Abstract
Various animal models of pain are dependent on activation of different glutamate receptor subtypes. First-degree burn of the paw elicits a secondary hyperalgesia that is dependent on Ca2++ permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), but not N-methyl-D-aspartate (NMDA) receptors. The present study takes advantage of that specificity by examining the effects of spinal pretreatments of agents on this secondary hyperalgesia. Rats with indwelling intrathecal catheters were pretreated with agents prior to paw injury. Mechanical withdrawal thresholds were measured before, and for three h after the injury.
Spinal pretreatment with cyclooxygenase (10 and 30 μg (S)-(+)-ibuprofen; and 3 and 30 μg ketorolac) and nitric oxide synthase (33 and 100 μg NG Nitro-L-arginine methyl ester hydrochloride (L-NAME) and 10 μg thiocitrulline) inhibitors resulted in no specific anti-allodynia. In contrast, ziconotide (0.3, 1.0 and 3 μg), the N-type voltage gated calcium channel antagonist was very effective in blocking burn-induced sensitivity at all doses used. L-type (Diltiazam 230 μg) and P-type (Agatoxin IVA 0.3 μg) calcium channel blockers produced intermediate effects. Thus, cyclooxygenase and nitric oxide synthase are assumed not to be downstream of Ca2++ permeable AMPA receptors. Voltage gated calcium channels blockers could exert their effects either pre- or post-synaptically.
Keywords: pain, Calcium permeable AMPA/kainite receptor, calcium channel, spinal cord, prostaglandin, Nitric Oxide (NO)
1. Introduction
Activation of spinal cord NMDA receptors is often an early and necessary step in induction of spinal facilitation and enhanced pain states. Accordingly, administration of N-methyl-D-aspartate (NMDA) receptor antagonists blocks both induction of electrophysiological and behavioral end points indicative of these conditions (Dougherty and Willis, 1992), (Woolf and Thompson, 1991), (Ren et al., 1992a). Permeability to Ca2+ and consequent activation of Ca2+ dependent second messenger cascades appears to be the major characteristic that links NMDA receptor activation to synaptic plasticity, hyperalgesia and allodynia (Mayer et al., 1987). There are many second messengers that function within this pathway, including the retrograde neurotransmitters, prostaglandins and nitric oxide. Spinal administration of NMDA induces the local release of both prostaglandin E2(PGE2) and Nitric Oxide (NO) (Sorkin, 1993; Sorkin and Moore, 1996). Blockade of prostaglandin synthesis via inhibition of cyclooxygenase reduces enhanced pain behavior in a variety of animal models including ones initiated by intrathecal glutamate or NMDA (Lizarraga et al., 2006; Malmberg and Yaksh, 1992a; Park et al., 2000). Blockade of nitric oxide production produces a similar blunting of spinal sensitization and pain behavior associated end points (Malmberg and Yaksh, 1993b; Park et al., 2000). Voltage gated calcium channels (VGCC) are also postulated to link NMDA receptor activation with spinal sensitization and pain behavior (Fossat et al., 2007; Heinke et al., 2004), but those with a pre-synaptic location on afferent terminals could exert much of their effect upstream of glutamate release and subsequent receptor activation, while post-synaptically located VGCC could be more closely associated with specific post-synaptic glutamate receptor subtypes.
The Ca2+ permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor participates in synaptic strengthening within the spinal cord dorsal horn (Gu et al., 1996) and is postulated to initiate an alternative pharmacological cascade leading to spinal sensitization. Work within our group indicates that intrathecal administration of antagonists to a subtype of non-NMDA receptor, Ca2+ permeable AMPA/kainate receptors, blocks or reverses secondary mechanical allodynia in a first degree burn model in which NMDA receptor antagonists have no effect (Jones and Sorkin, 2004; Nozaki-Taguchi and Yaksh, 2002a; Sorkin et al., 1999; 2001). Similar experiments using a paw incision model of post-operative pain demonstrate the same NMDA receptor independence for the area of primary hyperalgesia (Zahn and Brennan, 1998; Zahn et al., 1998) and a dependence on Ca2+ permeable AMPA/kainite, but not NMDA receptors for the area of secondary hyperalgesia (Pogatzki et al., 2003). The present study examines the pharmacology of allodynic reversal in the first degree burn model to determine if the non-NMDA receptor initiated changes are dependent on the same second messenger systems as NMDA dependent pain models. To this end, cyclooxygenase (COX) and nitric oxide synthase (NOS) inhibitors as well as specific VGCC blockers that are efficacious in many of the NMDA receptor sensitive models were given as spinal pretreatments prior to the thermal injury. As spinal dorsal horn Ca2+ permeable AMPA receptors seem to play a clear role in several clinically relevant pain models such as burn (Sorkin et al., 1999), post-surgical pain (Pogatzki et al., 2003) and inflammation (Sorkin, 2001; Vikman, 2008), elucidating the second messenger pathways downstream of these receptors will help us to identify relevant pharmacological targets for these pain states with the long term goal of developing analgesics tailored to a particular pain state.
2. Materials and Methods
2.1 Animals and Surgery
The Animal Care Committee of the University of California, San Diego, approved animal protocols that were in accordance with the guidelines adopted by the United States National Institutes of Health. Male Sprague Dawley rats (Harlan Industries, Indianapolis, IL) weighing 300–350 g were implanted with intrathecal cannulae (polyetheline (PE-10)). (Sorkin et al., 2003; Yaksh and Rudy, 1976). Briefly, a pre-measured length of stretched PE-10 tubing was inserted caudally through the atlanto-occipital membrane, through the subarachnoid space ending at the rostral end of the lumbar enlargement. A knot in the tubing was left outside of the membrane and was attached to the muscle, this limited the length of insertion. The rostral end of the tubing was then tunneled subcutaneously and externalized at the top of the head. Animals were given 5 ml of lactated Ringer’s i.p. after surgery and for each of the following two days. Experiments were performed at least seven days after cannulae implantation. Only animals that displayed no post-surgical motor or sensory deficits and had basal withdrawal thresholds above 120 mN on the mid plantar surface of the paw were used.
2.2 Behavior testing
Rats were brought to the testing room daily for three days prior to the experiment. They sat in their home cages for a minimum of 30 minutes and then acclimated to individual compartments with mesh bottoms for at least an additional 30 min prior to mechanical testing. Calibrated von Frey filaments (Stoelting, Wood Dale, IL) with buckling forces between 4 and 148 mN were applied through the mesh, perpendicular to the mid-paw plantar surface, just distal to the base of the toes, to determine 50% probability withdrawal thresholds using the up-down technique (Chaplan et al., 1994b). After two basal measurements, rats were administered varying doses of drug or the appropriate vehicle through the cannula. Five minutes post-injection, rats were lightly anesthetized with halothane or isoflurane and the heel of the left hind paw was held on a 52.5° C surface for 45 seconds using a 10 g weight to maintain constant pressure. Animals were returned to the test compartments; recovery from anesthesia took approximately 2–3 min. The heel turned red, but did not blister. The red area of the paw never encompassed the test site. Mechanical thresholds were then re-determined at 30 min intervals for three hours following the injury. The person performing the behavioral tests was unaware of the injected substance. Vehicle controls and inactive agents were tested at the same times as active compounds.
2.3 Intrathecal Drugs
Both the (S) - (+) - and (R) - (−) - isomers of the non-specific cycloxygenase inhibitor ibuprofen (10, 30 μg) were administered. Both isomers were made in a 5 % cyclodextran solution. Ketorolac (3 μg), another non-steroidal anti-inflammatory drug (NSAID) (Hoffman-La-Roche, Nutley, NJ) was diluted in saline before administration. NG Nitro-L-arginine methyl ester hydrochloride (L-NAME; 33, 100 μg), a non-specific nitric oxide synthase inhibitor, and its inactive isomer NG Nitro-D-arginine methyl ester hydrochloride (D-NAME) (RBI-Sigma; 100 μg) were mixed in sterile saline as was thiocitrulline (1, 3 and 10 μg), an inhibitor of both neuronal and endothelial NOS (RBI-Sigma). Ziconotide (0.3, 3 μg) an N-type voltage gated Ca2+ channel inhibitor was used as an intrathecal pretreatment as were the L-type voltage gated Ca2+ channel antagonists, verapamil (250 μg, racemic mixture) and diltiazem (76 and 230 μg). Agatoxin IVA (0.1, 0.3 μg), the P-type VGCC blocker (Peptide Institute, Osaka, Japan) was also given intrathecally 5 min before the injury to the paw. Cyclodextran and saline vehicles were injected for comparison where appropriate. Doses were based on previous experiments involving NMDA mediated pain models.
2.3 Statistical Analysis
Data are presented as mean ±S.E.M. for convenience in viewing. However, the mechanical threshold data is not normally distributed due to the frequent use of the 15 g cut-off, thus, these data were examined using nonparametric statistics, the Friedman test for repeated measures followed by post-hoc testing with the Dunnett test. In all cases P<0.05 was considered to be significant.
3. Results
3.1 Cyclooxygenase inhibitors
The first set of experiments asked if inhibition of spinal cyclooxygenases with NSAID prior to the injury reduced development of the burn-induced secondary hyperalgesia. Intrathecal pretreatment with 10 μg of the (S) - (+) - ibuprofen (the active isomer that blocks both COX 1 and 2; N=8) had no effect on burn-induced decreases in threshold compared to administration of the cyclodextrin (N=9) vehicle (Fig. 1A). In both groups, thermal injury induced a precipitous drop in 50% probability withdrawal threshold; this was significant over the entire period (P≤0.02; Friedman test for repeated measures) indicating allodynia. The decrease in withdrawal threshold plateaued for two hours post injury and still remained depressed compared to baseline at the 150 min timepoint. This dose of (S) - (+) - ibuprofen is sufficient to reduce phase 1 and 2 of the formalin test and to reduce intraplantar formalin induced increases in PGE2 (Malmberg and Yaksh, 1995); a dose of 5.6 μg (S ) - (+) - ibuprofen is sufficient to delay development of thermal hyperalgesia induced by intraplantar carageenan, while 16 μg (Dirig et al., 1998) substantially reduced hyperalgesia over a two h period. Increasing the pretreatment dose of (S) -(+) - ibuprofen to 30 μg (N=8) in the burn model was associated with a modest anti-allodynia. However an equimolar dose of (R) - (−) - ibuprofen, the inactive enantiomer, was equally efficacious in blocking the decrease in threshold; thus, it is likely that any antagonism observed in this study was due to nonspecific effects rather than COX inhibition. Intrathecal administration of ketorolac (3 and 30 μg; N= 6 each) prior to the thermal injury was also ineffective in altering the sensitization compared to saline (N=8; Fig. 1B). Following either dose of ketorolac, thresholds dropped from a mean of 143–146 mN to a low of 50mN at 90 min post-injury (P≤ 0.02 for both ketorolac groups; Friedman test for repeated measures). Two μg of spinal ketorolac causes 50% inhibition of phase 2a of the formalin test (Malmberg and Yaksh, 1992b) and 7.5 μg reduced both thermal and mechanical hyperalgesia following nerve injury by about 50% (Parris et al., 1996). Thus, two different NSAIDs failed to produce a specific anti-allodynic effect in this model of secondary hyperlagesia at doses that have significant effects in other NMDA-driven models of pain.
Fig. 1.
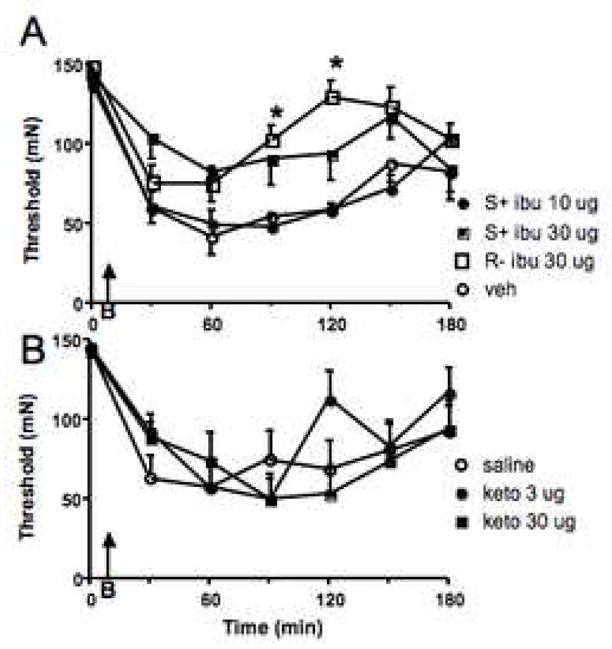
Inhibition of cycloyygenase enzymes had no effect on burn-induced secondary hyperalgesia. Agent was given through the intrathecal catheter as a pretreatment, 5 min prior to burning the paw (B, indicated by the arrow) and immediately after determination of withdrawal thresholds at time 0. All data is depicted as the mean ± S.E.M. A. The low dose of (S) - (+) - ibuprofen (ibu) resulted in the same degree of allodynia as was seen after vehicle (veh). Although, a high dose significantly reduced this behavior, the anti-allodynia was no different than that seen after administration of the same dose of the inactive form (R- ibu) of the agent. B. Spinal ketorolac (keto) pretreatments resulted in the same decrease of withdrawal thresholds in the area of secondary hyperalgesia, as did saline. * = P≤ 0.05 compared to the vehicle treated group at the specified timepoint.
3.2 NOS inhibitors
The second set of experiments examined the effects of intrathecal pretreatment with NOS inhibitors on the burn-induced secondary hyperalgesia (Fig. 2). Pretreatment with 33 μg of L-NAME was without effect. Increasing the dose to 100 μg resulted in a significant blunting of the pain behavior, but this anti-allodynia was equaled by intrathecal administration of an equivalent dose of D-NAME pointing to a non-specific effect. Lower doses of L-NAME (about 3 μg) successfully blocked mechanical allodynia induced by intrathecal fractalkine (Milligan et al., 2005) and 100 μg totally blocked phase 2 of the formalin test (Malmberg and Yaksh, 1993c). Intrathecal thiocitrulline, an inhibitor with relative selectivity for neuronal NOS (although it also has some efficacy for endothelial NOS (Frey et al., 1994)) was without effect at doses up to 10 μg (1 and 3 μg not shown; N=7 for each group). The Friedman test for repeated measures resulted in P values of ≤0.01 (indicative of allodynia) at every dose.
Fig. 2.
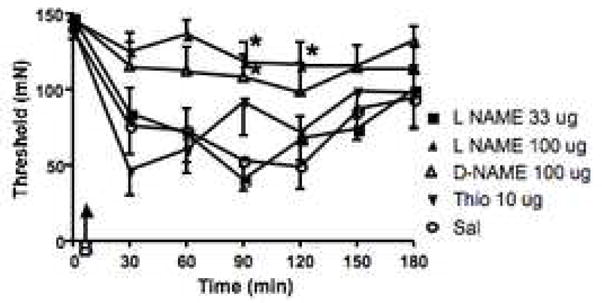
Inhibition of NOS enzymes has no effect on burn-induced secondary hyperalgesia. Agent was given through the intrathecal catheter as a pretreatment, 5 min prior to burning the paw (B, indicated by the arrow) and immediately after determination of withdrawal thresholds at time 0. All data is depicted as the mean ± S.E.M. Pretreatment with saline, the lower dose of L-NAME (33 μg) and thiocitrulline (Thio) resulted in indistinguishable magnitudes and time courses of the response indicating no anti-allodynic effects. While 100 μg of L-NAME totally prevented the development of the secondary hyperalgesia, this was paralleled by an equivalent result following administration of the same dose of the non-active agent D-NAME. * = P≤ 0.05 compared to the vehicle treated group at the specified timepoint.
3.3 VGCC Blockers
The third set of experiments examined the effects of various Ca2+ channel antagonists on the burn-induced secondary allodynia. We started with intrathecal ziconotide (0.3, 1 and 3 μg), an N type VGCC blocker (N=6–7). Chaplan and colleagues (Chaplan et al., 1994a) published that 3 μg was the highest usable spinal dose. All three doses of ziconotide totally prevented the burn-induced allodynia (Fig. 3). Only one animal given either of the 2 higher doses ever responded with a threshold below 100 mN. This contrasts sharply with the decrease in mechanical withdrawal threshold observed during the same trials when vehicle preceded the thermal injury to the paw; this difference was observed from 30–180 min post-injury (P≤0.01). Burn no longer caused a significant allodynia and all three doses produced an increased threshold compared to vehicle throughout the testing period. While some tail stiffness, tremors or skin twitching on the flanks was observed starting about 45 min following administration of the higher two doses, this was not apparent following 0.3 μg of ziconotide. Righting, placing and stepping reflexes appeared to be normal at all times.
Fig. 3.
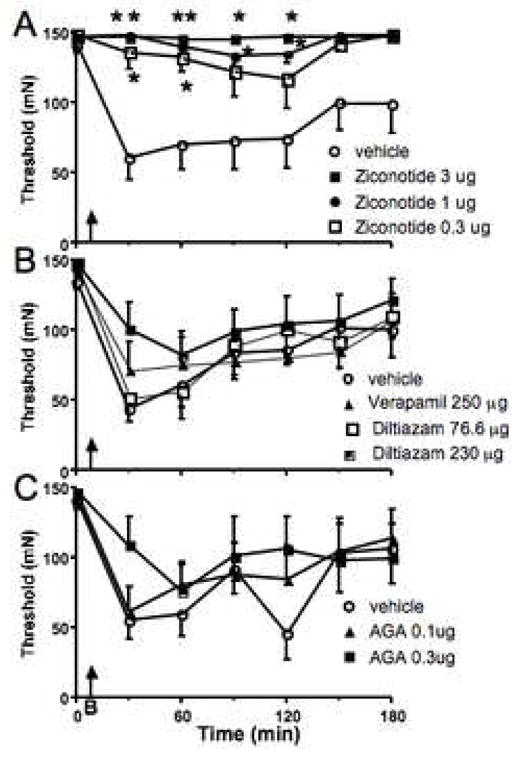
Subtypes of voltage gated Ca2+ channels are effective in blocking burn-induced allodynia. A. Spinal pretreatment with ziconotide, the N-type C2+ channel blocker prevented the development of sensitization. This appeared to be dose dependent and independent of the motor side effects elicited by the highest dose. B. Following spinal administration of L-type channel blockers there was still significant allodynia compared to baseline. However, the higher dose of diltiazam (230 μg) resulted in significantly less sensitization over the entire time period than vehicle pretreatment (P≤0.05) indicating an anti-allodynic effect. C. Spinal pretreatment with the higher dose of Agatoxin IVA (AGA) blocked the development of allodynia. * = P≤ 0.05 compared to the vehicle treated group at the specified timepoint.
Pretreatment with spinal diltiazam (76.6 and 230μg) and verapamil 250 μg, which antagonize L-type VGCC, also showed a marked tendency to block thermal injury-induced decreases in withdrawal thresholds. However, despite this tendency thresholds were still significantly reduced compared to baseline for all three of these groups (P≤0.0.5) and there was only a difference between the saline and diltiazam (230μg) treated animals at the 30 min timepoint (P≤0.0.5) and no differences between vehicle and either the lower dose of diltiazam or verapamil. Higher doses of intrathecal verapamil were attempted, but rats given doses of 500 μg verapamil or above frequently stopped breathing during the light halothane anesthesia required to burn their paw 5 min after injection.
The non P-type VGCC blocker Agatoxin IVA produced a low level of agitation upon being handled and segmental allodynia at the 0.3, but not the 0.1 μg dose. This is obviously a confounding factor, but despite this, careful testing indicated that thresholds were higher in the 0.3 μg Agatoxin IVA treated animals than those pretreated with saline. The Friedman- test for repeated measures indicated that these animals were no longer allodynic for the post-thermal injury period. While animals treated with 0.1 μg Agatoxin IVA were allodynic compared to their own baseline (P≤ 0.043), they were less sensitive than the saline treated animals (P≤ 0.003). Post-hoc testing revealed that 0.3 μg Agatoxin IVA treated animals had higher thresholds than those treated with saline at 30 and 120 min post injury. Thresholds of animals treated with the lower dose of channel blocker were not different from either saline or high dose treated animals.
4. Discussion
The first degree burn model of secondary hyperalgesia along with the primary hyperalgesia associated with the post-incisional model of pain share a common pharmacology in that both are sensitive to intrathecal administration of non-NMDA receptor antagonists and are insensitive to NMDA receptor antagonists (Nozaki-Taguchi and Yaksh, 2002a; Zahn and Brennan, 1998; Zahn et al., 1998). Additionally, the secondary hyperalgesia associated with both models is sensitive to Ca2+ permeable AMPA/kainite receptor antagonism (Pogatzki et al., 2003; Sorkin et al., 1999; 2001). We have recently demonstrated that phosokinase A and phosphokinase C, but not CaMkinase II alpha are downstream of Ca2+ permeable non-NMDA receptors in the first degree burn model (Jones and Sorkin, 2005). This suggests that Ca2++ entering through the different glutamate-mediated ionotropic receptors is ‘aimed’ at different cellular machinery and second messenger cascades. In the present series of experiments we sought to explore further the pharmacology of the burn-induced allodynia with an aim to understanding the second messengers that are activated downstream of Ca2+ permeable AMPA/kainite receptor activation. The main finding is that activation of COX and NO synthase do not appear to be downstream of Ca2+ permeable non-NMDA receptor activation in the first-degree burn model. In addition, we identified involvement of N and P type Ca2+ channels in this process.
When given either before or after spinal NMDA, intrathecal administration of both the mixed COX 1/2 inhibitor (S) - (+) - ibuprofen or the COX1 preferring inhibitor ketorolac block NMDA-induced hyperalgesia, indicating that COX activation is down-stream of NMDA receptor activation (Malmberg and Yaksh, 1992a; Park et al., 2000; Warner et al., 1999). A host of other studies have demonstrated the efficacy of NSAIDS in a variety of NMDA sensitive pain models (Ghilardi et al., 2004; Matsunaga et al., 2007) (Hefferan et al., 2003; Ren and Dubner, 1993; Ren et al., 1992b; Zhao et al., 2000) (Malmberg and Yaksh, 1993a) (Dirig et al., 1998). Taken together, these data strongly imply that products of cyclooxygenase activation (prostanoids) are frequently downstream of NMDA receptor activation in vivo.
In marked contrast, in our experiments similar intrathecal doses of the mixed COX 1 and 2 inhibitor (S) - (+) - ibuprofen, given as a pretreatment, did not alter the development or magnitude of the burn-induced secondary hyperalgesia. While a higher pretreatment dose (30 μg) reduced the burn-induced sensitization, this reduction also occurred following administration of the inactive isomer indicating that, at best, this was a non-specific effect. Intrathecal ketorolac was without effect in the burn model at bolus doses up to 30 μg. Admittedly, higher intrathecal doses of ketorolac have been used successfully to antagonize primary hyperalgesia in the paw incision model, where pretreatment with 50 μg ketorolac had a modest anti-hyperalgesic effect at some, but not the majority of post-injury time points tested (Zhu et al., 2003). However, this higher dose was used to antagonize primary hyperalgesia, which is unaffected by Ca2+ permeable non-NMDA antagonists (Pogatzki et al., 2000). Ketorolac and other COX1 preferring agents have not been tested against secondary hyperalgesia in the incisional model. Taken together, we believe that these data indicate that activation of COX and the resultant production of prostaglandins are unlikely to be activated downstream of Ca2+ permeable AMPA receptors.
Nitric oxide synthase is also downstream of NMDA receptor activation (Kawamata and Omote, 1999; Sorkin, 1993) and spinal administration of L-NAME blocks thermal hyperalgesia elicited by spinal NMDA (Malmberg and Yaksh, 1993c). Inhibitors of nitric oxide synthase were without anti-hyperalgesic properties in this model. Doses of L-NAME, that were ineffective in our model, were significantly higher than those used to block pain enhancement due to intrathecal fractalkine (Milligan et al., 2005) and equivalent to those used to block phase 2 formalin responses (Malmberg and Yaksh, 1993c). Although higher doses of intrathecal L-NAME (above 150 μg) are required to reverse thermal hyperalgesia induced by carrageenan (Osborne and Coderre, 1999; Sekiguchi et al., 2004), this model has both NMDA and Ca 2+ permeable AMPA/kainite receptor components (Sorkin et al., 2001). Importantly, intrathecal administration of the same dose that we employed (30 μg) was shown to inhibit local spinal nitric oxide synthase by more than 80% (Salter et al., 1996). The lack of anti-hyperalgesic effect of spinal thiocitrulline confirmed the non-participation of NOS. Nitric oxide synthase activation is reported to be downstream of not only NMDA receptors, but frequently, also of the prostaglandin receptor EP1 subtype (Minami et al., 1995) in the spinal cascade leading to pain behavior. Thus, it is not unexpected that the first degree burn model of secondary hyperalgesia that involves neither spinal NMDA receptors nor prostaglandin synthesis would also not be dependant on the generation of NO. Consistent with this hypothesis, spinal administration of L-NAME is without effect in the paw incision model (Zahn and Brennan, 1998) and does not reduce minimum alveolar anesthetic concentration of halothane (Adachi et al., 1994) indicating a lack of effect in both peri- and post-operative pain models.
Blockade of N type VGCC was highly effective in blocking the secondary hyperalgesia induced by the paw injury. Lower doses of ziconotide were effective in this model than was necessary for the spinal nerve ligation model of nerve injury, although it must be remembered that in that case the agent was administered as a post-treatment (Chaplan et al., 1994a), but see (Bowersox et al., 1996). As N-type VGCC on the central terminals of primary afferent fibers (Westenbroek et al., 1995) regulates neurotransmitter release in the spinal cord, including that of substance P into the superficial dorsal horn (Smith et al., 2002), it was expected that ziconotide would block allodynia in the burn model. Intrathecal morphine, which has the same presynaptic actions is also highly effective given as both pre- and post-treatment (Nozaki-Taguchi and Yaksh, 2002b). Ziconotide, within the same dose range also prevents mechanical allodynia in the zone of primary hyperalgesia following paw incision (Wang et al., 2000), as this model is insensitive to both NMDA and Ca2+-permeable AMPA/kainate receptor antagonism it is likely that it at least some of the effect is due to presynaptic actions. Pretreatment with the L-type voltage gated calcium channel blockers had only modest effects in the burn model, these were significant only 35 min after administration of the antagonist. This corresponds with the modest effects of verapamil and nifedipine within this time window in preventing the phase 2 formalin response (Coderre and Melzack, 1992). These same agents, given at similar dosages, were without effect given as postreatments in the spinal nerve ligation model (Chaplan et al., 1994c). Again the lower potency may be due to differences between agents given as pre-vs post-treatments.
In summary, neither cyclooxygenase inhibitors nor inhibitors of nitric oxide synthase appear to be involved as second messengers downstream of Ca2+-permeable AMPA/kainate receptors. Voltage gated calcium channels, N-type in particular, and P type to a lesser extent, may participate in this cascade. Thus, there is potential for Ca2+ channel specific antagonists as therapeutic agents in pain states that depend on Ca2+-permeable AMPA/kainate receptors rather than or in addition to NMDA receptors. In some pain states, targeting the Ca2+-permeable AMPA receptor and its downstream signal transduction pathway is also likely to be analgesic with perhaps a better side effect profile than has been the case with NMDA receptor antagonism.
Acknowledgments
This work was funded by the National Institutes of Health NS41580(LSS). We would like to thank Ms. Julie Nguyen for editorial assistance in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, Kurata J, Nakao S, Murakawa M, Shichino T, Shirakami G, Shinomura T, Mori K. Nitric oxide synthase inhibitor does not reduce minimum alveolar anesthetic concentration of halothane in rats. Anesth Analg. 1994;78:1154–1157. doi: 10.1213/00000539-199406000-00023. [DOI] [PubMed] [Google Scholar]
- Bowersox SS, Gadbois T, Singh T, Pettus M, Wang YX, Luther RR. Selective N-type neuronal voltage-sensitive calcium channel blocker, SNX-111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. J Pharmacol Exp Ther. 1996;279:1243–1249. [PubMed] [Google Scholar]
- Chaplan S, Pogrel J, Yaksh T. Role of Voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exptl Therapeut. 1994;269:1117–1123. [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994;269:1117–1123. [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3671–3675. doi: 10.1523/JNEUROSCI.12-09-03671.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirig DM, Isakson PC, Yaksh TL. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J Pharmacol Exper Therapeut. 1998;285:1031–1038. [PubMed] [Google Scholar]
- Dougherty PM, Willis WD. Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capsaicin-induced sensitization in the monkey. J Neurosci. 1992;12:883–894. doi: 10.1523/JNEUROSCI.12-03-00883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat P, Sibon I, Le Masson G, Landry M, Nagy F. L-type calcium channels and NMDA receptors: a determinant duo for short-term nociceptive plasticity. Eur J Neurosci. 2007;25:127–135. doi: 10.1111/j.1460-9568.2006.05256.x. [DOI] [PubMed] [Google Scholar]
- Frey C, Narayanan K, McMillan K, Spack L, Gross SS, Masters BS, Griffith OW. L-thiocitrulline. A stereospecific, heme-binding inhibitor of nitric-oxide synthases. J Biol Chem. 1994;269:26083–26091. [PubMed] [Google Scholar]
- Ghilardi JR, Svensson CI, Rogers SD, Yaksh TL, Mantyh PW. Constitutive spinal cyclooxygenase-2 participates in the initiation of tissue injury-induced hyperalgesia. J Neurosci. 2004;24:2727–2732. doi: 10.1523/JNEUROSCI.5054-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JG, Albuquerque C, Lee CJ, MacDermott AB. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature. 1996;381:793–796. doi: 10.1038/381793a0. [DOI] [PubMed] [Google Scholar]
- Hefferan MP, O’Rielly DD, Loomis CW. Inhibition of spinal prostaglandin synthesis early after L5/L6 nerve ligation prevents the development of prostaglandin-dependent and prostaglandin-independent allodynia in the rat. Anesthesiol. 2003;99:1180–1188. doi: 10.1097/00000542-200311000-00027. [DOI] [PubMed] [Google Scholar]
- Heinke B, Balzer E, Sandkuhler J. Pre- and postsynaptic contributions of voltage-dependent Ca2+ channels to nociceptive transmission in rat spinal lamina I neurons. Eur J Neurosci. 2004;19:103–111. doi: 10.1046/j.1460-9568.2003.03083.x. [DOI] [PubMed] [Google Scholar]
- Jones TL, Sorkin LS. Calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors mediate development, but not maintenance, of secondary allodynia evoked by first-degree burn in the rat. J Pharmacol Exp Ther. 2004;310:223–229. doi: 10.1124/jpet.103.064741. [DOI] [PubMed] [Google Scholar]
- Jones TL, Sorkin LS. Activated PKA and PKC, but not CaMKIIalpha, are required for AMPA/Kainate-mediated pain behavior in the thermal stimulus model. Pain. 2005;117:259–270. doi: 10.1016/j.pain.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Omote K. Activation of spinal N-methyl-D-aspartate receptors stimulates a nitric oxide/cyclic guanosine 3,5-monophosphate/glutamate release cascade in nociceptive signaling. Anesthesiol. 1999;91:1415–1424. doi: 10.1097/00000542-199911000-00035. [DOI] [PubMed] [Google Scholar]
- Lizarraga I, Chambers JP, Johnson CB. Depression of NMDA-receptor-mediated segmental transmission by ketamine and ketoprofen, but not L-NAME, on the in vitro neonatal rat spinal cord preparation. Brain Res. 2006;1094:57–64. doi: 10.1016/j.brainres.2006.03.117. [DOI] [PubMed] [Google Scholar]
- Malmberg A, Yaksh T. Hyperalgesia mediated by spinal glutamate or SP receptor blocked by cyclooygenase inhibition. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exper Ther. 1992;263:136–146. [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobolographic analysis of the NSAID interaction. Anesthesiol. 1993;79:270–281. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]; Malmberg AB, Yaksh TL. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. Pain. 1993;54:291–300. doi: 10.1016/0304-3959(93)90028-N. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. Pain. 1993;54:291–300. doi: 10.1016/0304-3959(93)90028-N. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Cyclooxygenase inhibition and the spinal release of prostaglandin E2 and amino acids evoked by paw formalin injection: a microdialysis study in unanesthetized rats. J Neurosci. 1995;15:2768–2776. doi: 10.1523/JNEUROSCI.15-04-02768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga A, Kawamoto M, Shiraishi S, Yasuda T, Kajiyama S, Kurita S, Yuge O. Intrathecally administered COX-2 but not COX-1 or COX-3 inhibitors attenuate streptozotocin-induced mechanical hyperalgesia in rats. Eur J Pharmacol. 2007;554:12–17. doi: 10.1016/j.ejphar.2006.09.072. [DOI] [PubMed] [Google Scholar]
- Mayer ML, MacDermott AB, Westbrook GL, Smith SJ, Barker JL. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987;7:3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan E, Zapata V, Schoeniger D, Chacur M, Green P, Poole S, Martin D, Maier SF, Watkins LR. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur J Neurosci. 2005;22:2775–2782. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- Minami T, Onaka M, Okuda-Ashitaka E, Mori H, Ito S, Hayaishi O. L-NAME, an inhibitor of nitric oxide synthase, blocks the established allodynia induced by intrathecal administration of prostaglandin E2. Neurosci Lett. 1995;201:239–242. doi: 10.1016/0304-3940(95)12176-5. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yaksh TL. Pharmacology of Spinal Glutamatergic Receptors in Post-Thermal Injury- evoked Tactile Allodynia and Thermal Hyperalgesia. Anesthesiol. 2002;96:617–626. doi: 10.1097/00000542-200203000-00018. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yaksh TL. Spinal and peripheral mu opioids and the development of secondary tactile allodynia after thermal injury. Anesth Analg. 2002;94:968–974. doi: 10.1097/00000539-200204000-00036. [DOI] [PubMed] [Google Scholar]
- Osborne MG, Coderre TJ. Effects of intrathecal administration of nitric oxide synthase inhibitors on carrageenan-induced thermal hyperalgesia. Br J Pharmacol. 1999;126:1840–1846. doi: 10.1038/sj.bjp.0702508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Shin CY, Lee TS, Huh IH, Sohn UD. The role of nitric oxide and prostaglandin E2 on the hyperalgesia induced by excitatory amino acids in rats. J Pharm Pharmacol. 2000;52:431–436. doi: 10.1211/0022357001774039. [DOI] [PubMed] [Google Scholar]
- Parris WC, Janicki PK, Johnson B, Jr, Horn JL. Intrathecal ketorolac tromethamine produces analgesia after chronic constriction injury of sciatic nerve in rat. Can J Anaesth. 1996;43:867–870. doi: 10.1007/BF03013041. [DOI] [PubMed] [Google Scholar]
- Pogatzki E, Niemeier J, Sorkin L, Brennan T. Effect of joro spider toxin (JSTX) on primary and secondary hyperalgesia after incision in the rat. Anesthesiol. 2000;93:A977. [Google Scholar]
- Pogatzki EM, Niemeier JS, Sorkin LS, Brennan TJ. Spinal glutamate receptor antagonists differentiate primary and secondary mechanical hyperalgesia caused by incision. Pain. 2003;105:97–107. doi: 10.1016/s0304-3959(03)00169-6. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. NMDA receptor antagonists attenuate mechanical hyperalgesia in rats with unilateral inflammation of the hindpaw. Neurosci Lett. 1993;163:22–26. doi: 10.1016/0304-3940(93)90220-f. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JL, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Ren K, Williams GM, Hylden JL, Ruda MA, Dubner R. The intrathecal administration of excitatory amino acid receptor antagonists selectively attenuated carrageenan-induced behavioral hyperalgesia in rats. Eur J Pharmacol. 1992;219:235–243. doi: 10.1016/0014-2999(92)90301-j. [DOI] [PubMed] [Google Scholar]
- Salter M, Strijbos PJ, Neale S, Duffy C, Follenfant RL, Garthwaite J. The nitric oxide-cyclic GMP pathway is required for nociceptive signalling at specific loci within the somatosensory pathway. Neurosci. 1996;73:649–655. doi: 10.1016/0306-4522(96)00060-7. [DOI] [PubMed] [Google Scholar]
- Sekiguchi F, Mita Y, Kamanaka Y, Kawao N, Matsuya H, Taga C, Kawabata A. The potent inducible nitric oxide synthase inhibitor ONO-1714 inhibits neuronal NOS and exerts antinociception in rats. Neurosci Lett. 2004;365:111–115. doi: 10.1016/j.neulet.2004.04.069. [DOI] [PubMed] [Google Scholar]
- Smith MT, Cabot PJ, Ross FB, Robertson AD, Lewis RJ. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain. 2002;96:119–127. doi: 10.1016/s0304-3959(01)00436-5. [DOI] [PubMed] [Google Scholar]
- Sorkin LS. NMDA evokes an L-NAME sensitive spinal release of glutamate and citrulline. Neuroreport. 1993;4:479–482. doi: 10.1097/00001756-199305000-00004. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Moore J, Boyle DL, Yang L, Firestein GS. Regulation of peripheral inflammation by spinal adenosine: role of somatic afferent fibers. Exp Neurol. 2003;184:162–168. doi: 10.1016/s0014-4886(03)00102-x. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Moore JH. Evoked Release of Amino Acids and Prostanoids in Spinal Cords of Anesthetized Rats: Changes During Peripheral Inflammation and Hyperalgesia. Am J Ther. 1996;3:268–275. doi: 10.1097/00045391-199604000-00003. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Yaksh TL, Doom CM. Mechanical allodynia in rats is blocked by a Ca2+ permeable AMPA receptor antagonist. Neuroreport. 1999;10:3523–3526. doi: 10.1097/00001756-199911260-00011. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Yaksh TL, Doom CM. Pain models display differential sensitivity to Ca2+-permeable non-NMDA glutamate receptor antagonists. Anesthesiol. 2001;95:965–973. doi: 10.1097/00000542-200110000-00028. [DOI] [PubMed] [Google Scholar]
- Vikman KS, Rycroft BK, Christie MJ. Switch to Ca2+permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol. 2008;586:515–527. doi: 10.1113/jphysiol.2007.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Pettus M, Gao D, Phillips C, Scott Bowersox S. Effects of intrathecal administration of ziconotide, a selective neuronal N-type calcium channel blocker, on mechanical allodynia and heat hyperalgesia in a rat model of postoperative pain. Pain. 2000;84:151–158. doi: 10.1016/s0304-3959(99)00197-9. [DOI] [PubMed] [Google Scholar]
- Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Zahn PK, Brennan TJ. Lack of effect of intrathecally administered N-methyl-D-aspartate receptor antagonists in a rat model for postoperative pain. Anesthesiol. 1998;88:143–156. doi: 10.1097/00000542-199801000-00022. [DOI] [PubMed] [Google Scholar]
- Zahn PK, Umali E, Brennan TJ. Intrathecal non-NMDA excitatory amino acid receptor antagonists inhibit pain behaviors in a rat model of postoperative pain. Pain. 1998;74:213–223. doi: 10.1016/s0304-3959(97)00181-4. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Chen SR, Eisenach JC, Busija DW, Pan HL. Spinal cyclooxygenase-2 is involved in development of allodynia after nerve injury in rats. Neurosci. 2000;97:743–748. doi: 10.1016/s0306-4522(00)00052-x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Conklin D, Eisenach JC. Cyclooxygenase-1 in the spinal cord plays an important role in postoperative pain. Pain. 2003;104:15–23. doi: 10.1016/s0304-3959(02)00465-7. [DOI] [PubMed] [Google Scholar]


