Abstract
Successful application of γδ T cells in adoptive cell therapies depends upon our ability to maintain these cells in vivo. Using an adoptive transfer model to study lymphopenia-induced homeostatic expansion, we show that CD8+ and NK1.1+ γδ T cell subsets are differentially regulated. While CD8+ γδ T cells have an early and sustained advantage following transfer into TCRβ−/−/δ−/− mice, NK1.1+ γδ T cells proliferate slowly and are maintained at low numbers. The advantage of the CD8+ subset could not be explained by increased bcl-2 or cytokine receptor expression but did correlate with Vγ4+ and Vδ5+ expression. Despite the role of CD8 in MHCI recognition by αβ T cells, β2m-associated MHCI molecules were not required for CD8+ γδ T cell homeostatic expansion. Surprisingly, all γδ T cells, including the CD8+ subset, exhibited enhanced proliferation following adoptive transfer into Rag1−/−/β2m−/− compared to Rag1−/− recipients. This effect was most notable for the NK1.1+ subset, which expresses high levels of NKG2A/CD94 and Ly49. Although expression of these inhibitory receptors correlated with poor homeostatic expansion in the presence of β2m, γδ T cell homeostatic proliferation in TCRβ−/−/δ−/− mice was not altered in the presence of Ly49C/I and NKG2 blocking antibodies. While the mechanism by which β2m negatively regulates γδ T cell homeostasis remains to be determined, this observation is unique to γδ T cells and confirms that multiple mechanisms are in place to maintain strict regulation of both the size and the composition of the γδ T cell pool.
Keywords: T cells, cell proliferation, cell differentiation, MHC
Introduction
γδ T cells are known for their immune-modulatory functions (1). As regulatory cells, γδ T cells are likely maintained at low numbers to allow for the generation of a balanced immune response. Maintenance of lymphocyte population size, or lymphocyte homeostasis, is achieved by balancing the generation of new cells and clonal expansion with cell death. Our previous studies revealed that the size of the γδ T cell pool is regulated by the availability of IL-15 and IL-7 (2). αβ T cells, primarily the CD8+ αβ T cell subset, potently inhibit γδ T cell expansion in lymphopenic hosts (2). This inhibitory effect occurs, at least in part, from competition for shared IL-15 resources. Similarly, NK cells, which also require IL-15 for their maintenance, are capable of inhibiting γδ T cell homeostasis when αβ T cells are absent or reduced in number (2). Of interest, γδ T cells are at a substantial disadvantage compared to αβ T cells during homeostatic expansion. When both cell types are adoptively transferred in equal numbers into TCRβ−/−/δ−/− mice, αβ T cells out-number the γδ T cell population by more than 15-fold one month after adoptive transfer (2). These data suggest that other factors limit the homeostatic potential of γδ T cells.
In addition to cytokines, MHC molecules are known to play an important role in αβ T cell homeostasis. Specifically, naïve CD4+ and naïve CD8+ αβ T cells require MHCII and MHCI molecules, respectively, for their survival and proliferation in lymphopenic hosts (3, 4). The role of MHC molecules in γδ T cell biology remains incompletely defined. Although γδ T cells are generally thought to be MHC-independent, a number of studies have revealed that certain γδ T cell clones and subpopulations recognize either classical or non-classical MHC molecules (5–7). The most well characterized example is the G8 γδ T cell clone, which recognizes the mouse non-classical MHCI molecule T22. T22 induces proliferation of transgenic G8 γδ T cells (8) and drives homeostatic expansion of G8 γδ T cells in a lymphopenic environment (9). T22-tetramer staining studies revealed that 0.5–2.0% of splenic γδ T cells recognized T22 (10).
γδ T cells are commonly grouped into subsets based on their expression of certain Vγ and/or Vδ TCR genes; however, this classification is complicated by expression of certain surface proteins that might also define functional subsets. For example, approximately 50% of human splenic and intraepithelial γδ T cells and 30% of circulating γδ T cells express CD8 (11, 12). In C57BL/6 mice, nearly 20% of splenic γδ T cells express CD8αβ, while CD8αα is expressed by 47% and 63% of γδ T cells in the liver and intestinal epithelium, respectively (13). Furthermore, both mouse and human γδ T cells express NK cell receptors. 70% of human peripheral blood γδ T cells express NKG2/CD94 heterodimers, while 10% express KIRs (14). In mice, some γδ T cells in the thymus and peripheral tissues are known to express NKG2/CD94, Ly49C/I, Ly49A, Ly49E and Ly49G2 (15–17). In addition, γδ T cells can express members of the NKR-P1 NK receptor family, which includes the well-known murine NK cell marker NK1.1 (18). Unlike the other families, NKR-P1 receptors do not recognize MHCI molecules, but instead bind to C-type lectin-related ligands (19, 20). Approximately 20% of mouse γδ T cells in the liver are NK1.1+ (15) and 25% of human peripheral blood γδ T cells express the equivalent marker, NKR-P1A (21).
Here, we show that CD8+ and NK1.1+ γδ T cell subsets are differentially regulated during homeostatic expansion. While the CD8+ subset proliferates rapidly in the lymphopenic environment, the NK1.1+ subset is considerably disadvantaged during homeostatic expansion. The advantage of CD8+ γδ T cells could not be attributed to an increase in bcl-2 or cytokine receptor expression. While Vγ4 and Vδ5 TCR gene expression was enriched in the CD8+ population following homeostatic expansion, β2m-associated MHCI molecules were not driving the expansion of this subset. Serendipitously, studies in β2m-deficient mice revealed that homeostatic expansion of the NK1.1+ γδ T cell subset was inhibited in the presence of β2m. This effect was observed, albeit to a lesser extent, in NK1.1− γδ T cells, suggesting that β2m-associated molecules act to limit the size of the entire γδ T cell pool.
Materials and Methods
Mice
C57BL/6 TCRβ−/−, C57BL/6 TCRβ−/−/δ−/−, and C57BL/6 Rag1−/− mice were purchased from The Jackson Laboratory. C57BL/6 Rag1−/−/β2m− mice were a generous gift from Dr. Philippa Marrack. All mouse strains were maintained and bred in our facility. Both male and female mice were used in these experiments at 6–12 weeks of age. These studies were approved by IACUC (AS-2504-09-09).
In Vivo γδ T Cell Proliferation and Reconstitution Studies
Donor γδ T cells were isolated from C57BL/6 TCRβ−/− mice. Single-cell suspensions were prepared from donor spleens. Following red blood cell lysis with Gey’s solution, cells were passed over nylon wool columns to enrich for T lymphocytes. Cell purity was determined by flow cytometry (see below) prior to adoptive transfer. The donor cell population contained, on average, 60% γδ T cells, 20% NK cells, and 20% non-T/non-NK cells. For short-term proliferation studies, cells were resuspended in PBS at 2 × 106 cells per ml and labeled with 0.1 µM 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes) at 37°C for 15 minutes. Cells were washed twice with PBS, resuspended in injection saline, and 5 × 105 – 1 × 106 cells were injected intravenously into recipient mice. Spleens were harvested at various time points after adoptive transfer, and T cells were isolated as described above. Total γδ T cell number per spleen was determined by multiplying the total number of cells recovered after nylon wool enrichment by the percentage of live cells and by the percentage of each cell type as determined by flow cytometry. γδ T cell proliferation was measured as a loss of CFSE fluorescence as detected by flow cytometry.
Flow Cytometry
Nylon wool-enriched T cells were resuspended in staining buffer (Hanks’ BSS/2%FBS/0.1% sodium azide, pH 7.1) and aliquoted into round-bottom 96-well polystyrene tissue culture plates (105–106 cell per well). Cells were preincubated with 2.4G2 to avoid nonspecific antibody binding by Fc receptors. γδ T cells were identified by staining with biotinylated or PE-conjugated anti-TCRδ antibodies (GL3, BD Biosciences) in combination with anti-CD3-PE-Cy5 (145-2C11, BD Biosciences). γδ T cell subsets were identified using anti-CD8α-APC (53.6.7, eBioscience) and anti-NK1.1-APC or PE (PK136, eBioscience). TCR Vγ and Vδ were detected using combinations of anti-Vγ1 (2.11), anti-Vγ4 (UC3), anti-Vδ4 (GL-2), anti-Vδ5 (F45-152), anti-Vδ6.3 (17-C), anti-Vδ6λ12 (F4.22), and anti-Vδ8/Vα2 (B20.1). All anti-TCR antibodies were produced from in vitro-cultured hybridomas and biotinylated. IL-7Rα-PE (A7R34, eBioscience), IL-2Rβ-PE (5H4, eBioscience), and IL-15Rα-biotin (R & D Systems) antibodies were used to assess cytokine receptor expression on gated γδ T cells or NK cells. Anti-CD94-PE (18d3), anti-NKG2A-PE (16a11), and anti-Ly49CIFH-PE (14B11) were purchased from eBioscience. Anti-Ly49A-fitc (A1), anti-Ly49F-PE (HBF-719), and anti-Ly49C/I-fitc (SW5E6) were purchased from BD Biosciences. Streptavidin-PE-Cy5 (BD Biosciences) or streptavidin-PE (BioSource International, Camarillo, CA) was used to detect biotinylated antibodies. Following extracellular staining, cells were fixed in 1% paraformaldehyde in PBS. To analyze bcl-2 expression, cells were then permeabilized with 0.05% saponin and stained with an anti-bcl-2-PE antibody (BD Biosciences). Immunofluorescence was detected by a FACScan (Becton Dickinson), and data sets were analyzed with FLOWJO 4.4.3 software (TreeStar, Inc.). Briefly, live γδ T cells or NK cells were gated and assessed for proliferation or surface-marker expression.
In Vivo NK Inhibitory Receptor Studies
Donor cells were prepared as described above, and 0.5–1 × 106 γδ T cells were transferred i.v. into TCRβ−/−/δ−/− mice. Recipient mice were treated with the 100 µg of each antibody on days 0, 2, 5, 8, and 11 relative to cell transfer. Anti-NKG2A/C/E (20d5, provided by David Raulet) (22) and anti-Ly49C/I (5E6, a generous gift from Vinay Kumar) (23) were isolated from in vitro-cultured hybridomas. 50 µg of Rat IgG and 50 µg of mouse IgG were injected together as a non-specific antibody control. Spleens were harvested from recipient mice on day 14 and the extent of γδ T cell reconstitution was determined by flow cytometry.
Statistical Analysis
In general, three mice were used per condition in each experiment. Each experiment was repeated to obtain an n ≥ 6 for each condition. To control for potential variability in donor cells, sham or untreated mice that received the same donor cells were included in relevant experiments and were analyzed at the same time point. Data shown are either representative of multiple experiments or display the combined data for all experiments. For combined data sets, the mean value is shown ± SEM. Statistical relevance was determined using the Mann-Whitney t test for nonparametric data, with a 95% confidence interval.
Results
CD8+ γδ T cells have an early advantage during homeostatic expansion, whereas NK1.1+ γδ T cells are slow to proliferate
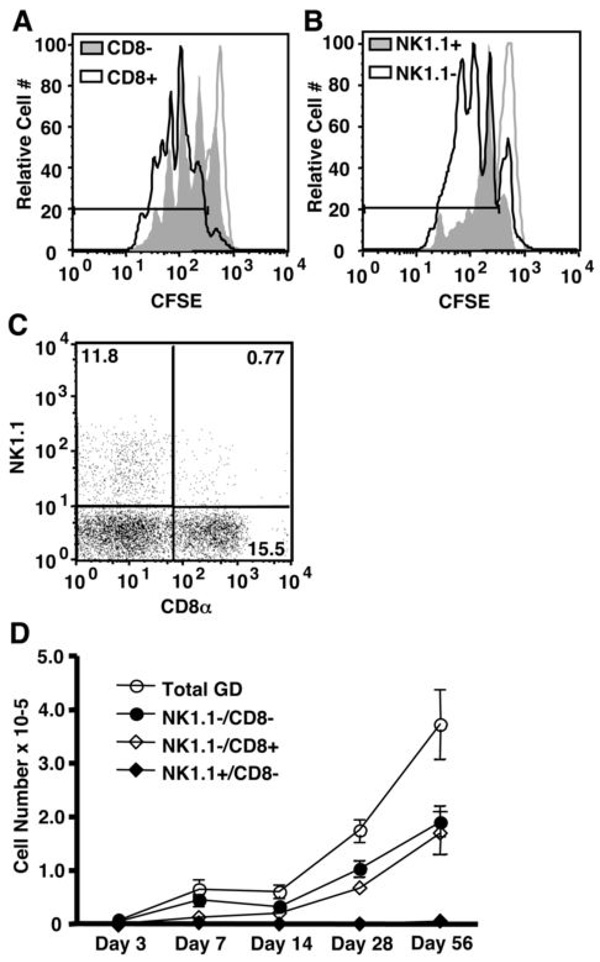
To determine the factors that regulate γδ T cell homeostasis, we developed a mouse model of γδ T cell homeostatic expansion (2). Briefly, splenic γδ T cells were obtained from TCRβ−/− mice, enriched by nylon wool purification, and injected i.v. into TCRβ−/−/δ−/− recipients. Short-term homeostatic expansion was measured as the loss of CFSE fluorescence in the donor cells three to six days following adoptive transfer. Long-term maintenance of the γδ T cell population was determined by cell yield as late as two months post adoptive transfer. Surprisingly, our studies revealed that subpopulations of γδ T cells displayed characteristically distinct proliferation profiles following adoptive transfer into TCRβ−/−/δ−/− recipients. Specifically, CD8α+ γδ T cells exhibited rapid expansion compared to their CD8α− counterparts (Figure 1A). 88.6 ± 6.0% of CD8+ γδ T cells compared to 73.8 ± 7.2% of CD8− γδ T cells had undergone at least one division five days after adoptive transfer. Furthermore, a larger percentage of CD8+ γδ T cells were found within the peak representing the second (30.6 ± 1.3%) and third (25.0 ± 4.5%) generations compared to CD8− γδ T cells (23.3 ± 1.3% and 15.5 ± 4.8%, respectively). Additionally, NK1.1+ γδ T cells proliferated poorly, compared to NK1.1− γδ T cells, in lymphopenic recipients (Figure 1B). While 76.4 ± 10.2% of NK1.1+ γδ T cells and 81.7 ± 6.6% NK1.1− γδ T cells had undergone at least one division, the majority of NK1.1+ cells were arrested after one division.
Figure 1. CD8+ and NK1.1+ γδ T cell subsets undergo homeostatic expansion in TCRβ−/−δ−/− mice at distinct rates.
(A and B) Splenic γδ T cells were isolated from TCRβ−/− mice, labeled with CFSE, and injected into TCRβ−/−/δ−/− recipients. Homeostatic proliferation of CD8− and CD8+ or NK1.1− and NK1.1+ γδ T cells subsets was assessed on day 5 by flow cytometry. Gates delineate those cells that have undergone at least one division, as determined by the CFSE levels of non-T cells within our donor population (unfilled grey histogram). (C) The frequency of NK1.1−/CD8−, NK1.1−/CD8+, and NK1.1+/CD8− in the donor γδ T cell population was determined by flow cytometry. Percentages of live CD3+/TCRδ+ cells are shown for each quadrant. (D) γδ T cell reconstitution was assessed from 3 days to 2 months after transfer into TCRβ−/−/δ−/− mice.
The observed difference in γδ T cell subset homeostatic expansion was even more pronounced at later time points. Although the CD8+ γδ T cells constituted only 12–15% of the donor population (Figure 1C), they made up nearly 50% of the total γδ T cell population one month after adoptive transfer (Figure 1D). This increase was due to enrichment of the existing CD8+ cells since CD8− cells did not upregulate CD8 following adoptive transfer into TCRβ−/−/δ−/− recipients (data not shown). Of note, greater than 90% of donor CD8α+ γδ T cells expressed the CD8αβ heterodimer, and this percentage was maintained after adoptive transfer (data not shown). While NK1.1+ γδ T cells constituted 10–15% of the initial donor cell population (Figure 1C), less than 2% of γδ T cells expressed NK1.1 two months after transfer (Figure 1D). As shown in Figure 3, NK1.1+/CD8α+ cells make up less than 1% of the donor population and do not increase significantly in number following adoptive transfer. Therefore, this population was not included in our analysis. Importantly, the spleen and lymph nodes were the primary destination of the donor γδ T cells, and NK1.1+ γδ T cells did not preferentially traffic to the lung, intestine, or liver (data not shown). Thus, the observed pattern of reconstitution cannot be explained by unique migration characteristics of γδ T cell subsets.
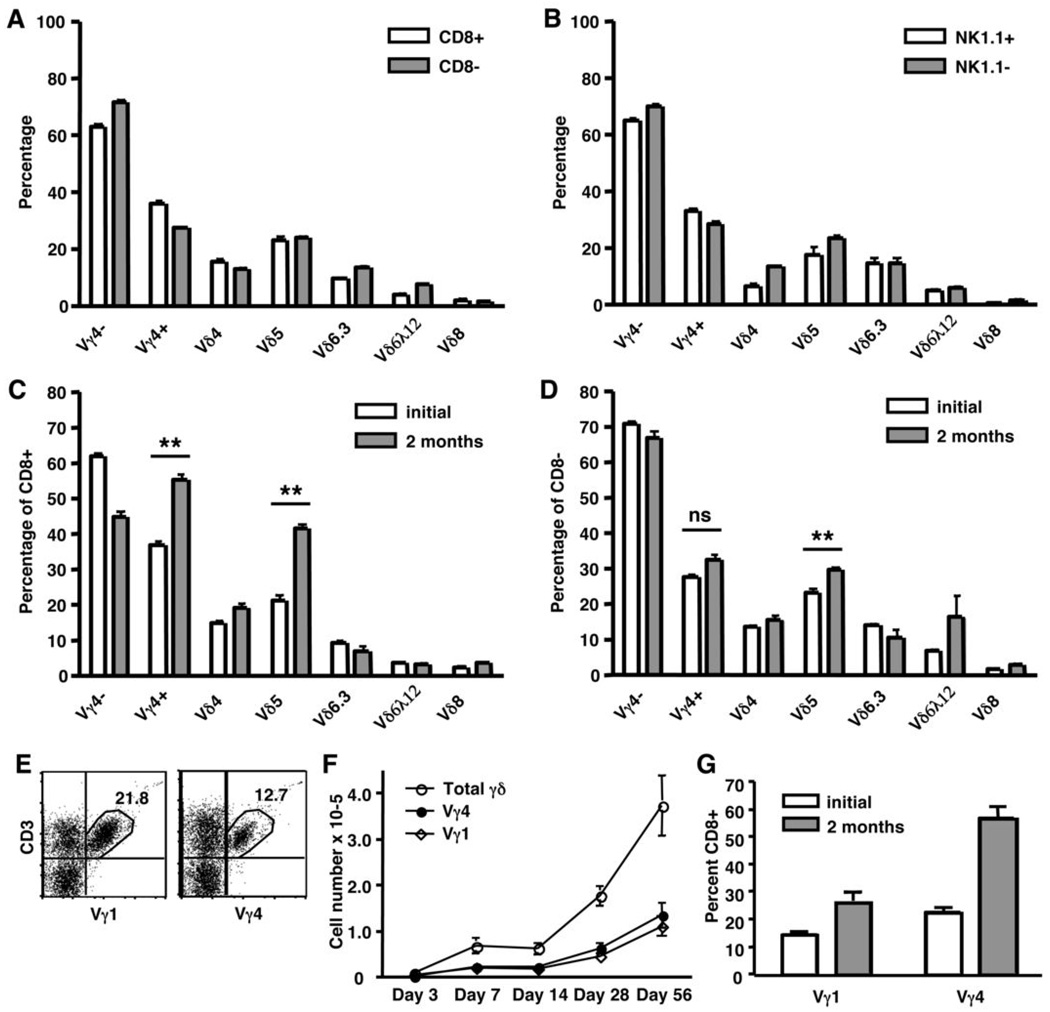
Figure 3. TCR variable gene expression by γδ T cell subsets.
Splenic γδ T cells were isolated from TCRβ−/− mice and analyzed by flow cytometry for Vγ and Vδ expression before (A and B) or after adoptive transfer into TCRβ−/−/δ−/− recipients (C and D). γδ T cell subsets were gated based on CD8α (A) or NK1.1 (B) expression, and the percentage of cells that express Vγ4, Vδ4, Vδ5, Vδ6.3, Vδ6λ12, or Vδ8 was determined. CD8+ (C) and CD8− (D) γδ T cell subsets were also characterized for Vγ and Vδ expression after undergoing homeostatic expansion in TCRβ−/−/δ−/− mice for two months. (E–G) TCRβ−/− splenic γδ T cells were analyzed by flow cytometry for expression of Vγ1, Vγ4, and CD8α either before (E and G) or after (F and G) adoptive transfer into TCRβ−/−/δ−/− recipients. (E) Percentage of live CD3+/TCRδ+ cells is shown. (ns = not statistically significant; ** P < 0.01)
Differential γδ T cell expansion does not correlate with bcl-2 or cytokine receptor expression
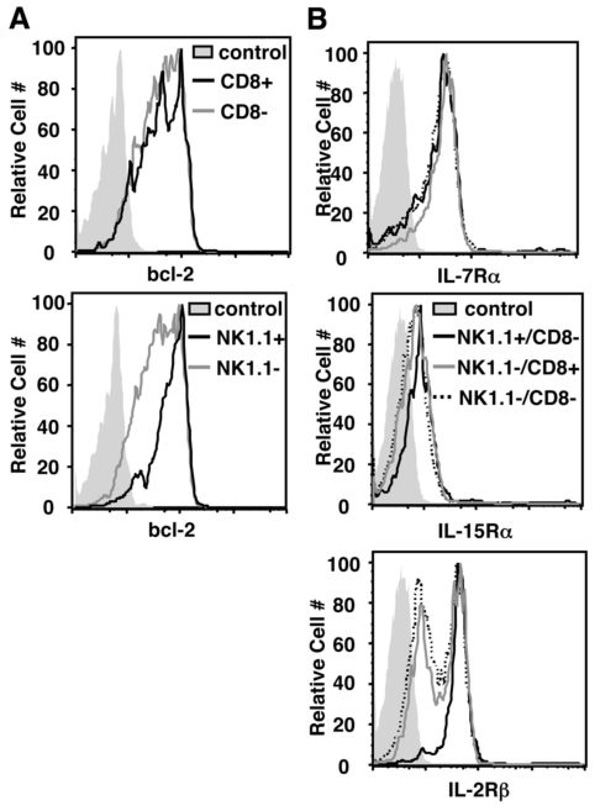
The observed advantage of CD8+ γδ T cells and disadvantage of NK1.1+ γδ T cells could be explained by an inherent difference in potential for survival and/or proliferation. To investigate this possibility, we next determined the relative expression of bcl-2 and homeostatic cytokine receptors by these γδ subsets. Splenic γδ T cells were isolated from TCRβ−/− mice and expression of bcl-2, IL-7Rα, IL-15Rα, and IL-2Rβ was determined by flow cytometry. As shown in Figure 2, CD8+ γδ T cells expressed similar levels of these survival factors compared to CD8− γδ T cells. In contrast, NK1.1+ γδ T cells expressed uniformly high levels of bcl-2 and IL-2Rβ while expression levels varied among the NK1.1− γδ T cells. Thus, the advantage of CD8+ γδ T cells during homeostatic expansion cannot be explained by bcl-2 or cytokine receptor expression levels. Moreover, despite having an inherent survival advantage (i.e., increased bcl-2 and IL-2Rβ expression), NK1.1+ γδ T cells compete poorly during homeostatic expansion. Of note, we did not observe any significant differences in bcl-2, IL-7Rα, IL-15Rα, or IL-2Rβ expression levels between CD8+ and CD8− subsets two months after adoptive transfer. Evaluation of the NK1.1+ subsets post-adoptive transfer was not possible due to inefficient reconstitution of these cells in TCRβ−/−/δ−/− mice.
Figure 2. Bcl-2 and cytokine receptor expression levels in γδ T cell subsets.
Splenic γδ T cells were enriched from TCRβ−/− mice and analyzed by flow cytometry. (A) CD8+ and CD8−or NK1.1+ and NK1.1− γδ T cell subsets were gated and assessed for intracellular expression of bcl-2. (B) NK1.1+/CD8−, NK1.1−/CD8+, and NK1.1−/CD8− γδ T cells were gated and assessed for expression of the homeostatic cytokine receptors, IL-7Rα, IL-15Rα, and IL-2Rβ. In both A and B, filled histograms represent unstained cells.
TCR and CD8 may work in concert to provide an advantage to γδ T cells during homeostatic expansion
Numerous studies suggest that γδ T cells can be divided into functionally distinct subsets based on TCR variable gene usage (i.e., Vγ4, Vγ1, Vγ6, and Vδ5). It is not known whether TCR gene usage correlates with expression of CD8 or NK1.1 in mature γδ T cells. Therefore, we next assessed TCR Vγ and Vδ gene expression by CD8+ and NK1.1+ γδ T cell subsets from TCRβ−/− mice. As shown in Figure 3A and 3B, TCR variable gene usage was similar in all donor γδ T cell subsets regardless of CD8 or NK1.1 expression. Thus, CD8+ and NK1.1+ γδ T cell subsets cannot be defined by TCR variable gene usage. We next questioned whether expression of certain Vγ or Vδ genes correlated with the advantage of the CD8+ subset during homeostatic expansion in TCRβ−/−/δ−/− recipients. While all Vγ and Vδ genes examined were represented in the γδ T cells that had undergone homeostatic expansion, both Vγ4+ and Vδ5+ populations were substantially enriched in the CD8+ subset (Figure 3C and 3D). Vγ4+/Vδ5+ and Vγ4−/Vδ5+ T cells were increased by 57% and 67%, respectively, in the CD8+ subset following adoptive transfer, suggesting that the Vδ5+ cell enrichment was not dependent upon Vγ4 expression (data not shown). While the percentages of Vγ4+ and Vδ5+ cells were slightly higher in the CD8− subset after homeostatic expansion, this increase was minimal compared to that observed in the CD8+ subset. To determine whether CD8+/Vγ4+ and/or CD8+/Vδ5+ cells had undergone clonal expansion, CD8+ γδ T cells were sorted two months after transfer into TCRβ−/−/δ−/− recipients and cDNA of each was sequenced across the V-J and V-D-J junction of the Vγ4 and Vδ5 transcripts, respectively. Both Vγ4+ and Vδ5+ T cells were composed of a diverse polyclonal population (data not shown). Thus, while expression of Vγ4 and/or Vδ5 may be associated with the homeostatic advantage among CD8+ γδ T cells, the hypervariable regions are not involved.
These data suggest that Vγ4 and Vδ5 work in concert with CD8 to provide an advantage during homeostatic expansion. In support of this hypothesis, Vγ4+ cells display a modest advantage over Vγ1+ cells during homeostatic expansion in TCRβ−/−/δ−/− mice. Although the Vγ4+ subset is out-numbered nearly two-fold by Vγ1+ cells in the donor population (Figure 3E), Vγ1+ and Vγ4+ cells reach equivalent numbers one week after adoptive transfer (Figure 3F). This advantage is maintained as long as two months and may be attributed, at least in part, to a higher composition of CD8+ cells in the Vγ4+ subset both before and after adoptive transfer (Figure 3G). Given the small size of the NK1.1+ subset following adoptive transfer, an accurate assessment of TCR gene expression could not be obtained for these cells.
γδ T cell homeostatic expansion is enhanced in the absence of β2-microglobulin
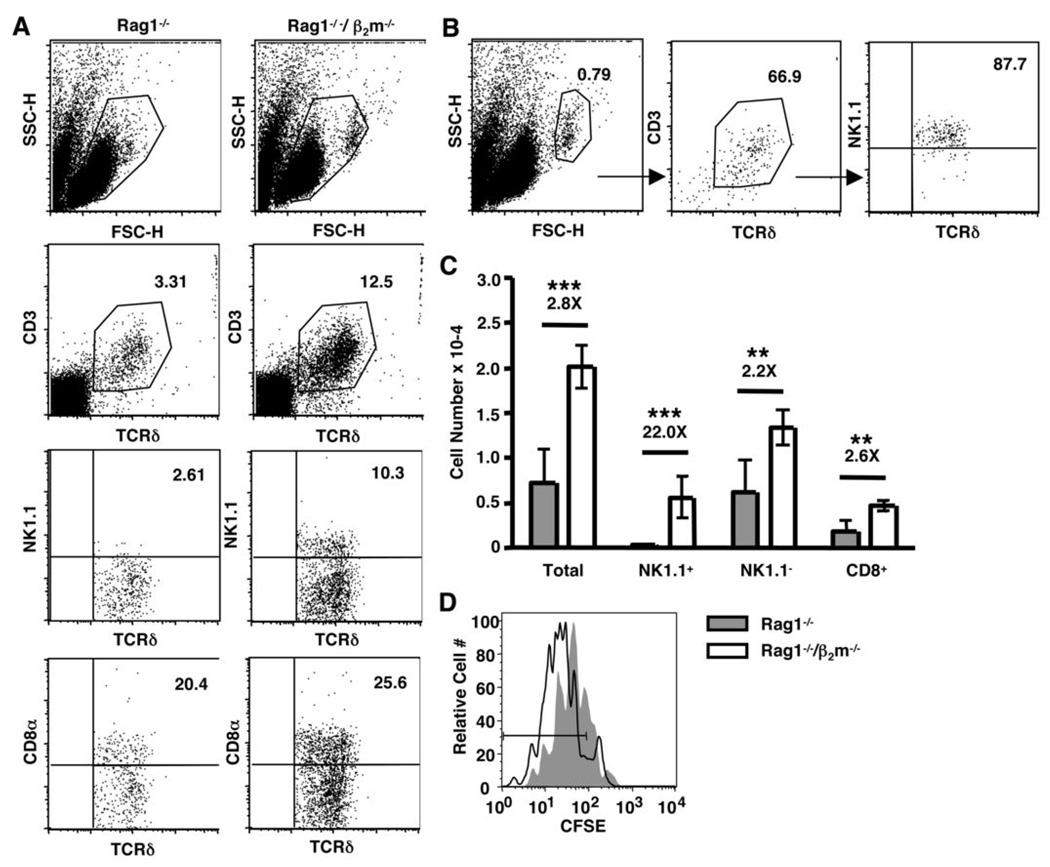
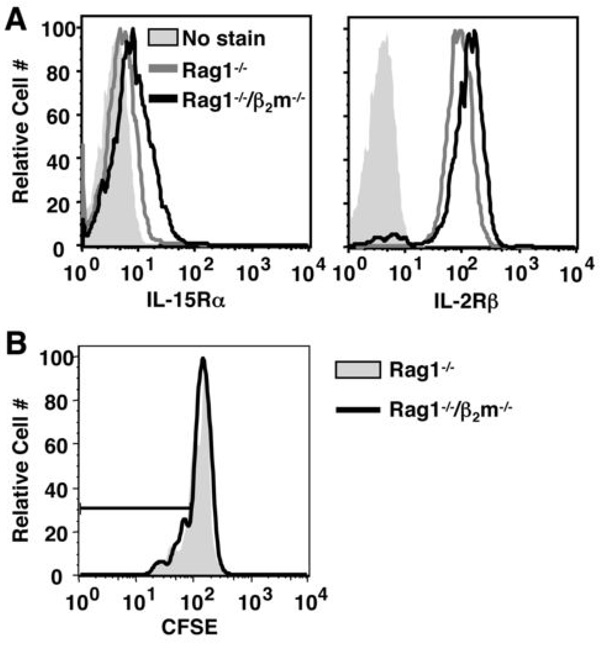
As noted above, naïve CD8+ αβ T cell homeostasis is dependent upon MHCI recognition. We next questioned whether MHCI molecules were similarly promoting CD8+ γδ T cell homeostatic expansion. Donor γδ T cells were transferred into Rag1−/− or Rag1−/−/β2m−/− recipients, and both long-term reconstitution and short-term proliferation were assessed by flow cytometry. As shown in Figure 4A, CD8+ γδ T cells reconstituted both Rag1−/− and Rag1−/−/β2m−/− mice efficiently. Unexpectedly, the NK1.1+ γδ T cell subset was dramatically increased in Rag1−/−/β2m−/− mice compared to Rag1−/− mice two weeks after adoptive transfer (Figure 4A). Of note, a significant percentage of cells recovered from Rag1−/−/β2m−/− recipients showed a heightened forward scatter, which is characteristic of lymphoblasts. This population consisted of more than 60% γδ T cells, which were almost entirely NK1.1+ (Figure 4B). NK cells and non-T cells were also found in the blast cell population, but were seen at a similar level in Rag1−/− recipients (data not shown). On average, NK1.1+ γδ T cells were increased more than twenty-fold in Rag1−/−/β2m−/− recipients compared to Rag1−/− hosts (Figure 4C). Furthermore, NK1.1− γδ T cells, including the CD8+ subset, were also increased in the absence of β2m, albeit to a lesser extent (Figure 4C). The overall proliferative advantage of γδ T cells in Rag1−/−/β2m−/− recipients was evident five days after adoptive transfer (Figure 4D). Importantly, the donor γδ T cells used in our model express β2m-associated molecules, which could affect the ability of these cells to proliferate even in the absence of host β2m. However, γδ T cells from C57BL/6 β2m−/− and C57BL/6 wild-type mice proliferated to a similar extent in Rag1−/−/β2m−/− recipients, suggesting that β2m-associated molecules expressed by the donor cells, themselves, had no measurable effect on their ability to undergo homeostatic expansion (data not shown). Thus, although β2m-associated molecules are not responsible for the observed advantage of the CD8+ γδ T cell population, they appear to negatively regulate the NK1.1+ γδ T cell subset and to have a general inhibitory effect on all γδ T cells.
Figure 4. γδ T cell homeostatic proliferation and reconstitution in Rag1−/−/β2m−/− recipients.
(A–C) Splenic γδ T cells were enriched from TCRβ−/− mice and injected into Rag1−/− or Rag1−/−/β2m−/− recipients. Reconstitution was assessed on day 14 by flow cytometry. (A) Live lymphocytes or (B) blasted lymphocytes were gated and CD3+/TCRδ+ cells were assessed for their expression of NK1.1+ or CD8+. Percentages of total live lymphocytes or live CD3+/TCRδ+ cells are shown. (C) The number of γδ T cells recovered from Rag1−/− and Rag1−/−/β2m−/− recipients was calculated by multiplying the total number of cells recovered by the percentage of live lymphocytes in each sample and by the percentage of each gated subpopulation. (D) Donor cells were labeled with CFSE prior to adoptive transfer into Rag1−/− and Rag1−/−/β2m−/− recipients, and homeostatic proliferation of total γδ T cells was assessed on day 5 by flow cytometry. (** P < 0.01, ***P < 0.001)
NK cells are homeostatically competent in Rag1−/−/β2m−/− mice
As noted above, NK cells are capable of inhibiting γδ T cell homeostatic expansion (2). Although NK cells are produced at normal numbers in β2m-deficient mice, their cytotoxic function is impaired (24). A defect in the ability of NK cells to compete for homestatic cytokines in Rag1−/−/β2m−/− mice could provide an advantage to γδ T cells during homestatic expansion. To determine whether endogenous NK cells are homeostatically competent in Rag1−/−/β2m−/− mice, we first assessed their ability to express the IL-15 receptor subunits IL-2Rβ and IL-15Rα. As shown in Figure 5A, splenic NK cells isolated from Rag1−/− and Rag1−/−/β2m−/− mice expressed comparable levels of IL-2Rβ and IL-15Rα. Adoptively-transferred NK cells undergo homeostatic expansion only in mice that lack endogenous NK cells due to the absence of IL-2Rγc or as a result of sub-lethal irradiation. The endogenous NK cell population in immune-competent mice out-competes adoptively-transferred NK cells for IL-15 resources that are required for homeostatic expansion and survival (25, 26). Importantly, in our model, CFSE-labeled donor NK cells did not undergo homeostatic proliferation in either Rag1−/− or Rag1−/−/β2m−/−mice (Figure 5B). Therefore, defective NK cells are not responsible for the observed advantage of γδ T cells in Rag1−/−/β2m−/− recipients.
Figure 5. Endogenous Rag1−/−/β2m−/− NK cells express IL-15 receptors and limit the expansion of donor NK cells.
(A) Splenocytes were harvested from Rag1−/− and Rag1−/−/β2m−/− mice and stained for NK1.1 and CD3 in combination with IL-15Rα or IL-2Rβ. NK1.1+/CD3− cells were gated and assessed for cytokine receptor expression. Filled histograms represent unstained cells. (B) Nylon wool-enriched lymphocytes from TCRβ−/− mice were labeled with CFSE and injected into Rag1−/− or Rag1−/−/β2m−/− recipients. Donor NK cells (NK1.1+/CD3−) were gated and their proliferation was assessed by flow cytometry on day 5.
NKG2A and Ly49C do not play a role in the negative regulation of γδ T cell homeostasis by MHCI molecules
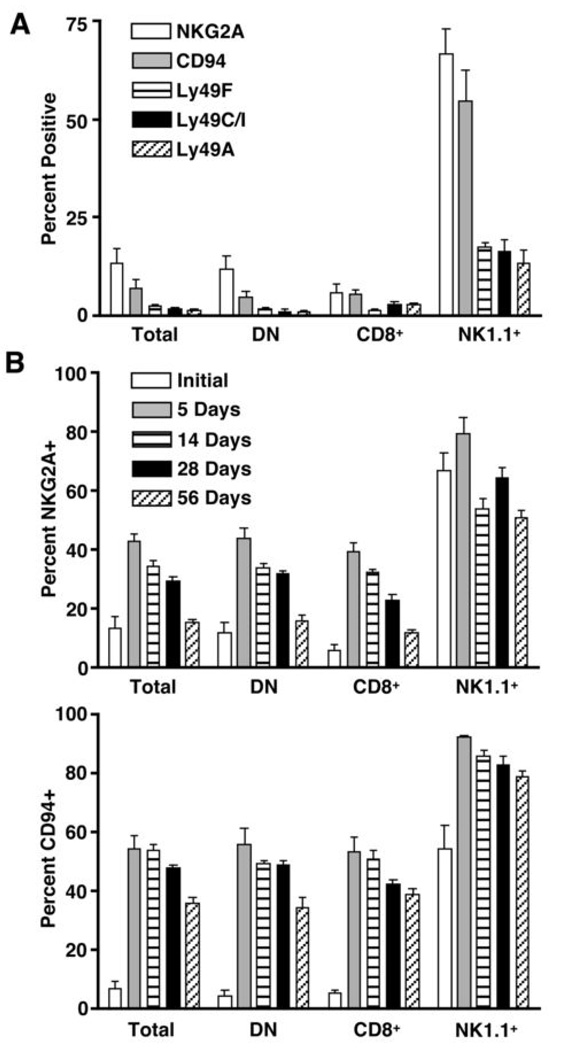
Ly49 family members and NKG2A/CD94 recognize classical MHCI molecules and the non-classical MHCI molecule, Qa-1b, respectively, and are known for their ability to inhibit NK cell and T cell function (27, 28). Both Ly49 family members and NKG2A/CD94 can be expressed by mouse γδ T cells, and their expression is more frequent within the NK1.1+ γδ T cell subset (15). Therefore, we next questioned whether NK inhibitory receptors could be responsible for mediating the inhibitory effect of MHCI molecules in our model. To address this possibility, we characterized TCRβ−/−-derived γδ T cells for expression of key NK inhibitory receptors both before and after adoptive transfer into TCRβ−/−/δ−/− mice. As shown in Figure 6A, NKG2A and CD94 are expressed by more than 60% of NK1.1+ γδ T cells. The Ly49 family members, Ly49F, Ly49I/C, and Ly49A, are each expressed by 20% of NK1.1+ γδ T cells. In contrast, less than 20% of NK1.1− γδ T cells express NKG2A/CD94 heterodimers, and the Ly49 family members are virtually absent (Figure 6A, DN). Surprisingly, NKG2A and CD94 were rapidly upregulated by all γδ T cell subsets following adoptive transfer. NKG2A levels peaked within the first week following adoptive transfer but dropped to near baseline levels by two months (Figure 6B). In contrast, CD94 levels remained high even after two months (Figure 6C).
Figure 6. NK inhibitory receptor expression by γδ T cells before and after homeostatic expansion.
Splenic γδ T cells were isolated from TCRβ−/− mice and analyzed for NK inhibitory receptor expression prior to adoptive transfer (A) or from days 5 to 56 following adoptive transfer into TCRβ−/−/δ−/− recipients (B). Total γδ T cells (TCRδ+/CD3+) and γδ T cell subsets were assessed for expression of NK inhibitory receptors by flow cytometry. (DN=CD8−/NK1.1−)
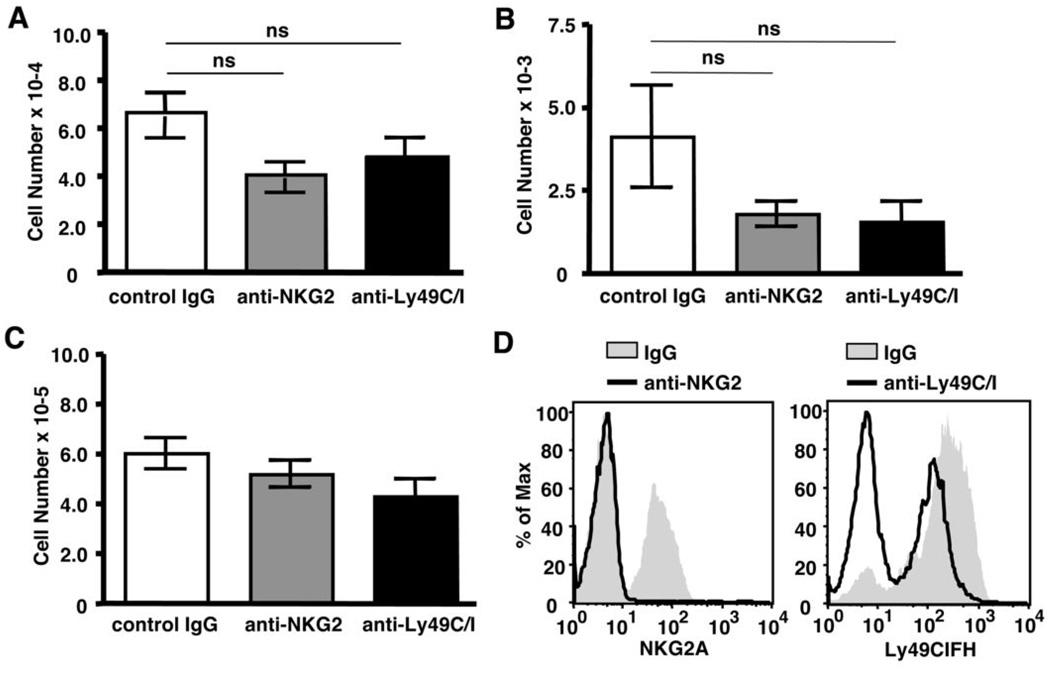
To determine whether these inhibitory receptors were involved in regulating γδ T cell homeostasis, we tested the effects of anti-NKG2 and anti-Ly49C/I antibodies that are known to block recognition of Qa-1b and H-2b by NKG2A and Ly49C/I, respectively (22, 29, 30). Treatment with either anti-NKG2 or anti-Ly49C/I did not enhance overall γδ T cell reconstitution compared to control mice treated with IgG (Figure 7A). Similarly, NK1.1+ γδ T cell numbers were not increased in the presence these blocking antibodies (Figure 7B). Rather, we observed a small but statistically insignificant decrease in the number of total γδ T cells and NK1.1+ γδ T cells following treatment. Furthermore, NK cell numbers were not significantly affected by treatment with these antibodies, suggesting cells expressing NK inhibitory receptors were not depleted (Figure 7C). As shown in Figure 4D, NKG2A could no longer be detected on the surface of NK cells following treatment with anti-NKG2 antibody, confirming that this treatment regimen was successful in down-regulating NKG2A expression (panel 1). While the anti-Ly49C/I antibody induced a partial down-regulation of Ly49 isoforms (Figure 4D, panel 2), this effect was inconsistent between experiments. Like NK cells, NK1.1+γδ T cells co-express multiple Ly49 isoforms (Figure 6A). The Ly49CIFH-specific antibody used to detect Ly49C/I also detects Ly49F and Ly49H, so some residual staining after anti-Ly49C/I treatment was expected in this experiment. Taken together, these data suggest that NKG2A and Ly49C do not mediate the inhibitory effects of β2m-associated molecules during γδ T cell homeostatic expansion.
Figure 7. Blocking NKG2A or Ly49C interactions with MHCI does not affect γδ T cell homeostatic expansion.
Splenic γδ T cells were enriched from TCRβ−/− mice and injected into TCRβ−/−/δ−/− recipients. Recipient mice received injections of control IgG, anti-NKG2, or anti-Ly49C/I antibodies on days 0, 2, 5, 8, and 11 relative to cell transfer. Reconstitution of total γδ T cells (A) and NK1.1+ γδ T cells (B), or total number of NK cells (C, NK1.1+/TCRδ−) was determined by flow cytometry on day 14. (D) NKG2A and Ly49CIFH expression by NK cells was determined by flow cytometry at the time of harvest. (ns = not statistically significant)
Discussion
While Vγ and Vδ TCR gene usage correlates with γδ T cell localization and function in numerous disease models, variable expression of CD8 and NK receptors within these subsets may warrant further sub-categorization. Indeed, we observed that CD8+ and NK1.1+ γδ T cell subsets were differentially regulated and conserved as distinct populations during homeostatic expansion, suggesting that these populations may serve unique functions. The observed enrichment of Vγ4+ and Vδ5+ γδ T cells in the CD8+ subset following homeostatic expansion suggest that TCR and CD8 may work in concert to provide a homeostatic advantage to these cells. If the CD8+ subset is driven by a TCR-specific ligand, we might expect to see a common sequence within the CDR3 region of either TCRγ or TCRδ. In support of this hypothesis, Shin et al. found that a W-SEGYEL motif in the TCRδ chain was required for T22 recognition (31, 32). In addition, our studies in a mouse arthritis model revealed that disease-exacerbating γδ cells expressed a common motif in the CDR3 region of both Vγ4 and Vδ4 (33). In contrast to these studies, we found no evidence of clonal expansion in CD8+ γδ T cells that had undergone homeostatic proliferation.
It remains possible, however, that conserved regions of Vγ4 and Vδ5 are responsible for recognition of a putative ligand. Despite the exhibited role of CD8 as a coreceptor in αβ T cell recognition of MHCI molecules, CD8+ γδ T cell homeostatic expansion was not reduced in recipient mice lacking β2m (Figure 4A). Thus, if CD8 is directly responsible for the homeostatic advantage of CD8+ γδ T cells, it does so by binding a ligand other than MHCI. While CD8 is not commonly studied outside the context of MHCI, gp180, a membrane glycoprotein expressed by human epithelial cells, binds human CD8 resulting in p56lck activation (34). It is not known whether a similar scenario exists in mice and could play a role in CD8+ γδ T cell homeostasis. Alternatively, CD8 could be a coincidental marker, and the advantage of this subset during homeostatic expansion may not depend upon CD8. Although CD8+ γδ T cells did not express higher levels of cytokine receptors or bcl-2, these cells may preferentially express other molecules that provide an inherent survival or mitotic advantage.
Previous studies, by our laboratory and others, revealed that γδ T cell homeostatic proliferation in irradiation-induced lymphopenic recipients was not affected by the absence of β2m (2, 35). However, irradiation has been found to cause a substantial inflammatory response which complicates the interpretation of these data (36). In fact, Gruber et al. discovered an “irradiation artifact” when comparing the requirements for CD8+ αβ T cell homeostasis in irradiation-dependent and -independent models (37). In light of these findings, we reassessed the role of β2m-associated molecules in γδ T cell homeostasis using, as recipients, β2m deficient mice on a Rag1−/− background. Unexpectedly, γδ T cell homeostatic expansion was substantially improved in the absence of β2m. Importantly, enhanced proliferation and reconstitution was not due to lack of competition from defective endogenous NK cells and could not be explained by an increased susceptibility of Rag1−/−/β2m−/− mice to infection. On the contrary, γδ T cell reconstitution was substantially reduced in recipients with increased spleen size, which is indicative of infection (data not shown).
Homeostatic expansion of the NK1.1+ γδ T cell subset was particularly enhanced in the absence of β2m, suggesting that the relative disadvantage of this subset following adoptive transfer into TCRβ−/−/δ−/− recipients was due to the presence of β2m-associated molecules. Consistent with these findings, Hara et al. showed that the NK1.1+ γδ T cell population is increased by 4-fold in liver of C576BL/6 β2m−/− mice (15). While the authors suggest that γδ T cell development is enhanced in β2m−/− mice, this increase may also reflect enhanced survival and/or homeostatic expansion of NK1.1+ γδ T cells in peripheral tissues in the absence of β2m. Homeostatic expansion of NK1.1− γδ T cells, including the CD8+ subset, was only slightly enhanced in the absence of β2m in our model, suggesting that NK1.1− and NK1.1+ subsets may be regulated by distinct mechanisms.
Despite the observed correlation between NK inhibitory receptor expression and reduced homeostatic expansion in β2m-competent hosts, our studies failed to support a definitive role for NKG2A/CD94 and Ly49C in γδ T cell homeostasis. NKG2A/CD94 is expressed by γδ T cells within all subsets, and the frequency of NKG2A/CD94 expression increases during homeostatic expansion. We hypothesized that NKG2A/CD94 is up-regulated during γδ T cell homeostatic expansion in order to limit the population size. NKG2A levels returned to baseline two months after adoptive transfer, when the population had reached its maximum size. At this point, other factors, such as limiting levels of IL-15 and IL-7, likely play a dominant role in regulating the size of the γδ T cell pool. However, blocking the interaction between NKG2A and Qa-1b with an anti-NKG2 antibody did not enhance γδ T cell reconstitution in TCRβ−/−/δ−/− recipients, suggesting that NKG2A and Qa-1b are not major players in the control of γδ T cell homeostasis. Of note, IL-15 is known to induce NKG2A expression in human CD8+ αβ T cells (38) and may also be responsible for NKG2A upregulation by γδ T cells during homeostatic expansion. Ly49 inhibitory receptors were expressed by 20% of NK1.1+ γδ T cells and could mediate the inhibitory effects of β2m-associated molecules on this subset. Of the Ly49 family members, only Ly49C is known to recognize the H-2b haplotype (39). However, blocking the interaction between Ly49C and H-2b with an anti-Ly49CIFH antibody had no effect on either NK1.1+ or NK1.1− γδ T cell homeostatic expansion. In agreement with these findings, anti-Ly49C/I also did not influence cytokine-induced proliferation of γδ T cells in vitro (data not shown).
Additional studies are required to determine whether β2m or β2m-associated molecules are responsible for negatively regulating γδ T cells during homeostatic expansion and the mechanism by which they exert their effects. Our studies suggest that NKG2A and Ly49C are not involved in regulating γδ T cell homeostasis, but it remains possible that β2m-associated Qa-1b and/or classical MHCI molecules exert an inhibitory effect through alternative receptors. The non-classical MHCI molecules H2-M3, T22, T10, Qa-2, and CD1 are viable candidates, given their dependence upon β2m and broad tissue expression (40, 41). Of interest, expression of Hfe, a non-classical MHCI molecule that regulates cellular iron uptake, is also dependent upon β2m (42). In β2m−/− mice, the absence of this receptor contributes to progressive iron overload in the liver (43). Numerous studies have shown that T cell proliferation is compromised when iron levels are reduced and enhanced in the presence of iron-loaded transferrin, suggesting that iron boosts T cell expansion (44–46). The effect of iron overload on γδ T cells is not known. Whether increased levels of iron in the absence of β2m contribute to the overall enhancement of γδ T cell homeostatic expansion in the Rag1−/−/β2m−/− mice remains to be determined.
The ability of β2m to inhibit γδ T cell homeostasis is a novel observation. Naïve CD8+ αβ T cell homeostatic expansion is driven by self-MHCI (47–49), while NK cell and NK T cell homeostatic expansion is neither driven nor inhibited by MHCI molecules (25, 26, 50, 51). An opposing role for β2m-associated molecules in αβ T cell and γδ T cell homeostasis may explain our previous observation that reconstitution of TCRβ−/−/δ−/− by γδ T cells is relatively inefficient compared to αβ T cells (2). While MHCI molecules provide an advantage to CD8+ naïve T cells, they appear to inhibit γδ T cell homeostasis, thus, limiting the maximum size of the population. Given their role in regulating the immune response, it is not surprising that multiple mechanisms are in place to restrict the quantity of γδ T cells in an organism.
γδ T cells have long been recognized for their potent anti-tumor effects both in vitro and in vivo. In human studies, in vivo γδ T cell expansions have been correlated with temporary tumor regression and increased survival of leukemia and myeloma patients (52–54). Thus, γδ T cell-based immunotherapies may be a viable option for such patients. Of interest, elevated serum β2m levels have been shown to correlate with reduced survival and tumor progression in myeloma patients following autologous hematopoietic stem cell transplant (55). Based on our findings, it is tempting to speculate that γδ T cell proliferation and/or survival may be inhibited in the presence of high serum β2m, contributing to the poor prognosis for these patients.
Footnotes
This work was supported by NIH grant 2RO1A144920 to R.L.O.
Disclosures: The authors have no financial interests or conflicts of interest pertaining to this work.
References
- 1.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 2.French JD, Roark CL, Born WK, O'Brien RL. {gamma}{delta} T cell homeostasis is established in competition with {alpha}{beta} T cells and NK cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J. Exp. Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 5.Chien YH, Jores R, Crowley MP. Recognition by gamma/delta T cells. Annual Review of Immunology. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 6.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. Journal of Immunology. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub BC, Jackson MR, Hedrick SM. Gamma delta T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. Journal of Immunology. 1994;153:3051–3058. [PubMed] [Google Scholar]
- 9.Tough DF, Sprent J. Lifespan of gamma/delta T cells. J. Exp. Med. 1998;187:357–365. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, Chien Y. A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- 11.Bucy RP, Chen CL, Cooper MD. Tissue localization and CD8 accessory molecule expression of T gamma delta cells in humans. J Immunol. 1989;142:3045–3049. [PubMed] [Google Scholar]
- 12.Deusch K, Luling F, Reich K, Classen M, Wagner H, Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur J Immunol. 1991;21:1053–1059. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Ohtsuka K, Watanabe H, Asakura H, Abo T. Detailed characterization of gamma delta T cells within the organs in mice: classification into three groups. Immunology. 1993;80:380–387. [PMC free article] [PubMed] [Google Scholar]
- 14.Poccia F, Cipriani B, Vendetti S, Colizzi V, Poquet Y, Battistini L, Lopez-Botet M, Fournie JJ, Gougeon ML. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive V gamma 9V delta 2 T lymphocytes. Journal of Immunology. 1997;159:6009–6017. [PubMed] [Google Scholar]
- 15.Hara T, Nishimura H, Hasegawa Y, Yoshikai Y. Thymus-dependent modulation of Ly49 inhibitory receptor expression on NK1.1+gamma/delta T cells. Immunology. 2001;102:24–30. doi: 10.1046/j.1365-2567.2001.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toyama-Sorimachi N, Taguchi Y, Yagita H, Kitamura F, Kawasaki A, Koyasu S, Karasuyama H. Mouse CD94 participates in Qa-1-mediated self recognition by NK cells and delivers inhibitory signals independent of Ly-49. Journal of Immunology. 2001;166:3771–3779. doi: 10.4049/jimmunol.166.6.3771. [DOI] [PubMed] [Google Scholar]
- 17.Van Beneden K, De Creus A, Stevenaert F, Debacker V, Plum J, Leclercq G. Expression of inhibitory receptors Ly49E and CD94/NKG2 on fetal thymic and adult epidermal TCR V gamma 3 lymphocytes. Journal of Immunology. 2002;168:3295–3302. doi: 10.4049/jimmunol.168.7.3295. [DOI] [PubMed] [Google Scholar]
- 18.Ryan JC, Turck J, Niemi EC, Yokoyama WM, Seaman WE. Molecular cloning of the NK1.1 antigen, a member of the NKR-P1 family of natural killer cell activation molecules. Journal of Immunology. 1992;149:1631–1635. [PubMed] [Google Scholar]
- 19.Carlyle JR, Jamieson AM, Gasser S, Clingan CS, Arase H, Raulet DH. Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3527–3532. doi: 10.1073/pnas.0308304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iizuka K, Naidenko OV, Plougastel BF, Fremont DH, Yokoyama WM. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nature Immunology. 2003;4:801–807. doi: 10.1038/ni954. [DOI] [PubMed] [Google Scholar]
- 21.Battistini L, Borsellino G, Sawicki G, Poccia F, Salvetti M, Ristori G, Brosnan CF. Phenotypic and cytokine analysis of human peripheral blood gamma delta T cells expressing NK cell receptors. Journal of Immunology. 1997;159:3723–3730. [PubMed] [Google Scholar]
- 22.Vance RE, Jamieson AM, Raulet DH. Recognition of the class Ib molecule Qa-1(b) by putative activating receptors CD94/NKG2C and CD94/NKG2E on mouse natural killer cells. Journal of Experimental Medicine. 1999;190:1801–1812. doi: 10.1084/jem.190.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sentman CL, Hackett J, Jr, Kumar V, Bennett M. Identification of a subset of murine natural killer cells that mediates rejection of Hh-1d but not Hh-1b bone marrow grafts. J Exp Med. 1989;170:191–202. doi: 10.1084/jem.170.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 25.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. Journal of Immunology. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 26.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–4893. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 27.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 28.McMahon CW, Raulet DH. Expression and function of NK cell receptors in CD8+ T cells. Curr Opin Immunol. 2001;13:465–470. doi: 10.1016/s0952-7915(00)00242-9. [DOI] [PubMed] [Google Scholar]
- 29.Brennan J, Mahon G, Mager DL, Jefferies WA, Takei F. Recognition of class I major histocompatibility complex molecules by Ly-49: specificities and domain interactions. J Exp Med. 1996;183:1553–1559. doi: 10.1084/jem.183.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George TC, Ortaldo JR, Lemieux S, Kumar V, Bennett M. Tolerance and alloreactivity of the Ly49D subset of murine NK cells. Journal of Immunology. 1999;163:1859–1867. [PubMed] [Google Scholar]
- 31.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien YH. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 32.Adams EJ, Chien YH, Garcia KC. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–231. doi: 10.1126/science.1106885. [see comment]. [DOI] [PubMed] [Google Scholar]
- 33.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yio XY, Mayer L. Characterization of a 180-kDa intestinal epithelial cell membrane glycoprotein, gp180. A candidate molecule mediating t cell-epithelial cell interactions. J Biol Chem. 1997;272:12786–12792. doi: 10.1074/jbc.272.19.12786. [DOI] [PubMed] [Google Scholar]
- 35.Baccala R, Witherden D, Gonzalez-Quintial R, Dummer W, Surh CD, Havran WL, Theofilopoulus AN. Gamma-delta T Cell Homeostasis is Controlled by IL-7 and IL-15 Together with Subset-Specific Factors. J. Immunol. 2005;174:4606–4612. doi: 10.4049/jimmunol.174.8.4606. [DOI] [PubMed] [Google Scholar]
- 36.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 37.Gruber A, Brocker T. MHC class I-positive dendritic cells (DC) control CD8 T cell homeostasis in vivo: T cell lymphopenia as a prerequisite for DC-mediated homeostatic proliferation of naive CD8 T cells. Journal of Immunology. 2005;175:201–206. doi: 10.4049/jimmunol.175.1.201. [DOI] [PubMed] [Google Scholar]
- 38.Mingari MC, Ponte M, Bertone S, Schiavetti F, Vitale C, Bellomo R, Moretta A, Moretta L. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1172–1177. doi: 10.1073/pnas.95.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanke T, Takizawa H, McMahon CW, Busch DH, Pamer EG, Miller JD, Altman JD, Liu Y, Cado D, Lemonnier FA, Bjorkman PJ, Raulet DH. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 1999;11:67–77. doi: 10.1016/s1074-7613(00)80082-5. [DOI] [PubMed] [Google Scholar]
- 40.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nature Reviews. Immunology. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 41.Howcroft TK, Singer DS. Expression of nonclassical MHC class Ib genes: comparison of regulatory elements. Immunol Res. 2003;27:1–30. doi: 10.1385/IR:27:1:1. [DOI] [PubMed] [Google Scholar]
- 42.Cardoso CS, de Sousa M. HFE, the MHC and hemochromatosis: paradigm for an extended function for MHC class I. Tissue Antigens. 2003;61:263–275. doi: 10.1034/j.1399-0039.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 43.de Sousa M, Reimao R, Lacerda R, Hugo P, Kaufmann SH, Porto G. Iron overload in beta 2-microglobulin-deficient mice. Immunol Lett. 1994;39:105–111. doi: 10.1016/0165-2478(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 44.Bowern N, Ramshaw IA, Badenoch-Jones P, Doherty PC. Effect of an iron-chelating agent on lymphocyte proliferation. Aust J Exp Biol Med Sci. 1984;62(Pt 6):743–754. doi: 10.1038/icb.1984.70. [DOI] [PubMed] [Google Scholar]
- 45.Brock JH, Mainou-Fowler T, Webster LM. Evidence that transferrin may function exclusively as an iron donor in promoting lymphocyte proliferation. Immunology. 1986;57:105–110. [PMC free article] [PubMed] [Google Scholar]
- 46.Mainou-Fowler T, Brock JH. Effect of iron deficiency on the response of mouse lymphocytes to concanavalin A: the importance of transferrin-bound iron. Immunology. 1985;54:325–332. [PMC free article] [PubMed] [Google Scholar]
- 47.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 48.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 49.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, Kronenberg M. Homeostasis of V alpha 14i NKT cells. Nat. Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 51.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2663–2668. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A. Induction of gamma delta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 53.Lamb LS, Jr, Henslee-Downey PJ, Parrish RS, Godder K, Thompson J, Lee C, Gee AP. Increased frequency of TCR gamma delta + T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J. Hematother. 1996;5:503–509. doi: 10.1089/scd.1.1996.5.503. [DOI] [PubMed] [Google Scholar]
- 54.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gamma delta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 55.Stella-Holowiecka B, Czerw T, Holowiecka-Goral A, Giebel S, Wojnar J, Holowiecki J. Beta-2-microglobulin level predicts outcome following autologous hematopoietic stem cell transplantation in patients with multiple myeloma. Transplant Proc. 2007;39:2893–2897. doi: 10.1016/j.transproceed.2007.08.052. [DOI] [PubMed] [Google Scholar]