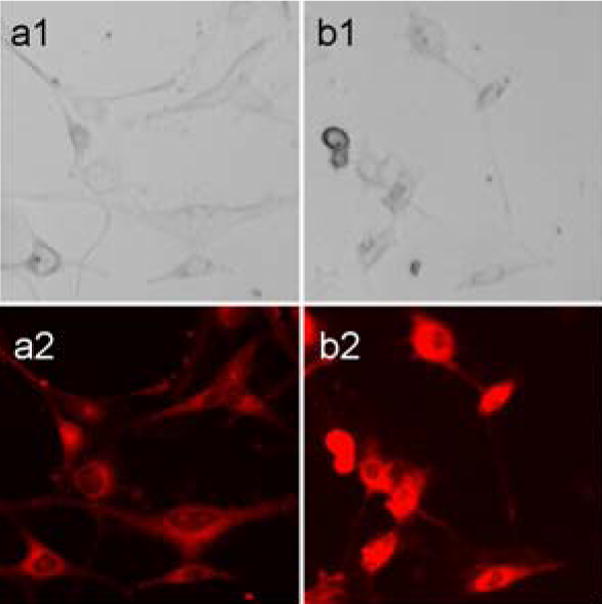
Localization of DOX upon treatment of DOX-loaded micelles by fluorescence microscopy. U87-MG-EGFRvIII-CBR cells were incubated with DOX-loaded micelle 2b. 1: phase contrast. 2: red fluorescence. a: 2 h after treatment. b: 24 h after treatment, more DOX were incorporated into the nucleus.
19F-NMR studies on the cellular uptake of the micelles
Fluorine-containing nanoscopic assemblies have been used for cell uptake and cell trafficking studies using 19F-NMR spectroscopy or 19F-MRI.(7, 29, 30) Since there is no background signal, the 19F-NMR/MRI technique is highly sensitive, with detection limits in the ng scale. In this study, 19F-NMR spectroscopy was employed to confirm and to quantify cell uptake of 2b in U87-MG-EGFRvIII-CBR cells. This micelle was selected because it had good loading capacity, desired release profile (Figure 2), and the best cytotoxicity result (Figure 3). Cells (4 million) were first incubated with the micelle solution for 2 h, followed by repeated washings with fresh PBS buffer and centrifugation to remove any free micelles. The cells were harvested and sonicated to lyse the cells. Trifluoroacetic acid (TFA) was used as an internal standard to quantify the fluorine concentration. Since the CF3 groups on the micelles have much shorter T1 relaxation times (ca. 500 ms) than that of TFA (ca. 2.5 s), it was necessary to adopt a long relaxation delay (10 s) to assure that both fluorine species were fully relaxed before the next acquisition scan. However, with such long delay times, ca. 24 h scanning time was required to acquire a spectrum with reasonable resolution (Figure 7). Using the same method as reported by Ahrens and coworkers,(30) it was possible to calculate that the total amount of fluorines in each cell was ca. 4.6 × 108 fluorines, indicating about 0.1% cell uptake efficiency. This very low cell uptake is possibly due to the negatively-charged micelle that limits cell uptake compared to neutral nanoemulsions, wherein the cell uptake of fluorine species can reach concentrations of 1011–1013.(30) Nevertheless, even with limited cell uptake, these nanostructures loaded with DOX exhibited a significant ability to reduce cell viability.
Figure 7.
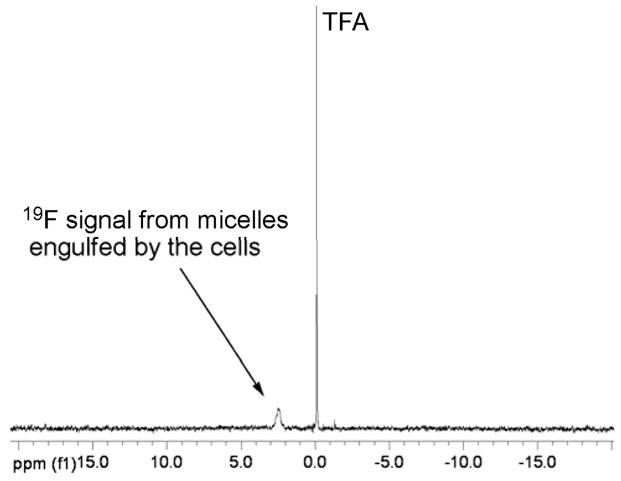
19F-NMR spectrum of U87-MG-EGFRvIII-CBR cell suspensions. 19F signal from micelles, engulfed by the cells, appears at 2.7 ppm.
CONCLUSIONS
In conclusion, we have successfully constructed fluorine and CB dual-labeled nanostructures, studied their loading and release characteristics for therapeutic guests, and applied 19F-NMR and fluorescence microscopy as tools to study their cellular uptake and cell viability reduction. The nanostructures were designed to possess an amphiphilic, multi-compartment morphology for packaging of therapeutic guests, based upon a hyperbranched-linear hybrid star-like structure that assembled into uni- or multi-molecular micelles having optimum hydrodynamic diameters of 20–30 nm. Reasonable quantities of DOX could be loaded into the structures, and they exhibited differential release profiles, in terms of rates and ultimate amounts, depending upon their composition. These DOX-loaded nanostructures exhibited potent cytotoxicity on U87-MG-EGFRvIII-CBR cells. We anticipate that the incorporation of ligands for cell targeting will improve the DOX delivery even further. 19F-NMR spectroscopy showed a partial degradation of the surface available fluorinated polymer segments, which could accelerate the release of DOX from the micelles in vitro and in vivo. The successful construction of this dual-labeled, multifunctional nanocarrier may find promising applications in nanomedicine. The key unmet challenge is to now prepare such nanoscale constructs with cell-specific targeting groups so that therapeutics can be efficiently delivered with minimal side effects.(5, 15, 31)
Supplementary Material
Acknowledgments
This material is based upon work supported by The Children’s Discovery Institute of St. Louis Children’s Hospital and Washington University School of Medicine, and the National Heart Lung and Blood Institute of the National Institutes of Health as a Program of Excellence in Nanotechnology (HL080729). Postdoctoral and assistant professor fellowship provided by the Knut and Alice Wallenberg Foundation is gratefully acknowledged (A.M.N). The authors thank Drs. Jeff Kao and André d’Avignon for assistance with NMR experiments.
Footnotes
Supporting Information Available: 1H and 19F NMR spectra of polymers, UV-vis spectra of DOX-loaded micelles, DLS data of the micelles, and data regarding the trifluoroethyl ester hydrolysis. This information is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 2.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 3.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Dutta RC. Drug carriers in pharmaceutical design: promises and progress. Curr Pharm Des. 2007;13:761–769. doi: 10.2174/138161207780249119. [DOI] [PubMed] [Google Scholar]
- 5.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 6.Almutairi A, Guillaudeu SJ, Berezin MY, Achilefu S, Fréchet JMJ. Biodegradable pH-sensing dendritic nanoprobes for near-infrared fluorescence lifetime and intensity imaging. J Am Chem Soc. 2008;130:444–445. doi: 10.1021/ja078147e. [DOI] [PubMed] [Google Scholar]
- 7.Partlow KC, Chen J, Brant JA, Neubauer AM, Meyerrose TE, Creer MH, Nolta JA, Caruthers SD, Lanza GM, Wickline SA. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007;21:1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 8.Gillies ER, Fréchet JMJ. pH-Responsive copolymer assemblies for controlled release of doxorubicin. Bioconjugate Chem. 2005;16:361–368. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]
- 9.Gillies ER, Jonsson TB, Fréchet JMJ. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. J Am Chem Soc. 2004;126:11936–11943. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- 10.Chan Y, Wong T, Byrne F, Kavallaris M, Bulmus V. Acid-labile core cross-linked micelles for pH-triggered release of antitumor drugs. Biomacromolecules. 2008;9:1826–1836. doi: 10.1021/bm800043n. [DOI] [PubMed] [Google Scholar]
- 11.Wang YC, Tang LY, Sun TM, Li CH, Xiong MH, Wang J. Self-assembled micelles of biodegradable triblock copolymers based on poly(ethyl ethylene phosphate) and poly(ε-caprolactone) as dug carriers. Biomacromolecules. 2008;9:388–395. doi: 10.1021/bm700732g. [DOI] [PubMed] [Google Scholar]
- 12.Nyström AM, Xu Z, Xu J, Taylor S, Nittisc T, Stewart SA, Leonard J, Wooley KL. SCKs as nanoparticle carriers of doxorubicin: investigation of core composition on the loading, release and cytotoxicity profiles. Chem Commun. 2008:3579–3581. doi: 10.1039/b805428b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi K, Ma Q, Remsen EE, Clark CG, Wooley KL. Determination of the bioavailability of biotin conjugated onto shell cross-linked (SCK) nanoparticles. J Am Chem Soc. 2004;126:6599–6607. doi: 10.1021/ja039647k. [DOI] [PubMed] [Google Scholar]
- 14.Sun G, Hagooly A, Xu J, Nyström AM, Li Z, Rossin R, Moore DA, Wooley KL, Welch MJ. Facile, efficient approach to accomplish tunable chemistries and variable biodistributions for shell cross-linked nanoparticles. Biomacromolecules. 2008;9:1997–2006. doi: 10.1021/bm800246x. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki Y, Maie H, Akiyoshi K. Cell-specific delivery of polymeric nanoparticles to carbohydrate-tagging cells. Biomacromolecules. 2007;8:3162–3168. doi: 10.1021/bm700606z. [DOI] [PubMed] [Google Scholar]
- 16.Fukukawa K, Rossin R, Hagooly A, Pressly ED, Hunt JN, Messmore BW, Wooley K, Welch M, Hawker CJ. Synthesis and characterization of core-shell star copolymers for in vivo PET imaging applications. Biomacromolecules. 2008;9:1329–1339. doi: 10.1021/bm7014152. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Ge Z, Xu J, Liu H, Liu S. Fabrication of multiresponsive shell cross-linked micelles possessing pH-controllable core swellability and thermo-tunable corona permeability. Biomacromolecules. 2007;8:3184–3192. doi: 10.1021/bm700743h. [DOI] [PubMed] [Google Scholar]
- 18.Rijcken CJF, Veldhuis TFJ, Ramzi A, Meeldijk JD, Van Nostrum CF, Hennink WE. Novel fast degradable thermosensitive polymeric micelles based on PEG-block-poly(N-(2-hydroxyethyl)methacrylamide-oligolactates) Biomacromolecules. 2005;6:2343–2351. doi: 10.1021/bm0502720. [DOI] [PubMed] [Google Scholar]
- 19.Orihara Y, Matsumura A, Saito Y, Ogawa N, Saji T, Yamaguchi A, Sakai H, Abe M. Reversible release control of an oily substance using photoresponsive micelles. Langmuir. 2001;17:6072–6076. [Google Scholar]
- 20.Pressly ED, Rossin R, Hagooly A, Fukukawa Ki, Messmore BW, Welch MJ, Wooley KL, Lamm MS, Hule RA, Pochan DJ, Hawker CJ. Structural effects on the biodistribution and positron emission tomography (PET) imaging of well-defined 64Cu-labeled nanoparticles comprised of amphiphilic block graft Copolymers. Biomacromolecules. 2007;8:3126–3134. doi: 10.1021/bm700541e. [DOI] [PubMed] [Google Scholar]
- 21.Sun G, Xu J, Hagooly A, Rossin R, Li Z, Moore DA, Hawker CJ, Welch MJ, Wooley KL. Strategies for optimized radiolabeling of nanoparticles for in vivo PET imaging. Adv Mater. 2007;19:3157–3162. [Google Scholar]
- 22.Xu J, Sun G, Rossin R, Hagooly A, Li Z, Fukukawa K, Messmore BW, Moore DA, Welch MJ, Hawker CJ, Wooley KL. Labeling of polymer nanostructures for medical imaging: importance of cross-linking extent, spacer length, and charge density. Macromolecules. 2007;40:2971–2973. doi: 10.1021/ma070267j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du W, Nyström AM, Zhang L, Powell KT, Li Y, Cheng C, Wickline SA, Wooley KL. Amphiphilic hyperbranched fluoropolymers as nanoscopic 19F-magnetic resonance imaging agent assemblies. Biomacromolecules. 2008;9:2826–2833. doi: 10.1021/bm800595b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CC, Gillies ER, Fox ME, Guillandeu SJ, Fréchet JMJ, Dy EE, Szoka FC. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci U S A. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavanpatil MD, Khdair A, Gerard B, Bachmeier C, Miller DW, Shekhar MPV, Panyam J. Surfactant–polymer nanoparticles overcome P-glycoprotein-mediated drug efflux. Mol Pharm. 2007;4:730–738. doi: 10.1021/mp070024d. [DOI] [PubMed] [Google Scholar]
- 26.Lasic DD. Doxorubicin in sterically stabilized liposomes. Nature. 1996;380:561–562. doi: 10.1038/380561a0. [DOI] [PubMed] [Google Scholar]
- 27.Uwatoku T, Shimokawa H, Abe K, Matsumoto Y, Hattori T, Oi K, Matsuda T, Kataoka K, Takeshita A. Application of nanoparticle technology for the prevention of restenosis after balloon Injury in rats. Circulation Res. 2003;92:62–69. doi: 10.1161/01.RES.0000069021.56380.E2. [DOI] [PubMed] [Google Scholar]
- 28.Zhang K, Fang H, Chen Z, Taylor JSA, Wooley KL. Shape effects of nanoparticles conjugated with cell-penetrating peptides (HIV tat PTD) on CHO cell uptake. Bioconjugate Chem. 2008;19:1880–1887. doi: 10.1021/bc800160b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med. 2007;58:725–734. doi: 10.1002/mrm.21352. [DOI] [PubMed] [Google Scholar]
- 30.Janjic JM, Srinivas M, Kadayakkara DKK, Ahrens ET. Self-delivering nanoemulsions for dual Fluorine-19 MRI and fluorescence detection. J Am Chem Soc. 2008;130:2832–2841. doi: 10.1021/ja077388j. [DOI] [PubMed] [Google Scholar]
- 31.Beduneau A, Saulnier P, Benoit JP. Active targeting of brain tumors using nanocarriers. Biomaterials. 2007;28:4947–4967. doi: 10.1016/j.biomaterials.2007.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.