Abstract
Osmotic stress protein 94 (OSP94), a member of the HSP110/SSE subfamily, is expressed in certain organs such as the kidney, testis, and brain where it can act as a molecular chaperon. In general, its alteration in expression is in response to hyper-ionic and osmotic stress as well as heat shock stress. Since many cells in the inner ear are involved in active ion transportation and are constantly exposed to two ionic different environments, we hypothesize that OSP94 may be expressed in the inner ear and its expression may be influenced by loud sound stress (LSS). With immunohistochemistry combined with confocal microscopy, immunoblotting, and RT-PCR techniques, we found that OSP94 was widely expressed in various cells in the murine cochlea including the stria vascularis (SV), the organ of Corti (OC), the interdental cells, spiral ganglion cells, the spiral ligament, and Reissner’s membrane. Under the unstressed condition, the transcription and protein level of OSP94 expression in the inner ear was quantitatively similar to that of the kidney. Furthermore, its expression in the inner ear by LSS from broadband noise at 117dB/SPL was upregulated, but remained unchanged in the kidney. In particular, the upregulation of OSP94 in the cochlear lateral wall tissue (CLW) was slowly elicited in a LSS time-dependent manner compared with the response of two other heat shock proteins (HSPs); HSP25 and HSP70 are considered to play a cytoprotective role under stressful conditions. Our results show that OSP94 is expressed in the inner ear and indicate this may be necessary for cells in a special ionic and osmotic environment such as endo- perilymphatic ion compartments. The organ-specific upregulation of OSP94 by acoustic overstimulation reveals that OSP94 in the inner ear is potentially important for cellular functional adaptation to LSS.
Keywords: stria vascularis (SV), spiral ligament, organ of Corti (OC), potassium recycling system, ionic and osmotic homeostasis, heat shock protein (HSP)
Osmotic stress protein 94 (OSP94), also named ATP and peptide-binding protein in germ cells (APG-1) or Heat shock 70kDa protein 4-like (HSPA4L), is a member of the HSP110/SSE subfamily (Kojima et al., 1996; Kaneko et al., 1997a; Held et al., 2006). To date, OSP94 is identified in the kidney, restricted to epithelial cells of the cortical segments of renal tubule (Held et al., 2006), ubiquitously in the testis, especially enhanced in spermatogenic cells (Kaneko et al., 1997a; Held et al., 2006), in germ cells (Kaneko et al., 1997b), moderately in the brain (Xue et al., 1998), and in sera of leukemia patients (Takahashi et al., 2007). Its main function is characterized as a molecular chaperone from the structural similarities to Hsp110 and HSP70RY (Kojima et al., 1996; Kaneko et al., 1997a; Fathallah et al., 1993; Lee-Yoon et al., 1995) and also as having a cytoprotective role from excessive stimulation from heat and hyper-ionic and osmotic stress(Kojima et al., 1996; Kaneko et al., 1997a), which cause marked perturbation of intracellular protein function including the suppression of protein synthesis (Cohen and Gullans, 1993).
In the kidney, renal epithelial cells are generally exposed to drastic ionic and osmotic stresses in the uriniferous tubule-collecting duct system, which regulates levels of electrolytes such as sodium, chloride, potassium, and hydrogen and controls blood volume and pH for maintenance of bodily fluid homeostatic balance. Therefore a series of studies showed that the stimulated cells respond by gradually accumulating organic osmolytes including betaine, inositol, sorbitol, and glycerophosphocholine, which help balance the high osmotic pressure without significantly perturbing intracellular macromolecules (Cohen and Gullans, 1993; Zablocki et al., 1991; Burg, 1996). Moreover recent studies revealed that OSP94 is promptly induced by hyper-ionic and osmotic stress in advance of the accumulation of organic osmolytes (Kojima et al., 1996; Kojima et al., 2004).
In the inner ear, the cochlea has two different ionic environments, endolymph and perilymph, produced by a number of ion channels and ion transporters in the organ of Corti and cochlear lateral wall. In particular, a network of active ion transport (known as the potassium recycling system) plays a critical role in maintaining the high concentration of potassium ions in endolymph. In the cochlear lateral wall, the stria vascularis has an abundance of various ion channels, active ion transporters, and well-developed capillaries, which are thought to strongly support potassium recycling as well as create the endocochlear potential (Wangemann, 2002). Normal ionic and osmotic homeostasis must be required for hair cells to convert sound energy into neuronal signals. Meanwhile, the cells involved in active ion transport in the inner ear are constantly exposed to an environment of high ionic strength that supports an intracellular flux of ions for a potassium recycling system. The expression of selected genes like OSP94 presumably is required for a cellular adaptation to an environment of high ionic stress.
In the present study, we determined the expression of OSP94 in the inner ear, and examined whether loud sound stress influences its expression in cochlear lateral wall tissue, which is enriched with active ion transporters and ion channels for potassium recycling system.
EXPRIMENTAL PROCEDURES
Female and male CBA/J mice aged 10–12 weeks with normal hearing were used. To determine the expression of OSP94 in a normal inner ear, twelve animals (six female and six male) were prepared for immunohistochemistry, immunoblot analysis, and reverse transcription polymerase chain reaction (RT-PCR). For quantitative real-time PCR analysis, twenty-one male animals were divided into seven groups; control group, 2, 4 and 6 hours loud sound stress groups (LSS 2h, LSS 4h, LSS 6h) and 4, 8 and 12 hours waiting after LSS 4h groups (LSS 4h+4h, LSS 4h+8h, LSS 4h+12h). Separately, six male animals were randomly assigned to serve as controls (n = 3) or as 12 hours after LSS 6h subjects (LSS 6h+12h, n = 3) and prepared for immunoblot analysis to evaluate the change of OSP94 in the whole cochlea. All groups were composed of three animals. Loud sound stress level was used at 117dB/SPL/broad band. After anesthesia with an overdose of ketamine hydrochloride (100 mg/kg, i.m.) and 2% xylazine hydrochloride (10 mg/kg) (Abbott Laboratories, N. Chicago, IL), the cochlear lateral wall tissues and kidneys were dissected in each group for RT-PCR and quantitative real-time PCR analysis. The procedures of this study were reviewed and approved by the Animal Use Committee of the Oregon Health & Science University.
Immunohistochemistry
For whole mount specimens, cochleas were dissected from normal animals (one female and one male) and fixed with 4% paraformaldehyde in phosphate buffer (PBS) for 4h. The cochlear lateral wall tissues and organ of Corti were then isolated and permeabilized with 0.5% Triton X – 100 for 1h. After being immunoblocked with blocking solution consisting of 10% goat serum/2% bovine serum albumin (BSA) in PBS for 1 h, the samples were incubated overnight at 4°C in an antibody for OSP94 (rabbit polyclonal anti-OSP94, diluted 1:100 with 5% BSA in PBS, Santa Cruz Biotechnology, USA). The specimens were also double labeled with either fluorescently labeled phallotoxins (Alexa fluor® 568 phalloidin, dilution 1:50, Molecular probes/Invitrogen, USA) or propidium iodide (PI; 500 nM, Molecular probes/Invitrogen, USA) for 1h at room temperature. The specimens were rinsed several times with PBS and incubated with a secondary antibody (Alexa Fluor® 488 goat anti- rabbit, diluted 1:100 with 5% BSA in PBS, Molecular probes/Invitrogen, USA) for 1h at room temperature. After rinsing, the tissues were mounted and observed on a Nikon Eclipse TE 300 inverted microscope fitted with a Bio-Rad MRC 1024 confocal laser microscope system. Negative control tissues were incubated with 5% BSA in PBS to replace the primary antibody and double labeled with either phalloidin or PI. For frozen sectioning, dissected and fixed cochleas were decalcificated for three days in the 4% EDTA in PBS. The decalcificated cochleas were transferred to PBS containing 10%, 20% and 30% sucrose in turn for cytoprotection and finally frozen in OCT at −20°C. Fourteen-micrometer-thick sections were cut with a cryostat microtome and stored at −20°C until used. The immunohistochemical procedures were the same as previously described for immunohistochemistry.
Immunoblot analysis
To compare the protein level of OSP94 expression between different organs and between genders, whole cochleas were isolated from normal animals (three female and three male). The kidney and testis tissues were also excised (kidney; one female and one male, testis; one male). Likewise, whole cochleas and the kidneys from the controls and the LSS 6h+12h were prepared to determine the change of OSP94 by LSS. All tissue samples were then homogenized in lysis buffer (RIPA Lysis buffer, Upstate, Cell Signaling Solutions, NY, USA) with protease inhibitor cocktails (Protease Inhibitor cocktails Set III, Calbiochem, Germany) for 30s. After centrifuging (4°C, 30min, and ×14000 rpm), the supernatants were collected and protein assay was done by using DC protein Assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of protein were loaded on 10% sodium dodecyl sulfate-polyacrylamide gels by electrophoresis (200V for 1h) and transferred to nitrocellulose membranes by electroblotting (100V/200mA for 1h). Subsequently, the nitrocellulose membranes were blocked in 5% non-fat milk and 0.1% Tween 20 in Tris-buffered saline (TBS-T) for 1h at room temperature and incubated overnight at 4°C with a primary antibody for OSP94 (diluted 1:100 with 5% BSA inTBS-T). The membranes were rinsed and incubated with a secondary antibody (Goat anti-Rabbit IgG conjugated with horseradish peroxidase, dilution 1:3,000, Bio-Rad, Hercules, CA, USA) at room temperature for 1h. After washing, the membranes were developed using enhanced chemiluminescence (ECL; Bio-Rad, Hercules, CA, USA). In this study, Actin (Rabbit anti-Actin, dilution 1:500, Sigma) served as an internal control.
Expression and Quantification of OSP94 from mouse tissues cDNA
Total RNA of whole cochleas was extracted from four ears of normal animals (two female and two male) by using RNeasy® Micro Kit (Qiagen, Valencia, CA, USA) following the standard protocol. Total RNA of the kidney and testis was also extracted from the same animals (one female and one male). First-strand cDNA of each sample was synthesized from 2 μg of total RNA by using RETROscript® Kit (Ambion, USA). The reaction was finally heat-inactivated. Conserved regions spanning introns were selected to design primers for OSP94 and Glyceraldehyde-3-phoshpate dehydrogenase (GAPDH) from mouse cDNA sequence encoding mouse OSP94 (mouse Chr11 NM_008300) and GAPDH (mouse Chr6 NM_008084). The primers used were as follows: OSP94 (forward, AATAGCAAGCTTAACCTGCAGAAC; reverse, AGCATGTTTTGGTTCCTCCTTT, 165 bp product), GAPDH (forward, ATGTGTCCGTCGTGGATCTGAC; reverse, AGACAACCTGGTCCTCAGTGTAG 132 bp product). Cycling conditions of RT-PCR were 95°C for 2min; up to 30 cycles of 95°C for 30s, 60°C for 30s, 72°C for 30s; and a final 4min extension at 72°C. All products were amplified usingTaqDNA polymerase (Promega, Madison, WI) by DNA Engine Dyad® (Bio-Rad, Hercules, CA, USA). The cDNA synthesized from total RNA was also diluted 6-fold with DNase-free water and each cDNA sample was independently subjected three times to real-time PCR with the same primers of RT-PCR and SYBR Green PCR Master Mix (Applied Biosystems, CA, USA) using the StepOnePlus™ Real-Time PCR System (Applied Biosystems, CA, USA). Cycling conditions of real-time PCR were 95°C for 2min; 40 cycles of 95°C for 30s, 60°C for 60s. GAPDH was used as a reference gene, and relative quantification analysis was determined via the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Comparative gene expression of OSP94 and other heat shock proteins in cochlea lateral wall tissues by loud sound stress
Cochlear lateral wall tissues were isolated from the control group, LSS 2h, 4h and 6h groups and LSS 4h+4h, 4h+8h and 4h+12h groups (three animals in each group). The kidney tissues were also excised from the control group and the LSS 2h, 4h and 6h groups (one animal in each group). After total RNA extraction and cDNA synthesis, RT-PCR and real-time PCR were performed by the same protocol and the data of real-time PCR was estimated by the 2−ΔΔCT method. Heat shock protein 25 (HSP25/HSPB1) and HSP70 (HSPA1A, HSPA1B) were selected as other heat shock proteins (HSPs) and their primers were designed from conserved regions in mouse cDNA sequence encoding mouse HSP25 (mouse Chr5 NM_013560) and HSP70 (HSPA1A: mouse Chr17 NM_010479). The primers of HSP70 (HSPA1A, HSPA1B) were designed from the same exon in HSPA1A, because cDNA sequences in HSPA1A (mouse Chr17 NM_010479) and HSPA1B (mouse Chr17 NM_010478) are homologous and they have only one exon in the open reading frame. The primers used were as follows: HSP25 (forward, GTCTCGGAGATCCGACAGAC; reverse, AGAGATGTAGCCATGTTCGTCCT, 153 bp), HSP70 (forward, ACAAGAAGAAGGTGCTGGACA; reverse, CTGGTACAGCCCACTGATGAT, 140 bp)
Statistical analysis
Numerical results are presented as means ± S.D. The significant differences between the data sets were assessed with the Student’s t-tests. The differences were considered significant at p < 0.05.
RESULTS
Expression and Distribution of OSP94 in the inner ear
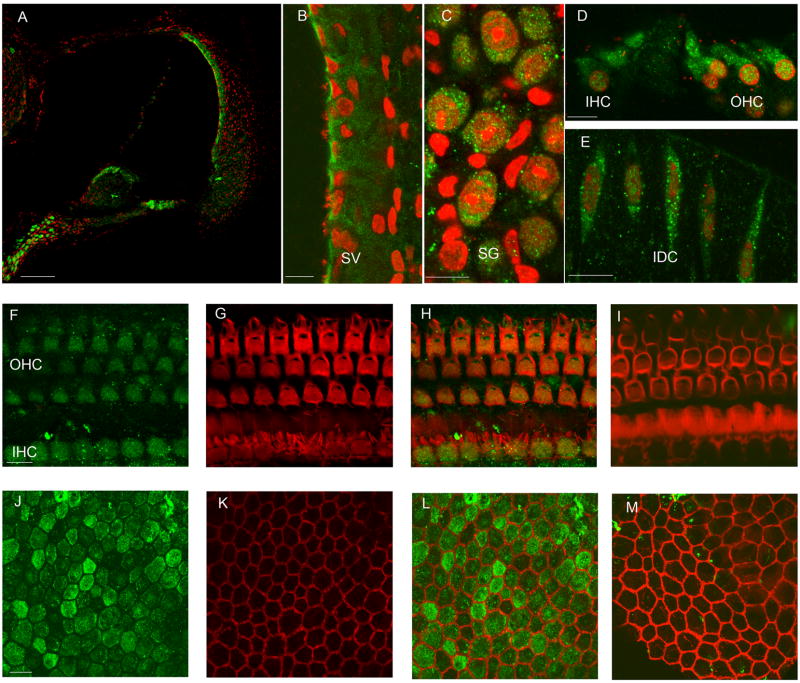
The immunohistochemistry showed that OSP94 was expressed in the murine inner ear including the stria vascularis (SV), the organ of Corti (OC), interdental cells (IDC), the type II, III fibrocytes and root cells area in the spiral ligament (SL), Reissner’s membrane, and distributed in the cells that face the endolymphatic space (Fig. 1). In particular, on the luminal side of the SV, marginal cells were the most labeled and the somas of inner and outer hair cells were intensely labeled in OC (Fig. 1). In addition, OSP94 was highly expressed in the spiral ganglion cells (SG) (Fig. 1). There was no difference in the distribution of OSP94 in the inner ear between female and male (data not shown).
Fig. 1.
Distribution of OSP94 in the cochlea. Panel A–E; fluorescent confocal images from frozen-sections of the cochlea. The tissues were immunolabeled with OSP94 (green) and the nuclei were stained with PI (red). Scale bars, 100μm (A), 10μm (B–E). Panels F–M; the fluorescent confocal images were from whole mount preparations. Cell structure was visualized by labeling with phalloidin (red). Merged images (H, L) were compared with negative controls (I, M). Immunoreaction for OSP94 (green) was found in the organ of Corti, especially both IHC and OHC soma (F, G, H). In the SV, OSP94 was prominently labeled on the luminal side of marginal cells (J, K, L). Scale bars, 10μm (F–I), 10μm (J–M).
The abbreviations used are as follows: SV, stria vascularis; SG, spiral ganglion cells; IDC, intradental cells; IHC, inner hair cell, and OHC, outer hair cell.
Comparison of OSP94 expression between organs
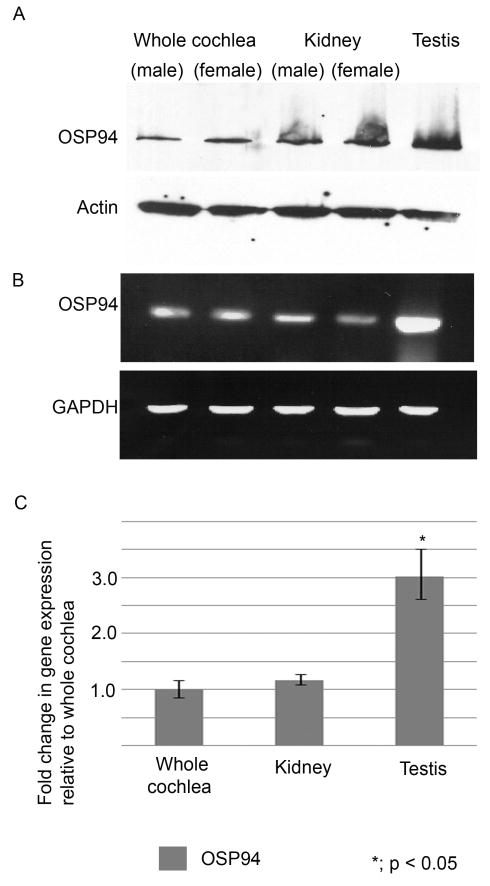
OSP94 was expressed in the whole cochlea, the kidney, and the testis at the protein level (Fig. 2A). Likewise, the RT-PCR analysis disclosed the presence of OSP94 transcription in the inner ear (Fig. 2B). Actin and GAPDH were used as a control and there were no apparent differences in the levels of actin and GAPDH among all samples (Fig. 2A,B). Quantitative real-time PCR analysis indicated the transcription level of OSP94 expression in the whole cochlea was quantitatively similar to that of the kidney. However, its expression in the testis was 3-fold more abundant than that of the other organs (p < 0.05) (Fig. 2C, Table 1).
Fig. 2.
OSP94 expression between whole cochlea (female and male), kidney (female and male) and testis. Panel A shows that the specific bands for OSP94 were observed at 120 kDa area in all samples by immunoblot analysis. (Actin was used as an internal control.) Panel B shows PCR products of OSP94 at 21cycles of the reaction by RT-PCR. Its intensity in the testis is higher than the other organ samples. (GAPDH was used as a reference gene.) Panel C shows the quantification of OSP94 expression in each organ by quantitative real-time PCR analysis. The bar graph shows the average of three independent experiments for each sample. Error bars represent the S.D. Each value represents a comparison with the whole cochlea sample defined as 1.0. There was a statistical difference in transcription level of OSP94 between the testis and the other organ samples (p < 0.05). There was no statistical difference between the whole cochlea and the kidney.
Table 1.
OSP94 expression in organs
| Material | CT OSP94 | CT GAPDH | ΔCT (OSP94 CT-GAPDH CT) | ΔΔCT (Ave.ΔCT- Ave.ΔCT whole cochlea) | 2−ΔΔCT Normalized OSP94 amount relative to whole cochlea |
|---|---|---|---|---|---|
| Whole | 24.48 | 17.89 | 6.59 | ||
| cochlea | 24.24 | 17.95 | 9.29 | ||
| 24.55 | 17.81 | 6.74 | |||
| Ave. | 24.43 | 17.89 | 6.54 ± 0.23 | 0.00 ± 0.23 | 1.00 (0.85 – 1.17) |
| Kidney | 26.81 | 20.41 | 6.40 | ||
| 26.91 | 20.73 | 6.18 | |||
| 26.88 | 20.53 | 6.35 | |||
| Ave. | 26.87 | 20.56 | 6.31 ± 0.12 | −0.23 ± 0.12 | 1.18 (1.08 – 1.27) |
| Testis | 26.84 | 21.91 | 4.93 | ||
| 26.63 | 21.89 | 4.74 | |||
| 26.91 | 21.75 | 5.16 | |||
| Ave. | 26.79 | 21.85 | 4.94 ± 0.21 | −01.60 ± 0.21 | *3.03 (2.62 – 3.51) |
CT: Threshold cycle, Ave: Average (± S.D.)
: p < 0.05
The change of OSP94 in the inner ear by loud sound stress
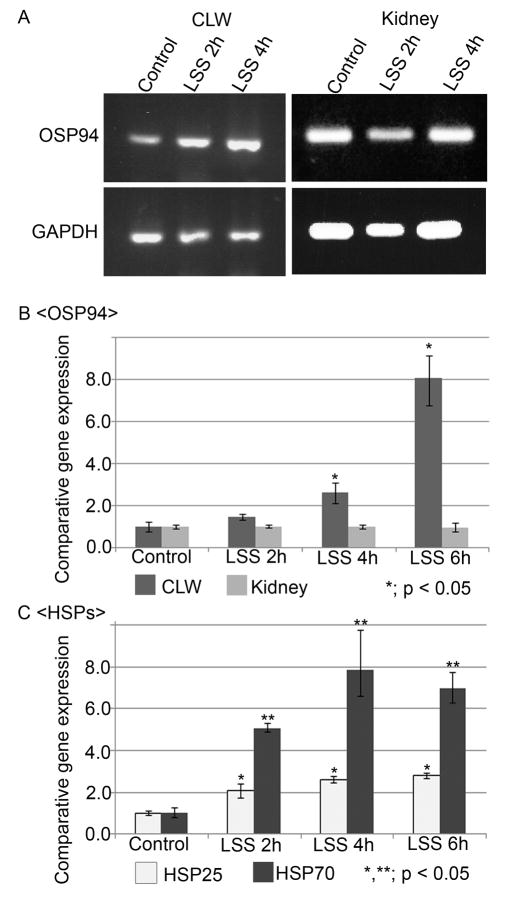
RT-PCR and quantitative real-time PCR analysis were used for determination of the change of OSP94 expression by loud sound stress (LSS). In RT-PCR, PCR products of OSP94 and GAPDH in the LSS groups were compared with the control group at 21 cycles of the reaction. The result exhibited that the band intensities of OSP94 in cochlear lateral wall tissue (CLW) were slightly enhanced in the LSS 2h group, but appreciably elevated in the LSS 4h group (Fig. 3A). Quantitative real-time PCR analysis also showed that transcription of the OSP94 gene in the CLW was significantly upregulated at 4h and 6h under LSS (Fig. 3B). Its upregulation reached 2.66-fold in the LSS 4h group and 8.11-fold in the LSS 6h group compared with the control group (p < 0.05) (Table 2). In contrast, the transcription level of OSP94 expression in the kidney was consistently unchanged by LSS (Fig. 3B, Table 2). Moreover, to compare with the response of other heat shock proteins (HSPs) by LSS, HSP25 and HSP70 were selected as stress resistant molecules (Yoshida et al., 1999; Bruey et al., 2000; Ravagnan et al., 2001). Although the transcriptions of HSP25 and HSP70 in the CLW were also significantly increased by LSS, their induction was more prompt than that of OSP94 and their upregulation came close to saturation between 4h and 6h under LSS (Fig. 3C, Table 3).
Fig. 3.
LSS time-dependent change of OSP94 and other HSPs in the CLW. Panel A shows PCR products of OSP94 and GAPDH in cochlear lateral wall (CLW) and the kidney by RT-PCR. Panel B and C show the value estimated by the 2−ΔΔCT method in quantitative real-time PCR analysis. Each value represents a comparison to either the CLW control or kidney control defined as 1.0. The bar graph shows the average of three independent experiments for each sample. Error bars indicate the S.D. In panel B, the expression of OSP94 in the CLW was time-dependently increased by LSS (p < 0.05). There was no statistical difference in the kidney between the control group and the LSS groups. In panel C, the significant increases of HSP25 and HSP70 were confirmed from 2h under LSS (p < 0.05), and their upregulation was settled after 4h.
Table 2.
LSS time-depedent change of OSP94 in CLW and Kidney
| CT OSP94 | CT GAPDH | ΔCT (OSP94 CT-GAPDH CT) | ΔΔCT (Ave.ΔCT-Ave.ΔCT CLW control) | 2−ΔΔCT Normalized OSP94 amount relative to CLW control | |
|---|---|---|---|---|---|
| CLW control | 23.86 | 18.22 | 5.64 | ||
| 23.07 | 17.97 | 5.10 | |||
| 22.97 | 17.89 | 5.08 | |||
| Ave. | 23.30 | 18.03 | 5.27 ± 0.32 | 0.00 ± 0.32 | 1.00 (0.80 – 1.25) |
| CLW LSS 2h | 22.55 | 17.81 | 4.74 | ||
| 22.76 | 18.21 | 4.55 | |||
| 22.83 | 18.00 | 4.83 | |||
| Ave. | 22.71 | 18.01 | 4.71 ± 0.14 | −0.56 ± 0.14 | 1.48 (1.34 – 1.64) |
| CLW LSS 4h | 23.31 | 19.16 | 4.15 | ||
| 23.55 | 19.94 | 3.61 | |||
| 23.42 | 19.60 | 3.82 | |||
| Ave. | 23.43 | 19.57 | 3.86 ± 0.27 | −1.41 ± 0.27 | *2.66 (2.20 – 3.20) |
| CLW LSS 6h | 23.62 | 21.48 | 1.10 | ||
| 23.60 | 21.54 | 0.83 | |||
| 24.00 | 21.45 | 1.18 | |||
| Ave. | 23.74 | 21.49 | 2.25 ± 0.26 | −3.02 ± 0.26 | *8.11 (6.77 – 9.71) |
| (Ave.ΔCT-Ave.ΔCT Kidney control) | relative to Kidney control | ||||
| Kidney control | 21.91 | 17.89 | 4.02 | ||
| 21.74 | 17.83 | 3.91 | |||
| 21.47 | 17.70 | 3.77 | |||
| Ave. | 21.71 | 17.81 | 3.90 ± 0.13 | 0.00 ± 0.13 | 1.00 (0.91 – 1.09) |
| Kidney LSS 2h | 21.82 | 17.87 | 3.95 | ||
| 21.71 | 17.91 | 3.80 | |||
| 21.71 | 17.89 | 3.82 | |||
| Ave. | 21.75 | 17.89 | 3.86 ± 0.08 | −0.05 ± 0.08 | 1.04 (0.98 – 1.09) |
| Kidney LSS 4h | 21.95 | 17.89 | 4.06 | ||
| 21.78 | 17.97 | 3.81 | |||
| 21.77 | 17.97 | 3.80 | |||
| Ave. | 21.83 | 17.94 | 3.89 ± 0.15 | −0.01 ± 0.15 | 1.01 (0.91 – 1.12) |
| Kidney LSS 6h | 21.73 | 17.88 | 3.85 | ||
| 21.65 | 17.92 | 3.73 | |||
| 21.41 | 17.18 | 4.23 | |||
| Ave. | 21.60 | 17.66 | 3.94 ± 0.26 | 0.04 ± 0.26 | 0.97 (0.86 – 1.23) |
CLW: Cochlear lateral wall, LSS: Loud sound stress, CT: Threshold cycle, Ave: Average (± S.D.)
: p < 0.05
Table 3.
LSS time-dependent change of HSPs in CLW
| CT HSPs | CT GAPDH | ΔCT (HSPs CT-GAPDH CT) | ΔΔCT (Ave. ΔCT -Ave.ΔCT CLW control) | 2−ΔΔCT Normalized HSPs amount relative to CLW control | |||||
|---|---|---|---|---|---|---|---|---|---|
| HSP25 | HSP70 | HSP25 | HSP70 | HSP25 | HSP70 | HSP25 | HSP70 | ||
| CLW control | 22.38 | 27.81 | 22.20 | 1.68 | 5.61 | ||||
| 23.77 | 27.42 | 22.01 | 1.76 | 5.41 | |||||
| 23.46 | 27.26 | 21.98 | 1.48 | 5.28 | |||||
| Ave. | 23.70 | 27.50 | 22.06 | 1.64 ± 0.14 | 5.43 ± 0.17 | 0.00 ± 0.14 | 0.00 ± 0.17 | 1.00 (0.91 – 1.10) | 1.00 (0.80 – 1.25) |
| CLW LSS 2h | 23.33 | 25.58 | 22.55 | 0.78 | 3.03 | ||||
| 23.10 | 25.89 | 22.76 | 0.34 | 3.17 | |||||
| 23.50 | 25.93 | 22.83 | 0.67 | 3.06 | |||||
| Ave. | 23.31 | 25.80 | 22.71 | 0.60 ± 0.23 | 3.09 ± 0.07 | −1.04 ± 0.23 | −2.34 ± 0.07 | *2.06 (1.75 – 2.41) | **5.06 (4.82 – 5.31) |
| CLW LSS 4h | 19.39 | 21.79 | 19.57 | 0.18 | 2.22 | ||||
| 19.43 | 22.07 | 19.66 | 0.23 | 2.41 | |||||
| 19.45 | 22.50 | 19.78 | 0.33 | 2.72 | |||||
| Ave. | 19.42 | 22.12 | 19.67 | 0.25 ± 0.08 | 2.45 ± 0.25 | −1.39 ± 0.08 | −2.98 ± 0.25 | *2.62 (2.48 – 2.77) | **7.89 (6.63 – 9.38) |
| CLW LSS 6h | 21.18 | 23.95 | 21.35 | 0.17 | 2.60 | ||||
| 21.72 | 23.86 | 21.51 | 0.21 | 2.35 | |||||
| 21.85 | 24.40 | 21.78 | 0.07 | 2.62 | |||||
| Ave. | 21.58 | 24.07 | 21.55 | 0.15 ± 0.07 | 2.52 ± 0.15 | −1.49 ± 0.07 | −2.81 ± 0.15 | *2.81 (2.68 – 2.95) | **7.01 (6.32 – 7.78) |
CLW: Cochlear lateral wall, HSP; Heat shock protein, LSS: Loud sound stress, CT: Threshold cylce, Ave: Average (± S.D.)
: p < 0.05
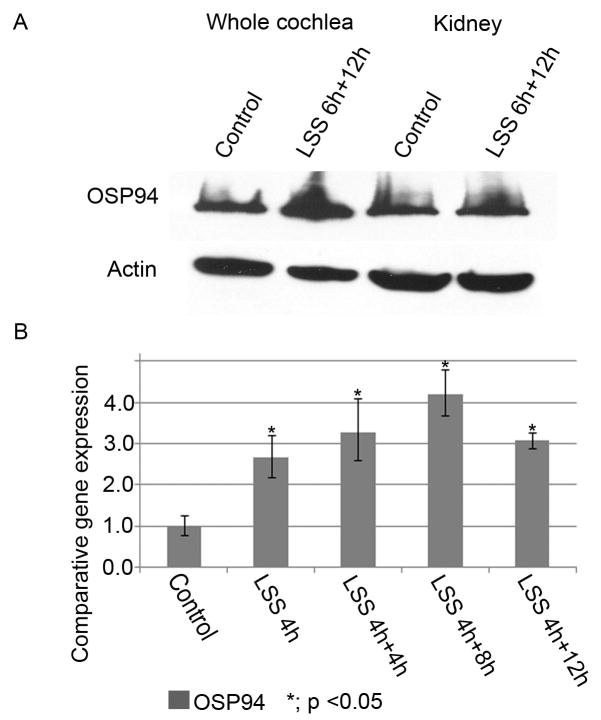
Additionally, to follow the change of OSP94 expression at the transcription and protein level after LSS, a time-course study was performed after either four hours or six hours noise-exposure (LSS 4h, LSS 6h), both of which significantly upregulate the OSP94 gene in the inner ear (Fig. 3B). At the protein level, the overexpression of OSP94 in the whole cochlea was observed at 12h after LSS 6h (LSS 6h+12h), while lacking any change in the kidney (Fig. 4A). At the transcription level, OSP94 expression was gradually increased until 8h after LSS 4h (LSS 4h+8h) and slowly declined at 12h (LSS 4h+12h) (Fig. 4B, Table 4). These results revealed that unlike the other HSPs, the upregulation of OSP94 in the CLW is elicited in a LSS time-dependent manner and continues for more than 12h after LSS.
Fig. 4.
The change of OSP94 in time-course after LSS. Panel A shows the change of OSP94 expression at the protein level by immunoblot analysis. In the whole cochlea, the enhancement of OSP94 protein was observed at 12h after LSS 6h (LSS 6h+12h) in comparison with the control. In contrast, there was no obvious difference in the kidney between the control and the LSS 6h+12 h. Panel B shows the time-course study of OSP94 change in the CLW after acoustic overstimulation (LSS 4h: 4hours noise-exposure at 117dB/SPL/broad band). At the transcription level, the upregulation of the OSP94 gene was until 12h after LSS 4h (p < 0.05).
Table 4.
Time-course study of OSP94 change in CLW after LSS 4h
| CT OSP94 | CT GAPDH | ΔCT (OSP94CT-GAPDHCT) | ΔΔCT (Ave.ΔCT -Ave.ΔCT CLW control) | 2−ΔΔCT Normalized OSP94 amount relative to CLW control | |
|---|---|---|---|---|---|
| CLW control | 23.86 | 18.22 | 5.64 | ||
| 23.07 | 17.97 | 5.10 | |||
| 22.97 | 17.89 | 5.08 | |||
| Ave. | 23.30 | 18.03 | 5.27 ± 0.32 | 0.00 ± 0.32 | 1.00 (0.80 – 1.25) |
| CLW LSS 4h | 23.31 | 19.16 | 4.15 | ||
| 23.55 | 19.94 | 3.61 | |||
| 23.42 | 19.60 | 3.82 | |||
| Ave. | 23.43 | 19.57 | 3.86 ± 0.27 | −1.41 ± 0.27 | *2.66 (2.20 – 3.20) |
| CLW LSS 4h+4h | 23.78 | 20.02 | 3.76 | ||
| 23.77 | 20.60 | 3.18 | |||
| 23.83 | 20.10 | 3.73 | |||
| Ave. | 23.79 | 20.24 | 3.56 ± 0.33 | −1.71 ± 0.33 | *3.27 (2.60 – 4.11) |
| CLW LSS 4h+8h | 22.81 | 19.48 | 3.33 | ||
| 23.03 | 19.75 | 3.28 | |||
| 22.93 | 19.95 | 2.98 | |||
| Ave. | 22.92 | 19.73 | 3.20 ± 0.19 | −2.07 ± 0.19 | *4.20 (3.68 – 4.79) |
| CLW LSS 4h+12h | 23.78 | 20.06 | 3.72 | ||
| 23.68 | 20.14 | 3.54 | |||
| 23.73 | 20.05 | 3.68 | |||
| Ave. | 23.73 | 20.08 | 3.65 ± 0.09 | −1.62 ± 0.09 | *3.07 (2.89 – 3.27) |
CLW: Cochlear lateral wall, LSS: Loud sound stress, CT: Threshold cycle, Ave: Average (± S.D.)
: p < 0.05
DISCUSSION
In the present study, we have shown that OSP94 is widely expressed in the murine inner ear with no difference in its expression between genders. The transcription and protein level of OSP94 expression in the whole cochlea is quantitatively comparable with that of the kidney, and less than that of the testis. It is noteworthy that the distribution of OSP94 in the inner ear overlapped with active ion transport regions such as the organ of Corti, the stria vascularis (SV), the spiral ligament (SL), and interdental cells. Moreover, our experimental data indicated that loud sound stress (LSS) induced the organ-specific enhancement of OSP94 in the inner ear at the transcription and protein level. In particular, the upregulation of OSP94 in the cochlear lateral wall tissue (CLW) was gradually initiated, but time-dependently advanced by LSS in comparison with the response of HSP25 and HSP70, both of which are considered to play a cytoprotective role under stressful conditions (Yoshida et al., 1999; Bruey et al., 2000; Ravagnan et al., 2001). Interestingly, the change of OSP94 expression is in accord with the study in the kidney, where the transcription of the OSP94 gene was slightly increased at 3h and continuously elevated until 24h in response to hyperosmotic NaCl (Kojima et al., 1996).
In the inner ear, the CLW composed of the SV and the SL is constantly exposed to the two ionic different environments and permits an intracellular flux of ions in order to maintain a potassium recycling system and endocochlear potential (EP). Past studies revealed that although there was no significant difference in the osmolality between endolymph and perilymph, the osmolality in the inner ear was altered according to the change of plasma osmotic pressure (Bosher and Warren, 1971), and the application of high concentration of NaCl on the round window membrane induced immediate reduction of EP (Hisashi et al., 1999). Additionally, LSS is known to cause the ionic imbalance between endolymph and perilymph (Konishi et al., 1979), disturb the vascular microcirculation system in the CLW (Thorne and Nuttall, 1987; Nakashima et al., 2003), and eventually lead to hearing dysfunction (Hirose and Liberman, 2003). This supports the view that the CLW requires a mechanism to maintain the ionic and osmotic homoeostasis as well as potassium recycling system in the inner ear. OSP94 presumably plays a role in adapting the cells involved in potassium recycling system to the ionic and osmotic stress and would have a response to its imbalance caused by LSS.
On the other hand, a gene targeting study provided the evidence that the phenotype of OSP94 deficient mice have about 40 percent male infertility by apoptosis of developing germ cells and a minor degree of hydronephrosis with no functional impairment (Held et al., 2006). In an osmotic pressure loading experiment, however, it was found that renal function was preferentially susceptible to osmotic stress (Held et al., 2006). This evidence leads us to speculate that depending on the expression level of OSP94 in organs, the deficiency of OSP94 has various influences on normal function in the testis and for osmotic tolerance in the kidney. The intolerance of osmotic stress on renal function suggests that OSP94 mutant animals would have poor tolerance to LSS, although they apparently have normal hearing function (Held et al., 2006).
In our study, the utilization of the CLW enabled us to obtain more accurate information about the relationship between OSP94 expression and ionic and osmotic imbalance by LSS than that of whole cochleas. The transcription study by RT-PCR and quantitative real-time PCR analysis was accomplished on a small sample volume reducing the number of experimental animals needed for this work. However, the possibility that the other factors rather than ionic and osmotic stress would be related to the expression of OSP94 in spiral ganglion cells cannot be ruled out and the molecular mechanism of OSP94 function in the inner ear still remains to be elucidated by further detailed studies in the future.
In conclusion, this study provides new information that OSP94 is widely expressed in the murine inner ear and induced in response to loud sound stress, suggesting that OSP94 would play a potential role related to cellular adaptation to the specific ionic and osmotic environment in the inner ear.
Acknowledgments
This study is supported by NIH NIDCD (DC00105)
Abbreviations
- APG-1
ATP and peptide-binding protein in germ cells
- GAPDH
Glyceraldehyde-3-phoshpate dehydrogenase
- HSPs
heat shock proteins
- HSPA4L
heat shock 70kDa protein 4-like
- LSS
loud sound stress
- SG
spiral ganglion cells
- SL
spiral ligament
- SV
stria vascularis
- OC
organ of Corti
- OSP94
osmotic stress protein 94
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burg MB. Coordinate regulation of organic osmolytes in renal cells. Kidney Int. 1996;49:1684–1685. doi: 10.1038/ki.1996.247. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–52. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Cohen DM, Gullans SR. Urea selectively induces DNA synthesis in renal epithelial cells. Am J Physiol. 1993;264:601–607. doi: 10.1152/ajprenal.1993.264.4.F601. [DOI] [PubMed] [Google Scholar]
- Fathallah DM, Cherif D, Dellagi K, Arnaout MA. Molecular cloning of a novel human hsp70 from a B cell line and its assignment to chromosome 5. J Immunol. 1993;151:810–813. [PubMed] [Google Scholar]
- Held T, Paprotta I, Khulan J, Hemmerlein B, Binder L, Wolf S, Schubert S, Meinhardt A, Engel W, Adham IM. Hspa4l-deficient mice display increased incidence of male infertility and hydronephrosis development. Mol Cell Biol. 2006;26:8099–8108. doi: 10.1128/MCB.01332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisashi K, Komune S, Nakagawa T, Kimitsuki T, Komiyama S. Regulation of inner ear fluid in the guinea pig cochlea after the application of saturated NaCl solution to the round window membrane. Eur Arch Otorhinolaryngol. 1999;256(Suppl 1):S2–5. doi: 10.1007/pl00014147. [DOI] [PubMed] [Google Scholar]
- Konishi T, Salt AN, Hamrick PE. Effects of exposure to noise on ion movement in guinea pig cochlea. Hear Res. 1979;1:325–342. doi: 10.1016/0378-5955(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Nishiyama H, Nonoguchi K, Higashitsuji H, Kishishita M, Fujita J. A novel hsp110-related gene, apg-1, that is abundantly expressed in the testis responds to a low temperature heat shock rather than the traditional elevated temperatures. J Biol Chem. 1997;272:2640–2645. doi: 10.1074/jbc.272.5.2640. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Kimura T, Nishiyama H, Noda Y, Fujita J. Developmentally regulated expression of APG-1, a member of heat shock protein 110 family in murine male germ cells. Biochem Biophys Res Commun. 1997;233:113–116. doi: 10.1006/bbrc.1997.6410. [DOI] [PubMed] [Google Scholar]
- Kojima R, Randall J, Brenner BM, Gullans SR. Osmotic stress protein 94 (Osp94). A new member of the HSP110/SSE gene subfamily. J Biol Chem. 1996;271:12327–12332. doi: 10.1074/jbc.271.21.12327. [DOI] [PubMed] [Google Scholar]
- Bosher SK, Warren RL. A study of the electrochemistry and osmotic relationships of the cochlear fluids in the neonatal rat at the time of the development of the endocochlear potential. J Physiol. 1971;212:739–761. doi: 10.1113/jphysiol.1971.sp009354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima R, Randall JD, Ito E, Manshio H, Suzuki Y, Gullans SR. Regulation of expression of the stress response gene, Osp94: identification of the tonicity response element and intracellular signalling pathways. Biochem J. 2004;380:783–794. doi: 10.1042/BJ20040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Yoon D, Easton D, Murawski M, Burd R, Subjeck JR. Identification of a major subfamily of large hsp70-like proteins through the cloning of the mammalian 110-kDa heat shock protein. J Biol Chem. 1995;270:15725–15733. doi: 10.1074/jbc.270.26.15725. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Naganawa S, Sone M, Tominaga M, Hayashi H, Yamamoto H, Liu X, Nuttall AL. Disorders of cochlear blood flow. Brain Res Rev. 2003;43:17–28. doi: 10.1016/s0165-0173(03)00189-9. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Furukawa T, Yano T, Sato N, Takizawa J, Kurasaki T, Abe T, Narita M, Masuko M, Koyama S, Toba K, Takahashi M, Aizawa Y. Identification of an overexpressed gene, HSPA4L, the product of which can provoke prevalent humoral immune responses in leukemia patients. Exp Hematol. 2007;35:1091–1099. doi: 10.1016/j.exphem.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–43. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Thorne PR, Nuttall AL. Laser Doppler measurements of cochlear blood flow during loud sound exposure in the guinea pig. Hear Res. 1987;27:1–10. doi: 10.1016/0378-5955(87)90021-9. [DOI] [PubMed] [Google Scholar]
- Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- Xue JH, Fukuyama H, Nonoguchi K, Kaneko Y, Kido T, Fukumoto M, Fujibayashi Y, Itoh K, Fujita J. Induction of Apg-1, a member of the heat shock protein 110 family, following transient forebrain ischemia in the rat brain. Biochem Biophys Res Commun. 1998;247:796–801. doi: 10.1006/bbrc.1998.8894. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Kristiansen A, Liberman MC. Heat stress and protection from permanent acoustic injury in mice. J Neurosci. 1999;19:10116–24. doi: 10.1523/JNEUROSCI.19-22-10116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablocki K, Miller SP, Garcia-Perez A, Burg MB. Accumulation of glycerophosphocholine (GPC) by renal cells: osmotic regulation of GPC:choline phosphodiesterase. Proc Natl Acad Sci U S A. 1991;88:7820–7824. doi: 10.1073/pnas.88.17.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]