Abstract
AIM: To explore clinicopathologic characteristics of intrahepatic cholangiocarcinoma (ICC) in patients with positive serum a-fetoprotein (AFP).
METHODS: One hundred and thirty one patients who underwent surgical dissection for pathologically confirmed ICC were divided into a positive AFP (> 20 ng/mL) group (n = 32) and a negative AFP group (n = 99), whose clinicopathologic features were analyzed and compared.
RESULTS: The positive rate of HBsAg and liver cirrhosis of the positive AFP group was higher than that of the negative AFP group, while the positive rate of CA19-9 (> 37 U/mL) and the lymph node metastasis rate was lower.
CONCLUSION: ICC patients with positive AFP share many clinicopathologic similarities with hepatocellular carcinoma.
Keywords: Intrahepatic cholangiocarcinoma, A-fetoprotein, Hepatitis B virus, Liver cirrhosis, Hepatic stem cells
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is a tumor originating from peripheral intrahepatic biliary epithelia, ranking as the second most common primary hepatic malignant tumor next to hepatocellular carcinoma (HCC), accounting for 5% of all primary hepatic malignant tumors[1]. The incidence and mortality of ICC is on the rise in recent years[2]. Serum a-fetoprotein (AFP), as a tumor marker of HCC[3–5], and carbohydrate antigen 19-9 (CA19-9), as a tumor marker of ICC, have been widely used in clinical practice[6]. In about 19% ICC patients, serum AFP is also positive (> 20 ng/mL)[1], but there is little knowledge about the clinicopathologic features of such patients. The purpose of this study was to define clinicopathologic features of ICC patients with positive AFP by comparing them with ICC patients with negative AFP.
MATERIALS AND METHODS
Patients
Included in this study were 131 ICC patients who received surgical dissection at the Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University (Shanghai, China) from March 2002 to June 2003, including 90 males and 41 females ranging in age from 23 to 73 years with a mean of 53 years. Of the 131 ICC patients, serum AFP was positive in 32 patients (24.4%), of whom AFP was > 200 ng/mL in 13 patients (9.9%), and > 1000 ng/mL in 6 patients (4.5%). Their clinical manifestations, pathological findings and surgical outcomes were compared with those of ICC patients whose serum AFP was negative. Positive serum hepatitis B surface antigen (HBsAg) and hepatitis C antibody were biomarkers of chronic viral hepatitis.
The diagnosis of ICC was confirmed by pathology. All the excised specimens were fixed in 4% neutral formaldehyde routinely, paraffin embedded, sliced into 4 μm sections, and haematoxylin and eosin (HE) stained. Pathological study included the size, number and location of the tumors, background of cirrhosis, portal or hepatic vein invasion, lymph node metastasis, formation of tumor capsules, and histological grade. Tumors whose diameter was smaller than 3 cm were classified as small ICC.
Immunohistochemistry for HCC marker hepatocyte paraffin 1 (Hep Par 1)[7] and ICC marker cytokeratin 19 (CK-19)[8] was performed using a polymer-based method with the Envision Kit (Fuzhou Maxim Biotech, China). Formalin-fixed, paraffin-embedded serial tissue sections (4 μm) were deparaffinised and rehydrated in xylene and grade-diluted ethanol. Tissue sections were then incubated in methanol containing 0.3% hydrogen peroxide at room temperature for 20 min to block endogenous peroxidase. Sections were then incubated overnight at 4°C with anti-Hep Par 1 antibody (Dako, Denmark) or anti- CK-19 antibody(NeoMarkers, USA), followed by incubation with Envision reagent at room temperature for 30 min, and color developed with 3, 3’-diaminobenzidine tetrahydrochloride. Finally, the sections were counterstained with haematoxylin, and hyalinized water. For negative controls, the sections were processed the same way, except they were incubated with phosphate-buffered saline instead of the primary antibody.
All patients were followed up after discharge from the hospital, with a median follow-up period of 31 mo (range 5-52 mo).
Statistical analysis
Data were analyzed with SPSS 11.0 statistical software. Quantitative inter-group comparison was tested by t test, and classification inter-group comparison was tested by χ2 test. Survival analysis was done by Kaplan-Meier method. Inter-group comparison was done by log-rank method. P < 0.05 was considered statistically significant.
RESULTS
Clinical features
The mean age of the positive AFP group was lower than that of the negative AFP group (P = 0.007). There was no significant difference in sex distribution between the two groups. The positive rate of HBsAg (78.1%) and transaminase of the positive AFP group was higher than that of the negative AFP group (P = 0.000 and P = 0.036 respectively), while the positive rate of CA19-9 was lower (> 37 U/mL, P = 0.007; Table 1).
Table 1.
Clinical features of ICC patients with positive AFP
|
AFP |
|||
| + (n = 32) | - (n = 99) | P value | |
| Gender (M/F) | 24/8 | 66/33 | NS |
| Age (yr) | 48.7 ± 11.7 | 54.5 ± 9.9 | 0.007 |
| HBsAg + (%) | 25 (78.1) | 38 (38.3) | 0.000 |
| Anti-HCV + (%) | 0 | 1 (0.01) | NS |
| CA19-9 (> 37 U/mL) (%) | 10 (31.4) | 58 (58.6) | 0.007 |
| TBIL (> 17.1 μmol/L) (%) | 17 (53.1) | 39 (39.4) | NS |
| ALT (> 40 IU/L) (%) | 16 (50.0) | 26 (26.3) | 0.012 |
| AST (> 40 IU/L) (%) | 14 (43.8) | 23 (23.2) | 0.025 |
NS: Not significant; M: Male; F: Female; HBsAg: Hepatitis B surface antigen; HCV: Hepatitis C virus antibody; TBIL: Total bilirubin; AST: Aspartate transaminase; ALT: Alanine transaminase.
Pathological features
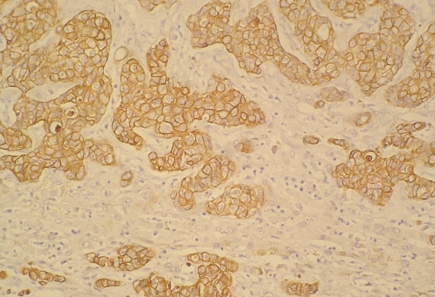
Backgrounds of liver cirrhosis were elicited in 13 ICC patients with positive AFP, which was significantly higher than that of the negative AFP group (40.6% vs 22.2%, P = 0.041), but the lymph node metastasis rate was significantly lower (15.6% vs 35.4%, P = 0.035). There were no significant differences in the location, size and number of tumors, tumor capsule defect, histological differentiation, portal venous invasion and microvascular invasion (Table 2). Immunohistochemical staining showed that Hep Par 1 expression was negative and CK-19 expression was positive in all 131 cases (Figure 1).
Table 2.
Pathologic features of ICC patients with and without positive AFP
|
AFP |
|||
| + (n = 32) | - (n = 99) | P value | |
| Tumor location (%) | NS | ||
| Right lobe | 21 (65.6) | 57 (57.6) | |
| Left lobe | 8 (25.0) | 33 (33.3) | |
| Both lobes | 3 (9.4) | 9 (9.1) | |
| Tumor size (cm) | NS | ||
| mean ± SD | 7.97 ± 4.12 | 6.83 ± 2.98 | |
| ≤ 3 (%) | 2 (0.6) | 9 (0.9) | |
| Tumor number (%) | NS | ||
| Single | 25 (78.2) | 62 (62.7) | |
| ≥ 2 | 7 (21.8) | 37 (37.3) | |
| Liver cirrhosis (%) | 13 (40.6) | 22 (22.2) | 0.041 |
| Capsule formation (%) | 8 (25.0) | 15 (15.2) | NS |
| Histological grades (%) | NS | ||
| Well- Moderately | 21 (65.6) | 68 (68.6) | |
| Poorly | 11 (34.4) | 31 (31.4) | |
| Lymph node metastasis (%) | 5 (15.6) | 35 (35.4) | 0.035 |
| Portal invasion (%) | 1 (3.1) | 5 (5.1) | NS |
| Microvascular invasion (%) | 18 (56.2) | 62 (62.6) | NS |
| Immunohistochemical examinations | NS | ||
| Hepatocyte paraffin | 1 | 0 | 0 |
| Cytokeratin 19 (%) | 32 (100) | 99 (100) | |
NS: Not significant.
Figure 1.
Representative sections showing immunohistochemical expression of CK-19 in intrahepatic cholangiocarcinoma (× 200).
Outcomes
No hospital death occurred in all the 131 ICC cases. The median postoperative survival of the ICC patients with positive AFP and with negative AFP was 37 mo and 28 mo respectively. The cumulative 1-year and 3-year survival rate of the positive AFP group was 68.7% and 46.8% respectively, both higher than 64.6% and 40.4% of the negative AFP group, though the difference was not statistically significant. Possible risk factors affecting survival included tumor size > 3 cm (P = 0.014), lymph node metastasis (P < 0.0001), portal venous invasion (P = 0.006), and the number of tumors ≥ 2 (P < 0.0001).
DISCUSSION
Human AFP is a fetal glucoprotein with a molecular weight of about 72 kDa. Under physiological conditions, it is synthesized by fetal hepatocytes, yolk sac cells and gastrointestinal cells. AFP level begins to decrease gradually to < 10 ng/mL by 300 d of birth. Since detection of AFP in the serum of HCC patients in 1963, AFP has been widely used for screen examination and clinical diagnosis as an HCC tumor marker. In 60%-70% HCC patients, serum AFP is higher than the normal range[1,3,4]. In addition, increased AFP is also found in other pathological conditions such as hepatic cirrhosis, extensive hepatic necrosis, chronic hepatitis, pregnancy, gonadal fetal tumors and digestive tract tumors including gastric carcinoma, pancreatic carcinoma and gallbladder carcinoma. Positive AFP is rarely seen in ICC patients. A series of studies from a Japanese liver cancer research team showed that 19% ICC patients had a serum AFP level > 20 ng/mL, 10.3% > 200 ng/mL, and only 6.3% ICC patients had a serum AFP level > 1000 ng/mL[1]. In our series of 131 ICC patients, 32 patients (24.4%) had positive AFP, including 13 patients (9.9%) > 200 ng/mL, and 6 patients (4.5%) > 1000 ng/mL. The exact mechanism of how AFP is synthesized in ICC is not clear.
We found that ICC patients with positive AFP were associated with HBV infection and cirrhosis. This clinical feature is similar to that of HCC. What is consistent with ICC is that transaminase (a biomarker reflecting hepatic impairment) was higher in the positive AFP group than in the negative AFP group. Yamamoto et al[9] reported that ICC patients who were preoperatively diagnosed as having HCC had a relatively high rate of HCV infection. In ICC patients presenting with a high level of AFP and a low level of CA19-9, surgical treatment similar to HCC should be considered. Okuda et al[10] found that in ICC patients with positive Lens culinaris agglutinin-A-reactive AFP (AFP-L3), the hepatitis viruses infection rate was as high as 60%.
Lymph node metastasis is a common event in ICC; while it occurs rarely in HCC[11]. The data obtained from our study showed that the lymph node metastasis rate was low in ICC patients with positive AFP. What is consistent with previous studies is that lymph node metastasis is an important factor affecting the prognosis of ICC[12]. We found that the 1-year and 3-year survival rate of the positive AFP group was higher than that of the negative AFP group. This may be due to the lower lymph node metastasis rate of ICC patients with positive AFP. However, as the capacity of our cases is small and the follow-up period is not long enough, this statistical difference may not be significant.
The pathogenesis of ICC remains unclear. Recent studies show that HCC, ICC and many other tumors may originate from stem cells[13]. It is generally accepted that adult hepatic stem cells are hepatic oval cells. They are a group of intrahepatobiliary multi-potential differentiation cells, capable of differentiating to hepatobiliary cells and to hepatic cells. These cells are mainly located in the fetal liver or the hepatobiliary terminal Hering tube in adults. In normal physiological conditions, the number of oval cells is very small, and they are in a resting state. When the hepatic parenchyma is severely damaged, or regeneration of the hepatic cells is inhibited by virus, drugs, hepatic toxins or carcinogens, oval cells are activated, proliferating in large numbers and differentiating to hepatic and hepatobiliary cells to repair and reconstruct the liver[13]. AFP is not only an indicator of cell de-differentiation or immaturity but an important sign of hepatic stem cells[14]. Wang et al[15] reported that the expression rate of hepatic stem cell marker CK7 and CK19 was 100% in 12 ICC patients, while the expression rate of c-kit, Thy-1 and AFP was 41.7%, 33.3% and 33.3%, respectively. Transformation of oval cells to ICC cells was also observed. AFP synthesis in ICC suggests that ICC may originate from hepatic stem cells that underwent malignant transformation[16]. However, this presumption awaits verification by more studies.
Liver fluke infection (Clonorchis sinensis or Opisthorchi viverrini)[17,18], primary sclerosing cholangitis (PSC)[19,20], and hepatolithiasis are thought to be the risk factors for ICC[21,22]. Multiple studies in recent years show that viral hepatitis and hepatic cirrhosis are not only closely related to HCC but to ICC[23–26]. Our most recent case-control study showed that HBV infection is the possible pathogenic factor causing ICC in Chinese populations[27]. Proliferation of large numbers of oval cells was seen in chronic HBV and hepatic cirrhosis[28–30]. HBV infection may induce activation of oval cells, and this process may be accompanied with abnormal genetic alteration[31], which in turn triggers malignant transformation of oval cells.
In summary, ICC patients with positive AFP share many clinicopathologic similarities with HCC.
COMMENTS
Background
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignant tumor next to hepatocellular carcinoma (HCC). Serum a-fetoprotein (AFP), as a tumor marker of HCC, has been widely used in clinical practice. In about 19% ICC patients, serum AFP is also positive (> 20 ng/mL), but there is little knowledge about the clinicopathologic features of such patients.
Research frontiers
One hundred and thirty one patients who underwent surgical dissection for pathologically confirmed ICC were divided into a positive AFP (> 20 ng/mL) group (n = 32) and a negative AFP group (n = 99), whose clinicopathologic features were analyzed and compared.
Innovations and breakthroughs
The positive rate of HBsAg and liver cirrhosis of the positive AFP group was higher than that of the negative AFP group, while the positive rate of CA19-9 (> 37 U/mL) and the lymph node metastasis rate was lower.
Applications
AFP synthesis in ICC suggests that ICC may originate from hepatic stem cells that underwent malignant transformation[16]. However, this presumption awaits verification by more studies.
Peer review
This paper by Yan-Ming Zhou is an interesting study that describes the clinicopathologic characteristics of patients affected by intrahepatic cholangiocarcinoma with positive and negative serum AFP. This study includes 131 patients who underwent surgical dissection for pathologically confirmed ICC. The authors, concluding that ICC patients with positive AFP share many clinicopathologic similarities with HCC, suggest new perspectives in the management of intrahepatic cholangiocarcinoma.
Peer reviewer: Giammarco Fava, MD, via Gervasoni 12, 60129 Ancona, Italy
S- Editor Zhong XY L- Editor Alpini GD E- Editor Ma WH
References
- 1.Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Liver Cancer Study Group of Japan. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 2.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 3.Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 4.He P, Tang ZY, Ye SL, Liu BB. Relationship between expression of alpha-fetoprotein messenger RNA and some clinical parameters of human hepatocellular carcinoma. World J Gastroenterol. 1999;5:111–115. doi: 10.3748/wjg.v5.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abelev GI, Eraiser TL. Cellular aspects of alpha-fetoprotein reexpression in tumors. Semin Cancer Biol. 1999;9:95–107. doi: 10.1006/scbi.1998.0084. [DOI] [PubMed] [Google Scholar]
- 6.Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004;24:139–154. doi: 10.1055/s-2004-828891. [DOI] [PubMed] [Google Scholar]
- 7.Chu PG, Ishizawa S, Wu E, Weiss LM. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol. 2002;26:978–988. doi: 10.1097/00000478-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Stroescu C, Herlea V, Dragnea A, Popescu I. The diagnostic value of cytokeratins and carcinoembryonic antigen immunostaining in differentiating hepatocellular carcinomas from intrahepatic cholangiocarcinomas. J Gastrointestin Liver Dis. 2006;15:9–14. [PubMed] [Google Scholar]
- 9.Yamamoto M, Ariizumi S, Otsubo T, Katsuragawa H, Katagiri S, Nakano M, Takasaki K. Intrahepatic cholangiocarcinoma diagnosed preoperatively as hepatocellular carcinoma. J Surg Oncol. 2004;87:80–83; discussion 83-84. doi: 10.1002/jso.20091. [DOI] [PubMed] [Google Scholar]
- 10.Okuda H, Shiratori K, Yamamoto M, Takasaki K, Nakano M. Clinicopathologic features of patients with intrahepatic cholangiocarcinoma who are seropositive for alpha-fetoprotein-L3 and those with combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol. 2006;21:869–873. doi: 10.1111/j.1440-1746.2006.04257.x. [DOI] [PubMed] [Google Scholar]
- 11.Shirabe K, Shimada M, Harimoto N, Sugimachi K, Yamashita Y, Tsujita E, Aishima S. Intrahepatic cholangiocarcinoma: its mode of spreading and therapeutic modalities. Surgery. 2002;131:S159–S164. doi: 10.1067/msy.2002.119498. [DOI] [PubMed] [Google Scholar]
- 12.Isa T, Kusano T, Shimoji H, Takeshima Y, Muto Y, Furukawa M. Predictive factors for long-term survival in patients with intrahepatic cholangiocarcinoma. Am J Surg. 2001;181:507–511. doi: 10.1016/s0002-9610(01)00628-6. [DOI] [PubMed] [Google Scholar]
- 13.Alison MR. Liver stem cells: implications for hepatocarcino-genesis. Stem Cell Rev. 2005;1:253–260. doi: 10.1385/SCR:1:3:253. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Oyamada M, Fujimoto Y, Satoh MI, Hattori A, Dempo K, Mori M, Tanaka T, Watabe H, Masuda R. Elevation of serum alpha-fetoprotein and proliferation of oval cells in the livers of LEC rats. Jpn J Cancer Res. 1988;79:821–827. doi: 10.1111/j.1349-7006.1988.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Suo JY, Deng J, Yang J, Zheng JJ, Wang HZ, Hu Q, Li ZP, Xiao HL, Wang D. Origin of hepatic stem cells in human hepatocellular carcinoma. Acta Academiae Medicinae Militaris Teriae. 2006;28:114–116. [Google Scholar]
- 16.Ishikawa K, Sasaki A, Haraguchi N, Yoshikawa Y, Mori M. A case of an alpha-fetoprotein-producing intrahepatic cholangiocarcinoma suggests probable cancer stem cell origin. Oncologist. 2007;12:320–324. doi: 10.1634/theoncologist.12-3-320. [DOI] [PubMed] [Google Scholar]
- 17.Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, Ahn YO, Shigemastu T. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933–940. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- 18.Parkin DM, Srivatanakul P, Khlat M, Chenvidhya D, Chotiwan P, Insiripong S, L’Abbe KA, Wild CP. Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. Int J Cancer. 1991;48:323–328. doi: 10.1002/ijc.2910480302. [DOI] [PubMed] [Google Scholar]
- 19.Broome U, Lofberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404–1408. doi: 10.1002/hep.1840220511. [DOI] [PubMed] [Google Scholar]
- 20.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzen H, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 21.Chen MF. Peripheral cholangiocarcinoma (cholangiocellular carcinoma): clinical features, diagnosis and treatment. J Gastroenterol Hepatol. 1999;14:1144–1149. doi: 10.1046/j.1440-1746.1999.01983.x. [DOI] [PubMed] [Google Scholar]
- 22.Su CH, Shyr YM, Lui WY, P’Eng FK. Hepatolithiasis associated with cholangiocarcinoma. Br J Surg. 1997;84:969–973. doi: 10.1002/bjs.1800840717. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, Arase Y, Murashima N, Chayama K, Kumada H. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471–2477. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959–964. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- 25.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 26.Takegoshi K, Su Q, Omata M, Taira S, Okada E, Bannasch P. Cholangiocarcinoma with a background of hepatitis B virus-associated cirrhosis. Intern Med. 2001;40:382–385. doi: 10.2169/internalmedicine.40.382. [DOI] [PubMed] [Google Scholar]
- 27.Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: A case-control study in China. World J Gastroenterol. 2008;14:632–635. doi: 10.3748/wjg.14.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsia CC, Evarts RP, Nakatsukasa H, Marsden ER, Thorgeirsson SS. Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology. 1992;16:1327–1333. doi: 10.1002/hep.1840160604. [DOI] [PubMed] [Google Scholar]
- 29.Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao JC, Jin XL, Ruck P, Adam A, Kaiserling E. Hepatic progenitor cells in human liver cirrhosis: immunohistochemical, electron microscopic and immunofluorencence confocal microscopic findings. World J Gastroenterol. 2004;10:1208–1211. doi: 10.3748/wjg.v10.i8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang T, Chesnokov V, Yokoyama KK, Carr BI, Itakura K. Expression of the Hoxa-13 gene correlates to hepatitis B and C virus associated HCC. Biochem Biophys Res Commun. 2001;281:1041–1044. doi: 10.1006/bbrc.2001.4470. [DOI] [PubMed] [Google Scholar]