Abstract
AIM: To determine the accuracy of computed tomography (CT) and magnetic resonance (MR) for presurgical characterization of paraaortic lymph nodes in patients with pancreatico-biliary carcinoma.
METHODS: Two radiologists independently evaluated CT and MR imaging of 31 patients who had undergone lymphadenectomy (9 metastatic and 22 non-metastatic paraaortic nodes). Receiver operating characteristic (ROC) curve analysis was performed using a five point scale to compare CT with MRI. To re-define the morphologic features of metastatic nodes, we evaluated CT scans from 70 patients with 23 metastatic paraaortic nodes and 47 non-metastatic ones. The short axis diameter, ratio of the short to long axis, shape, and presence of necrosis were compared between metastatic and non-metastatic nodes by independent samples t-test and Fisher’s exact test. P < 0.05 was considered statistically significant.
RESULTS: The mean area under the ROC curve for CT (0.732 and 0.646, respectively) was slightly higher than that for MRI (0.725 and 0.598, respectively) without statistical significance (P = 0.940 and 0.716, respectively). The short axis diameter of the metastatic lymph nodes (mean = 9.2 mm) was significantly larger than that of non-metastatic ones (mean = 5.17 mm, P < 0.05). Metastatic nodes had more irregular margins (44.4%) and central necrosis (22.2%) than non-metastatic ones (9% and 0%, respectively), with statistical significance (P < 0.05).
CONCLUSION: The accuracy of CT scan for the characterization of paraaortic nodes is not different from that of MRI. A short axis-diameter (> 5.3 mm), irregular margin, and presence of central necrosis are the suggestive morphologic features of metastatic paraaortic nodes.
Keywords: Paraaortic lymph node, Pancreatico-biliary carcinoma, Computed tomography, Magnetic resonance imaging
INTRODUCTION
Paraaortic lymph node metastasis in the patients with pancreatico-biliary carcinoma has been reported as a definite predictor of early recurrence and shorter survival term, despite differences between individual tumors[1–3]. It is very difficult to preoperatively predict paraaortic node metastasis with imaging, palpation, or intraoperative sonography. Therefore it is recommended that sampling and pathologic confirmation of paraaortic nodes should be performed before starting radical operation. Many surgeons, including those in our hospital, do paraaortic node dissection before radical surgery[4–7]. Although lymphadenectomy followed by histologic examination of the lymph nodes is still the gold standard for determination of metastasis, this procedure is invasive and could cause many surgical complications[8–11]. Therefore preoperative, noninvasive imaging diagnosis of paraaortic node metastasis is very important[12].
Lymph node staging in the various carcinomas has been extensively discussed in the previous literature[13–19]. Dorfman et al[20] reported that the upper limits of the normal nodes in the upper abdomen are site-specific. Therefore, site-specific nodal evaluation is necessary not only due to different clinical importance but also due to different morphologic criteria for malignancy[21]. To our knowledge, however, there have been no radiologic reports on preoperative imaging diagnosis with a focus on the paraaortic node.
The purpose of our study was to compare computed tomography (CT) and magnetic resonance (MR) for pre-operative detecting paraaortic lymph node metastasis in the patients with pancreatico-biliary carcinoma and to re-define the significant morphologic features of metastatic ones.
MATERIALS AND METHODS
Patient population
The protocol for this study was approved by the Institutional Review Board at our institution and informed consent for this retrospective study was not required. From February 2000 to June 2006, 70 patients (37 men, 33 women; mean age, 62.9 years) with pancreatic head cancer (n = 22), ampulla of vater cancer (n = 16), distal common bile duct cancer (n = 24), or gallbladder (GB) cancer (n = 8) underwent CT (n = 63) and/or MR (n = 38) imaging. The mean interval time between lymphadenectomy and imaging evaluation was 16.7 d after CT and 18.3 d after MRI. Paraaortic lymphadenectomy was performed in all of the patients before or during surgical resection operations. Histological examinations revealed metastatic paraaortic nodes in 23 patients and non-metastatic nodes in 47 patients. Both CT and MRI were performed in 31 patients with pancreatic head cancer (n = 11), distal common bile duct cancer (n = 13), ampulla of vater cancer (n = 6) or GB cancer (n = 1). Nine patients had metastatic paraaortic nodes and 22 patients had non-metastatic nodes.
Imaging acquisition
All CT scans were obtained with one of the following commercially available multidetector or single detector CT scanners (Somatom Sensation 64, Somatom Sensation 64, Somatom Plus 4; Siemens Medical Solutions, Erlangen, Germany; Lightspeed Plus or QX/i, GE Medical Systems, Milwaukee, Wisconsin). Each patient received 120-150 mL of iopromide (Ultravist 300 or Ultravist 370; Schering, Berlin, Germany) at a rate of 3 mL/s. CT scans were obtained during the arterial phase (using a 25-35-s delay), portal venous phase (using a 70-75-s delay), and equilibrium phase (using a 3-min delay) after IV administration with 3-5-mm section thickness and 3-5-mm reconstruction interval.
MRI examinations were performed using a 1.5-T imaging system (Gyroscan Intera, Philips Medical Systems Best, Netherlands), equipped with commercially available phased-array coils (Synergy; Philips Medical Systems, Best, Netherlands). Four-hour fasting was recommended before the examinations. Antiperistaltic agents or oral contrast agents were not used. The MRI protocol consisted of a breath-hold axial T1-weighted dual fast-gradient-recalled-echo sequence [(TR/in-phase TE, 180/4.6 ms; out-of-phase TE = 2.3 ms; flip angle, 90°; field of view, 32-36 cm × 25-29 cm; matrix, 240 × 240; section thickness, 7 mm; slice spacing, 7.7 mm; one signal acquired; number of slices = 24)]; a single shot turbo spin echo (TR/TE, 452/80 and 160; field of view, 32-36 cm × 25-29 cm; matrix, 288 × 230; section thickness, 7 mm; slice spacing, 5 mm; scan slices were overlapped by 2 mm using an interleaved acquisition technique) with spectral fat suppression and respiratory triggering technique; and a breath-hold transverse 3D gradient echo sequence with fat saturation (TR/TE, 3.9/1.1 msec; flip angle, 25°; field of view, 32-36 cm × 25-36 cm; matrix, 320/224; section thickness, 3 mm).
Contrast-enhanced MRI was performed using a breath-hold 3D gradient echo sequence with fat saturation sequence, following an IV bolus of 0.1 mmol gadobenate dimeglumine (MultiHance, Bracco SpA, Milan, Italy) per kilogram of body weight followed by a saline flush of 30 mL. This sequence was repeated four times with data acquisition in the hepatic arterial, portal venous, and equilibrium phases. An automatic infusion system (Spectris MR injection system, Medrad Europe, Maastricht, Netherlands) operating at an injection rate of 2 mL/s was used. The actual pulse sequence was started manually when the fluoroscopic sequence revealed that the contrast material bolus had reached the abdominal aorta.
Image analysis
All of the imaging analysis was performed on a picture archiving and communication system (PACS) workstation (Centricity 1.0; GE Medical Systems). This retrospective study was composed of two parts. To compare the diagnostic accuracy of CT and MRI, two radiologists independently evaluated preoperative CT and MR images within a 3-wk interval in 31 patients, without knowledge of final pathologic diagnosis. They considered the following criteria as the primary findings for metastatic nodes: (1) short diameter > 9 mm; (2) long axis diameter > 13 mm; (3) presence of necrosis; (4) irregular margin. Reviewers graded the paraaortic lymph node on a five-point scale of diagnostic confidence: 1, no node; 2, definitely benign; 3, probably benign; 4, probably metastatic; and 5, definitely metastatic. Diagnostic accuracy was evaluated using receiver operating characteristic (ROC) curve analysis with a calculation of the area (Az) under the ROC curve. Degree of interobserver agreement was expressed by a Kappa value; a kappa value greater than 0.60 indicated excellent agreement, between 0.40 and 0.60 was good, and less than 0.40 was poor[22].
Using the CT and MR images, we redefined the morphologic criteria of metastatic nodes by comparing them with non-metastatic nodes. Two radiologists evaluated the CT scan in consensus for 63 patients (18 metastatic paraaortic nodes and 45 non-metastatic ones) to record the short and long axial diameter and their ratio, margin (smooth or irregular), and the presence of necrosis in the detected paraaortic lymph nodes. The short and long axis diameter and their ratio were compared between metastatic paraaortic and non-metastatic lymph nodes by the independent samples t-test. The margin and presence of necrosis of metastatic paraaortic lymph nodes were compared to those of non-metastatic nodes by Fisher’s exact test. P < 0.05 was considered statistically significant. A ROC curve was used to determine the best cut-off value for the short and long axis diameter for differentiation of metastatic from non-metastatic nodes. When multiple nodes in the paraaortic region were detected, the largest, irregular-shaped, and/or necrotic node was selected and defined as a metastatic node. The imaging findings were compared with histopathologic results on a per-case basis.
RESULTS
Accuracy of CT and MRI for detecting metastatic paraaortic lymph nodes
Interobserver agreement between the two readers for CT was excellent (kappa value 0.674; standard error 0.088), but that for MRI was poor (kappa value 0.359 ; standard error 0.157).
The mean area under the two readers’ ROC curve for CT (0.732 and 0.646, respectively) was slightly higher than that for MRI (0.725 and 0.598, respectively) without statistical significance (P = 0.940 and 0.716, respectively) (Figures 1 and 2).
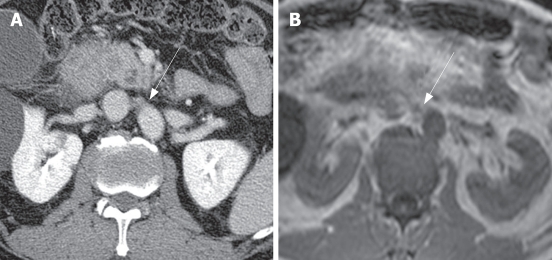
Figure 1.
Metastatic right paraaortic lymph node in a 63-year-old man with pancreatic head cancer. A: Contrast-enhanced CT shows an irregularly shaped lymph node (arrow) with a short axis dimension of 11.5 mm that was interpreted as a definitely metastatic lymph node; B: Axial T1-weighted MRI shows an irregularly shaped lymph node (arrow) with a short axis dimension of 8.5 mm that was interpreted as a definitely benign lymph node. Pathologic examination revealed that this lymph node was metastatic.
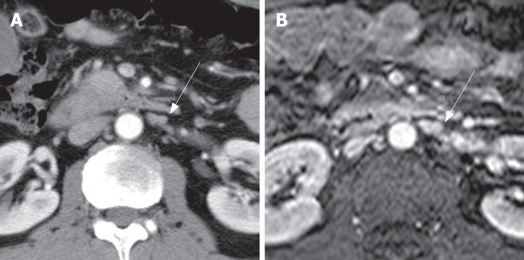
Figure 2.
Metastatic left paraaortic lymph node in a 51-year-old man with pancreatic head cancer. A: Contrast-enhanced CT shows an irregularly shaped lymph node (arrow) with a short axis dimension of 7.2 mm that was interpreted as a probably metastatic lymph node; B: Axial contrast-enhanced T1-weighted MRI shows an irregularly shaped lymph node (arrow) with a short axis dimension of 7 mm that was interpreted as a probably metastatic lymph node; this diagnosis was confirmed by lymphadenectomy and pathological examination.
Features of metastatic paraaortic lymph nodes on CT
The comparison between non-metastatic and metastatic paraaortic lymph nodes on CT is summarized in Table 1. The short axis diameter of metastatic lymph nodes (mean = 9.2 mm, 3.8-28.1 mm) was significantly larger than that of non-metastatic lymph nodes (mean = 5.17 mm, 2.1-11.8 mm, P < 0.05). The long axis diameter of metastatic lymph nodes (mean = 13.18 mm, 5-32.1 mm) was significantly larger than that of non-metastatic lymph nodes (mean = 8.72 mm, 4.6-22.9 mm, P < 0.05). However, the ratio of the short to long axis of metastatic lymph nodes (mean = 0.70) was slightly larger than that of non-metastatic lymph nodes (mean = 0.58) without statistical significance (P = 0.284). The margins of the paraaortic lymph nodes were irregular in 8 of 18 patients (44.4%) with metastasis (Figures 1 and 2), and 4 of 45 patients (8.9%) without metastasis. The presence of central necrosis was seen in 4 of 18 patients (22.2%) with metastasis, but was not seen in patients without metastasis. Metastatic nodes had more irregular margins (44%) and central necrosis (28%) than non-metastatic nodes (9% and 0%, respectively), with statistical significance (P < 0.05).
Table 1.
Features of metastatic paraaortic lymph nodes on CT
| Non-metastatic | Metastatic | P value | |
| Mean short diameter | 5.17 mm | 9.2 mm | < 0.05 |
| Mean long diameter | 8.72 mm | 13.18 mm | < 0.05 |
| Mean ratio (short/long) | 0.58 | 0.7 | 0.284 |
| Irregular margin | 9% | 44% | < 0.05 |
| Necrosis | 0% | 28% | < 0.05 |
Based on the ROC curve, we determined that the best cut-off values for differentiating metastatic nodes from non-metastatic nodes were > 5.3 mm for the short axis diameter and > 11.6 mm for the long axis diameter (Figure 3). According to the short axis cutoff of > 5.3 mm, the diagnostic values for metastatic nodes were 77.8% sensitivity (95% confidence interval (CI): 52.4%-93.5%) and 66.4% specificity (95% CI: 48.8%-78.1%). According to the cutoff of > 11.6 mm for the long axis diameter, the diagnostic values for metastatic nodes were 50.0% sensitivity (95% CI: 26.1%-73.9%) and 91.1% specificity (95% CI: 78.8%-97.5%).
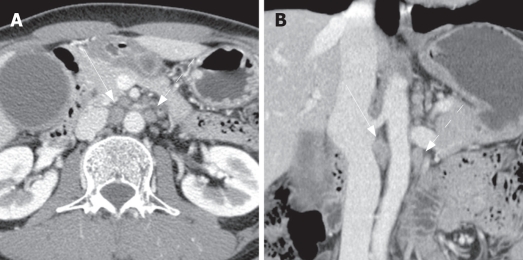
Figure 3.
Two metastatic paraaortic lymph nodes in a 49-year-old man with gallbladder cancer. Axial (A) and coronal (B) contrast-enhanced CT shows several paraaortic lymph nodes. Among them, the right largest node (straight arrow) shows 10 mm and 18.8 mm of short and long axis diameters with irregular margin (on coronal image), compatible with metastatic node. The left one (dot arrow) shows 8.2 mm and 12.2 mm of short and long axis diameters, less than the mean value of metastatic ones (9.2 mm and 13.2 mm, respectively). According to the best cut-off value of short diameter more than 5.3 mm and long axis diameter more than 11.6 mm, The left one is also metastatic one rather than non-metastatic one. Pathologic examination revealed that two lymph nodes were metastatic ones among six resected paraaortic lymph nodes.
DISCUSSION
Although CT and MR imaging are well established for the staging and follow-up of patients with malignancy, the rates of accuracy for the detection of metastatic lymph nodes vary widely. It has been reported that the accuracy of CT and MRI for the detection of lymph nodes in patients with cervical carcinoma[23–25] and the evaluation of regional nodes in the patients with rectal cancer[26,27] is comparable. Other studies have suggested that CT is more specific for detecting positive lymph nodes in gynecologic cancers, whereas MR imaging is more sensitive[23]. In some reports on the evaluation of cervical cancer, MRI (60%) was reported to be more sensitive than CT (43%), whereas the specificities of the two modalities were comparable[28]. Focusing on paraaortic nodes in the patients with pancreatico-biliary cancer, our study showed that the accuracy of CT and MRI were comparable. Our results revealed that the interobserver agreement for CT was excellent, whereas that for MR was poor. This finding suggests that the radiologist’s experience is more important for evaluating by MRI than CT, although it is generally accepted that the tissue contrast with MRI is better than that with CT.
Size criteria have been used in the differentiation of metastatic from non-metastatic nodes, despite much dispute[29]. In past, the maximum short axis diameter of a normal lymph node was known to vary on abdominal computed tomography, according to the node's location; the upper paraaortic region is 9 mm and the lower paraaortic region is 11 mm[20]. A recent study for metastatic paraaortic nodes in pancreatic cancer shows that the size criteria combined with a long axis diameter (12, 10, 8, or 6 mm) and the axial ratio (0.5, 0.7, or 1.0) have a positive predictive value of 13% to 36% and an overall accuracy of 66.7% to 78.9%[21]. Therefore it has been concluded that morphologic criteria are not useful in the evaluation of metastatic paraaortic nodes. A previous study of gallbladder carcinoma, on the other hand, demonstrated a high positive predictive value (86%) in the evaluation of metastatic interaortocaval nodes based on the size and shape criteria; anterior posterior dimensions of 10 mm or larger and ring-like or heterogeneous contrast enhancement[30]. In our study, there was a statistically significant difference between two groups: the mean values for the short and long axis diameter of metastatic paraaortic nodes were 9.2 mm and 13.18 mm, respectively, whereas those of nonmetastatic ones were 5.17 mm and 8.27 mm, respectively. In our study, the best cut-off value for differentiating metastatic nodes from non-metastatic nodes was a short axis diameter of more than 5.3 mm (77.8% sensitivity and 66.4% specificity) and a long axis diameter of more than 11.6 mm (50.0% sensitivity and 91.1% specificity).
It is well known that central necrosis has a very high positive predictive value (almost 100%) in the diagnosis of metastasis. Our study also demonstrated that central necrosis was seen only in metastatic nodes. However, central necrosis may be seen with tuberculosis. Moreover, the sensitivity of central necrosis is very low. In our study, irregular margin had a high positive predictive value, although it was not pathognomic.
Our study had some limitations. First, it was a retrospective study and the parameters of the CT and MRI were not uniform. Second, the imaging findings were compared with histopathologic results on a per-case basis not on a per-node basis.
In conclusion, we found that the accuracy of CT and MRI were comparable for the evaluation of paraaortic nodes in the patients with pancreatico-biliary cancer. Central necrosis, irregular margin, and a cut-off value of more than 5.3 mm for the short axis diameter and 11.6 mm for the long axis diameter may be used as the criteria for diagnosing metastatic paraaortic nodes on CT scan. However, functional studies, such as high-resolution MRI with lymphotropic contrast agent, are necessary to overcome the limitation of morphologic evaluation of nodes.
COMMENTS
Background
In patients with pancreatico-biliary carcinoma, paraaortic lymph node metastasis has a crucial impact on surgical indication or extent of operation. At present, many surgeons perform paraaortic lymphadenectomy for accurate assessment and decision for adequate extent of operation. However, because of its invasiveness and complications, paraaortic lymphadenectomy for pancreatico-biliary carcinoma is controversial.
Research frontiers
Although a comparison between computed tomography (CT) and magnetic resonance (MR) has already been performed in cervical cancer, colorectal cancer and other malignancy, no studies to date have compared CT with MR in terms of detecting paraaortic lymph node metastases from pancreatico-biliary carcinoma. The aim of this study is to determine the accuracy of CT and MR for presurgical characterization of paraaortic lymph nodes in patients with pancreatico-biliary carcinoma.
Innovations and breakthroughs
The results of this study indicate that the accuracy of CT and MR were comparable for the evaluation of paraaortic nodes in the patients with pancreatico-biliary cancer. The lymph node diameter > 5.3 mm, irregular margin, and central necrosis are the suggestive morphologic features of metastatic paraaortic nodes.
Applications
CT and MR could be used for the selection of candidates for lymphadenectomy in the patients with pancreatico-biliary carcinoma.
Terminology
Paraaortic lymph node metastasis in the patients with pancreatico-biliary carcinoma has been reported as a definite predictor of early recurrence and shorter survival term.
Peer review
This is a very interesting paper, although it is a retrospective study. The idea of paraaortic lymph node in pancreatico-biliary is important for evaluation. This unique study will be a first step to confirm the results of a prospective study in the future.
Peer reviewers: Joseph D Boujaoude, Assistant Professor, Department of Gastroenterology, Hotel-Dieu de FranceHospital, Saint-Joseph University, Beirut 961, Lebanon; Aydin Karabacakoglu, Dr, Assistant Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Ma L L- Editor Roberts SE E- Editor Lu W
References
- 1.Yoshida T, Matsumoto T, Sasaki A, Shibata K, Aramaki M, Kitano S. Outcome of paraaortic node-positive pancreatic head and bile duct adenocarcinoma. Am J Surg. 2004;187:736–740. doi: 10.1016/j.amjsurg.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Shimada K, Sakamoto Y, Sano T, Kosuge T. The role of paraaortic lymph node involvement on early recurrence and survival after macroscopic curative resection with extended lymphadenectomy for pancreatic carcinoma. J Am Coll Surg. 2006;203:345–352. doi: 10.1016/j.jamcollsurg.2006.05.289. [DOI] [PubMed] [Google Scholar]
- 3.Niedergethmann M, Rexin M, Hildenbrand R, Knob S, Sturm JW, Richter A, Post S. Prognostic implications of routine, immunohistochemical, and molecular staging in resectable pancreatic adenocarcinoma. Am J Surg Pathol. 2002;26:1578–1587. doi: 10.1097/00000478-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki K. Surgical strategy based on the spread mode of gallbladder carcinoma. Nippon Geka Gakkai Zasshi. 2005;106:286–290. [PubMed] [Google Scholar]
- 5.Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Kanai M, Uesaka K, Yuasa N, Sano T. Value of paraaortic lymphadenectomy for gallbladder carcinoma. Nippon Geka Gakkai Zasshi. 1998;99:728–732. [PubMed] [Google Scholar]
- 6.Miyazaki I, Kayahara M, Nagakawa T. Changes in lymph node dissection for pancreatic cancer. Nippon Geka Gakkai Zasshi. 1997;98:610–614. [PubMed] [Google Scholar]
- 7.Kondo S, Nimura Y, Kamiya J, Nagino M, Kanai M, Uesaka K, Hayakawa N. Mode of tumor spread and surgical strategy in gallbladder carcinoma. Langenbecks Arch Surg. 2002;387:222–228. doi: 10.1007/s00423-002-0318-6. [DOI] [PubMed] [Google Scholar]
- 8.Recht A, Houlihan MJ. Axillary lymph nodes and breast cancer: a review. Cancer. 1995;76:1491–1512. doi: 10.1002/1097-0142(19951101)76:9<1491::aid-cncr2820760902>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Harika L, Weissleder R, Poss K, Papisov MI. Macromolecular intravenous contrast agent for MR lymphography: characterization and efficacy studies. Radiology. 1996;198:365–370. doi: 10.1148/radiology.198.2.8596833. [DOI] [PubMed] [Google Scholar]
- 10.Moghimi SM, Bonnemain B. Subcutaneous and intravenous delivery of diagnostic agents to the lymphatic system: applications in lymphoscintigraphy and indirect lymphography. Adv Drug Deliv Rev. 1999;37:295–312. doi: 10.1016/s0169-409x(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 11.Alexakis N, Halloran C, Raraty M, Ghaneh P, Sutton R, Neoptolemos JP. Current standards of surgery for pancreatic cancer. Br J Surg. 2004;91:1410–1427. doi: 10.1002/bjs.4794. [DOI] [PubMed] [Google Scholar]
- 12.Endo I, Shimada H, Tanabe M, Fujii Y, Takeda K, Morioka D, Tanaka K, Sekido H, Togo S. Prognostic significance of the number of positive lymph nodes in gallbladder cancer. J Gastrointest Surg. 2006;10:999–1007. doi: 10.1016/j.gassur.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Heriot AG, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17–28. doi: 10.1046/j.1365-2168.1999.00996.x. [DOI] [PubMed] [Google Scholar]
- 14.Bipat S, Glas AS, van der Velden J, Zwinderman AH, Bossuyt PM, Stoker J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: a systematic review. Gynecol Oncol. 2003;91:59–66. doi: 10.1016/s0090-8258(03)00409-8. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda H, Nakagawa T, Shibuya H. Metastases to pelvic lymph nodes from carcinoma in the pelvic cavity: diagnosis using thin-section CT. Clin Radiol. 1999;54:237–242. doi: 10.1016/s0009-9260(99)91158-3. [DOI] [PubMed] [Google Scholar]
- 16.De Gaetano AM, Vecchioli A, Minordi LM, Parrella A, Gaudino S, Masselli G, Savino G. Role of diagnostic imaging in abdominal lymphadenopathy. Rays. 2000;25:463–484. [PubMed] [Google Scholar]
- 17.Wallis F, Gilbert FJ. Magnetic resonance imaging in oncology: an overview. J R Coll Surg Edinb. 1999;44:117–125. [PubMed] [Google Scholar]
- 18.Schima W, Fugger R, Schober E, Oettl C, Wamser P, Grabenwoger F, Ryan JM, Novacek G. Diagnosis and staging of pancreatic cancer: comparison of mangafodipir trisodium-enhanced MR imaging and contrast-enhanced helical hydro-CT. AJR Am J Roentgenol. 2002;179:717–724. doi: 10.2214/ajr.179.3.1790717. [DOI] [PubMed] [Google Scholar]
- 19.Misselwitz B. MR contrast agents in lymph node imaging. Eur J Radiol. 2006;58:375–382. doi: 10.1016/j.ejrad.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 20.Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–322. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- 21.Noji T, Kondo S, Hirano S, Tanaka E, Ambo Y, Kawarada Y, Morikawa T. CT evaluation of paraaortic lymph node metastasis in patients with biliary cancer. J Gastroenterol. 2005;40:739–743. doi: 10.1007/s00535-005-1618-8. [DOI] [PubMed] [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 23.Bellomi M, Bonomo G, Landoni F, Villa G, Leon ME, Bocciolone L, Maggioni A, Viale G. Accuracy of computed tomography and magnetic resonance imaging in the detection of lymph node involvement in cervix carcinoma. Eur Radiol. 2005;15:2469–2474. doi: 10.1007/s00330-005-2847-1. [DOI] [PubMed] [Google Scholar]
- 24.Scheidler J, Hricak H, Yu KK, Subak L, Segal MR. Radiological evaluation of lymph node metastases in patients with cervical cancer. A meta-analysis. JAMA. 1997;278:1096–1101. [PubMed] [Google Scholar]
- 25.Choi HJ, Kim SH, Seo SS, Kang S, Lee S, Kim JY, Kim YH, Lee JS, Chung HH, Lee JH, et al. MRI for pretreatment lymph node staging in uterine cervical cancer. AJR Am J Roentgenol. 2006;187:W538–W543. doi: 10.2214/AJR.05.0263. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka H, Nakamura A, Masaki T, Sugiyama M, Takahara T, Hachiya J, Atomi Y. A prospective comparison between multidetector-row computed tomography and magnetic resonance imaging in the preoperative evaluation of rectal carcinoma. Am J Surg. 2003;185:556–559. doi: 10.1016/s0002-9610(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 27.Blomqvist L. Preoperative staging of colorectal cancer--computed tomography and magnetic resonance imaging. Scand J Surg. 2003;92:35–43. doi: 10.1177/145749690309200106. [DOI] [PubMed] [Google Scholar]
- 28.Yang WT, Lam WW, Yu MY, Cheung TH, Metreweli C. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol. 2000;175:759–766. doi: 10.2214/ajr.175.3.1750759. [DOI] [PubMed] [Google Scholar]
- 29.Grubnic S, Vinnicombe SJ, Norman AR, Husband JE. MR evaluation of normal retroperitoneal and pelvic lymph nodes. Clin Radiol. 2002;57:193–200; discussion 201-204. doi: 10.1053/crad.2001.0893. [DOI] [PubMed] [Google Scholar]
- 30.Ohtani T, Shirai Y, Tsukada K, Muto T, Hatakeyama K. Spread of gallbladder carcinoma: CT evaluation with pathologic correlation. Abdom Imaging. 1996;21:195–201. doi: 10.1007/s002619900045. [DOI] [PubMed] [Google Scholar]