Abstract
AIM: To investigate biological prevention with flavonoids the recurrence risk of neoplasia was studied in patients with resected colorectal cancer and after adenoma polypectomy.
METHODS: Eighty-seven patients, 36 patients with resected colon cancer and 51 patients after polypectomy, were divided into 2 groups: one group was treated with a flavonoid mixture (daily standard dose 20 mg apigenin and 20 mg epigallocathechin-gallat, n = 31) and compared with a matched control group (n = 56). Both groups were observed for 3-4 years by surveillance colonoscopy and by questionnaire.
RESULTS: Of 87 patients enrolled in this study, 36 had resected colon cancer and 29 of these patients had surveillance colonoscopy. Among the flavonoid-treated patients with resected colon cancer (n = 14), there was no cancer recurrence and one adenoma developed. In contrast the cancer recurrence rate of the 15 matched untreated controls was 20% (3 of 15) and adenomas evolved in 4 of those patients (27%). The combined recurrence rate for neoplasia was 7% (1 of 14) in the treated patients and 47% (7 of 15) in the controls (P = 0.027).
CONCLUSION: Sustained long-term treatment with a flavonoid mixture could reduce the recurrence rate of colon neoplasia in patients with resected colon cancer.
Keywords: Flavonoids, Colorectal cancer, Recurrence risk, Intestinal neoplasia, Colon polyps
INTRODUCTION
Patients with resected colon cancer are at risk of cancer recurrence which depends mainly on the tumor stage[1]. Within 4-5 years after a curative surgical resection about 40%-50% of patients suffer from a tumor recurrence when their initial tumor stage was II or III according to the International Union against Cancer (UICC) classification[2–4]. Tumor recurrence can manifest itself as a local recurrence at the site of resection, as metachronous tumor growth somewhere else in the colon or as local or distant metastasis. Recurrence in the colon can take three forms of neoplasia: either as incident carcinoma, as incident adenoma or as a mixture of both.
Patients with colon polyps (adenomas, hyperplastic polyps or serrated polyps) who had a polypectomy are also at risk of recurrence[5] .After an index polypectomy these patients can develop incident adenomas in 40% of cases within 3 years depending on the histology of the polyp. The adenoma recurrence is highest for large and multiple adenomas with dysplastic changes of the adenoma structure[5].
There is much controversy about what can be done to reduce the risk or recurrence of neoplasia in tumor and polyp patients. Secondary prevention is urgently needed in these patients; however, it is not yet clear what measures are most effective. Epidemiological studies indicate that dietary interventions with ballast augmented food can be successful for primary prevention of colorectal carcinomas[6]. On the other hand diets supplemented with bran[7] and fruits and vegetables[8] do not suppress the evolution of colorectal adenomas after polypectomy. Other dietary components such as folic acid, calcium, vitamin D and selenium either have shown only marginal beneficial effects or no effects for prevention[9–12]. Antioxidative vitamins could not prevent gastrointestinal cancer[13]. Beside dietary factors (bioprevention) chemically defined intervention with aspirin[14–17] and nonsteroidal anti-inflammatory drugs (NSAIDs) seem to be effective for primary and secondary prevention of colon neoplasia[18,19]. However, their unwanted side effects and complications (ulcerations, bleedings and thromboembolic events) prevent their general use for risk reduction[20].
Various modes of bioprevention with dietary components have been tested mostly in epidemiological studies[21–23] and in studies with cell culture work and other in vitro tests[24]. Few clinical intervention studies were reported. Candidates for use in clinical studies include secondary plant products such as flavonoids, indols, isothiocyanates, glucosinolates, allyes, resveratrol, curcumin, saponins and terpenes. Some of these plant products can be applied as nutritional supplements as tablets, thereby facilitating long-term use without side effects or problems of compliance. Tea flavonoids from green tea and camomile contain flavons, flavonols and flavanols. They have been shown to display various anticarcinogenic, antiproliferative, antimutagenic and antioxidative properties in vivo and in vitro[24]. Certain species of dietary flavonoids were able to reduce the risk of colorectal cancer[25], even in a dose dependent manner[26]. This is not true for colon neoplasia in general[27,28]. Recently, a clinical study[29] suggested, that the flavonol quercetin taken together with curcumin suppressed the growth of adenomatous polyps in patients with hereditary colon polyposis syndrome (familiar adenomatous polyposis, FAP patients).
We decided to study prospectively the effects of a sustained treatment with a tea-based flavonoid mixture on the evolution of neoplastic alteration in a cohort of high risk patients with resected colon cancer as well as patients after polypectomy. In this proof of principle study we found that a flavonoid intervention can reduce the recurrence rate of neoplasia in patients with sporadic colorectal neoplasia, in comparison with an untreated matched control group.
MATERIALS AND METHODS
Study subjects
Between January 2000 and December 2003 a total of 160 patients with colorectal neoplasia (index patients) were recruited and their data were collected from the clinical charts of the Community Hospital Groß-Gerau, Germany (Department of Internal Medicine and General Surgery) and included into the tumor registry for this clinical study. All patients with the diagnosis of colorectal cancer and colon polyps confirmed by pathology reports were eligible for this study if they completed the clinical questionnaire and had surgical tumor resection or polypectomy. By the end of 2003, recruitment was terminated and until December 2005, 87 patients were followed up by surveillance colonoscopy. Patients who were still in the study at this time were considered to be censored cases for overall survival. All data were extracted from the local tumor registry of the clinic and further pseudonymized for evaluation. In this prospective observational cohort study the investigators had no role in the clinical management; all treatment decisions (except the assignment of the flavonoid nutritional supplementation) and the schedule of surveillance colonoscopies were left at the discretion of the treating physician. Surveillance data were collected prospectively. All 160 patients were treated according to the clinical guidelines for follow-up investigations for colorectal cancer and colon adenomas published by the German Association for Digestive and Metabolic Diseases[30]. This study was approved by the Ethics Committee of the Technical University of Dresden, Germany. The patients provided information using a self-administered questionnaire and in this way written informed consent was obtained authorizing use of their data for this study.
Study protocol
Figure 1 explains the trial profile, the outcome and the patient flow of this controlled study. One hundred and sixty patients were registered and of these 87 patients were enrolled to test the efficacy of flavonoids. During the four years from January 2000 to December 2003 we recruited 31 of the 160 patients to agree to take the flavonoid supplement for tumor prevention. Following assignment to treatment these 31 patients were matched to 56 controls. Matching by gender, age (10 years intervals) and type of neoplasia (resected carcinoma vs polypectomized adenomas) was performed by using the data of the 129 untreated patients. The remaining 73 patients of the total of 160, who did not fulfil the matching criteria, were not followed further in this study. The flavonoid- treated patients took a daily dose of 2 tablets of the flavonoid mixture[24] containing 10 mg apigenin and 10 mg epigallocatechin-gallate per tablet. This nutritional supplement (tea bioflavonoids) was produced according to the principles of Good Manufacturing Practice by Köhler-Pharma, Alsbach-Hähnlein, Germany. The content of active ingredients in each batch of the product was tested by chemical analysis (HPLC technique). Flavonoids were taken for 2-5 years; the treatment compliance was evaluated by questionnaire.
Figure 1.
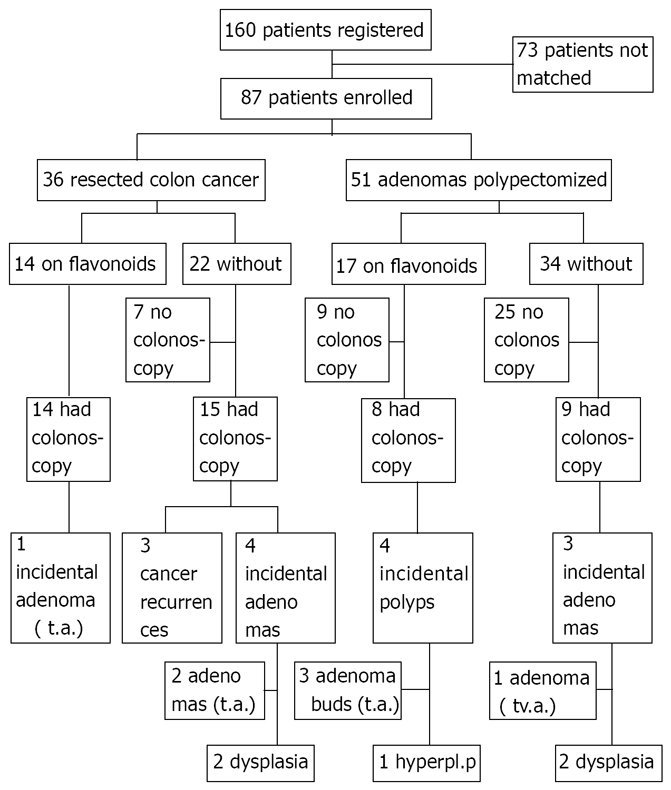
Trial profile and outcomes. t.a.: Tubular adenoma; tv.a.: Tubulovillous adenoma; hyperpl.p: Hyperplastic polyp, buds diminutive polyp (< 5 mm).
Outcomes were evaluated according to the per protocol principle: data of patients using flavonoids (n = 31) were analyzed regardless of how long they had been treated. The primary endpoints of this study were the incident neoplasia (cancer and/or adenomas) observed by surveillance colonoscopy.
The self-administered questionnaire provided information on relevant clinical variables which might influence the clinical outcome. These included life style variables, body mass index (BMI), a dietary food frequency questionnaire, information on medical treatment, cancer and adenoma histories of relatives and tea consumption (Table 1). Data on colonoscopy findings were taken from the standardized clinical endoscopy protocols and transferred to the registry. Histological findings of neoplasia provided by the clinical pathologist were rated according to the guidelines as mentioned above[30]. Tumor stage was assessed from the surgical protocols and rated according to the UICC classification[4].
Table 1.
Demographic and outcome data of patients treated with flavonoids and controls
| Treated (n = 31) | Controls (n = 56) | P value | |
| Males/females | 17/14 | 31/25 | > 0.9 |
| Age (yr) median (IQR) | 74 (68-80) | 77 (69-82) | 0.35 |
| BMI (kg/m²) median (IQR) | 26.1 (24.4-28.2) (n = 28) | 27.5 (25.0-30.3) (n = 45) | 0.32 |
| Resected colon cancer/polypectomy | 14/17 | 22/34 | 0.65 |
| Surveillance colonoscopy/no | 22/9 | 24/32 | 0.0141 |
| Surveillance time by colonoscopy | |||
| Years: Median (IQR) | 3.5 (3-4.75) (n = 22) | 3.0 (2-3) (n = 24) | 0.0191 |
| Surveillance time by questionnaire | |||
| Years: Median (IQR) | 3.6 (3.1-4.7) | 2.9 (2.5-3.4) | 0.0041 |
| Cancer recurrence/no | 0/20 | 3/18 | 0.23 |
| Polyp recurrence/no | 5/15 | 7/14 | 0.73 |
| Neoplasia recurrence/no | 5/15 | 10/11 | 0.20 |
| Smoker/non-smoker | 2/27 | 6/48 | 0.71 |
| Alcohol/no | 24/5 | 33/20 | 0.08 |
| Black tea/no | 16/15 | 27/26 | > 0.9 |
| Green tea/no | 13/16 | 21/27 | > 0.9 |
| Fruit intake < 3/≥ 3 × weekly | 8/20 | 17/35 | 0.80 |
| Vegetable intake < 3/≥ 3 × weekly | 15/13 | 29/22 | 0.82 |
| Aspirin use/no | 11/20 | 18/37 | 0.82 |
| NSAID use/no | 2/29 | 3/52 | > 0.9 |
| Colon cancer in family/no | 1/30 | 6/49 | 0.41 |
| Adenomas in family/no | 2/29 | 1/54 | 0.29 |
IQR: Interquartile range (25%-75%); BMI: Body mass index; n: Number of patients;
Significantly different at P < 0.05.
Statistical analysis
The data of the total cohort of 160 patients were subdivided into the two basic sub-cohorts: patients only observed (n = 73) and patients surveyed for secondary prevention (n = 87). The latter group was divided into a treatment group (n = 31) and a control group (n = 56) as described in the Study Protocol. The patient characteristics of the two surveillance groups, the per protocol group of the treated patients (n = 31) and their controls (n = 56) were compared on baseline as well as for their outcome variables by using descriptive and confirmatory statistical methods. Categorical variables were analyzed using the chi-square test or the 2-sided Fisher Exact Test in the case of small frequencies. Continuous variables (age, BMI) were analyzed using the non-parametric Wilcoxon-Mann-Whitney U-Test. They are described by their median and the interquartile range (IQR). The IQR is defined as the range between the 25th and the 75th percentile of the empirical distribution of the data.
Differences of recurrence were expressed in percen-tages as absolute differences. The relative risk ratio (RRR) and the number needed to treat (NNT) were computed. Because of the observational nature of this study no adjustments for multiplicity were applied and P < 0.05 was considered statistically significant.
RESULTS
The prognostically relevant clinical variables of the treated patients were compared with those of the matched patients (Table 1). During the study period one patient in the treatment group and two patients in the control group died of causes not related to tumor recurrence. The patients in the treated group had significantly higher numbers of follow-up colonoscopies than patients in the control group (Table 1).The time under surveillance both by colonoscopy and by questionnaire was significantly longer for the treatment group (Table 1). The ratio of cancer to polyp patients was not significantly different (45% vs 39%) among treatment and control group.
Recurrence rates of cancer were 0 in 20 in the treated group vs 3 in 21 in the control group (P = 0.23) Polyp recurrence rates were 5 in 20 in the treatment group vs 7 in 21 in the control group (P = 0.73). The combined rate of recurrence for neoplasia was 5 in 20 in treated vs 10 in 21 in the controls (P = 0.20). These differences are not statistically significant, but there is a trend for more favourable outcomes in the flavonoid exposed patients. Note that both groups were not adjusted according to surveillance colonoscopy and according to neoplasia type. The sample size of this proof of principle study is small. Also, it can be seen in Figure 1, that the incident polyps in the control group were high grade adenomas (4 adenomas with dysplasia, one tubulovillous adenoma); there were only 2 tubular adenomas. Among the treated patients there were 3 diminutive tubular adenomas (polyp buds), one hyperplastic polyp and one tubular adenoma (with 10 mm diameter). This shows that there were more advanced adenomas present in the control group than in the treatment group.
Fruit consumption of less than 3 d a week was considered as low intake and was found in 29% (8 in 28) of the treatment group as compared to 33% (17 in 52) of the control group (P = 0.80). Habitual vegetable intake of less than 3 d a week was reported by 54% (15 in 28) of patients in the treatment group vs 57% (29 in 51) in the control group. Habitual drinking of green and black tea was not significantly different among both groups; about 44% drank green tea and 51% black tea. About 10% of the patients in both groups smoked and about 30% of them took aspirin regularly. NSAIDs were taken long term by 5%-6% of the patients in both groups. Habitual alcohol use was reported by 83% in the treatment group as compared to 62% in the control group (P = 0.08). Gender, age and BMI were approximately evenly distributed among the two groups.
Most patients in the flavonoid group (20 in 31) took the nutritional supplement for more than 12 mo, 8 patients took it less than 3 mo, 2 up to 6 mo and one patient up to 12 mo. Three in 27 (11%) reported slight discomfort and discontinued the flavonoid treatment within 3 mo. The majority of 65% (17 in 26) took the flavonoids continuously on a daily basis.
As the data in Table 1 suggested that there is a possible treatment effect of the use of flavonoids we analyzed our data in the well adjusted group of patients with curative colon cancer resection. There were 14 patients with resected colon cancer in the treatment group compared to 15 control patients (Table 2); all had surveillance colonoscopies. None of the treated patients had cancer recurrence vs 20% (3 in 15) of the controls. Among the controls two patients had metastatic colorectal cancer and one had local cancer recurrence at the surgical anastomosis. The time to relapse was 2-3 years after surgery in patients with cancer recurrence. Adenomas developed in 7% (1 in 14) of the treated patients and in 27% (4 in 15) of the controls including two adenomas with dysplasia (Table 3). There was a statistically significant difference (P = 0.02) between the two groups when the combined endpoint of neoplasia recurrence (incident cancer and incident adenomas) was evaluated. The potentially confounding patient characteristics of both groups did not differ significantly except for habitual alcohol consumption, which was significantly more prevalent in the treated patients than in controls. For neoplasia recurrence the prognostically most important factor is the previous tumor stage, which was not significantly different between the two groups.
Table 2.
Comparison of clinical variables in patients with resected colon cancer on surveillance colonoscopy treated with flavonoids vs controls
| Flavonoid treatment (n = 14, %) | Controls (n = 15, %) | P value | |
| Males/females | 7/7 | 7/8 | > 0.9 |
| Age (yr) median (IQR) | 75.0 (77-82) | 81.0 (77-86) | 0.12 |
| BMI (kg/m²) median (IQR) | 26.2 (24.6-28.0) (n = 13) | 25.9 (24.5-27.5) (n = 10) | 0.57 |
| Smoker/non-smoker | 0/13 (0) | 1/12 (8) | > 0.9 |
| Alcohol habitual/no | 13/0 (100) | 7/5 (58) | 0.0151 |
| Black tea/no | 5/9 (36) | 8/5 (61) | 0.26 |
| Green tea/no | 5/8 (36) | 5/6 (45) | > 0.9 |
| Fruit intake < 3/≥ 3 d a week) | 2/11 (15) | 2/10 (17) | > 0.9 |
| Vegetable intake < 3/≥ 3 d a week) | 6/7 (46) | 8/4 (67) | 0.43 |
| Aspirin/no | 4/10 (28) | 7/7 | 0.44 |
| NSAID/no | 0/14 (0) | 1/13 (7) | > 0.9 |
| Colon vs rectum cancer | 13/1 (93) | 9/6 (60) | 0.080 |
| Low vs high tumor stage (I and II/III) | 9/5 (64) | 9/6 (60) | > 0.9 |
| Surveillance time by colonoscopy | |||
| Years: median (IQR) | 4.0 (3.25-5) | 3.0 (2-3) | 0.0221 |
IQR: Interquartile range (25%-75%).
Significantly different at P < 0.05.
Table 3.
Recurrence rates of colon neoplasia in patients with resected colon cancer treated with flavonoids compared to controls
| Treated (% of total, n = 14) | Controls (% of total, n = 15) | Absolute difference (%) | RRR | NNT | P value | |
| Cancer recurrence/no | 0/14 (0) | 3/12 (20) | 20 | 5 | 0.125 | |
| Adenoma recurrence/no | 1/13 (7) | 4/11 (27) | 20 | 3.9 | 5 | 0.101 |
| Neoplasia recurrence/no | 1/13 (7) | 7/8 (47) | 40 | 6.7 | 2.5 | 0.0271 |
RRR: Relative risk ratio; NNT: Number needed to treat.
Significantly different at P < 0.05.
DISCUSSION
Recurrence risk is the main concern of patients with previous resected colorectal cancer[1–4]. On follow-up about 40% of surgically curable colorectal cancers with stage II and stage III (according to the UICC staging system) will suffer recurrent cancers within 3-4 years. The best outcomes were reported for stage I and stage II tumors (around 90% survival without recurrences). The prognosis of stage III cancer (with cancerous regional lymph nodes) is less favourable, but can be improved by adjuvant chemotherapy. Treated cases and controls in our study did not differ regarding the initial tumor stage at surgery; about 40% in both groups were stage III tumors, only 2 of them (controls) had adjuvant chemotherapy because the surgeon felt confident that most of these patients would not be suitable for adjuvant chemotherapy. The tumor recurrence in the controls was not observed in the patients on chemotherapy, but there were too few patients to judge whether this could influence outcomes. We found the expected recurrence rate in the controls (Table 3), but no incident cancers and only one incident adenoma in the flavonoid exposed patients. Eighty-seven of the 160 patients from the registry were enrolled because we detected only 56 controls that could be properly adjusted to the 31 treated patients. The matching ratio of about one to two (31 treated vs 56 controls) seems to be appropriate. In this real world study 76% of the treated and 43% of the controls had surveillance colonoscopies; among the resected patients 80% had surveillance by colonoscopy but only 33% of the polypectomized patients. This fact might influence the reliability of the conclusions regarding the adenoma recurrence.
Our controlled clinical trial was a prospective and observational cohort study performed with the aim of finding out whether long-term flavonoid exposure of patients from a tumor registry alters the outcome compared to untreated control patients. This proof of principle study suggests that flavonoids can be used to reduce the recurrence rate in patients with resected colorectal cancers. Flavonoids are good candidates for primary and secondary prevention of colorectal cancer, since numerous in vitro studies and animal work report on their beneficial activities in terms of suppression of cancer proliferation, antioxidative and antiangiogenetic properties[24]. Epidemiological investigations[22,25,26], in vivo and in vitro experiments[31–35] and one clinical intervention study[29] support this concept. Other authors could not find protective effects of flavonoids on colorectal cancer incidence[21,27,36]. Flavonoids derived from tea plants can be used as a mean of bioprevention and have been manufactured and marketed as nutritional supplements[24]. Other methods of prevention are not effective (e.g. vitamins except folic acid), show only marginal efficacy (e.g. calcium, selenium) or cannot be used in general because of their unwanted side effects and complications (aspirin, NSAIDs)[20].
We tested the efficacy of flavonoid supplementation in a high risk population (resected colorectal cancer) to examine its effect in a relatively small number of patients, which were carefully adjusted for various clinical variables with prognostic relevance. However, there are prognostic clinical factors which were not taken into account such as penetration depth into the colonic wall and histological grading. Clinical studies with a larger sample size and a higher statistical power are necessary to show that flavonoid exposure alters the outcome in terms of tumor recurrence. Flavonoids could prevent recurrences of neoplasia by protecting the genome of colonocytes from genotoxic insults such as oxidative damage, free radical attacks and adduct formation[37]. Flavonoids are secondary plant products which could be responsible for some of the healthy effects of fruits and vegetables. It is still unknown which components of vegetables and fruits are effective for tumor prevention; ballast, fibres and secondary plant products play a major role[6,10,38]. Flavonoids, indols, isothiocyanates, curcumin, resveratrol, glucosinolates and other plant products affect carcinogenic, mutagenic and neoplastic mechanisms[24], but could also induce protective enzymes of the intestinal mucosa[39]. Beside the type of chemical and biological prevention lifestyle factors, type and amount of tea consumption, genetic factors, aspirin and NSAID medication could influence the outcome. These variables have to be considered when evaluating the effects of flavonoid intervention. As shown in Tables 1 and 2 these variables were well balanced among cases and controls. However, alcohol use was more prevalent in the treated patients with resected colorectal cancer than in controls. We do not think that differences of habitual alcohol drinking can explain the difference of recurrence since ethanol is thought of as a carcinogenic risk factor and would rather increase the recurrence risk of the flavonoid exposed patients.
Patient compliance with the flavonoid treatment was evaluated using information derived from a questionnaire given to 31 treated patients in the treatment group. 67% of these treated patients took the nutritional supplement longer than 12 mo, only 10% discontinued the intake within the first 3 mo. No side effects or unwanted symptoms were reported.
The habitual vegetable intake of the patients in both treatment and control groups (Tables 1 and 2) was rather low (< 3 d a week ) and only about 40%-50% of the patients consumed vegetables ≥ 3 d a week, which still is not sufficient for tumor prevention. About 16%-30% of the patients (cases and controls, Tables 1 and 2) reported low fruit content in their diet (< 3 d a week). Thus, no significant differences of the dietary habits were observed among treated and untreated patients. The self-administered questionnaire which was used to assess dietary habits provided only a crude estimate and was not validated; it is however a simple and practical tool that was well accepted and understood by the patients.
Flavonoids are part of human nutrition and are contained in vegetables and fruits, especially in apples, onions, berries, citrus fruits and teas but also in chocolate. Tea consumption of the patients was moderate and was reported in most cases only as occasional tea drinking.
More patients with resected colorectal carcinoma of the control group (7 of 14, 50%) took aspirin compared to the cases (4 of 14, 28%) but this difference was not statistically significant (Table 2).
Surveillance by colonoscopy was performed in more cases (65%) than in controls (38%) and the time interval covered by colonoscopy was longer in treated patients than controls (Table 1). Thus the treated patients had a better chance for detection of neoplasia which would be a bias against a treatment effect. If the controls had more surveillance intensity, their recurrence rate would have been even higher.
In patients with prior adenomas that were removed by polypectomy and had surveillance colonoscopies, those treated with flavonoid treatment had a polyp recurrence rate similar to that of controls (about 50%). However, flavonoid treatment was associated with low risk incident adenomas while the control group included polyp recurrence of two adenomas with dysplasia (Figure 1). These differences were not statistically significant but could indicate that flavonoids could also suppress adenoma development and evolution. Cruz-Correa et al have recently reported that a combined treatment with quercetin (a flavonol) and curcumin (from curry) inhibited proliferation of adenomas in patients with familiar adenomatous polyposis coli[29]. These point to the possibility that flavonoids taken as long-term treatment could suppress neoplasia recurrence in high risk patients.
In conclusion, this pilot study which was controlled, prospective and observational, suggests that long-term flavonoid treatment could reduce the recurrence rate of colon neoplasia in high risk patients particularly in those with resected colorectal cancer. Therefore flavonoid supplementation should be investigated by further clinical studies to prove the efficacy and validity of this concept.
COMMENTS
Background
Recurrence of cancer after a curative surgical resection in patients with colorectal cancer is a common problem that occurs in about 20%-40% depending on the previous tumor stage. It is essential for these patients to find ways to prevent this disaster.
Research frontiers
Prevention of recurrence can be achieved by adherence to a diet containing lots of fruits and vegetables or for higher tumor stages by cytostatic chemotherapy (adjuvant chemotherapy). Chemotherapy is very demanding and prone to unpleasant side effects. Dietary measures are difficult to implement and could give rise to bloating, gas and pain of the abdomen.
Innovations and breakthroughs
Other authors and articles seem to suggest that flavonoids could prevent colorectal cancers by healthy dietary habits, e.g. intake of foods with a high content of flavonoids. All these studies rely on epidemiological data and these are not always consistent and sometimes controversial. Our study uses an interventional approach with a nutritional supplement (as tablets) and this has not been done previously. Our data suggest that all patients at risk of recurrence of colorectal cancer should be treated with flavonoid supplements.
Peer review
It is a well-designed paper. The authors showed that sustained long-term treatment with a flavonoid mixture could reduce the recurrence rate of colon neoplasia in patients with resected colon cancer. This is an interesting article.
Acknowledgments
We are grateful for the cooperation with the department of general surgery of the Community Hospital Groβ-Gerau, Germany (Head Michael Kahl, MD) which provided the data from the clinical charts of the patients. We also thank Renate Rausch from the Department of Biostatistics at the German Cancer Research Center for continuing support in the data analysis.
Supported by Technical University of Dresden and this work
Peer reviewer: Walter E Longo, Professor, Department of Surgery, Yale University School of Medicine, 205 Cedar Street, New Haven 06510, United States
S- Editor Zhong XY L- Editor Alpini GD E- Editor Yin DH
References
- 1.Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D, Burt RW, Byers T, Fletcher RH, Hyman N, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130:1865–1871. doi: 10.1053/j.gastro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Berman JM, Cheung RJ, Weinberg DS. Surveillance after colorectal cancer resection. Lancet. 2000;355:395–399. doi: 10.1016/S0140-6736(99)06552-6. [DOI] [PubMed] [Google Scholar]
- 3.Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 4.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. doi: 10.1016/s0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- 7.Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, van Leeuwen JB, Reid ME, Ritenbaugh C, Vargas PA, Bhattacharyya AB, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians' Network. N Engl J Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 8.Schatzkin A, Lanza E, Corle D, Lance P, Iber F, Caan B, Shike M, Weissfeld J, Burt R, Cooper MR, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342:1149–1155. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg ER, Baron JA, Tosteson TD, Freeman DH Jr, Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl HD, Haile RW. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med. 1994;331:141–147. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 10.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR. 2007:280–288. [Google Scholar]
- 11.Park SY, Murphy SP, Wilkens LR, Nomura AM, Henderson BE, Kolonel LN. Calcium and vitamin D intake and risk of colorectal cancer: the Multiethnic Cohort Study. Am J Epidemiol. 2007;165:784–793. doi: 10.1093/aje/kwk069. [DOI] [PubMed] [Google Scholar]
- 12.Terry P, Baron JA, Bergkvist L, Holmberg L, Wolk A. Dietary calcium and vitamin D intake and risk of colorectal cancer: a prospective cohort study in women. Nutr Cancer. 2002;43:39–46. doi: 10.1207/S15327914NC431_4. [DOI] [PubMed] [Google Scholar]
- 13.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 14.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 15.Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, Couturier D, Coste T, Little J, Chaussade S. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–336. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 16.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 17.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 18.Rahme E, Barkun AN, Toubouti Y, Bardou M. The cyclooxygenase-2-selective inhibitors rofecoxib and celecoxib prevent colorectal neoplasia occurrence and recurrence. Gastroenterology. 2003;125:404–412. doi: 10.1016/s0016-5085(03)00880-1. [DOI] [PubMed] [Google Scholar]
- 19.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 20.Kerr DJ, Dunn JA, Langman MJ, Smith JL, Midgley RS, Stanley A, Stokes JC, Julier P, Iveson C, Duvvuri R, et al. Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med. 2007;357:360–369. doi: 10.1056/NEJMoa071841. [DOI] [PubMed] [Google Scholar]
- 21.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary flavonoids and cancer risk in the Zutphen Elderly Study. Nutr Cancer. 1994;22:175–184. doi: 10.1080/01635589409514342. [DOI] [PubMed] [Google Scholar]
- 22.Arts IC, Jacobs DR Jr, Folsom AR. Dietary catechins and cancer incidence: the Iowa Women's Health Study. IARC Sci Publ. 2002;156:353–355. [PubMed] [Google Scholar]
- 23.Witte JS, Longnecker MP, Bird CL, Lee ER, Frankl HD, Haile RW. Relation of vegetable, fruit, and grain consumption to colorectal adenomatous polyps. Am J Epidemiol. 1996;144:1015–1025. doi: 10.1093/oxfordjournals.aje.a008872. [DOI] [PubMed] [Google Scholar]
- 24.Hoensch HP, Kirch W. Potential role of flavonoids in the prevention of intestinal neoplasia: a review of their mode of action and their clinical perspectives. Int J Gastrointest Cancer. 2005;35:187–195. doi: 10.1385/IJGC:35:3:187. [DOI] [PubMed] [Google Scholar]
- 25.Rossi M, Negri E, Talamini R, Bosetti C, Parpinel M, Gnagnarella P, Franceschi S, Dal Maso L, Montella M, Giacosa A, et al. Flavonoids and colorectal cancer in Italy. Cancer Epidemiol Biomarkers Prev. 2006;15:1555–1558. doi: 10.1158/1055-9965.EPI-06-0017. [DOI] [PubMed] [Google Scholar]
- 26.Theodoratou E, Kyle J, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M, Campbell H. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:684–693. doi: 10.1158/1055-9965.EPI-06-0785. [DOI] [PubMed] [Google Scholar]
- 27.Borrelli F, Capasso R, Russo A, Ernst E. Systematic review: green tea and gastrointestinal cancer risk. Aliment Pharmacol Ther. 2004;19:497–510. doi: 10.1111/j.1365-2036.2004.01884.x. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Zhang SM, Wu K, Willett WC, Fuchs CS, Giovannucci E. Flavonoid intake and colorectal cancer risk in men and women. Am J Epidemiol. 2006;164:644–651. doi: 10.1093/aje/kwj296. [DOI] [PubMed] [Google Scholar]
- 29.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Schmiegel W, Pox C, Adler G, Fleig W, Folsch UR, Fruhmorgen P, Graeven U, Hohenberger W, Holstege A, Junginger T, et al. S3-Guidelines Conference "Colorectal Carcinoma" 2004. Z Gastroenterol. 2004;42:1129–1177. doi: 10.1055/s-2004-813699. [DOI] [PubMed] [Google Scholar]
- 31.Yamane T, Nakatani H, Kikuoka N, Matsumoto H, Iwata Y, Kitao Y, Oya K, Takahashi T. Inhibitory effects and toxicity of green tea polyphenols for gastrointestinal carcinogenesis. Cancer. 1996;77:1662–1667. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1662::AID-CNCR36>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 32.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 33.Hara Y. Green tea, health benefits and applications. Food Science and Technology. Marcel Dekker Inc. Basel: Marcel Dekker; 2001. pp. 26–41. [Google Scholar]
- 34.Steele VE, Kelloff GJ, Balentine D, Boone CW, Mehta R, Bagheri D, Sigman CC, Zhu S, Sharma S. Comparative chemopreventive mechanisms of green tea, black tea and selected polyphenol extracts measured by in vitro bioassays. Carcinogenesis. 2000;21:63–67. doi: 10.1093/carcin/21.1.63. [DOI] [PubMed] [Google Scholar]
- 35.Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 36.Goldbohm RA, Hertog MG, Brants HA, van Poppel G, van den Brandt PA. Consumption of black tea and cancer risk: a prospective cohort study. J Natl Cancer Inst. 1996;88:93–100. doi: 10.1093/jnci/88.2.93. [DOI] [PubMed] [Google Scholar]
- 37.Duthie SJ, Dobson VL. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur J Nutr. 1999;38:28–34. doi: 10.1007/s003940050043. [DOI] [PubMed] [Google Scholar]
- 38.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78:559S–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 39.Hoensch H, Morgenstern I, Petereit G, Siepmann M, Peters WH, Roelofs HM, Kirch W. Influence of clinical factors, diet, and drugs on the human upper gastrointestinal glutathione system. Gut. 2002;50:235–240. doi: 10.1136/gut.50.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]


