Abstract
Several lines of evidence point towards a biological role of mucin and particularly MUC1 in colorectal cancer. A positive correlation was described between mucin secretion, proliferation, invasiveness, metastasis and bad prognosis. But, the role of MUC1 in cancer progression is still controversial and somewhat confusing. While Mukherjee and colleagues developed MUC1-specific immune therapy in a CRC model, Lillehoj and co-investigators showed recently that MUC1 inhibits cell proliferation by a β-catenin-dependent mechanism. In carcinoma cells the polarization of MUC1 is lost and the protein is over expressed at high levels over the entire cell surface. A competitive interaction between MUC1 and E-cadherin, through β-catenin binding, disrupts E-cadherin-mediated cell-cell interactions at sites of MUC1 expression. In addition, the complex of MUC1-β-catenin enters the nucleus and activates T-cell factor/leukocyte enhancing factor 1 transcription factors and activates gene expression. This mechanism may be similar to that just described for DCC and UNC5H, which induced apoptosis when not engaged with their ligand netrin, but mediate signals for proliferation, differentiation or migration when ligand bound.
Keywords: Mucin, MUC1, Glycoprotein, Colorectal cancer, Gastrointestinal oncology, Carcinogenesis, Metastasis, Tumorigenicity
MUC1 is a structural membranous bound mucin, expressed on the apical borders of secretory epithelial cells which previously had many names such as DF3, episialin, CA5-3, PAS-O, polymorphic epithelial mucin (PEM), or epithelial membrane antigen[1].
MUC1 gene is located on chromosome 1q21 and has 1201 nucleotides. The N-terminal ectodomain of MUC1 (MUC1-N) consists of variable numbers of 20-amino-acid tandem repeats (VNTRs), with the number of repeats varying from 20 to 120 in different individuals[2]. These sites are subject to O-glycosylation that contributes to form a structure that extends beyond the glycocalyx of the cell. The protein has a protective function by binding to pathogens and also functions in a cell signaling capacity[3].
MUC1-N is tethered to the cell membrane as a heterodimer with the MUC1 C-terminal subunit (MUC1-C), which includes a 58-amino acid extracellular domain, a 28-amino acid transmembrane domain, and a 72-amino acid cytoplasmic tail that contains sites for tyrosine and serine phosphorylation[4]. Over expression, aberrant intracellular localization, and changes in glycosylation of this protein as found in most human carcinomas, confers anchorage-independent growth and tumorigenicity[5]. Other studies have demonstrated that over expression of MUC1 confers resistance to apoptosis induced by oxidative stress and anti-cancer agents[6]. Multiple alternatively spliced transcript variants that encode different isoforms of MUC1 have been reported, but the full-length nature of only some of them has been determined[7].
Several lines of evidence point towards a biological role of mucin and particularly MUC1 in colorectal cancer (CRC). A positive correlation was described between mucin secretion, proliferation, invasiveness, metastasis and bad prognosis[8–10]. When MUC1 was expressed at the deepest tumor invasive portion, lymphatic and venous invasion was more pronounced as well as lymph nodes and liver metastasis[11]. Correlation with bad prognosis was found in mismatch repair (MMR) - proficient colorectal tumors, but not in MLH1 negative tumors or in Lynch syndrome (HNPCC)[12].
But, the role of MUC1 in cancer progression is still controversial and somewhat confusing. While Mukherjee and colleagues, in a very sophisticated way, developed MUC1-specific immune therapy in a CRC model, Lillehoj and co-investigators showed recently that MUC1 inhibits cell proliferation by β-catenin-dependent mechanism[13,14]. A similar observation was described by Yuan and co-workers[15].
Interaction of the cytoplasmic tail of MUC1 with β-catenin has a significant effect on cell cycle and proliferation. This process hardly happens in normal polarized epithelium, because MUC1 resides on the apical surface while β-catenin resides on the lateral surface. Loss of polarity during transformation creates a permissive environment for MUC1 and β-catenin interaction[7].
β-catenin can bind directly to the amino acid sequence 50-SAGNGGSSL-59 of the MUC1 cytoplasmic domain (a similar binding site is found on E-cadherin and APC proteins). The binding is promoted by phosphorylation of T41 by Ser/Thr kinase PKCζ and of Y46 by Src or EGFR[16]. Inhibition of β-catenin binding to MUC 1 is the result of phosphorylation of S44 by GSK3β that can also directly degrades β-catenin. Disruption of the β-catenin binding site in MUC1 suppresses its ability to induce anchorage-dependent and independent growth, indicating β-catenin binding to MUC1 is a critical component of its tumorigenc activity. MUC1 also protects β-catenin from degradation by GSK3β, and when co-localized with β-catenin in the nucleus co activates transcription of Wnt target genes[17–19]. MUC1 binding to β-catenin suppresses its ability to interact with E-cadherin at adherent junctions, leading to a breakdown in cell-cell interactions. GSK3β-mediated disruption of the complex restores the E-cadherin/β-catenin interaction[20].
In carcinoma cells the polarization of MUC1 is lost, and the protein is over expressed at high levels over the entire cell surface. A competitive interaction between MUC1 and E-cadherin, through β-catenin binding, disrupts E-cadherin-mediated cell-cell interactions at sites of MUC1 expression. In addition, the complex of MUC1-β-catenin enters the nucleus and activates T-cell factor/leukocyte enhancing factor 1 (Tcf/LEF-1) transcription factors and activates gene expression[18]. This enhances proliferation, and decreases cell-cell adhesion which may both increase carcinogenesis and metastasis. GSK3β interacts with the STDRSPYE motif in MUC1, phosphorylates the serine in this domain, and prevents binding of β-catenin[16].
It is proposed that APC binding prevents the formation of β-catenin-Tcf complex, and that MUC1 binding prevents β-catenin-α-catenin-E-cadherin complex. GSK3β interacts with β-catenin to restore β-catenin-E-cadherin complex, and with APC to bind β-catenin and prevent β-catenin-Tcf complex (Figure 1). The exact mechanism of MUC1 associated cancer cell proliferation and carcinogenesis is not well understood. MUC1 can bind β-catenin, prevents its entering the nucleus or activating Tcf/LEF-1, and inhibits proliferation. On the other hand, MUC1-C complex with β-catenin may enter the nucleus and the opposite action will take place. In both cases, β-catenin binding to MUC1 will prevent its binding to E-cadherin or to APC.
Figure 1.
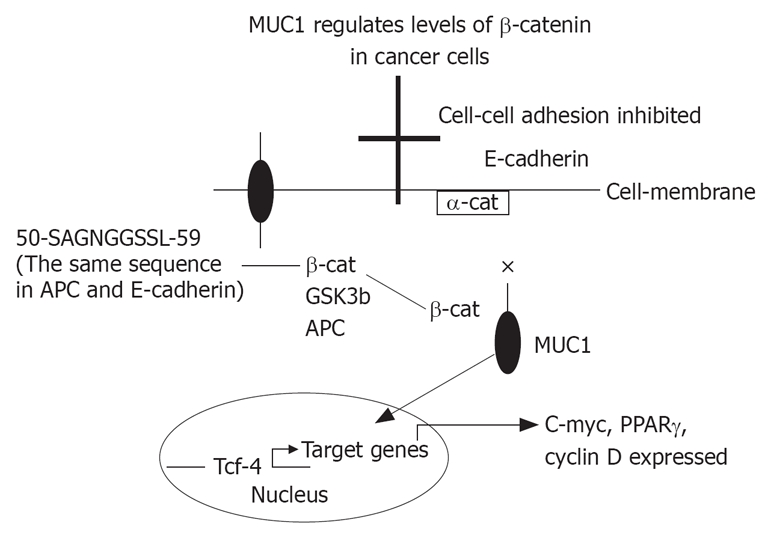
It is proposed that APC binding prevents the formation of β-catenin-Tcf complex, and that MUC1 binding prevents β-catenin-α-catenin-E-cadherin complex. GSK3β interacts with β-catenin to restore β-catenin-E-cadherin complex, and with APC to bind β-catenin and prevent β-catenin-Tcf complex. × = inhibition; arrow = enhancing.
The cytoplasmic tail of MUC1 contains 4 tyrosine residues that are phosphorylated before binding β-catenin. It is speculated that MUC1 is a membranous receptor which maintains cell cycle progression or enhances apoptosis. Activating MUC1 will result in phosphorylation of the tyrosines on the cytoplasmic tail and binding β-catenin. This will prevent β-catenin from binding E-cadherin or activating Tcf/LEF-1 pathway. This mechanism may be similar to that just described for DCC and UNC5H, which induced apoptosis when not engaged with their ligand netrin, but mediate signals for proliferation, differentiation or migration when ligand bound[21].
Peer reviewers: Finlay A Macrae, MD, Professor, Royal Melbourne Hospital, Po Box 2010, Victoria 3050, Australia; Filip Braet, Associate Professor, Australian Key Centre for Microscopy and Microanalysis, Madsen Building (F09), The University of Sydney, Sydney NSW 2006, Australia
S- Editor Zhu WL L- Editor Rippe RA E- Editor Lu W
References
- 1.Patton S, Gendler SJ, Spicer AP. The epithelial mucin, MUC1, of milk, mammary gland and other tissues. Biochim Biophys Acta. 1995;1241:407–423. doi: 10.1016/0304-4157(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 2.Muller S, Alving K, Peter-Katalinic J, Zachara N, Gooley AA, Hanisch FG. High density O-glycosylation on tandem repeat peptide from secretory MUC1 of T47D breast cancer cells. J Biol Chem. 1999;274:18165–18172. doi: 10.1074/jbc.274.26.18165. [DOI] [PubMed] [Google Scholar]
- 3.McAuley JL, Linden SK, Png CW, King RM, Pennington HL, Gendler SJ, Florin TH, Hill GR, Korolik V, McGuckin MA. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007;117:2313–2324. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merlo GR, Siddiqui J, Cropp CS, Liscia DS, Lidereau R, Callahan R, Kufe DW. Frequent alteration of the DF3 tumor-associated antigen gene in primary human breast carcinomas. Cancer Res. 1989;49:6966–6971. [PubMed] [Google Scholar]
- 5.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 6.Yin L, Kharbanda S, Kufe D. Mucin 1 oncoprotein blocks hypoxia-inducible factor 1alpha activation in a survival response to hypoxia. J Biol Chem. 2007;282:257–266. doi: 10.1074/jbc.M610156200. [DOI] [PubMed] [Google Scholar]
- 7.Carraway KL 3rd, Funes M, Workman HC, Sweeney C. Contribution of membrane mucins to tumor progression through modulation of cellular growth signaling pathways. Curr Top Dev Biol. 2007;78:1–22. doi: 10.1016/S0070-2153(06)78001-2. [DOI] [PubMed] [Google Scholar]
- 8.Niv Y, Schwartz B, Amsalem Y, Lamprecht SA. Human HT-29 colon carcinoma cells: mucin production and tumorigenicity in relation to growth phases. Anticancer Res. 1995;15:2023–2027. [PubMed] [Google Scholar]
- 9.Duncan TJ, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J Surg Oncol. 2007;5:31. doi: 10.1186/1477-7819-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bresalier RS, Niv Y, Byrd JC, Duh QY, Toribara NW, Rockwell RW, Dahiya R, Kim YS. Mucin production by human colonic carcinoma cells correlates with their metastatic potential in animal models of colon cancer metastasis. J Clin Invest. 1991;87:1037–1045. doi: 10.1172/JCI115063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko I, Tanaka S, Oka S, Yoshida S, Hiyama T, Arihiro K, Shimamoto F, Chayama K. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol. 2007;13:3829–3835. doi: 10.3748/wjg.v13.i28.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lugli A, Zlobec I, Baker K, Minoo P, Tornillo L, Terracciano L, Jass JR. Prognostic significance of mucins in colorectal cancer with different DNA mismatch-repair status. J Clin Pathol. 2007;60:534–539. doi: 10.1136/jcp.2006.039552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee P, Pathangey LB, Bradley JB, Tinder TL, Basu GD, Akporiaye ET, Gendler SJ. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25:1607–1618. doi: 10.1016/j.vaccine.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillehoj EP, Lu W, Kiser T, Goldblum SE, Kim KC. MUC1 inhibits cell proliferation by a beta-catenin-dependent mechanism. Biochim Biophys Acta. 2007;1773:1028–1038. doi: 10.1016/j.bbamcr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan Z, Wong S, Borrelli A, Chung MA. Down-regulation of MUC1 in cancer cells inhibits cell migration by promoting E-cadherin/catenin complex formation. Biochem Biophys Res Commun. 2007;362:740–746. doi: 10.1016/j.bbrc.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. J Biol Chem. 2001;276:6061–6064. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- 17.Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005;65:10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- 18.Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Holscher AH, Dienes HP. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790–2796. doi: 10.1158/1078-0432.ccr-03-0163. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Ren J, Chen D, Li Y, Kharbanda S, Kufe D. MUC1 cytoplasmic domain coactivates Wnt target gene transcription and confers transformation. Cancer Biol Ther. 2003;2:702–706. [PubMed] [Google Scholar]
- 20.Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol Cell Biol. 1998;18:7216–7224. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin SK, Nagasaka T, Jung BH, Matsubara N, Kim WH, Carethers JM, Boland CR, Goel A. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology. 2007;133:1849–1857. doi: 10.1053/j.gastro.2007.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]


